Abstract
Achaetescute-like 2 (ASCL2), a basic helix-loop-helix (bHLH) transcription factor, plays an important role in the determination of neuronal precursors in the central and peripheral nervous system and involves in tumor progression. However, the role of ASCL2 expression in the osteosarcoma prognosis has not been elaborated. This study aimed to evaluate ASCL2 expression level in osteosarcoma and assess its prognostic value for patients. ASCL2 protein expression was detected by immunohistochemistry (IHC) in 73 cases of osteosarcoma. Kaplan-Meier analysis and Cox regression analysis were performed to evaluate the prognostic significance of ASCL2. Immunohistochemistry analysis showed that the overall survival and metastasis-free survival of patients with positive ASCL2 expression were significantly shorter than patients with negative expression (both P<0.01). Multivariate Cox analysis identified ASCL2 expression as an independent prognostic factor to predict poor overall survival and metastasis-free survival (both P<0.01). Overexpression of ASCL2 expression greatly promoted cell proliferation and enhanced migration and invasion in vitro. This study indicates that increased expression of ASCL2 in primary osteosarcoma is a novel biomarker for predicting the development of metastases and poor outcomes of the patients.
Keywords: Osteosarcoma, Achaetescute-like 2, prognosis, metastasis
Introduction
Osteosarcoma is the most common primary malignant bone tumor and the second highest cause of cancer-related death in children and adolescents [1]. In recent years, advanced progress in diagnosis and neoadjuvant chemotherapy for osteosarcoma, the 5-year survival remains poor due to metastasis and local relapse after resection of primary osteosarcoma and with a very high case fatality and disability rate [2]. Therefore, a novel and more reliable gene marker is imperative to be identified to facilitate the correlation with clinicopathologic characteristics and prognosis of osteosarcoma.
Achaetescute-like 2 (ASCL2) is a transcription factor containing the basic helix-loop-helix (bHLH) domain and a downstream target of Wnt signaling in intestinal stem cells [3]. Several studies have demonstrated that ASCL2 is overexpressed in colorectal cancer [4-6]. In addition, overexpression of ASCL2 has the potential to shift the hierarchy of stem/progenitor cells in liver metastases, and affect the clinical outcome of these tumors [6]. Desregulated expression of ASCL2 is thought to be involved in cell proliferation, invasion, migration, and epithelial-mesenchymal transition in colorectal cancer [7,8]. However, the significance of ASCL2 in osteosarcoma remains unknown. In the present study, we first investigated ASCL2 expression in human osteosarcoma patients by immunohistochemistry (IHC) assays and determined whether the expression of ASCL2 can be used as a prognostic and metastasis marker of human osteosarcoma. Subsequently, we evaluated the functional role of ASCL2 in proliferation, migration and invasion of osteosarcoma cells. Our results suggest that elevated expression of ASCL2 is associated with metastasis of osteosarcoma and poor prognosis.
Materials and methods
Ethics statement
The Medical Ethics Committee of the Xinqiao Hospital of Third Military Medical University has approved this protocol, and all patients provided written informed consent. All specimens were handled and stored anonymously according to ethical and legal standards.
Patients and tissue samples
Primary osteosarcoma samples were collected from 73 patients who had undergone surgical treatment for osteosarcoma with pathologic identification in Xinqiao Hospital, Third Military Medical University from 1995 to 2014. Patients were enrolled in this retrospective study based on the following criteria: diagnosis of osteosarcoma with histopathological assessment, no prior anticancer treatment (radiotherapy or chemotherapy), and the availability of complete clinicopathologic and follow-up data. Tumor tissue specimens were grouped according to the sixth edition of the TNM classification of the International Union against Cancer (UICC).
Immunohistochemistry and evaluation
Immunohistochemical examination of ASCL2 was performed as described previously [9]. Briefly, after deparaffinising, rehydrating and blocking, the tissue sections of osteosarcoma were boiled in EDTA buffer (pH 8.0) for 15 min. The sections were then incubated with anti-ASCL2 (dilution 1:75; Millipore, USA) at 4°C overnight. Following incubation with secondary antibody (DAKO, Denmark), the sections were visualised using diaminobenzidine solution (DAKO) and lightly counterstained with haematoxylin. Negative control slides with the primary antibodies omitted were included for all assays.
The staining of ASCL2 was determined semi-quantitatively according to the intensity (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining) and the percentage of positive cells (0: none or <5%; 1: 5% to 20%; 2: 21% to 40%; 3: >40%). Scores of 0 to 2 were expected negative, and scores of 3 to 6 were expected positive. Cells were counted in at least three fields (at × 400 magnification) in the tumor areas. The scores were evaluated by two independent pathologists who were blinded to the pathological parameters and clinical outcomes of the patients.
Cell line and cell culture
The human osteosarcoma cell line SaOS and MG-63 were purchased from the American Type Culture Collection (Rockville, USA). The osteosarcoma cell line SaOS and MG-63 were incubated in RPMI-1640 (Life Technologies, USA) supplemented with 10% fetal bovine serum (Gibco, USA). All cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
ASCL2 overexpression assay
Lentivirus particles expressing ASCL2 were produced by GenePharma (Shanghai, China). SaOS and MG-63 cells were transfected with lentivirus particles using LV5 (EF-1aF/GFP&Puro) vector with ASCL2 insert. Stably transfected cells with GFP were isolated under puromycin selection (Solarbio, Beijing, China) or sorted with a flow-cytometric sorting system (BD FACS Aria II; BD Biosciences, USA).
Cell proliferation assay
Cell proliferation was performed by using Cell Counting Assay Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s protocol. Briefly, 5 × 103 cells were harvested after 24, 48, 72 and 96 hours. 10 μL CCK-8 solution was added to each well, the cells were incubated for 1 hour, and the absorbance at 490 nm was measured by spectrophotometer (Bio-Rad, USA). All experiments were performed three times.
Wound healing migration and Matrigel invasion assays
For wound-healing migration assays, 1 × 105 cells were plated into in each well of 12-well plates. Three parallel lines were made in confluent cell cultures with a 200 ml tip. The wounds were observed at 0 and 48 h after scratching separately, and photographed via microscope at each time point. The distance between the two edges of the wound width was randomly quantified at 10 sites in all images. The average distance migrated by the cells was measured using a microscope calibrated with an ocular micrometer. The Matrigel invasion assay was performed with the BD BioCoatTM MatrigelTM Invasion Chamber (BD, USA) following the manufacturer’s instructions. The cells (5 × 104 cell/plate) were seeded into the upper chamber of each well in serum-free medium, and complete medium was put into the bottom chamber. After incubated for 24 h, non-invading cells were removed by scrubbing from the upper surface of the membrane with cotton-tipped swabs. Following the processes of fixation in 100% methanol and staining in hematoxylin, the invading cells were counted in three images of each membrane under a microscope using a × 200 objective.
Western blotting
Western blotting was carried out by standard procedures as described previously. In brief, protein samples were separated on 10% (w/v) SDS gels and transferred onto PVDF membranes (Millipore, USA). The membranes were blocked with 5% milk proteins in TBST for 1 h at 37°C and then incubated with primary antibodies (MMP2, 1:500 dilution, Sigma-Aldrich, USA) overnight at 4°C. The membranes were then incubated with HRP-conjugated anti-mouse secondary antibodies for 1 h at 37°C. Immunolabeling was detected using ECL reagent (Thermo Scientific, USA).
Statistical analysis
All group data are presented as mean ± SD. All statistical analyses were performed using SPSS version 13.0 statistical programs (SPSS, Chicago, IL). For all the analyses, a two-sided P value <0.05 was considered statistically significant. For the IHC analysis, the correlation of ASCL2 expression with the clinicopathologic data was analyzed using a Chi-square test. The prognostic values were evaluated by univariate Kaplan-Meier survival analysis and multivariate Cox proportional hazard model analysis. Overall survival (OS) was defined as the time from diagnosis until the date of either death or the last follow-up. For the metastasis-free survival (MFS) analysis, the duration was defined as the time from diagnosis until the occurrence of metastasis. If these patients had metastatic disease at diagnosis, this was considered time 0.
Result
Patient characteristics
In this study, it was shown that the demographic and clinicopathological data of the cases in Table 1. Our study comprised of 73 osteosarcoma patients with 34 (46.6%) males and 39 (53.4%) females. The mean age was 27.4 ± 11.3 years. All patients featured osteosarcoma with I-III Enneking stage and received the same curative resection treatment. Follow-up was ava-ilable for all patients, with a median time of 37.4 months (range: 0 months to 89 months). Seventeen patients (17/73, 23.3%) died during the follow-up period, primarily due to metastases (14/17, 82.4%). Forty-five patients (45/73, 61.6%) develo-ped metastases at a mean of 16.2 months (range 0-129 months). Of these patients, 26 had metastases in the lung, and 5 had metastases in bones (five patients had both lung and bone metastases).
Table 1.
Patients’ characteristics
| Patients information | Number (n) |
|---|---|
| Sex | |
| Male | 34 |
| Female | 39 |
| Age at diagnosis | |
| Average | 27.4 years |
| Median | 18.3 years |
| Range | 9-72 years |
| Localization | |
| Femur | 29 |
| Tibia | 16 |
| Humerus | 11 |
| Fibula | 8 |
| Others | 9 |
| Metastases | |
| Yes (total) | 45 |
| Yes (at initial diagnosis) | 9 |
| No | 28 |
| Time to metastases average | 16.2 |
| Time to metastases median | 11.7 |
| Time to metastases range | 0-67 months |
| Follow-up | |
| Average | 37.4 |
| Median | 48.3 |
| Range | 0-89 months |
| Survival | |
| Died | 17 |
| Alive | 56 |
| Histological classification | |
| Osteoblastic | 57 |
| Chondroblastic | 12 |
| Others | 4 |
Clinicopathologic correlation of ASCL2 expression in osteosarcoma patients
To examine the expression of ASCL2 in human osteosarcoma patients, ASCL2 staining was positive in 48 osteosarcoma patients (65.8%). Microscopic observations indicated that ASCL2 was both expressed in the cytoplasm and nucleus of tumor cells (Figure 1). To elucidate the biologic significance, we investigated the association of patient clinicopathologic features and ASCL2 expression levels (Table 2). Although positive staining of ASCL2 was present in both non-metastatic and metastatic tumors, the percentage of ASCL2-positive staining in osteosarcoma with metastases (39/45, 86.7%) was significantly higher than in the cases without metastases (9/28, 32.1%; P<0.001, Table 2). Positive ASCL2 expression was more frequent in osteosarcoma tissues with high Enneking staging (P<0.05). However, no significant difference was observed between the expression of ASCL2 and patient gender, age, tumor location, histological classification, and histological grade.
Figure 1.
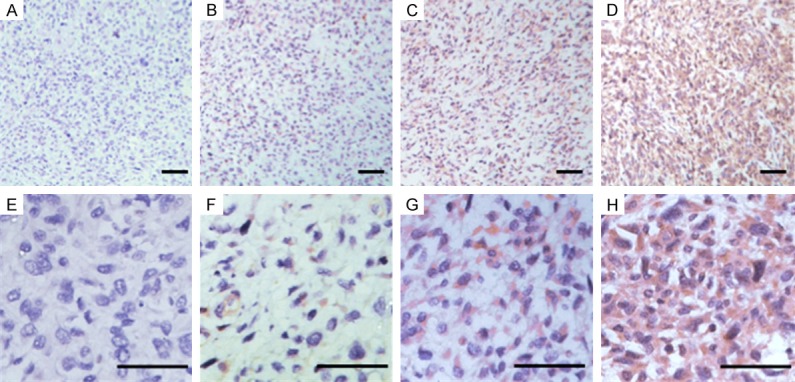
Representative immunohistochemical staining for ASCL2 in osteosarcoma tissues. Representative ASCL2 staining samples at low magnification at levels of 0, 1, 2, and 3 (A-D). Representative ASCL2 staining samples at high magnification at levels of 0, 1, 2, and 3 (E-H).
Table 2.
Relationship between ASCL2 and clinicopathologic factors of Patients
| Variable | ASCL2 expression | ||||
|---|---|---|---|---|---|
|
| |||||
| Total (N=73) | Positive | Negative | X2 | P-values | |
| Gender | |||||
| Male | 44 (60.3%) | 26 | 18 | 2.183 | 0.140 |
| Female | 29 (39.7%) | 22 | 7 | ||
| Age | |||||
| ≤20 | 42 (57.5%) | 25 | 17 | 1.705 | 0.192 |
| >20 | 31 (42.5%) | 23 | 8 | ||
| Tumor Location | |||||
| Femur | 29 (39.7%) | 18 | 11 | 3.971 | 0.410 |
| Tibia | 16 (21.9%) | 11 | 5 | ||
| Humerus | 11 (15.1%) | 8 | 3 | ||
| Fibula | 8 (11.0%) | 7 | 1 | ||
| Others | 9 (12.3%) | 4 | 5 | ||
| Histological classification | |||||
| Osteoblastic | 57 (78.1%) | 37 | 20 | 4.615 | 0.100 |
| Chondroblastic | 12 (16.4%) | 10 | 2 | ||
| Others | 4 (5.5%) | 1 | 3 | ||
| Metastasis | |||||
| Yes | 45 (61.6%) | 39 | 6 | 22.787 | <0.001 |
| No | 28 (38.4%) | 9 | 19 | ||
| Histological grade | |||||
| I | 19 (26.0%) | 10 | 9 | 2.992 | 0.224 |
| II | 36 (49.3%) | 27 | 9 | ||
| III | 18 (24.7%) | 11 | 7 | ||
| Enneking staging | |||||
| I | 8 (11.0%) | 3 | 5 | 8.759 | 0.033 |
| IIA | 27 (37.0%) | 11 | 16 | ||
| IIB | 32 (43.8%) | 23 | 9 | ||
| III | 6 (8.2%) | 5 | 1 | ||
Abbreviation: ASCL2, Achaetescute-like 2.
Prognostic effect of ASCL2 expression in patients with osteosarcomas
Further analyses of the patient samples indicated that the 3-year OS and MFS rates were 77.8% and 41.0% for the total study cases, respectively. Importantly, the negative ASCL2 group had significantly greater survival rates for 3-year OS and MFS than did the positive ASCL2 group (87.2% vs. 61.8%, P = 0.006, and 85.7% vs. 48.7%, P = 0.004, Figure 2) by the Kaplan-Meier method and a log-rank test. Interestingly, histological grade and Enneking staging were still significant prognostic factors for both OS and MFS (P<0.01; Table 3), suggesting that ASCL2 was an independent biomarker for OS and MFS in addition to histological grade and Enneking staging. We then performed multivariate Cox regression analysis, which revealed that ASCL2 expression, histological grade and Enneking staging were indeed independent prognostic factors for OS and MFS (P<0.05; Table 4). These data indicated that ASCL2 may be a significant and novel biomarker for evaluating the prognoses of osteosarcoma patients.
Figure 2.
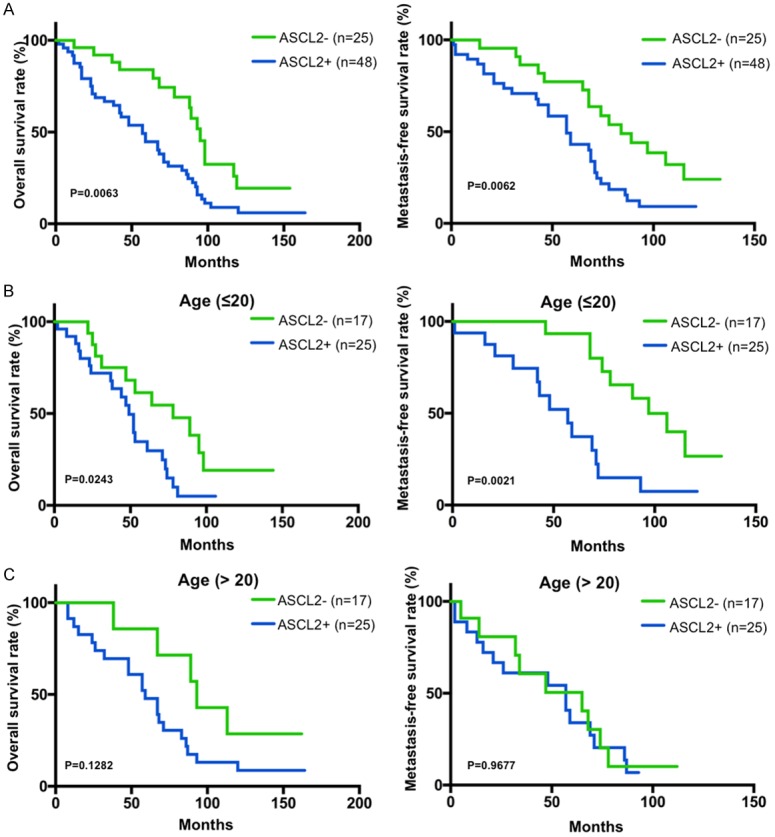
Positive ASCL2 immunohistochemical staining was associated with poor prognosis and survival. A. Overall survival (OS) and metastasis-free survival (MFS) of ASCL2 positive or negative patients was determined by Kaplan-Meier analysis. B. OS and MFS of ASCL2 positive or negative patients younger than 20 years old (≤ 20). C. OS and MFS of ASCL2 positive or negative patients elder than 20 years old (>20).
Table 3.
Clinicopathologic patient characteristics and univariate survival analysis
| Variable | