Abstract
Plants in their natural habitats adapt to drought stress in the environment through a variety of mechanisms, ranging from transient responses to low soil moisture to major survival mechanisms of escape by early flowering in absence of seasonal rainfall. However, crop plants selected by humans to yield products such as grain, vegetable, or fruit in favorable environments with high inputs of water and fertilizer are expected to yield an economic product in response to inputs. Crop plants selected for their economic yield need to survive drought stress through mechanisms that maintain crop yield. Studies on model plants for their survival under stress do not, therefore, always translate to yield of crop plants under stress, and different aspects of drought stress response need to be emphasized. The crop plant model rice ( Oryza sativa) is used here as an example to highlight mechanisms and genes for adaptation of crop plants to drought stress.
Keywords: Adaptation, Drought tolerance, drought resistance, grain yield, rice, photosynthesis
Introduction
Drought stress is the most prevalent environmental factor limiting crop productivity 1, and global climate change is increasing the frequency of severe drought conditions 2. The sheer diversity of plant species grown across climatic regions that include extreme dry conditions suggests that, in nature, plants have evolved to endure drought stress with an array of morphological, physiological, and biochemical adaptations 3. ‘Drought resistance’ (DR) is a broader term applied to plant species with adaptive features that enable them to escape, avoid, or tolerate drought stress 4. ‘Drought escape’ is the ability of a plant species to complete its life cycle before the onset of drought. Thereby, plants do not experience drought stress, as they are able to modulate their vegetative and reproductive growth according to water availability, essentially through two different mechanisms: rapid phenological development and developmental plasticity 5. Rapid phenological development involves rapid plant growth, producing a minimal number of seeds before the soil water depletes, and these plants are considered not to have any special morphological, physiological, or biochemical adaptations. Plants with mechanisms of developmental plasticity show little growth during the dry season, with very few flowers and seeds, but in wet seasons they grow indeterminately, producing a large amount of seed. ‘Drought avoidance’ is the ability of plants to maintain (relatively) higher tissue water content despite reduced water content in the soil 4. This is achieved through a variety of adaptive traits involving the minimization of water loss (water savers) and optimization of water uptake (water spenders). Water spenders achieve higher tissue water status by maintaining the water uptake through increased rooting, hydraulic conductance, etc. under drought stress. In contrast, water savers use water effectively through reduced loss of water by reducing transpiration, transpiration area, radiation absorption, etc. under drought stress. ‘Drought tolerance’ (DT) is the ability of plants to endure low tissue water content through adaptive traits. These adaptive traits involve maintenance of cell turgor through osmotic adjustment and cellular elasticity, and increasing protoplasmic resistance 6.
Improvement of yield and maintaining yield stability of crops, under normal as well as drought stress conditions, is essential for the food security of the growing global population. It is difficult to resolve the role of different components of DR in the stability of the crop yield as the major objective. However, there exist a variety of different mechanisms for drought escape, avoidance, or tolerance in natural populations that can improve DR and maintain grain yield in crop plants. In nature, extreme DR is found in resurrection plants 7, 8 which possess strong drought escape mechanisms. Resurrection plants can be exposed to severe drought for months, extending up to years, forcing them to optimize their growth for survival, but not for seed production, in the long term 9. Therefore, the DR mechanisms that enable plants to merely survive longer lead to subsistence yield, which is much lower than that which is observed under normal conditions. Crop plants, on the other hand, are grown by humans in environments under conditions for high agricultural production and will be exposed to only a random short-term drought stress of days to weeks, from which they must quickly respond to limit the damage caused by short-term drought stress while they continue to grow and yield in the stressful environments. Therefore, bringing in the drought adaptive mechanisms from plants adapted to grow in extreme dry conditions may not be a feasible option, as it may result in growth and/or yield penalty in crop plants under drought as well as normal conditions.
Although plant survival is very critical in the early growth stages, the mechanisms have little relevance to increasing grain yield directly. The emphasis to improve DR of crop plants should therefore be based on stability of yield components and not on plant survival alone. So far, most of the efforts to improve grain yield under drought stress were focused on secondary traits such as root architecture, leaf water potential, osmotic adjustment, and relative water content at the vegetative stage, which are often not highly correlated with grain yield 10, 11. Looking forward in crops, the effective drought improvement approach should be selection for yield and its component traits under reproductive-stage drought stress 12. Additionally, little importance has also been given to simultaneous improvement of grain yield under normal and drought conditions. Selection for DT has been suggested to have a yield drag under normal conditions. It has been proposed that the yield potential of crop plants should be simultaneously selected for under favorable and environmental stress conditions, as there is a positive correlation between yield potential under normal and drought stress conditions 13. Combining high yield potential under normal conditions with good yield under drought stress is the ideal trait. Identification of mechanisms, traits, and genes regulating yield under drought stress that are free from yield drag under normal conditions should be the focus. For example, regulation of yield under normal as well as drought stress conditions has been shown for three NAC family transcription factors (TFs). Transgenic plants expressing OsNAC5, OsNAC9, and OsNAC10 TFs showed an increase in grain yield of 5-26% under normal conditions 14– 16. Nevertheless, in these studies, yield under normal conditions has been overlooked with more emphasis given to yield under drought stress. Two of our recent studies show the potential of simultaneously improving and stabilizing grain yield, both under normal as well as drought stress conditions, using two regulatory genes, namely GUDK and HYR in rice 17, 18. These studies indicate that it might be advantageous to identify mechanisms and genes for increasing grain yield that are also stable or maintained under drought stress conditions.
Despite the complexity of DR, tremendous progress has been made in understanding the drought-adaptive mechanisms of plants 1, 19, 20. Adaptation through DR mainly involves morpho-physiological alterations. These alterations in adaptive processes are controlled by molecular mechanisms that regulate the expression of genes 21. There exists a large diversity in drought adaptation within a crop species, as some genotypes are able to cope with drought better than others. Genotypes that differ in drought adaptive mechanisms serve as an important resource to study the variation in drought adaption in crop plants. This natural variation needs to be exploited to simultaneously improve DR and yields of cultivated varieties through better understanding of the underlying mechanisms and to aid in selection for these traits 22. In the following sections, we describe the widely known morpho-physiological processes and recent molecular advances in regulating these drought-adaptive processes leading to increased yield in crop plants.
Photosynthesis
Drought stress is known to reduce photosynthesis by decreasing both leaf area and photosynthetic rate per unit leaf area. Reduced photosynthetic rate is mainly through stomatal closure or metabolic impairment 23. Continued photosynthetic light reactions during drought stress under limited intercellular CO 2 concentration results in the accumulation of reduced photosynthetic electron transport components, which can potentially reduce molecular oxygen, resulting in the production of reactive oxygen species (ROS). ROS can cause severe damage to the photosynthetic apparatus 24. The adaptive responses that plants have developed to reduce drought-induced damage to photosynthesis include thermal dissipation of light energy, the xanthophyll cycle, the water-water cycle, and dissociation of the light-harvesting complexes from photosynthetic reaction centers 25– 27. The metabolic impairment during drought stress is mainly caused by changes in photosynthetic carbon metabolism 24. The biochemical efficiency of photosynthesis under drought stress mainly depends on ribulose-1,5-bisphosphate (RuBP) regeneration and the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) 28, 29. Considerable progress has been made in improving the stomatal components for CO 2 diffusion, photosynthetic light reaction, and metabolic changes, including the expression of photosynthesis-related genes to regulate photosynthesis under drought towards the improvement of grain yield 30.
The C4 pathway of carbon assimilation has been suggested to be the major adaptation of the C3 pathway to limit water loss, reduce photorespiration, and improve photosynthetic efficiency under drought stress 31. However, many important crops—including rice, wheat, soybean, and potato—use the C3 pathway of photosynthesis. Although the transfer of the C4 pathway into C3 crops is underway, so far its contribution to increased grain yield is very limited 32. Photosynthetic adaptation of plants to drought stress involves a complex interaction of hormones, ROS, sugars, and other metabolic events 33. Combinations of computational models, which integrate the physiological and metabolic processes with gene expression data, along with modern breeding and transgenic technologies hold promise in improving photosynthesis and hence crop yield under normal as well as drought stress conditions.
In recent studies, we used a rice gene regulatory network to identify a TF termed HYR (HIGHER YIELD RICE), which was highly associated with primary carbon metabolism 17, and on overexpression in rice enhanced photosynthesis under normal conditions as well as under drought and high temperature stress. HYR regulates several morpho-physiological processes leading to higher yield under normal and environmental stress conditions. Our study showed that HYR is a master regulator of photosynthesis, directly activating photosynthesis genes, cascades of TFs, and other downstream genes involved in photosynthetic carbon metabolism, resulting in improved yield.
Hormonal regulation
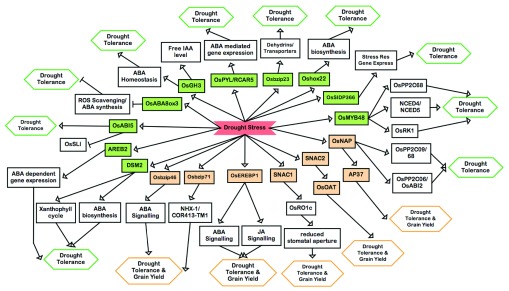
Major phytohormones, such as abscisic acid (ABA), cytokinin (CK), gibberellic acid (GA), auxin, and ethylene, regulate diverse processes which enable plant adaptation to drought stress 34. Upon exposure of plants to drought stress, ABA is the major hormone synthesized in roots and translocated to leaves to initiate adaptation of plants to drought stress through stomatal closure and reduced plant growth 35. However, modulating the ABA-induced drought adaptation of plants for better yield remains a greater challenge because of the potential inadvertent reduction in carbon gain upon stomatal closure and ABA-induced senescence, especially if the drought occurs at the reproductive stage 36. There are ABA signaling genes, such as OsNAP, OsNAC5, and DSM2, which promote improved yield under reproductive drought 37– 40. These ABA-induced non-stomatal adaptations of plants under drought stress can be exploited to improve grain yield under reproductive drought ( Figure 1).
Figure 1. The abscisic acid (ABA)-dependent gene regulatory pathway in rice.
This pathway is required for drought stress tolerance and grain yield under drought. The drought stress ABA-dependent signal is shown perceived directly by the regulatory genes described in the text, followed by transcriptional regulation of downstream genes and underlying stress response mechanisms. Genes regulating drought tolerance (DT) at the vegetative stage are shaded green, and genes regulating DT and grain yield under drought are shaded orange. The resulting phenotypes are represented for DT at the vegetative level only (green diamonds), or DT and grain yield (orange diamonds). The genes described here are OsGH3 92, OsNAP, OsABI2 39, AP37 93, OsPP2C09, OsPP2C06 94, OsPYL/ RCAR5, OsSIDP366 95, OsMYB48 96, OsRK1 97, Oshox22 98, SNAC2 99, 100, OsOAT 101, OsbZIP23 102, SNAC1 99, OsEREBP1 103, OsbZIP71 104, OsbZIP46 105, OsABI5 106, DSM2 40, AREB2 107, OsSRO1c 108, and OsABA8OX3 109.
Under drought stress, CKs are known to delay premature leaf senescence and death, adaptive traits very useful for increasing grain yield. An increase in the endogenous levels of CK through expression of isopentenyltransferase ( IPT), a CK biosynthetic pathway gene, leads to stress adaptation by delaying drought-induced senescence and an increase in yield 41, 42. Generally, auxin has been shown to negatively regulate drought adaptation in plants. Decrease in indole-3-acetic acid (IAA) content was shown to be associated with up-regulation of genes encoding late embryogenesis abundant (LEA) proteins, leading to drought adaptation in plants 43, 44. Recently, the DEEPER ROOTING 1 ( DRO1) gene determining a quantitative trait locus (QTL) controlling root growth angle was shown to be negatively regulated by auxin. Higher expression of DRO1 in a shallow-rooting rice cultivar resulted in drought avoidance and high yield under drought 45. GA is suggested to positively regulate plant adaptation to drought stress. A rapid decline in levels of endogenous GA was observed in plants subjected to drought stress, resulting in growth inhibition 46. The role of GA in regulating grain yield of crop plants is thus an important area that can be further explored. Ethylene is a negative regulator of drought stress response by promoting leaf senescence and inhibiting root growth and development, shoot/leaf expansion, and photosynthesis 47– 51. Ethylene can also directly affect yield by increasing embryo and grain abortion and reducing the grain-filling rate 52. In addition to the major hormones, other hormones such as brassinosteroids, jasmonic acid (JA), salicylic acid (SA), and strigolactone also have an equally important role in plant growth and development. However, their function under drought stress is relatively less characterized. Tillering in rice has been suggested to be the outcome of an interaction among three hormones, CK, auxin, and strigolactone, with CK promoting branching and the other two inhibiting it 53, 54, suggesting that all hormones do not act in isolation but instead interact and modulate each other’s biosynthesis and responses. Therefore, the net outcome of the drought stress response is regulated by a balance between hormones that promote and those that inhibit the trait, rather than individual hormones.
Transpiration and stomatal conductance
The immediate response of plants on being exposed to drought stress is stomatal closure. However, stomatal closure not only diminishes water loss through transpiration but also reduces CO 2 and nutrient uptake, and hence alters metabolic pathways such as photosynthesis 55. Plants growing in dry areas have developed xeromorphic traits to reduce transpiration under drought stress. Reduction in transpiration under drought stress conditions can also be achieved through leaf shedding (i.e. deciduous species in drought) as well as decrease in leaf number, leaf size, and branching. Another adaptation to counter drought stress is sclerophylly, where plants form hard leaves that will not suffer permanent damage due to wilting and can be restored to full functionality when normal conditions resume 56. Recent research has shown that decreased stomatal conductance in response to drought stress is related not only to reduced expression of aquaporin genes but also to anatomical traits leading to reduction of chloroplast surface area exposed to intercellular space per unit leaf area 57, 58. Several other factors, including leaf developmental stage and light availability, are also known to interact with drought in modulating mesophyll and chloroplast differentiation, ultimately affecting conductance and photosynthetic capacity 58. Reduction in stomatal size and number on exposure to drought is another adaptation for survival under drought conditions. Previous studies have shown that while there is an increase in stomatal density under mild drought stress, there is a decrease during severe drought 59. Thus, all these adaptations in plants reduce the negative impacts of drought stress on photosynthesis and thereby have a positive effect on water use efficiency (WUE), which in turn will result in high yield potential and high yield 60. Such an adaptation was shown in rice by overexpression of the Arabidopsis AP2/ERF TF HARDY that improved WUE (the ratio of biomass produced to water used) by enhancing photosynthesis and reducing transpiration 61. The above reported traits therefore exemplify adaptive mechanisms in plants to survive under drought stress without loss of productivity or yield.
Root morphology
In many agriculturally important crops, drought stress is perceived first by the root system, which continues to grow underneath the soil even though shoot growth is inhibited under these conditions 62. Although the growth of the primary root is not affected by drought stress, the growth of lateral roots is significantly reduced, mainly by suppression of the activation of the lateral root meristems 63. The Arabidopsis R2R3-type MYB TF MYB96 has been shown to regulate activation of lateral root meristem through an ABA signaling cascade, with an activation-tagged mutant showing enhanced DR with reduced lateral root formation 64. The plant microRNA miR393 has also been shown to play a role in root-mediated adaptation to drought stress response through attenuation of auxin signaling 65. In addition to the lateral roots, the presence of small roots is also considered as an adaptive strategy to increase water uptake by providing more absorptive surface. Presence of specialized tissues like rhizodermis, with a thickened outer cell wall or suberized exodermis, or reduction in the number of cortical layers are considered an adaptive advantage for drought stress survival. Hydrotropism is another adaptive measure taken by plants to counter stress, where studies have shown that degradation of amyloplasts in the columella cells of plant roots on exposure to drought stress increases hydrotropism 66, 67. Hormonal cross-talk mediated by auxin, CK, GA, and ABA has been implicated as a potential chemical signal in response to water stress to modulate root system architecture 68.
The expression of enzymes related to root morphology (e.g. xyloglucan endotransglucosylase) is induced upon mild drought stress, while other structural proteins are down-regulated, which is strongly correlated with root growth and hence an augmentation in the surface area for water uptake. The alterations in the expression of these proteins correlate positively with lateral development that in turn also affects photosynthesis 69. More lateral root and root hair formation was found in lines possessing a QTL, qDTY12.1, only when under drought 70. Such traits, which are expressed only under drought stress, have higher potential to increase grain yield under drought. Moreover, it has also been shown that drought stress triggers a wide variety of anatomical traits expressed to different levels and patterns in different species and even in different cultivars within species 71– 73. For example, suberization and compaction of sclerenchyma layer cells were shown to decrease in rice under drought, which increases retention of water under drought stress 71.
Osmotic adjustment
Osmotic adjustment (OA) is defined as a process of solute accumulation in dividing cells when the water potential is reduced, and thereby helps in maintaining the turgor 74. Cell enlargement and growth in plants is highly dependent on water availability and helps in maintaining the turgor. Turgor measurement in growing regions of plants, especially the leaves and stems, shows little or no reduction, though cell enlargement is inhibited during drought stress and is believed to be due to OA 75, 76. Under conditions of drought stress, OA has been implicated in maintaining stomatal conductance, photosynthesis, leaf water volume, and growth 74, 77. At times of drought stress, in addition to the reduction in water content, there are also other associated changes, such as increases in salt concentration and mechanical impedance 78. Inorganic cations, organic acids, carbohydrates, and free amino acids are the known predominant solutes that accumulate in response to water stress. Previous studies have shown that drought-resistant wheat varieties, with yield stability under drought stress, have a greater capacity for osmoregulation than less resistant varieties 76. The accumulation of compatible solutes such as proline and glycine betaine help in protecting the plants from detrimental effects of drought stress not only by OA but also by detoxification of ROS, protection of membrane integrity, and stabilization of enzymes or proteins 79. Enzymes such as betaine aldehyde dehydrogenase (BADH), pyrroline-5-carboxylate reductase (P5CR), and ornithine δ-aminotransferase (OAT) have been shown to play major roles in OA. Overexpression of Arabidopsis EDT1/HDG11 was shown to increase DT of poplar and cotton through increased accumulation of solutes such as proline and soluble sugars and also increase the yield of cotton in the field 80. However, there are some plants in which sugars are the main osmolytes that play a significant role in OA, including sucrose, trehalose, glucose, and fructose. Previous studies have shown that overexpression of the sucrose:fructan-6-fructosyltransferase ( 6-SFT) gene from Psathyrostachys huashanica in tobacco and the trehalose-6-phosphate phosphatase gene OsTPP1 in rice confers abiotic stress tolerance 81, 82. Researchers have also identified a QTL for OA on chromosome 8 in rice that is homeologous with a segment of wheat chromosome 7 83.
Source-sink relationships
Source-sink relationships largely determine the grain yield of cereal crops, with developing grains being primary sinks while the top two leaves, the flag leaf in particular, are the primary source 84, 85. Drought stress affects the source-sink relationship by reducing the source strength, leading to yield reduction. Sufficient sugar supply through photosynthesis, transport, and conversion of sugars is regarded as the most critical component in determining the viability of reproductive organs in rice 86– 88. Drought stress dramatically affects pollen viability due to abnormal starch accumulation 89. Insufficient starch synthesis and arrested pollen development have been linked to reduced invertase activity under drought stress 88. In addition to invertases, active grain filling involves other key enzymes, such as sucrose synthase, ADP glucose pyrophosphorylase (AGPase), and starch synthase as well as starch branching and debranching enzymes, which are also affected by drought stress 88. Therefore, enhancing rice yield through source-sink relationships involves not only sugar metabolism but also the regulated mobilization of metabolic resources from source to sink tissue.
Future research perspectives
Improving DR in crop plants is a challenge for plant breeders and crop physiologists, as it is a complex genetic trait with multiple pathways involved. Effective development of drought-resistant crop plants thus requires the pyramiding and interaction of many mechanisms, traits, and genes that are appropriate to individual crops and their growing environments. Success in this direction not only extends the growing area of crop plants but also achieves stable yield in drought-prone areas. Identifying genetic variation for DR is the first step towards development of drought-resistant crop plants. Such variation is often present in wild species and adapted genotypes that have evolved under natural selection and these are the best source of DR traits. Evaluation of these resources through an integrated phenotyping and genotyping approach under field conditions alongside identification of traits that are directly associated with yield is key to improving DR 90. Comparative omics analyses between the diverse germplasm could aid in bettering our understanding of the variety of crop adaptations to drought stress. In addition, analysis of specific genes focussed on increasing DR while stabilizing the yield is crucial for understanding the broad basis of complex traits such as DR 91. The development of high-yielding resilient crops 74 that maintain yield stability under drought and other environmental stresses due to climate change is also currently needed. Drought-resistant plants should combine a better root system, stomatal regulation, WUE, and hormonal balance while avoiding the negative effects on grain yield under both normal and drought stress conditions. Therefore, a holistic crop improvement strategy should involve the deployment of high crop yield potential and the utilization of a combination of morpho-physiological, biochemical, and anatomical adaptive responses to drought stress.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Arvind Kumar, International Rice Research Institute, Metro Manila, Philippines
Zhulong Chan, Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
Krishna Jagadish, Kansas State University, Manhattan, KS, USA
Funding Statement
This work was supported by the National Science Foundation award numbers DBI–0922747 and ABI1062472.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Bray EA: Plant responses to water deficit. Trends Plant Sci. 1997;2(2):48–54. 10.1016/S1360-1385(97)82562-9 [DOI] [Google Scholar]
- 2. Dai A: Increasing drought under global warming in observations and models. Nat Clim Chang. 2012;3:52–8. 10.1038/nclimate1633 [DOI] [Google Scholar]
- 3. Bohnert HJ, Nelson DE, Jensen RG: Adaptations to Environmental Stresses. Plant Cell. 1995;7(7):1099–111. 10.1105/tpc.7.7.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levitt J: Responses of plants to environmental stresses. Volume II. Water, radiation, salt, and other stresses. (Academic Press),1980. Reference Source [Google Scholar]
- 5. Jones MM, Turner NC, Osmond CB: Mechanisms of drought resistance. The physiology and biochemistry of drought resistance in plants.1981;1:15–37. [Google Scholar]
- 6. Morgan JM: Osmoregulation and Water Stress in Higher Plants. Annu Rev Plant Physiol. 1984;35:299–319. 10.1146/annurev.pp.35.060184.001503 [DOI] [Google Scholar]
- 7. Gaff DF: Desiccation tolerant plants in South America. Oecologia. 1987;74(1):133–6. 10.1007/BF00377357 [DOI] [PubMed] [Google Scholar]
- 8. Gaff DF: Desiccation-tolerant flowering plants in southern Africa. Science. 1971;174(4013):1033–4. 10.1126/science.174.4013.1033 [DOI] [PubMed] [Google Scholar]
- 9. Porembski S: Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000;151(1):19–28. 10.1023/A:1026565817218 [DOI] [Google Scholar]
- 10. Jongdee B, Fukai S, Cooper M: Leaf water potential and osmotic adjustment as physiological traits to improve drought tolerance in rice. Field Crops Res. 2002;76(2–3):153–63. 10.1016/S0378-4290(02)00036-9 [DOI] [Google Scholar]
- 11. Pantuwan G, Fukai S, Cooper M, et al. : Yield response of rice ( Oryza sativa L.) genotypes to drought under rainfed lowland: 3. Plant factors contributing to drought resistance. Field Crops Res. 2002;73(2–3):181–200. 10.1016/S0378-4290(01)00194-0 [DOI] [Google Scholar]
- 12. Venuprasad R, Lafitte HR, Atlin GN: Response to Direct Selection for Grain Yield under Drought Stress in Rice. Crop Sci. 2007;47(1):285–293. 10.2135/cropsci2006.03.0181 [DOI] [Google Scholar]
- 13. Guan YS, Serraj R, Liu SH, et al. : Simultaneously improving yield under drought stress and non-stress conditions: a case study of rice ( Oryza sativa L.). J Exp Bot. 2010;61(15):4145–56. 10.1093/jxb/erq212 [DOI] [PubMed] [Google Scholar]
- 14. Jeong JS, Kim YS, Redillas MC, et al. : OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol J. 2013;11(1):101–14. 10.1111/pbi.12011 [DOI] [PubMed] [Google Scholar]
- 15. Redillas MC, Jeong JS, Kim YS, et al. : The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol J. 2012;10(7):792–805. 10.1111/j.1467-7652.2012.00697.x [DOI] [PubMed] [Google Scholar]
- 16. Jeong JS, Kim YS, Baek KH, et al. : Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010;153(1):185–97. 10.1104/pp.110.154773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ambavaram MM, Basu S, Krishnan A, et al. : Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat Commun. 2014;5: 5302. 10.1038/ncomms6302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramegowda V, Basu S, Krishnan A, et al. : Rice GROWTH UNDER DROUGHT KINASE is required for drought tolerance and grain yield under normal and drought stress conditions. Plant Physiol. 2014;166(3):1634–45. 10.1104/pp.114.248203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yordanov I, Velikova V, Tsonev T: Plant responses to drought, acclimation, and stress tolerance. Photosynthetica. 2000;38(2):171–86. 10.1023/A:1007201411474 [DOI] [Google Scholar]
- 20. Bray EA: Molecular Responses to Water Deficit. Plant Physiol. 1993;103(4):1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osakabe Y, Osakabe K, Shinozaki K, et al. : Response of plants to water stress. Front Plant Sci. 2014;5:86. 10.3389/fpls.2014.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reynolds M, Tuberosa R: Translational research impacting on crop productivity in drought-prone environments. Curr Opin Plant Biol. 2008;11(2):171–9. 10.1016/j.pbi.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 23. Tezara W, Mitchell VJ, Driscoll SD, et al. : Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–7. 10.1038/44842 [DOI] [Google Scholar]
- 24. Lawlor DW, Cornic G: Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002;25(2):275–94. 10.1046/j.0016-8025.2001.00814.x [DOI] [PubMed] [Google Scholar]
- 25. Niyogi KK: PHOTOPROTECTION REVISITED: Genetic and Molecular Approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–59. 10.1146/annurev.arplant.50.1.333 [DOI] [PubMed] [Google Scholar]
- 26. Demmig-Adams B, Adams WW, III: The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1(1):21–6. 10.1016/S1360-1385(96)80019-7 [DOI] [Google Scholar]
- 27. Demmig-Adams B, Adams WW, 3rd: Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 2006;172(1):11–21. 10.1111/j.1469-8137.2006.01835.x [DOI] [PubMed] [Google Scholar]
- 28. Lawlor DW: Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Ann Bot. 2002;89(Spec No):871–85. 10.1093/aob/mcf110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medrano H, Parry MAJ, Socias X, et al. : Long term water stress inactivates Rubisco in subterranean clover. Ann Appl Biol. 1997;131(3):491–501. 10.1111/j.1744-7348.1997.tb05176.x [DOI] [Google Scholar]
- 30. Chaves MM, Flexas J, Pinheiro C: Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot. 2009;103(4):551–60. 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edwards G, Walker D: C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis. Univ of California Press,1983. Reference Source [DOI] [PubMed] [Google Scholar]
- 32. Gowik U, Westhoff P: The path from C 3 to C 4 photosynthesis. Plant Physiol. 2011;155(1):56–63. 10.1104/pp.110.165308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pinheiro C, Chaves MM: Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot. 2011;62(3):869–82. 10.1093/jxb/erq340 [DOI] [PubMed] [Google Scholar]
- 34. Wilkinson S, Kudoyarova GR, Veselov DS, et al. : Plant hormone interactions: innovative targets for crop breeding and management. J Exp Bot. 2012;63(9):3499–509. 10.1093/jxb/ers148 [DOI] [PubMed] [Google Scholar]
- 35. Wilkinson S, Davies WJ: Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 2010;33(4):510–25. 10.1111/j.1365-3040.2009.02052.x [DOI] [PubMed] [Google Scholar]
- 36. Ji X, Dong B, Shiran B, et al. : Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 2011;156(2):647–62. 10.1104/pp.111.176164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sperotto RA, Ricachenevsky FK, Duarte GL, et al. : Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta. 2009;230(5):985–1002. 10.1007/s00425-009-1000-9 [DOI] [PubMed] [Google Scholar]
- 38. Liang C, Wang Y, Zhu Y, et al. : OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci U S A. 2014;111(27):10013–8. 10.1073/pnas.1321568111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X, Wang Y, Lv B, et al. : The NAC family transcription factor OsNAP confers abiotic stress response through the ABA pathway. Plant Cell Physiol. 2014;55(3):604–19. 10.1093/pcp/pct204 [DOI] [PubMed] [Google Scholar]
- 40. Du H, Wang N, Cui F, et al. : Characterization of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol. 2010;154(3):1304–18. 10.1104/pp.110.163741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peleg Z, Reguera M, Tumimbang E, et al. : Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J. 2011;9(7):747–58. 10.1111/j.1467-7652.2010.00584.x [DOI] [PubMed] [Google Scholar]
- 42. Peleg Z, Blumwald E: Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol. 2011;14(3):290–5. 10.1016/j.pbi.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 43. Xie Z, Jiang D, Cao W, et al. : Relationships of endogenous plant hormones to accumulation of grain protein and starch in winter wheat under different post-anthesis soil water statusses. Plant Growth Regul. 2003;41(2):117–27. 10.1023/A:1027371906349 [DOI] [Google Scholar]
- 44. Zhang S, Li C, Cao J, et al. : Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol. 2009;151(4):1889–901. 10.1104/pp.109.146803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uga Y, Sugimoto K, Ogawa S, et al. : Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet. 2013;45(9):1097–102. 10.1038/ng.2725 [DOI] [PubMed] [Google Scholar]
- 46. Wang C, Yang A, Yin H, et al. : Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J Integr Plant Biol. 2008;50(4):427–34. 10.1111/j.1774-7909.2008.00638.x [DOI] [PubMed] [Google Scholar]
- 47. Munné-Bosch S, Alegre L: Die and let live:Leaf senescence contributes to plant survival under drought stress. Funct Plant Biol. 2004;31(3):203–216. 10.1071/FP03236 [DOI] [PubMed] [Google Scholar]
- 48. Fukao T, Xu K, Ronald PC, et al. : A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18(8):2021–34. 10.1105/tpc.106.043000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perata P, Voesenek LA: Submergence tolerance in rice requires Sub1A, an ethylene-response-factor-like gene. Trends Plant Sci. 2007;12(2):43–6. 10.1016/j.tplants.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 50. Rajala A, Peltonen-Sainio P: Plant Growth Regulator Effects on Spring Cereal Root and Shoot Growth. Agron J. 2001;93(4):936–943. 10.2134/agronj2001.934936x [DOI] [Google Scholar]
