Abstract
This study aims to determine the risk factors for epileptogenesis and characteristics of seizures in patients with progressive multifocal leukoencephalopathy (PML) who survive more than 1 year from onset of neurological symptoms (PML survivors). We reviewed clinical data including seizure history and MR imaging studies from PML survivors evaluated at our institution between 1997 and 2014. PML progressors who passed away within 1 year and patients with a history of seizures prior to PML diagnosis were excluded from the analysis. Of 64 PML survivors, 28 (44 %) developed seizures. The median time from the onset of PML symptoms to the first seizure was 5.4 months (range 0–159) and 64 % of patients with seizures had them within the first year. The presence of juxtacortical PML lesions was associated with a relative risk of seizures of 3.5 (p<0.02; 95 % confidence interval (CI) 1.3– 9.4) in multivariate analyses. Of all seizure types, 86 % were focal and 60 % most likely originated from the frontal lobes. Among seizure patients, 89 % required treatment, including one (54 %), two (25 %), or three (10.5 %) antiepileptic drugs. Seizures are a frequent complication in PML and can develop throughout the entire course of the disease. However, late onset seizures did not signify PML relapse. Seizures may require treatment with multiple antiepileptic medications and are a significant co-morbidity in PML.
Keywords: Seizures, Epilepsy, Progressive multifocal leukoencephalopathy, JC virus, Survivors
Introduction
Progressive multifocal leukoencephalopathy (PML) is an often fatal demyelinating disease of the brain which occurs in immunosuppressed individuals and is caused by the polyoma virus JC (JCV). JCV causes a lytic infection of oligodendrocytes and astrocytes, and PML is characterized histologically by confluent demyelinating lesions commonly located in the subcortical white matter. While seizures are considered to be a manifestation of cortical dysfunction, and are not expected in a leukoencephalopathy, we and others have described cortical involvement of PML and extension of the lesions into the adjacent gray matter. In addition, we have shown that JCV can also infect cortical pyramidal neurons in PML cases (Moll et al. 2008; Wuthrich and Koralnik 2012).
In an earlier study, we found that seizures occurred in 18 % of PML patients seen at our institution from 1995 to 2005 (Lima et al. 2006). However, PML mortality was high, and 69 % of those patients were PML progressors, who died within 1 year from disease onset. Therefore, there is only limited knowledge on the long-term incidence of seizures in patients who survive PML (Lima et al. 2006). We have recently reported that hyperintense cortical signal (HCS) seen on pre-contrast T1-weighted images on MRI were associated with seizures in PML (Khoury et al. 2014). In addition, immune reconstitution inflammatory syndrome (IRIS), which can manifest itself by contrast enhancement of PML lesions on MRI, is also suspected to be a risk factor for seizures in some cases (Clifford et al. 2010; Gheuens et al. 2012b). We therefore undertook this study, focused only on survivors of PML, where we sought to determine the incidence and characteristics of seizures as well as identify predisposing factors for epileptogenesis.
Methods
Study population
We performed a retrospective analysis of patients with PML enrolled consecutively in clinical studies at Beth Israel Deaconess Medical Center between October 1997 and May 2014. Patients surviving beyond 1 year of PML onset were considered PML survivors. Sixty-four PML survivors were included in the analysis. PML progressors and patients with a history of seizures prior to PML diagnosis were excluded from the analysis.
Clinical data
PML diagnosis was established according to consensus terminology criteria (Berger et al. 2013). Possible PML was defined by the presence of clinical and imaging findings consistent with PML, but with negative or unknown CSF JCV PCR and no histologic confirmation. Definite PML was confirmed by the detection of JCV DNA in the CSF by PCR or by histologic diagnosis. We included cases of possible PML since we have shown that they have the same clinical, immunological, and radiologic evolution as patients with definite PML (Gheuens et al. 2011; Gheuens et al. 2012a).
MRI
We reviewed the T1, fluid-attenuated inversion recovery (FLAIR), and T1 post-contrast sequences of the 64 patients. The imaging studies were obtained at various facilities using different MRI protocols. Lesions were classified as being either immediately adjacent to the hemispheric cortex (juxtacortical) or deep within the white matter (subcortical). The presence or absence of HCS adjacent to PML lesions was determined using comparison with normal-appearing cortical gray matter in the same T1-weighted image. The presence of contrast enhancement of PML lesions was determined on T1 post-contrast images.
Seizure and epilepsy classification and EEG
Patients were classified as having epilepsy if they had two unprovoked seizures or one unprovoked seizure and a probability of further seizures of at least 60 % over the next 10 years (Fisher et al. 2014). Seizures and epilepsy were classified according to the revised ILAE system (Berg et al. 2010). Acute symptomatic seizure was defined by a clinical seizure occurring within 2 weeks of PML symptom onset (Beghi et al. 2010). A patient was classified as having focal epilepsy if they went on to have additional unprovoked seizures. We reviewed EEGs for focal slowing, epileptiform activity, and seizures, and determined if EEG findings were concordant with MRI lesions.
Statistical analysis
We recorded relevant demographic and clinical variables as well as radiologic and electrodiagnostic findings. We first performed descriptive analyses to assess the distributions of the binary outcome variable seizure and potential predictors. We reported median and range for continuous variables, and percent for categorical variables. We used non-parametric median test to compare median estimates, and chi-square and Fisher’s exact test for contingency analysis. We used multivariate generalized linear models with log link and binary error to obtain independent predictors of seizure. We reported unadjusted and adjusted relative risk estimates and 95 % confidence intervals which were obtained from the models. We set type 1 error rate at 0.05. All analyses were performed using the SAS/STAT software version 9.13.
Results
Characteristics of PML survivors with and without seizures
The median follow-up of PML survivors at our institution was 8.6 years (range 1.1–22.7 years). The clinical characteristics of all 64 PML survivors are listed in Table 1. Of all PML survivors, 28/64 (44 %) had seizures. There were no significant differences between patients with and without seizures for median age at PML onset (41.5 vs 44.5 %), gender (86 vs 78 % male), methods of PML diagnosis (75 vs 64 % virologic/histologic), etiology of immunosuppression (75 vs 64 % HIV) or exposure to experimental treatments for PML. The median time from the onset of PML symptoms to seizures was 5.4 months (range 0–159). Of those with seizures, the percent-age of patients who developed seizures immediately at onset of PML symptoms, and within 4 months and 1 and 10 years was 25, 50, 64, and 96 %, respectively (Fig. 1). Late development of seizures (>1 year from PML onset) was never associated with a relapse of PML.
Table 1.
Demographics and clinical characteristics of PML survivors with and without seizures
| Seizures | No seizures | Univariate analysisi | Multivariate analysisj |
||
|---|---|---|---|---|---|
| N=28 | N=36 | P value | Relative risk (95 % confidence interval) |
P value | |
| Median age at PML onset, year (range) | 41.5 (23–84) | 44.5 (20–84) | 0.13 | ||
| Gender, N (%) | |||||
| Male | 24 (86 %) | 28 (78 %) | 0.52 | ||
| Female | 4 (14 %) | 8 (22 %) | |||
| Diagnosis, N (%) | |||||
| Clinical-radiologic | 7 (25 %) | 13 (36 %) | 0.55 | ||
| Virologic | 14 (50 %) | 17 (47 %) | |||
| Histologic | 7 (25 %) | 6 (17 %) | |||
| Etiology of immunosuppression, N (%) | |||||
| HIV | 21 (75 %) | 23 (64 %) | 0.34 | ||
| Hematologic malignancy | 5 (18 %)a | 8 (22 %)b | |||
| Other | 2 (7 %)c | 5 (14 %)d | |||
