Abstract
Emerging studies have demonstrated that EMT phenotype is closely related with tumor progression and drug resistance in a variety of human cancers. Recently, it has been extensively demonstrated that microRNAs (miRNAs) play a pivotal role in regulating EMT. In our previously reports, we have reported that inhibition of miR-223 could reverse EMT phenotype and improve chemotherapeutic drug sensitivity. We also reported that genistein down-regulated miR-223 expression in gemcitabine-resistant (GR) pancreatic cancer cells. Here, we explored whether there was the synergistic effect between miR-223 inhibitor and genistein on cell growth, migration, invasion and reversal of EMT in GR pancreatic cancer. We found that the combination of miR-223 inhibitor and genistein synergistically reduced cell motility and invasion and enhanced gemcitabine sensitivity in GR cells. In addition, we further observed that miR-223 inhibitor and genistein reversed EMT features in GR cells. This study suggests that the combination of miR-223 inhibitor and genistein may be a potential therapeutic strategy for the treatment of pancreatic cancer.
Keywords: Gemcitabine, genistein, miR-223, EMT, invasion, pancreatic cancer
Introduction
Pancreatic cancer is one of lethal malignant tumor with high morbidity and mortality with the estimated 48,960 new cancer cases and 40,560 deaths in 2015 [1]. The incidence rates and death rates have slowly increasing during the past ten years in the United States [1]. The standard chemotherapy was gemcitabine alone or in combination with paclitaxel for advanced Pancreatic cancer (PC) patients [2]. However, chemotherapy treatment only extends the median survival of pancreatic cancer patients with a very slight extension [3]. It was well known that the important reason for this high mortality was due to highly drug resistance to chemotherapy [4]. Thus, it is vital to understand the drug resistance to gemcitabine which can help to improve more effective therapies for PC.
Emerging evidence indicates that EMT is essential for the progression of pancreatic cancer [5-7]. EMT is a process that allows the epithelial cells to acquire mesenchymal phenotype, leading to enhanced migration and invasion capacity [8,9]. As the characteristic of EMT, the expression of epithelial markers such as E-cadherin is reduced, whereas some mesenchymal markers expressions are increased including vimentin, Snail, Slug, zinc-finger E-box binding home-box 1 (ZEB1) and ZEB2 [10,11]. There is extended evidence that EMT process is related to drug resistance to gemcitabine in human tumors including PC [12,13]. Furthermore, accumulating evidence has revealed that microRNAs (miRNAs) play a critically role in regulation of drug resistance-mediated EMT [14-16]. Consistently, we have reported that gemcitabine resistance to PC is associated with EMT and induction of miR-223 expression [17].
Genistein is an isoflavonid found in high amounts in soy beans and other soy products. It has been demonstrated that genistein exerts anticancer activity in diverse human cancers with a low toxicity to normal cells [18]. Specifically, genistein treatment leads to inhibition of cell proliferation and induction of cell apoptosis through regulation of several signaling pathways [19-21]. Furthermore, it has been reported that genistein plays a key role in suppression of cell migration and metastasis [22]. For example, Xiao et al. found that genistein suppresses the human colorectal cancer metastasis through inhibiting the FLT4 expression [23]. Furthermore, we previously found that genistein inhibited pancreatic cancer cell growth and migration through down-regulation of miR-223 expression [24]. Moreover, a recently study showed that genistein suppressed EMT process and migration capacity of ovarian cancer cells via down-regulation of TGF-β signaling [25]. More importantly, genistein has been extensively studied in cancer therapy, particularly in combination with other anticancer drugs, suggesting a potential role in combination therapy for cancers [26-28].
In the current study, we explored whether genistein could regulate drug resistance-mediated EMT in GR PC cells. We further explored whether the combinations of genistein and miR-223 inhibition could have the synergistic effects in GR PC cells. Our findings suggest that the combination of miR-223 inhibitor and genistein might be a potential therapeutic strategy for pancreatic cancer.
Materials and methods
Cell culture, reagents and antibodies
The AsPC-1 GR and BxPC-3 GR cells were cultured in DMEM (Gibco, Gaithersburg, MD, USA) and RPMI 1600 (Invitrogen, Carlsbad, CA, USA), respectively, supplemented with 10% fetal bovine serum (FBS) in humidified airs with 5% CO2 at 37°C. Genistein (Toronto Research Chemicals, North York, ON, Canada) was dissolved in 0.1 M Na2CO3 to make a 10 mM stock solution and was added directly to the media at different concentrations. MTT [3-(4,5-dimethythiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] was obtained from Sigma (St. Louis, Mo, USA). Antibodies against Vimentin, E-cadherin, Snail, Slug, ZEB1, ZEB2, Fbw7, Notch-1, β-actin and the secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Transwell inserts and Matrigel were purchased from BD Biosciences.
MiR-223 inhibitor transfection
The cells were seeded in six-well plates and transfected with miR-223 inhibitor (GenePharma, Shanghai, China) or the non-specific control (GenePharma) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The sequence of miR-223 inhibitor: 5’-UGG GGU AUU UGA CAA ACU GAC A-3’. The cells were subjected to further analysis as presented under the results section. The miR-223 expression was analyzed using the TaqMan miRNA assays (Applied Biosystems, CA, USA) according to the manufacturer’s instructions [17].
Cell viability assay
Cells were seeded in 96-well plates (5 × 103 cells/well) for 24 h incubation and treated with different final concentrations for 72 h. Specifically, AsPC-1 GR was treated with genistein at 10 µM, 60 µM, 120 µM and 180 µM for 72 h, respectively. BxPC-3 GR was treated with 10 µM, 20 µM, 40 µM or 60 µM genistein for 72 h. AsPC-1 GR cells were treated with 60 µM genistein or a final concentration of 20 nmol/L miR-223 inhibitor or the combination for 72 h. BxPC-3 GR cells were treated with 20 µM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 h. MTT assay were applied for cell viability analysis as described earlier [29].
Transwell migration and invasion assay
The cells migration and invasion capacities were determined by Transwell assay according to the manufacturer protocol as described previously [30]. The GR cells were seeded into an upper-chamber of inserts in a 24-well plate. In addition, the Transwell inserts need precoat with Matrigel (BD Biosciences) in the invasion assay before the cells were seeded. After the cells were treated with genistein or miR-223 inhibitor for 12 h, the upper cells of the chambers were wiped by the cotton buds and the bottom surface cells of the chambers were fixed and dyed with Giemsa solution.
Wound healing assay
The BxPC-3 GR cells were seeded into a six-well plate and incubated until the cells reach to about 95% confluence. Then, the scratch wound was produced by scraping the surface cells of the plates with a pipette tip. After the detached cells were rinsed with PBS, the BxPC-3 GR cells were treated with 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 16 h. The wound healing images were photographed at 0 h and 16 h, respectively.
Cell attachment and detachment assays
Cell attachment and detachment assays were implemented according to previously published protocols [31]. Briefly, the GR cells were seeded in a 24-well plate and pretreated described as above. For the attachment assay, the cells were incubated for 1 h, subsequently removed the unattached cells and counted the attached cells after trypsinization. For the detachment assay, the seeded cells were incubated for 24 h, subsequently counted the detached cells with 0.05% trypsin for 3 minutes. Data were calculated as a percentage of the attached or detached cells to total cells.
Real-time RT-PCR (RT-PCR)
The total RNA was extracted using Trizol reagent from the cells and transcribed into cDNA according to the manufacturer’s protocol. The miR-223 expression was detected using TaqMan miRNA assay kit (Applied Biosystems, CA, USA) and U6 level was used for normalization. The mRNA level of EMT associated markers, including Snail, E-cadherin, Vimentin, Slug, ZEB1 and ZEB2, was performed using SYBR green assay kit (Takara, Dalian, China) and GAPDH level was applied for normalization. The primers used in PCR reaction were described as before [17].
Western blotting analysis
The cells were lysed in RIPA buffer with protease inhibitors. Then, the BCA protein assay was performed to detect the protein concentrations. In addition, the proteins were separated from SDS-PAGE electrophoresis and subsequently transferred to PVDF membranes. Furthermore, the membranes were immunoblotted with proper antibodies as described earlier [32].
In vivo experiments
Five-week-old male nude mice were subcutaneously inoculated 1 × 107 BxPC-3 GR cells. We allowed 2 weeks for palpable subcutaneous tumors to form before administering genistein and miR-223 inhibitor. When tumors were >100 mm3 in size, mice were separated into four groups (each group, n=5), namely control antagomir (100 μl control antagomir, diluted in PBS), genistein alone, miR-223 inhibitor alone, and genistein plus miR-223 inhibitor. The genistein was dissolved in 0.1 M Na2CO3 and mixed with sesame seed oil at a 2:1 ratio just prior to treatment to facilitate gavage and avoid irritation of the esophagus by Na2CO3. Mice were treated by gavage with genistein at 15 mg/kg body weight/day for 20 days. Control and miR-223 inhibitor-only treated groups received Na2CO3 and sesame seed oil. The control antagomir or 100 μl miR-223 inhibitor (diluted in PBS at 2 mg/ml), was injected twice a week for 3 weeks by way of multiple-center intratumor injection, separately. The tumor size was measured with a caliper every 5 days. Tumor volume was calculated as length × width × height/2. Mice of each group were killed after three weeks injections, and the xenografted tumors were excised and examined Western blotting. All animal studies and procedures were approved by the Institutional Animal Care and Use Committee.
Statistical analysis
The data were statistical analyzed by Student’s t-test using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA). p<0.05 was considered statistically significant.
Results
Genistein treatment caused the inhibition of GR cell growth
The establishment of AsPC-1 GR and BxPC-3 GR cell lines was described as previously [17]. We first explore whether genistein induced inhibition of cell proliferation in GR cells. MTT assay was implemented to detect the cells proliferation after genistein treatment for 72 h. We found that genistein inhibited cell growth in a dose-dependent manner in both AsPC-1 GR cells and BxPC-3 GR cells (Figure 1A). Specifically, 60 μM genistein significantly suppressed cell growth in AsPC-1 GR cells. Interestingly, 20 μM genistein remarkably inhibited cell growth in BxPC-3 GR cells, indicating that BxPC-3 GR cells are sensitive to genistein treatment. We used 60 μM genistein for AsPC-1 GR and 20 μM genistein for BxPC-3 GR cells in the following study.
Figure 1.