- 51. Sharp RE: Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002;25(2):211–22. 10.1046/j.1365-3040.2002.00798.x [DOI] [PubMed] [Google Scholar]
- 52. Yang JC, Zhang JH, Ye YX, et al. : Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ. 2004;27(8):1055–64. 10.1111/j.1365-3040.2004.01210.x [DOI] [Google Scholar]
- 53. Gomez-Roldan V, Fermas S, Brewer PB, et al. : Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–94. 10.1038/nature07271 [DOI] [PubMed] [Google Scholar]
- 54. Xing Y, Zhang Q: Genetic and molecular bases of rice yield. Annu Rev Plant Biol. 2010;61:421–42. 10.1146/annurev-arplant-042809-112209 [DOI] [PubMed] [Google Scholar]
- 55. Xiong L, Zhu J: Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002;25(2):131–9. 10.1046/j.1365-3040.2002.00782.x [DOI] [PubMed] [Google Scholar]
- 56. Micco VD, Aronne G: in Plant Responses to Drought Stress. (ed Aroca. Ricardo). Springer,2002. [Google Scholar]
- 57. Miyazawa S, Yoshimura S, Shinzaki Y, et al. : Deactivation of aquaporins decreases internal conductance to CO 2 diffusion in tobacco leaves grown under long-term drought. Funct Plant Biol. 2008;35(7):553–564. 10.1071/FP08117 [DOI] [PubMed] [Google Scholar]
- 58. Tosens T, Niinemets U, Vislap V, et al. : Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 2012;35(5):839–56. 10.1111/j.1365-3040.2011.02457.x [DOI] [PubMed] [Google Scholar]
- 59. Xu Z, Zhou G: Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot. 2008;59(12):3317–25. 10.1093/jxb/ern185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blum A: Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agric Res. 2005;56(11):1159–1168. 10.1071/AR05069 [DOI] [Google Scholar]
- 61. Karaba A, Dixit S, Greco R, et al. : Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci U S A. 2007;104(39):15270–5. 10.1073/pnas.0707294104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spollen WG, Sharp RE: Spatial distribution of turgor and root growth at low water potentials. Plant Physiol. 1991;96(2):438–43. 10.1104/pp.96.2.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deak KI, Malamy J: Osmotic regulation of root system architecture. Plant J. 2005;43(1):17–28. 10.1111/j.1365-313X.2005.02425.x [DOI] [PubMed] [Google Scholar]
- 64. Seo PJ, Park C: Auxin homeostasis during lateral root development under drought condition. Plant Signal Behav. 2009;4(10):1002–4. 10.4161/psb.4.10.9716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen H, Li Z, Xiong L: A plant microRNA regulates the adaptation of roots to drought stress. FEBS Lett. 2012;586(12):1742–7. 10.1016/j.febslet.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 66. Jaffe MJ, Takahashi H, Biro RL: A pea mutant for the study of hydrotropism in roots. Science. 1985;230(4724):445–7. 10.1126/science.230.4724.445 [DOI] [PubMed] [Google Scholar]
- 67. Takahashi N, Yamazaki Y, Kobayashi A, et al. : Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol. 2003;132(2):805–10. 10.1104/pp.102.018853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Blilou I, Xu J, Wildwater M, et al. : The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433(7021):39–44. 10.1038/nature03184 [DOI] [PubMed] [Google Scholar]
- 69. Sengupta D, Reddy AR: Water deficit as a regulatory switch for legume root responses. Plant Signal Behav. 2011;6(6):914–7. 10.4161/psb.6.6.15340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dixit S, Kumar Biswal A, Min A, et al. : Action of multiple intra-QTL genes concerted around a co-localized transcription factor underpins a large effect QTL. Sci Rep. 2015;5: 15183. 10.1038/srep15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Henry A, Cal AJ, Batoto TC, et al. : Root attributes affecting water uptake of rice ( Oryza sativa) under drought. J Exp Bot. 2012;63(13):4751–63. 10.1093/jxb/ers150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang B, Fry JD: Root Anatomical, Physiological, and Morphological Responses to Drought Stress for Tall Fescue Cultivars. Crop Sci. 1998;38(4):1017–1022. 10.2135/cropsci1998.0011183X003800040022x [DOI] [Google Scholar]
- 73. Steudle E: Water uptake by roots: effects of water deficit. J Exp Bot. 2000;51(350):1531–42. 10.1093/jexbot/51.350.1531 [DOI] [PubMed] [Google Scholar]
- 74. Chaves MM, Oliveira MM: Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot. 2004;55(407):2365–84. 10.1093/jxb/erh269 [DOI] [PubMed] [Google Scholar]
- 75. Meyer RF, Boyer JS: Sensitivity of cell division and cell elongation to low water potentials in soybean hypocotyls. Planta. 1972;108(1):77–87. 10.1007/BF00386508 [DOI] [PubMed] [Google Scholar]
- 76. Serraj R, Sinclair TR: Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002;25(2):333–41. 10.1046/j.1365-3040.2002.00754.x [DOI] [PubMed] [Google Scholar]
- 77. Oosterhuis DM, Wullschleger SD: Osmotic Adjustment in Cotton ( Gossypium hirsutum L.) Leaves and Roots in Response to Water Stress. Plant Physiol. 1987;84(4):1154–7. 10.1104/pp.84.4.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sauter A, Davies WJ, Hartung W: The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot. 2001;52(363):1991–7. 10.1093/jexbot/52.363.1991 [DOI] [PubMed] [Google Scholar]
- 79. Ashraf M, Foolad MR: Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59(2):206–16. 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- 80. Yu LH, Wu SJ, Peng YS, et al. : Arabidopsis EDT1/ HDG11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnol J. 2016;14(1):72–84. 10.1111/pbi.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. He X, Chen Z, Wang J, et al. : A sucrose: Fructan-6-fructosyltransferase (6-SFT) gene from Psathyrostachys huashanica confers abiotic stress tolerance in tobacco. Gene. 2015;570(2):239–47. 10.1016/j.gene.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 82. Ge L, Chao D, Shi M, et al. : Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta. 2008;228(1):191–201. 10.1007/s00425-008-0729-x [DOI] [PubMed] [Google Scholar]
- 83. Ahn S, Anderson JA, Sorrells ME, et al. : Homoeologous relationships of rice, wheat and maize chromosomes. Mol Gen Genet. 1993;241(5–6):483–90. [DOI] [PubMed] [Google Scholar]
- 84. Hirota O, Oka M, Takeda T: Sink activity estimation by sink size and dry matter increase during the ripening stage of barley ( Hordeum vulgare) and rice ( Oryza sativa). Ann Bot. 1990;65(4):349–353. Reference Source [Google Scholar]
- 85. Gladun IV, Karpov EA: Production and partitioning of assimilates between the panicle and vegetative organs of rice after flowering. Russ J Plant Physiol. 1993;40(5):629-633. Reference Source [Google Scholar]
- 86. Li X, Lawas LMF, Malo R, et al. : Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ. 2015;38(10):2171–92. 10.1111/pce.12545 [DOI] [PubMed] [Google Scholar]
- 87. Nguyen GN, Hailstones DL, Wilkes M, et al. : DROUGHT STRESS: Role of Carbohydrate Metabolism in Drought-Induced Male Sterility in Rice Anthers. J Agron Crop Sci. 2010;196(5):346–57. 10.1111/j.1439-037X.2010.00423.x [DOI] [Google Scholar]
- 88. Sheoran IS, Saini HS: Drought-induced male sterility in rice: Changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sexual Plant Reprod. 1996;9(3):161–9. 10.1007/BF02221396 [DOI] [Google Scholar]
- 89. Jin Y, Yang H, Wei Z, et al. : Rice male development under drought stress: phenotypic changes and stage-dependent transcriptomic reprogramming. Mol Plant. 2013;6(5):1630–45. 10.1093/mp/sst067 [DOI] [PubMed] [Google Scholar]
- 90. Tuberosa R: Phenotyping for drought tolerance of crops in the genomics era. Front Physiol. 2012;3:347. 10.3389/fphys.2012.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nguyen TT, Klueva N, Chamareck V, et al. : Saturation mapping of QTL regions and identification of putative candidate genes for drought tolerance in rice. Mol Genet Genomics. 2004;272(1):35–46. 10.1007/s00438-004-1025-5 [DOI] [PubMed] [Google Scholar]
- 92. Du H, Wu N, Fu J, et al. : A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J Exp Bot. 2012;63(18):6467–80. 10.1093/jxb/ers300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Oh SJ, Kim YS, Kwon CW, et al. : Overexpression of the Transcription Factor AP37 in Rice Improves Grain Yield under Drought Conditions. Plant Physiol. 2009;150(3):1368–79. 10.1104/pp.109.137554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. He Y, Hao Q, Li W, et al. : Identification and Characterization of ABA Receptors in Oryza sativa. PLoS One. 2014;9(4):e95246. 10.1371/journal.pone.0095246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guo C, Luo C, Guo L, et al. : OsSIDP366, a DUF1644 gene, positively regulates responses to drought and salt stresses in rice. J Integr Plant Biol. 2016;58(5):492–502. 10.1111/jipb.12376 [DOI] [PubMed] [Google Scholar]
- 96. Xiong H, Li J, Liu P, et al. : Overexpression of OsMYB48-1, a Novel MYB-Related Transcription Factor, Enhances Drought and Salinity Tolerance in Rice. PLoS One. 2014;9(3):e92913. 10.1371/journal.pone.0092913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chae M, Lee JS, Nam MH, et al. : A rice dehydration-inducible SNF1-related protein kinase 2 phosphorylates an abscisic acid responsive element-binding factor and associates with ABA signaling. Plant Mol Biol. 2007;63(2):151–69. 10.1007/s11103-006-9079-x [DOI] [PubMed] [Google Scholar]
- 98. Zhang S, Haider I, Kohlen W, et al. : Function of the HD-Zip I gene Oshox22 in ABA-mediated drought and salt tolerances in rice. Plant Mol Biol. 2012;80(6):571–85. 10.1007/s11103-012-9967-1 [DOI] [PubMed] [Google Scholar]
- 99. Hu H, Dai M, Yao J, et al. : Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci U S A. 2006;103(35):12987–92. 10.1073/pnas.0604882103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu H, You J, Fang Y, et al. : Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol. 2008;67(1–2):169–81. 10.1007/s11103-008-9309-5 [DOI] [PubMed] [Google Scholar]
- 101. You J, Hu H, Xiong L: An ornithine δ-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci. 2012;197:59–69. 10.1016/j.plantsci.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 102. Xiang Y, Tang N, Du H, et al. : Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008;148(4):1938–52. 10.1104/pp.108.128199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jisha V, Dampanaboina L, Vadassery J, et al. : Overexpression of an AP2/ERF Type Transcription Factor OsEREBP1 Confers Biotic and Abiotic Stress Tolerance in Rice. PLoS One. 2015;10(6):e0127831. 10.1371/journal.pone.0127831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu C, Mao B, Ou S, et al. : OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol Biol. 2014;84(1–2):19–36. 10.1007/s11103-013-0115-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Tang N, Zhang H, Li X, et al. : Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012;158(4):1755–68. 10.1104/pp.111.190389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zou M, Guan Y, Ren H, et al. : A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol Biol. 2008;66(6):675–83. 10.1007/s11103-008-9298-4 [DOI] [PubMed] [Google Scholar]
- 107. Yoshida T, Fujita Y, Sayama H, et al. : AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010;61(4):672–85. 10.1111/j.1365-313X.2009.04092.x [DOI] [PubMed] [Google Scholar]
- 108. You J, Zong W, Li X, et al. : The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice. J Exp Bot. 2013;64(2):569–83. 10.1093/jxb/ers349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cai S, Jiang G, Ye N, et al. : A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. PLoS One. 2015;10(2):e0116646. 10.1371/journal.pone.0116646 [DOI] [PMC free article] [PubMed] [Google Scholar]