| Treatment for PML, N (%) | |||||
| 5HT2a blockers | 16 (57 %)e | 21 (58 %)f | 0.73 | ||
| Anti-virals | 5 (18 %)g | 4 (11 %)h | |||
| None | 7 (25 %) | 11 (31 %) | |||
| Lesion location, N (%) | |||||
| Juxtacortical | 22 (79 %) | 11 (31 %) | <0.001 | 3.5 | 0.02 |
| Subcortical only | 4 (14 %) | 20 (56 %) | (1.3–9.4) | ||
| Insufficient imaging data available | 2 (7 %) | 5 (13 %) | |||
| Hyperintense cortical signal, N (%) | |||||
| Present | 14 (50 %) | 7 (19.5 %) | 0.02 | 1.0 | 0.98 |
| Absent | 12 (43 %) | 22 (61 %) | (0.58–1.76) | ||
| Insufficient imaging data available | 2 (7 %) | 7 (19.5 %) | |||
| Enhancement, N (%) | |||||
| Present | 15 (54 %) | 14 (39 %) | 0.20 | 1.07 | 0.81 |
| Absent | 9 (32 %) | 17 (47 %) | (0.61–1.87) | ||
| Insufficient imaging data available | 4 (14 %) | 5 (14 %) | |||
Chronic lymphocytic leukemia (n = 2), idiopathic lymphocytopenia (n = 2), Hodgkin’s lymphoma (n = 1)
Chronic lymphocytic leukemia (n = 2), idiopathic lymphocytopenia (n = 2), non-Hodgkin’s lymphoma (n = 2), follicular cell lymphoma (n = 2)
Multiple sclerosis on natalizumab (n = 1), combined variable immunodeficiency (n = 1)
Dermatomyositis (n = 1), rheumatoid arthritis (n = 1), chemotherapy for brainstem glioma (n= 1), Waldenstrom’s macroglobulinemia (n = 1), alcoholic cirrhosis (n = 1)
Mirtazapine (n =11), mirtazapine + mefloquine (n = 3), mirtazapine + cytarabine (n = 2)
Mirtazapine (n = 16), mirtazapine + mefloquine (n = 3), mefloquine (n = 1), Mefloquine + IL-2 (n = 1)
Cidofovir (n = 4), cidofovir + interferon alpha (n = 1)
Cidofovir (n = 3), cytarabine + interferon alpha (n = 1)
Non-parametric median test was used for comparing age distributions, while chi-square and Fisher’s exact test were used for categorical variables with type 1 error set at 0.05
Relative risks were estimated from multivariate generalized linear model with log link and binary error term
Fig. 1.
Time from PML symptom onset to seizure onset. The time of first seizure onset of each of the 28 PML survivors with seizures is marked on the x-axis, which is displayed in months
Association of lesion location, HCS, and contrast enhancement with seizures
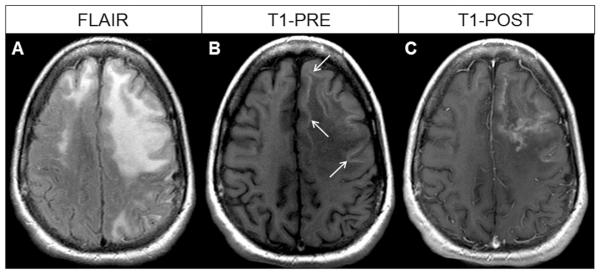
Juxtacortical lesions were present in 22/28 (79 %) of patients with seizures compared to 11/36 (31 %) of those without seizures (p <0.001) and conferred a relative risk of seizures of 4.0 (p < 0.003). In addition, HCS was present in 14/28 (50 %) of patients with seizures compared to 7/36 (19.5 %) of those without seizures (p = 0.02) and conferred a relative risk of seizures of 1.9 (p < 0.02). However, contrast enhancement, present in 15/28 (54 %) of patients with seizures was not significantly different compared to 14/36 (39 %) of those without seizures (p = 0.20). Brain MRI of a patient with juxtacortical lesions, HCS, and contrast enhancement is depicted in Fig. 2. We then performed multivariate analyses to determine the respective contributions of lesion location, HCS, and contrast enhancement in the development of seizures. In this predictive model, the relative risk of seizures in patients with juxtacortical lesions remained statistically significant at 3.5 (p < 0.02; 95 % confidence interval (CI) 1.3–9.4), whereas HCS and contrast enhancement were no longer contributory (relative risk (RR) = 1.0, p<0.98 and RR=1.1, p <0.81), respectively.
Fig. 2.
MRI of the brain in a PML survivor with seizures. a Axial fluid-attenuated inversion recovery (FLAIR) sequence demonstrating left greater than right frontal juxtacortical white matter hyperintense PML lesions. b Axial T1 pre-contrast image demonstrating hyperintense cortical signal (HCS, arrows). c Axial T1-post-contrast image demonstrating diffuse enhancement of the left frontal PML lesion
Seizure characteristics in PML survivors
Seizure-related classifications are detailed in Table 2. Of all PML survivors with seizures, 23 (82 %) had no other seizure risk factors than PML. Twenty-two (78.5 %) had focal onset, including 16 (57 %) with focal epilepsy and 6 (21.5 %) with both acute symptomatic seizures and focal epilepsy. Of eight patients with acute symptomatic seizures, six (75 %) developed epilepsy. Focal epilepsy constituted the great majority of all seizure types (31/36, 86 %) and consisted of focal motor seizures (14 patients), focal seizure with impairment of consciousness (4 patients), focal seizure without impairment of consciousness (1 patient), focal seizure evolving to a bilateral convulsive seizure (9 patients), and epilepsy partialis continua (3 patients). Only four patients had generalized seizures (unclear if focal or generalized onset), and one patient had an unclassifiable seizure type. Based on the seizure semiology of predominantly motor manifestations, 60 % of seizures were felt to originate in the frontal lobes.
Table 2.
Seizures characteristics in PML survivors
| PML survivors with seizures (N=28) | |
|---|---|
| Median age at seizure onset, year (range) | 43 (23–85) |
| Median time from PML onset to seizure onset, m (range) | 5.4 (0–159) |
| Epilepsy classification, N (%) | |
| Focal epilepsy | 16 (57 %) |
| Acute symptomatic seizures and focal epilepsy | 6 (21.5 %) |
| Epilepsy (unclassifiable) | 4 (14.5 %) |
| Acute symptomatic seizures | 2 (7 %) |
| New seizure classification, N | |
| Focal motor seizure | 14 |
| Focal seizure evolving to a bilateral convulsive seizure | 9 |
| Focal seizure with impairment of consciousness | 4 |
| Generalized seizure (unclear if focal or generalized) | 4 |
| Epilepsy partialis continua | 3 |
| Focal seizure without impairment of consciousness | 1 |
| Unclassifiable | 1 |
| Risk factor for seizures, N (%) | |
| PML | 23 (82 %) |
| Othera | 5 (18 %) |
| Localization of seizures, N (%) | |
| Left frontal | 13 (46 %) |
| Right frontal | 4 (14 %) |
| Left parietal | 1 (4 %) |
| Right parietal | 2 (7 %) |
| Unknown | 8 (29 %) |
| EEG concordant or diagnostic, N (%) | |
| Yesb | 9 (32 %) |
| No | 8 (29 %) |
| No EEG data available | 11 (39 %) |
| Treatment of Seizures with anti-epileptic drugsc, N (%) | |
| 1 AED | 15 (54 %) |
| 2 AED | 7 (25 %) |
| 3 AED | 3 (10.5 %) |
| No AED | 3 (10.5 %) |
Alcohol intoxication or withdrawal (n = 1), dehydration (n = 1), brain biopsy (n = 2), medication side-effect (n = 1)
Focal slowing (n = 4), focal epileptiform/localizing (i = 3), focal slowing, epileptiform/localizing (n = 1), focal seizures (n = 1)
Levetiracetam (n = 21), gabapentin (n = 11), phenytoin (n = 9), clonazepam (n = 4), topiramate (n = 3), valproic acid (n = 2), oxcarbazepine (n = 2), carbamazepine (n = 1), phenobarbital (n = 1), lacosamide (n = 1)
Electrodiagnostic findings
EEG data was available in 17/28 patients with seizures. Most had only a single EEG study available for review. Nine (53 %) of the EEGs demonstrated focal slowing, focal epileptiform discharges, or focal seizures concordant with neuroimaging findings. EEG was normal or showed nonspecific abnormalities in eight (47 %) of the available studies.
Treatment of seizures
Of 28 PML patients who developed seizures, 25 (89 %) were treated with antiepileptic drugs (AED). Seizure control was established with monotherapy in 15 (54 %), but 7 (25 %) needed 2, and 3 (10.5 %) needed 3 medications to achieve seizure control. The most commonly prescribed AEDs in our study included levetiracetam (75 %) and gabapentin (39 %).
Discussion
The population at risk for PML is continuously expanding. In addition to patients with AIDS, hematologic malignancies, and transplant recipients, PML has been reported in a growing number of individuals with autoimmune diseases treated with immunomodulatory medications such as natalizumab (Clifford et al. 2010; Dahlhaus et al. 2013; Gheuens et al. 2012b; Hoepner et al. 2014; Marousi et al. 2012; Tan et al. 2011), dimethyl fumarate (Bartsch et al. 2015; Dammeier et al. 2015; Khatri et al. 2015), or efalizumab (Carson et al. 2009; Gadzia and Turner 2010; Korman et al. 2009; Kothary et al. 2011). Since seizures frequently occurred at or soon after PML onset, PML should be included in the differential diagnosis of new onset seizures in the aforementioned high-risk patients. This study provides novel information compared to previous reports. The 44 % incidence of seizures in PML survivors is higher than 18 % reported in our initial study, which included a majority of PML progressors who passed away within 1 year of disease onset (Lima et al. 2006). The difference can be explained in part by the fact that more than one third of PML patients develop seizures later than 12 months from PML onset. In addition, advances in treatment of HIV infection with antiretroviral medications occurring over the past 10 years may have improved PML survival in HIV-infected patients, thereby increasing the population of PML survivors (Casado et al. 2014).
The overwhelming majority of seizures was focal, consistent with the location of PML lesions, and most had a motor component with suspected origin in the frontal lobes. AEDs were required in most cases, and one third of patients needed two or three medications to achieve seizure control. Therefore, seizures significantly contributed to the morbidity of PML among survivors. Choice of AED was often dictated by HIV status, as many AEDs, such as carbamazepine, phenobarbital, and phenytoin, induce the cytochrome P450 enzyme system and decrease the efficacy of certain antiretroviral drugs (ARVs). Conversely, certain ARVs, such as lopinavir, ritonavir, efavirenz, nevirapine, and maraviroc, may reduce serum levels of AEDs (Okulicz et al. 2011). Lacosamide, gabapentin, and pregabalin are recommended AEDs in patients with focal epilepsy and/or those failing levetiracetam (Siddiqi and Birbeck 2013).
The main risk factors associated with seizures in univariate analyses were the location of lesions in juxtacortical areas, which confirms our previous observation (Lima et al. 2006), and the presence of HCS (Khoury et al. 2014). HCS, found in half of PML seizure patients on pre-contrast T1 MRI images, corresponds histologically to JCV-induced cortical demyelination, cortical astrogliosis and infiltration by phagocytic macrophages clearing up the myelin debris. This triad recapitulates the histological hallmark of PML, albeit in the hemispheric cortex rather than in the white matter itself. We have named this entity focal leukocortical encephalitis (Khoury et al. 2014). However, multivariate analyses performed to delineate the relative contributions of seizure risk factors indicated that the juxtacortical location of the lesions was the predominant element, while HCS was not an added contributor. This is understandable since HCS usually occurs in cortical areas located over underlying white matter lesions of PML.
There was only a trend for contrast enhancement in seizure vs non-seizure patients (54 vs 39 %). Contrast enhancement is one of the manifestations of the immune reconstitution inflammatory syndrome (IRIS), which occurs during immune recovery (Collazos et al. 1999; Du Pasquier and Koralnik 2003; Gray et al. 2005; Hoffmann et al. 2003; Kotecha et al. 1998; Martinez et al. 2006; Miralles et al. 2001; Nuttall et al. 2004; Silva et al. 2006) and has been associated with seizures (Dahlhaus et al. 2013; Tan et al. 2009). However, contrast enhancement is only present in 56 % of PML/IRIS cases (Gheuens et al. 2012a). Therefore, contrast enhancement will underestimate occurrence of IRIS, which is otherwise defined by immunological, virological, and clinical markers (Lima and Koralnik 2005; Tan et al. 2011). Those data were not available in all patients in this retrospective analysis. A prospective study is currently in progress at our institution, to better define the role of IRIS in epileptogenesis.
Our study has limitations. Due to the retrospective design, most of the MRI and EEG data available for review were performed only as dictated by the clinical setting, and may have missed transient phenomena such as HCS, contrast enhancement, or epileptiform discharges. Prospective studies including serial EEGs and neuroimaging may provide additional information on the time course of epileptiform abnormalities in relationship to seizure occurrence. In addition, the PML population is heterogeneous, with both HIV+ and HIV− individuals, including only one natalizumab-treated MS patient. It is possible that subgroups of PML may be at a higher risk of seizures. However, there was no difference in the seizure frequency between PML survivors with or without seizures irrespective of HIV status. In particular, natalizumab-treated MS patients who develop PML are often treated with plasmapheresis, which hastens normalization of lymphocyte migration, but can also promote IRIS and thereby trigger seizures.
Our findings have important clinical implications. Clinicians should be aware that seizures are a frequent occurrence in PML. They may develop throughout the entire course of the disease, including years after symptom onset, when PML is essentially burnt out, with no molecular, clinical, or radiologic evidence of ongoing JCV replication. Hence, late onset seizures should not be interpreted as a sign of disease relapse, a very rare occurrence in PML. When available, EEG data was concordant with the imaging findings in more than half of seizure patients.
This study also provides insight into possible mechanisms of epileptogenesis in PML. Juxtacortical lesions often involve cortical areas microscopically, and could serve as excitatory foci. In addition, undercutting or deafferentation of the cortex by white matter lesions may lead to seizures, as has been well demonstrated in animal models (Nita et al. 2006; Topolnik et al. 2003; Xiong et al. 2011). Finally, we have shown that in addition to glial cells, JCV can also infect cortical pyramidal neurons, either within areas of cortical demyelination in up to half of PML cases, or in isolation in 11 % of them (Wuthrich and Koralnik 2012). Most neurons express the JCV regulatory T Ag, rather than the viral capsid protein VP1, consistent with early or restrictive infection. Indeed, JCV infection of neurons was recently documented in the brain biopsy sample of a psoriasis patient who developed a juxtacortical right frontal PML lesion after treatment of dimethyl fumarate (Bartsch et al. 2015). This lesion was responsible for left-sided focal motor seizures, serving as further evidence that a non-lytic JCV infection of neurons may be a trigger for seizures.
Acknowledgments
Funding This study was supported by NIH grants R01NS 047029 and 074995.
Dr. Herman is funded by NIH grants R01 047029 and 074409; received research grants from UCB Pharma, Sage Pharmaceuticals, Acorda Therapeutics, and the Epilepsy Therapy Development Project; received consulting fees from Eisai, Inc. and Biotie, Inc. Dr. Ngo is funded by NIH grant R01 047029. Dr. Koralnik is funded by NIH grants R01 047029 and 074995; has received a research grant from Biogen Idec and the National Multiple Sclerosis Society; served on scientific advisory boards for Hoffmann La Roche, GlaxoSmithKline, Merck Serono, and MedImmune; received consulting fees from Bristol Myers Squibb, Ono Pharmaceuticals, Merck Serono, Hoffmann La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceuticals, and Johnson & Johnson; is an Associate Editor for the Annals of Neurology; and receives royalties from UpToDate for topics on the management of HIV and CNS mass lesions and on PML.
Footnotes
Authors’ contributions Dr. Miskin was involved in the conceptualization or design of the study, acquisition, analysis, and interpretation of the data and drafting of the manuscript. Dr. Herman was involved in the conceptualization or design of the study, analysis, and interpretation of the data and drafting and critical revision of the manuscript. Dr. Ngo was involved in the analysis and interpretation of the data, critical revision of the manuscript, and statistical analysis. Dr. Koralnik was involved in the conceptualization of the study, analysis and interpretation of the data, critical revision of the manuscript, obtaining funding, and supervision.
Compliance with ethical standards
Conflict of interest Dr. Miskin reports no disclosures or conflicts of interest.
Contributor Information
Dhanashri P. Miskin, Email: dmiskin@bidmc.harvard.edu.
Susan T. Herman, Email: sherman2@bidmc.harvard.edu.
Long H. Ngo, Email: lngo@bidmc.harvard.edu.
Igor J. Koralnik, Email: ikoralni@bidmc.harvard.edu.
References
- Bartsch T, Rempe T, Wrede A, Leypoldt F, Adams O, Rohr A, Jansen O, Bruck W, Wuthrich C, Deuschl G, Koralnik IJ. Progressive hemiparesis in a psoriasis patient treated with dimethyl fumarate. Ann Neurol. 2015;78:501–14. doi: 10.1002/ana.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, Tomson T, Hauser WA. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–675. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, Bartt R, Major EO, Nath A. PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, Bennett CL. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10:816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- Casado JL, Corral I, Garcia J, Martinez-San Millan J, Navas E, Moreno A, Moreno S. Continued declining incidence and improved survival of progressive multifocal leukoencephalopathy in HIV/AIDS patients in the current era. Eur J Clin Microbiol Infect Dis. 2014;33:179–187. doi: 10.1007/s10096-013-1941-6. [DOI] [PubMed] [Google Scholar]
- Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- Collazos J, Mayo J, Martinez E, Blanco MS. Contrast-enhancing progressive multifocal leukoencephalopathy as an immune reconstitution event in AIDS patients. Aids. 1999;13:1426–1428. doi: 10.1097/00002030-199907300-00032. [DOI] [PubMed] [Google Scholar]
- Dahlhaus S, Hoepner R, Chan A, Kleiter I, Adams O, Lukas C, Hellwig K, Gold R. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013;84:1068–1074. doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammeier N, Schubert V, Hauser TK, Bornemann A, Bischof F. Case report of a patient with progressive multifocal leukoencephalopathy under treatment with dimethyl fumarate. BMC Neurol. 2015;15:108. doi: 10.1186/s12883-015-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Koralnik IJ. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? J Neurovirol. 2003;9(Suppl 1):25–31. doi: 10.1080/13550280390195315. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshe SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Gadzia J, Turner J. Progressive multifocal leukoencephalopathy in two psoriasis patients treated with efalizumab. J Drugs Dermatol. 2010;9:1005–1009. [PubMed] [Google Scholar]
- Gheuens S, Bord E, Kesari S, Simpson DM, Gandhi RT, Clifford DB, Berger JR, Ngo L, Koralnik IJ. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85:7256–7263. doi: 10.1128/JVI.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheuens S, Ngo L, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Metabolic profile of PML lesions in patients with and without IRIS: an observational study. Neurology. 2012a;79:1041–1048. doi: 10.1212/WNL.0b013e318268465b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheuens S, Smith DR, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Simultaneous PML-IRIS after discontinuation of natalizumab in a patient with MS. Neurology. 2012b;78:1390–1393. doi: 10.1212/WNL.0b013e318253d61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Bazille C, Adle-Biassette H, Mikol J, Moulignier A, Scaravilli F. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol. 2005;11(Suppl 31):16–22. doi: 10.1080/13550280500511741. [DOI] [PubMed] [Google Scholar]
- Hoepner R, Ahlbrecht J, Faissner S, Schneider R, Dahlhaus S, Adams O, Raab P, Lukas C, Chan A, Stangel M, Gold R. Clinical and paraclinical findings in natalizumab-associated infratentorial progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2014;85:1177–1178. doi: 10.1136/jnnp-2014-307582. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Horst HA, Albrecht H, Schlote W. Progressive multifocal leucoencephalopathy with unusual inflammatory response during antiretroviral treatment. J Neurol Neurosurg Psychiatry. 2003;74:1142–1144. doi: 10.1136/jnnp.74.8.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri BO, Garland J, Berger J, Kramer J, Sershon L, Olapo T, Sesing J, Dukic M, Rehn E. The effect of dimethyl fumarate (Tecfidera) on lymphocyte counts: a potential contributor to progressive multifocal leukoencephalopathy risk. Mult Scler Relat Disord. 2015;4:377–379. doi: 10.1016/j.msard.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Khoury MN, Alsop DC, Agnihotri SP, Pfannl R, Wuthrich C, Ho ML, Hackney D, Ngo L, Anderson MP, Koralnik IJ. Hyperintense cortical signal on magnetic resonance imaging reflects focal leukocortical encephalitis and seizure risk in progressive multifocal leukoencephalopathy. Ann Neurol. 2014;75:659–669. doi: 10.1002/ana.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937–942. doi: 10.1001/archdermatol.2009.175. [DOI] [PubMed] [Google Scholar]
- Kotecha N, George MJ, Smith TW, Corvi F, Litofsky NS. Enhancing progressive multifocal leukoencephalopathy: an indicator of improved immune status? Am J Med. 1998;105:541–543. doi: 10.1016/s0002-9343(98)00321-0. [DOI] [PubMed] [Google Scholar]
- Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal Pan G. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546–551. doi: 10.1016/j.jaad.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Lima MA, Koralnik IJ. New features of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy and natalizumab. J Neurovirol. 2005;11(Suppl 3):52–57. doi: 10.1080/13550280500513325. [DOI] [PubMed] [Google Scholar]
- Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- Marousi S, Travasarou M, Karageorgiou CE, Gheuens S, Koralnik IJ. Simultaneous PML-IRIS after discontinuation of natalizumab in a patient with MS. Neurology. 2012;79:2160. doi: 10.1212/01.wnl.0000423208.11698.16. author reply 2160. [DOI] [PubMed] [Google Scholar]
- Martinez JV, Mazziotti JV, Efron ED, Bonardo P, Jordan R, Sevlever G, Martinez M, Verbanaz SC, Salazar ZS, Pardal MF, Reisin R. Immune reconstitution inflammatory syndrome associated with PML in AIDS: a treatable disorder. Neurology. 2006;67:1692–1694. doi: 10.1212/01.wnl.0000242728.26433.12. [DOI] [PubMed] [Google Scholar]
- Miralles P, Berenguer J, Lacruz C, Cosin J, Lopez JC, Padilla B, Munoz L, Garcia-de-Viedma D. Inflammatory reactions in progressive multifocal leukoencephalopathy after highly active antiretroviral therapy. Aids. 2001;15:1900–1902. doi: 10.1097/00002030-200109280-00028. [DOI] [PubMed] [Google Scholar]
- Moll NM, Rietsch AM, Ransohoff AJ, Cossoy MB, Huang D, Eichler FS, Trapp BD, Ransohoff RM. Cortical demyelination in PML and MS: similarities and differences. Neurology. 2008;70:336–343. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- Nita DA, Cisse Y, Timofeev I, Steriade M. Increased propensity to seizures after chronic cortical deafferentation in vivo. J Neurophysiol. 2006;95:902–913. [Google Scholar]
- Nuttall JJ, Wilmshurst JM, Ndondo AP, Yeats J, Corcoran C, Hussey GD, Eley BS. Progressive multifocal leukoencephalopathy after initiation of highly active antiretroviral therapy in a child with advanced human immunodeficiency virus infection: a case of immune reconstitution inflammatory syndrome. Pediatr Infect Dis J. 2004;23:683–685. doi: 10.1097/01.inf.0000130954.41818.07. [DOI] [PubMed] [Google Scholar]
- Okulicz JF, Grandits GA, French JA, George JM, Simpson DM, Birbeck GL, Ganesan A, Weintrob AC, Crum-Cianflone N, Lalani T, Landrum ML. Virologic outcomes of HAART with concurrent use of cytochrome P450 enzyme-inducing antiepileptics: a retrospective case control study. AIDS Res Ther. 2011;8:18. doi: 10.1186/1742-6405-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi O, Birbeck GL. Safe treatment of seizures in the setting of HIV/AIDS. Curr Treat Options Neurol. 2013;15:529–543. doi: 10.1007/s11940-013-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT, Pacheco MC, Jr, Vaz B. Inflammatory progressive multifocal leukoencephalopathy after antiretroviral treatment. Aids. 2006;20:469–471. doi: 10.1097/01.aids.0000196175.88421.eb. [DOI] [PubMed] [Google Scholar]
- Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72:1458–1464. doi: 10.1212/01.wnl.0000343510.08643.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77:1061–1067. doi: 10.1212/WNL.0b013e31822e55e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 2003;13:883–893. doi: 10.1093/cercor/13.8.883. [DOI] [PubMed] [Google Scholar]
- Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012;71:54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Ping X, Gao J, Jin X. Preparing undercut model of posttraumatic epileptogenesis in rodents. J Vis Exp. 2011 doi: 10.3791/2840. [DOI] [PMC free article] [PubMed] [Google Scholar]