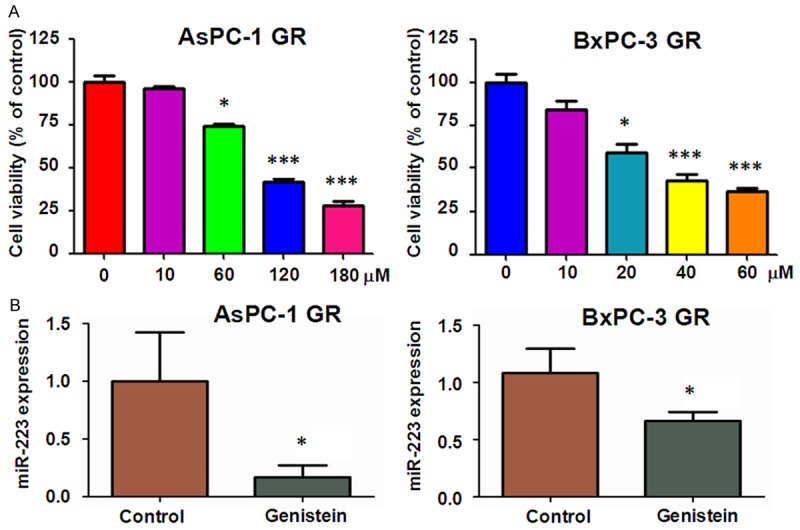
Effect of genistein on miR-223 expression and cell growth. A. MTT assay was conducted to detect cell proliferation in AsPC-1 GR and BxPC-3 GR cells after different concentrations of genistein treatment for 72 hours. B. Left panel: The expression of miR-223 was measured by real-time RT-PCR in AsPC-1 GR cells after 120 μM genistein treatment for 72 hours. Right panel: miR-223 expression was detected by real-time RT-PCR in BxPC-3 GR cells treated with 40 μM genistein for 72 hours.
Genistein down-regulated miR-223 expression in GR cells
Our previous study has shown that genistein could inhibit miR-223 expression in PC parental cells [24]. Then, we investigated whether genistein treatment causes down-regulation of miR-223 expression in GR cells. To achieve this goal, we performed RT-PCR analysis to measure the expression of miR-223 in GR cells after genistein treatment for 72 h. As shown in Figure 1B, miR-223 level was remarkably down-regulated in AsPC-1 GR cells and BxPC-3 GR cells treated with 120 μM genistein and 40 μM genistein, respectively (Figure 1B).
Genistein and miR-223 inhibitor reversed EMT in GR cells
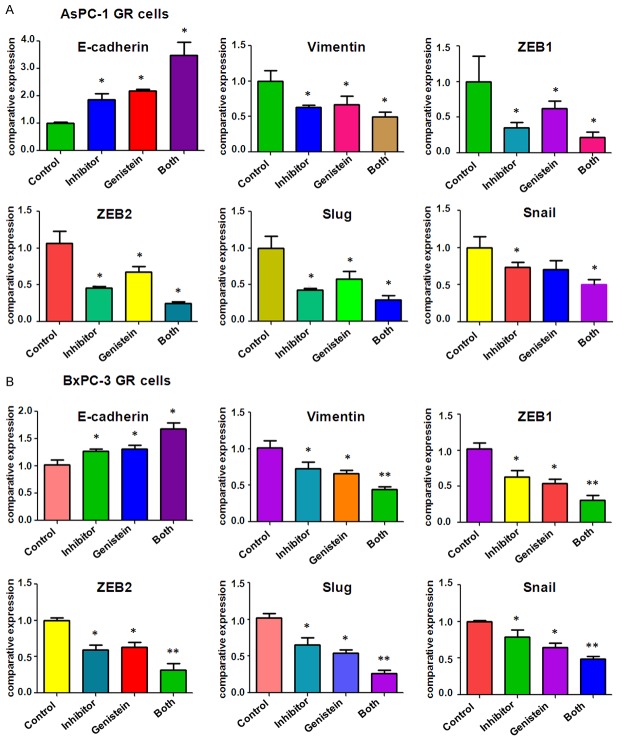
To investigate whether genistein could sensitize miR-223 inhibitor to reverse EMT in GR cells, we observed the cell morphology change in GR cells after genistein or miR-223 inhibitor or combination treatment. We found both genistein treatment and miR-223 inhibitor caused round cell morphology change from elongated, fibroblastoid morphology in GR cells (data not shown). Furthermore, combination of genistein with miR-223 inhibitor led to the remarkable morphology change in GR cells compared with genistein or miR-223 inhibitor alone. We further detected the expression of EMT molecular markers at mRNA and protein levels by real-time RT-PCR and Western blotting assays in GR cells after genistein or miR-223 inhibitor or combination treatment. We observed that both mRNA and protein expressions of E-cadherin were obviously up-regulated by genistein and miR-223 inhibitor treatments compared with the single treatment (Figures 2, 3). However, the expressions of mesenchymal markers, such as Slug, Vimentin, Snail, ZEB1 and ZEB2, were down-regulated to a greater degree in the combination treatment (Figures 2, 3).
Figure 2.
Effect of genistein and miR-223 inhibitor on EMT markers mRNA levels. A. The expression of EMT markers was measured by real-time RT-PCR in AsPC-1 GR treated with 60 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 hours. B. The expression of EMT markers was determined by real-time RT-PCR in BxPC-3 GR cells treated with 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 hours.
Figure 3.
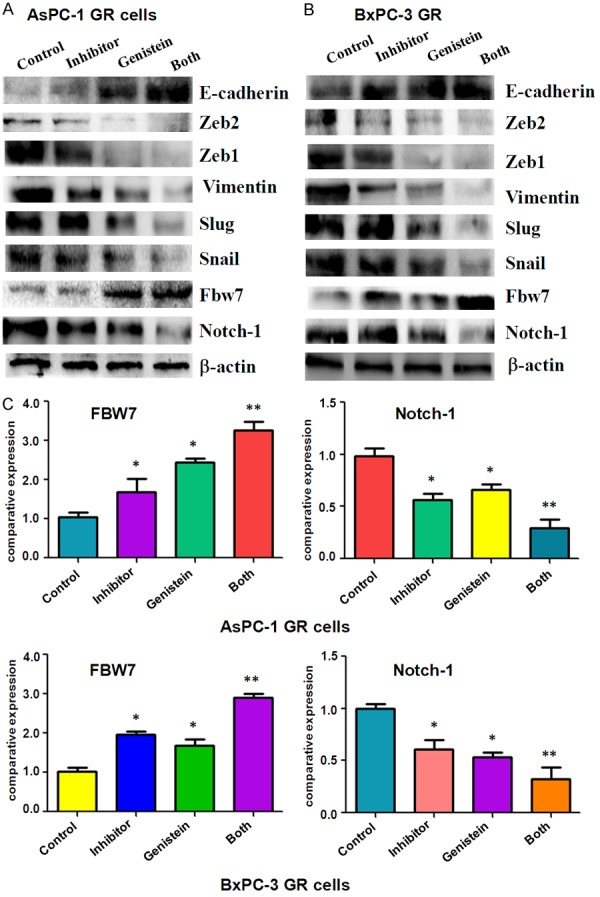
Effect of genistein and miR-223 inhibitor on EMT markers protein levels. A. The expression of EMT markers was measured by Western blotting analysis in AsPC-1 GR treated with 60 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 hours. B. The expression of EMT markers was detected by Western blotting analysis in BxPC-3 GR cells treated with 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 hours. C. Quantitative results are illustrated for expression of FBW7 and Notch-1 in panel A and panel B. *P<0.05; **P<0.01.
Genistein and miR-223 inhibitor increased Fbw7 expression
Fbw7 (F-box and WD repeat domain-containing 7) has been proved as a miR-223 target [33,34]. It has been known that Fbw7 suppressed EMT and subsequently increased chemo-sensitivity in hepatocellular carcinoma cells [35]. Notch-1 has been validated as one of FBW7 targets [36], and induces EMT in human cancers [37,38]. Thus, we further investigated the mRNA and protein expression of FBW7 and Notch-1 in GR cells treated with genistein and/or miR-223 inhibitor. We observed that genistein plus miR-223 treatment caused up-regulation of Fbw7 and down-regulation of Notch-1 in a deeper degree compared with the single treatment (Figure 3).
Genistein and miR-223 inhibitor reduces cell migration and invasion in GR cells
It is well known that during the process of EMT, cells acquire increased invasion and metastasis capacity [39]. Therefore, we performed the cells migration and invasion analysis after genistein and (or) miR-223 inhibitor treatment. Our Transwell migration and invasion assays showed that genistein and miR-223 inhibitor inhibited cell migration and invasion capacity (Figure 4A, 4B). Consistently, the wound healing assay revealed that the migration of GR cells was significantly inhibited by genistein and miR-223 inhibitor (Figure 4C). Next, the cells attachment and detachment assays were performed to further confirm the inhibition of motility effect by miR-223 inhibitor and genistein. As expected, genistein and miR-223 inhibitor treatment inhibited the cells detachment and attachment capacity to a greater degree compared to single treatment alone (Figure 5A, 5B).
Figure 4.
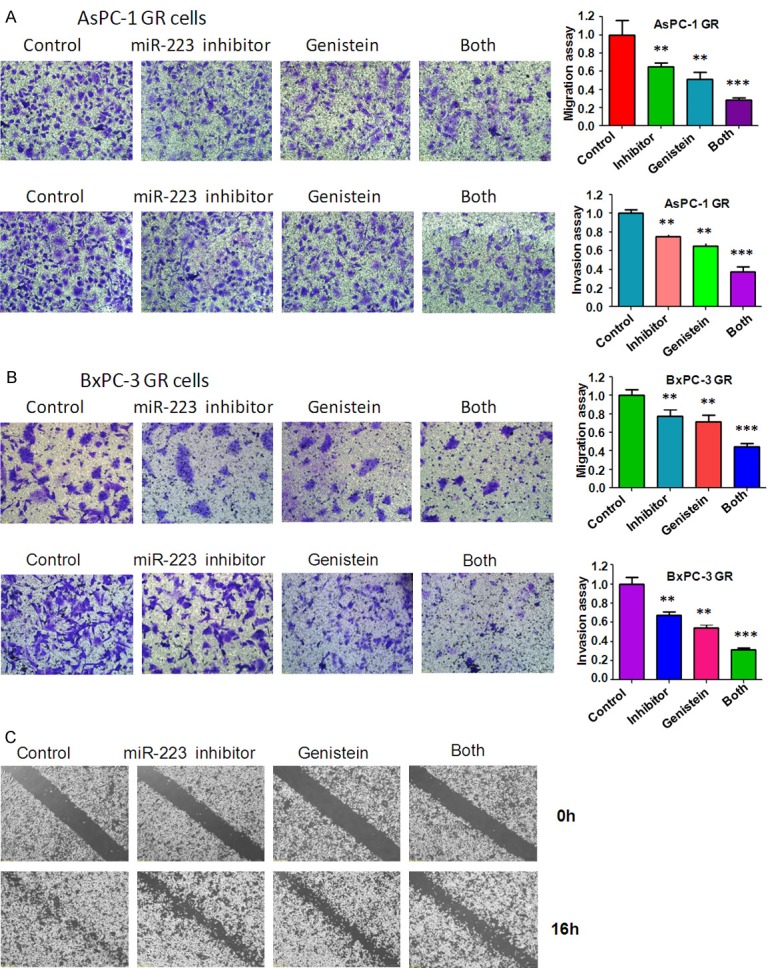
Effect of genistein and miR-223 inhibitor on cell migration and invasion. A. Cell migration and invasion were measured using Transwell in AsPC-1 GR cells after 60 μM genistein or 20 nmol/L miR-223 inhibitor or the combination treatments for 24 hours. B. Cell migration and invasion assays were performed using Transwell in BxPC-3 GR cells after 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination treatments for 24 hours. C. Cell migration was detected using Wound-healing assay in BxPC-3 GR cells after 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 16 hours.
Figure 5.
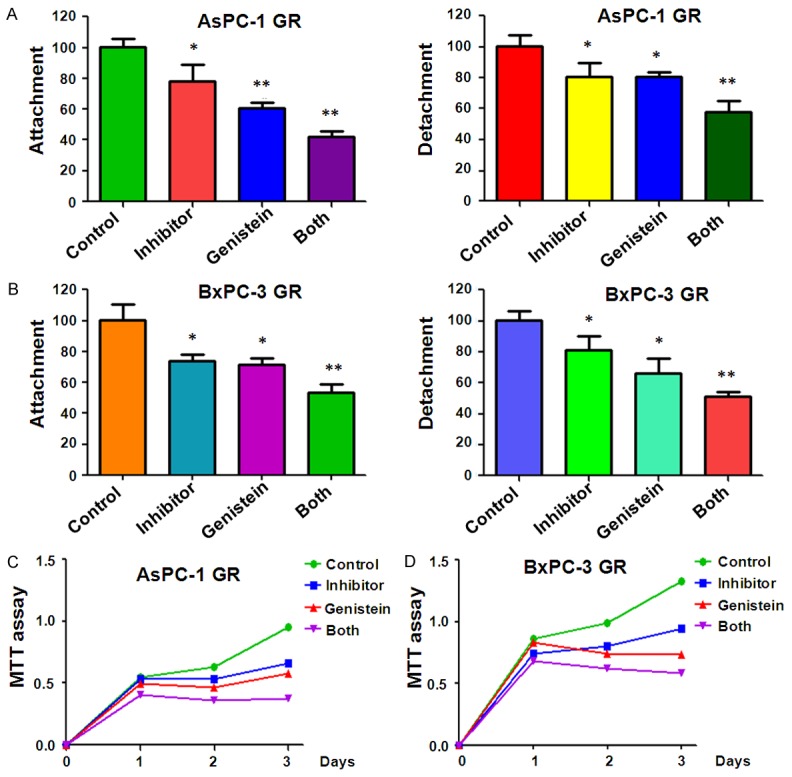
Effect of genistein and miR-223 inhibitor on cell attachment. A. The cell attachment and detachment were measured in AsPC-1 GR cells treated with 60 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 hours. B. The cell attachment and detachment were detected in BxPC-3 GR cells treated with 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination for 72 hours. C. MTT assay was conducted to detect cell sensitivity to gemcitabine in AsPC-1 GR cells after 60 μM genistein or 20 nmol/L miR-223 inhibitor or the combination treatments for 72 hours. D. MTT assay was performed to detect cell sensitivity to gemcitabine in BxPC-3 GR cells after 20 μM genistein or 20 nmol/L miR-223 inhibitor or the combination treatments for 72 hours.
Genistein and miR-223 inhibitor enhanced the GR cells to gemcitabine sensitivity
We performed MTT assay in GR cells after both genistein and miR-223 inhibitor treatments to determine whether genistein and miR-223 inhibitor enhanced the GR cells to gemcitabine sensitivity. Our MTT results showed that both genistein and miR-223 inhibitor caused the more significant growth inhibition than genistein alone and miR-223 inhibitor alone in GR cells (Figure 5C). These findings suggested combination of miR-223 inhibitor and genistein could significantly increase sensitivity of the GR cells to gemcitabine.
Genistein enhances the antitumor effects of miR-223 inhibitor in vivo
To assess the effects of genistein in combination of miR-223 inhibitor in vivo, nude mice were injected subcutaneously with BxPC-3 GR cells. We observed that administration of both genistein and miR-223 inhibitor clearly suppressed subcutaneous tumor growth compared with genistein or miR-223 inhibitor alone (Figure 6A). These results indicate that genistein could sensitize the anti-tumor activity of miR-223 inhibitor in vivo. We also investigated the expression of key EMT markers and Notch-1 pathway. We found that the expression of E-cadherin and Fbw7 was increased, but the expression of Notch-1 and other mesenchymal markers was decreased in BxPC-3 GR cells treated with genistein and miR-223 inhibitor in mice tumors (Figure 6B). The results validated that genistein could promote the antitumor function of miR-223 inhibitor through regulation of EMT and Notch-1 pathway.
Figure 6.
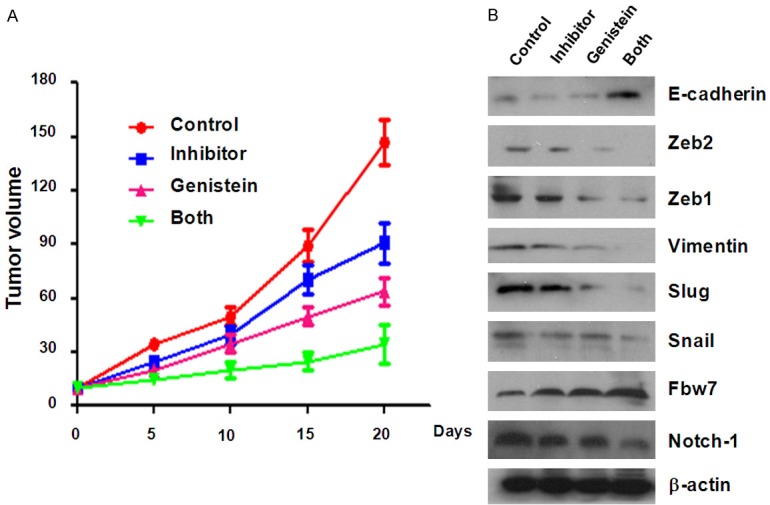
Genistein enhances the antitumor effects of miR-223 inhibitor in vivo. A. BxPC-3 GR cells were subcutaneously injected into the right flank of five-week-old male nude mice. After 2 weeks for palpable subcutaneous tumors to form, themice were separated into four groups (each group, n=5), namely vehicle (100 μl control antagomir, diluted in PBS) by injection to intratumor), genistein alone, miR-223 inhibitor alone, and genistein plus miR-223 inhibitor. Genistein was given by gavage (15 mg/kg daily for 20 days). The control antagomir or 100 μL miR-223 inhibitor (diluted in PBS at 2 mg/ml) was injected twice a week for 3 weeks by way of multiple-center intratumor injection, separately. The tumor size was measured with a caliper every 5 days. Tumor volume was calculated as length × width × height/2. B. Mice of each group were killed after three weeks injections, and the xenografted tumors were excised and examined Western blotting. The expression of Notch-1 and EMT markers was measured by Western blotting in mice tumors.
Discussion
MicroRNAs including miR-223 are well known to be involved in the development and progression of human cancers. Increasing evidence has shown that miR-223 could be an oncogene in various human cancers. To support the role of miR-223, one study has demonstrated that miR-223 was overexpressed in human gastric cancer tissues and enhanced the cisplatin resistance through down-regulation of Fbw7 [40]. Moreover, it has been found that miR-223 could increase the proliferation and invasion capacity of glioblastoma cells by suppressing tumor suppressor PAX6 [41]. Similarly, miR-223 was up-regulated during PC progression using the KrasG12D; Pdx1-Cre mouse model [42]. Importantly, increased expression of miR-223 was observed in PC tissues and PC patients plasma [43], suggesting that miR-223 could be an oncogene in PC.
Recently, multiple studies have indicated that miRNAs regulate EMT transcription factors or EMT-related pathway [44,45]. For example, miR-34 has been reported to inhibit EMT procession through suppressing Snail in colorectal cancer cells [46]. Another study has shown that IL6-dependent STAT3 activation has obviously decreased miR-34 expression [9]. Interestingly, miR-34 subsequently targeted to decrease the expression of IL-6 receptor (IL-6R), which mediates IL6/STAT3 activation in human colorectal cancer cells. Thus, EMT was mediated by IL-6R/STAT3/miR-34a feedback loop [9]. One group also reported that increased miR-409 expression is correlated with EMT progression and prostate cancer metastasis [47,48]. Additionally, a direct correlation between the expression of miR-101 and the inhibition of ZEB1 and ZEB2 has been validated in ovarian carcinoma [49]. In line with these findings, our previous study showed that down-regulation of miR-223 significantly inhibited cell growth and induced apoptosis in PC cells [24]. We reported that down-regulation of miR-223 reversed drug resistance-mediated EMT and enhanced gemcitabine sensitivity in PC GR cells [17]. It is indicated that miR-223 may be a potential target for pancreatic cancer therapy.
Genistein has been studied to explore the mechanism of anticancer activity in PC [50,51]. However, genistein as well as other phytochemicals showed two deficiencies: high pleiotropy and low bioavailability, suggesting a synergistic effect with other anti-cancer drugs that may enhance the chemotherapeutic efficacy [51]. But very little work has been done on the combination of genistein and miRNA for the pancreatic cancer therapy. In this study, we focus on the synergistic effect of genistein and miR-223 inhibitor in the PC GR cells. We observed genistein plus miR-223 inhibitor led to a more extensively reversal effect from EMT to MET compared to genistein alone and miR-223 inhibitor alone. Furthermore, genistein and miR-223 inhibitor treatment inhibited the cell motility and invasion capacity and enhanced gemcitabine sensitivity to a greater degree compared to single treatment alone. In summary, these findings suggest that the combination of miR-223 inhibitor and genistein could be a potential therapeutic strategy for pancreatic cancer.
Acknowledgements
This work was supported by grant from National Natural Science Foundation of China (NSFC 81172087, 81502126 and 81572936) and the priority academic program development of Jingsu higher education institutions. This work was also supported in part by the Natural Science Research key Project of Education Office of Anhui Province (KJ2012A196 and KJ2014A153) and Natural Science Foundation of Anhui Province (1508085SMH232) and the program for graduate research of Bengbu Medical College (Byycxz 1404 and Byycxz 1405).
Disclosure of conflict of interest
None.
References
- 1.Society AC. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Thota R, Pauff JM, Berlin JD. Treatment of metastatic pancreatic adenocarcinoma: a review. Oncology (Williston Park) 2014;28:70–74. [PubMed] [Google Scholar]
- 3.Plate JM. Advances in therapeutic vaccines for pancreatic cancer. Discov Med. 2012;14:89–94. [PubMed] [Google Scholar]
- 4.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Guan J, Zhang H, Wen Z, Gu Y, Cheng Y, Sun Y, Zhang T, Jia C, Lu Z, Chen J. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014;345:132–139. doi: 10.1016/j.canlet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, Miele L, Sarkar FH, Wang Z. The Role of EMT in Pancreatic Cancer Progression. Pancreat Disord Ther. 2013;2 doi: 10.4172/2165-7092.1000e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Wang D, Zhang L, Xie X, Wu Y, Liu Y, Shao G, Su Z. Upregulation of autophagy by hypoxia-inducible factor-1alpha promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep. 2014;32:935–942. doi: 10.3892/or.2014.3298. [DOI] [PubMed] [Google Scholar]
- 8.Meng F, Wu G. The rejuvenated scenario of epithelial-mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev. 2012;31:455–467. doi: 10.1007/s10555-012-9379-3. [DOI] [PubMed] [Google Scholar]
- 9.Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, Slotta-Huspenina J, Bader FG, Greten FR, Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellanos JA, Merchant NB, Nagathihalli NS. Emerging targets in pancreatic cancer: epithelial-mesenchymal transition and cancer stem cells. Onco Targets Ther. 2013;6:1261–1267. doi: 10.2147/OTT.S34670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, VandenBoom TG 2nd, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 14.Bai WD, Ye XM, Zhang MY, Zhu HY, Xi WJ, Huang X, Zhao J, Gu B, Zheng GX, Yang AG, Jia LT. MiR-200c suppresses TGF-beta signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer. 2014;135:1356–1368. doi: 10.1002/ijc.28782. [DOI] [PubMed] [Google Scholar]
- 15.Izumchenko E, Chang X, Michailidi C, Kagohara L, Ravi R, Paz K, Brait M, Hoque MO, Ling S, Bedi A, Sidransky D. The TGFbeta-miR200-MIG6 pathway orchestrates the EMT-associated kinase switch that induces resistance to EGFR inhibitors. Cancer Res. 2014;74:3995–4005. doi: 10.1158/0008-5472.CAN-14-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M, Zhang W, Chen W, Pan C, Liu Q, Song E, Li J. MiR-200b and miR-15b regulate chemotherapy-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene. 2012;31:432–445. doi: 10.1038/onc.2011.263. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, Wang Z. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740–1749. doi: 10.18632/oncotarget.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiyomaru T, Yamamura S, Zaman MS, Majid S, Deng G, Shahryari V, Saini S, Hirata H, Ueno K, Chang I, Tanaka Y, Tabatabai ZL, Enokida H, Nakagawa M, Dahiya R. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS One. 2012;7:e43812. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Wang SX, Zhou ZQ, Wang Z, Zhang YG, Zhang Y, Zhao P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-kappaB) pathway. Tumour Biol. 2014;35:11483–11488. doi: 10.1007/s13277-014-2487-7. [DOI] [PubMed] [Google Scholar]
- 21.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29:465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao X, Liu Z, Wang R, Wang J, Zhang S, Cai X, Wu K, Bergan RC, Xu L, Fan D. Genistein suppresses FLT4 and inhibits human colorectal cancer metastasis. Oncotarget. 2014;6:3225–3239. doi: 10.18632/oncotarget.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng F, Miele L, Sarkar FH, Xia J, Wang Z. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr Drug Targets. 2013;14:1150–1156. doi: 10.2174/13894501113149990187. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Choi KC, Hwang KA. Genistein suppressed epithelial-mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-beta signaling pathway. Phytomedicine. 2015;22:993–999. doi: 10.1016/j.phymed.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Arzuman L, Beale P, Proschogo N, Yu JQ, Huq F. Combination of Genistein and Cisplatin with Two Designed Monofunctional Platinum Agents in Human Ovarian Tumour Models. Anticancer Res. 2015;35:6027–6039. [PubMed] [Google Scholar]
- 27.Chen QH, Yu K, Zhang X, Chen G, Hoover A, Leon F, Wang R, Subrahmanyam N, Addo Mekuria E, Harinantenaina Rakotondraibe L. A new class of hybrid anticancer agents inspired by the synergistic effects of curcumin and genistein: Design, synthesis, and anti-proliferative evaluation. Bioorg Med Chem Lett. 2015;25:4553–4556. doi: 10.1016/j.bmcl.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 28.Montales MT, Simmen RC, Ferreira ES, Neves VA, Simmen FA. Metformin and soybean-derived bioactive molecules attenuate the expansion of stem cell-like epithelial subpopulation and confer apoptotic sensitivity in human colon cancer cells. Genes Nutr. 2015;10:49. doi: 10.1007/s12263-015-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Fang B, Zeng F, Pang H, Zhang J, Shi Y, Wu X, Cheng L, Ma C, Xia J, Wang Z. Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol Lett. 2014;231:82–91. doi: 10.1016/j.toxlet.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X, Chen S, Chen C, Wang Z. Acquisition of epithelial-mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br J Cancer. 2014;110:1958–1967. doi: 10.1038/bjc.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou Y, Chen C, Yang Y, Miele L, Sarkar FH, Chen Y, Wang Z. Chemoresistance to gemcitabine in hepatoma cells induces epithelial-mesenchymal transition and involves activation of PDGF-D pathway. Oncotarget. 2013;4:1999–2009. doi: 10.18632/oncotarget.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan Y, Qin S, Hou X, Qian X, Xia J, Li Y, Wang R, Chen C, Yang Q, Miele L, Wu Q, Wang Z. Proteomic-based analysis for identification of proteins involved in 5-fluorouracil resistance in hepatocellular carcinoma. Curr Pharm Des. 2014;20:81–87. doi: 10.2174/138161282001140113125143. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Sengupta T, Kukreja L, Minella AC. MicroRNA-223 regulates cyclin E activity by modulating expression of F-box and WD-40 domain protein 7. J Biol Chem. 2010;285:34439–34446. doi: 10.1074/jbc.M110.152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Guo Y, Liang X, Sun M, Wang G, De W, Wu W. MicroRNA-223 functions as an oncogene in human gastric cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol. 2012;138:763–774. doi: 10.1007/s00432-012-1154-x. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Zhang W, Gao F, Liu YX, Chen ZY, Cheng LY, Xie SF, Zheng SS. FBW7 increases chemosensitivity in hepatocellular carcinoma cells through suppression of epithelial-mesenchymal transition. Hepatobiliary Pancreat Dis Int. 2014;13:184–191. doi: 10.1016/s1499-3872(14)60029-1. [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Azmi AS, Aboukameel A, Kauffman M, Shacham S, Abou-Samra AB, Mohammad RM. Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget. 2014;5:3444–3454. doi: 10.18632/oncotarget.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao XJ, Liu JW, Zhang QG, Zhang JJ, Xu HT, Liu HJ. Nobiletin inhibited hypoxia-induced epithelial-mesenchymal transition of lung cancer cells by inactivating of Notch-1 signaling and switching on miR-200b. Pharmazie. 2015;70:256–262. [PubMed] [Google Scholar]
- 38.Fender AW, Nutter JM, Fitzgerald TL, Bertrand FE, Sigounas G. Notch-1 Promotes Stemness and Epithelial to Mesenchymal Transition in Colorectal Cancer. J Cell Biochem. 2015;116:2517–2527. doi: 10.1002/jcb.25196. [DOI] [PubMed] [Google Scholar]
- 39.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Jin W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34:28. doi: 10.1186/s13046-015-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang BS, Luo QZ, Han Y, Li XB, Cao LJ, Wu LX. microRNA-223 promotes the growth and invasion of glioblastoma cells by targeting tumor suppressor PAX6. Oncol Rep. 2013;30:2263–2269. doi: 10.3892/or.2013.2683. [DOI] [PubMed] [Google Scholar]
- 42.Rachagani S, Macha MA, Menning MS, Dey P, Pai P, Smith LM, Mo YY, Batra SK. Changes in microRNA (miRNA) expression during pancreatic cancer development and progression in a genetically engineered KrasG12D; Pdx1-Cre mouse (KC) model. Oncotarget. 2015;6:40295–309. doi: 10.18632/oncotarget.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komatsu S, Ichikawa D, Miyamae M, Kawaguchi T, Morimura R, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H, Shiozaki A, Ikoma H, Okamoto K, Taniguchi H, Otsuji E. Malignant potential in pancreatic neoplasm; new insights provided by circulating miR-223 in plasma. Expert Opin Biol Ther. 2015;15:773–785. doi: 10.1517/14712598.2015.1029914. [DOI] [PubMed] [Google Scholar]
- 44.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, Siefker-Radtke A, Dinney C. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diaz-Lopez A, Moreno-Bueno G, Cano A. Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives. Cancer Manag Res. 2014;6:205–216. doi: 10.2147/CMAR.S38156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, Rowe RG, Lee S, Maher CA, Weiss SJ, Yook JI. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol. 2011;195:417–433. doi: 10.1083/jcb.201103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josson S, Gururajan M, Hu P, Shao C, Chu GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, Lichterman J, Nandana S, Li Q, Rogatko A, Berel D, Posadas EM, Fazli L, Sareen D, Chung LW. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin Cancer Res. 2014;20:4636–4646. doi: 10.1158/1078-0432.CCR-14-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Josson S, Gururajan M, Sung SY, Hu P, Shao C, Zhau HE, Liu C, Lichterman J, Duan P, Li Q, Rogatko A, Posadas EM, Haga CL, Chung LW. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34:2690–2699. doi: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 49.Guo F, Cogdell D, Hu L, Yang D, Sood AK, Xue F, Zhang W. MiR-101 suppresses the epithelial-to-mesenchymal transition by targeting ZEB1 and ZEB2 in ovarian carcinoma. Oncol Rep. 2014;31:2021–2028. doi: 10.3892/or.2014.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azimi H, Khakshur AA, Abdollahi M, Rahimi R. Potential New Pharmacological Agents Derived From Medicinal Plants for the Treatment of Pancreatic Cancer. Pancreas. 2015;44:11–15. doi: 10.1097/MPA.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 51.Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]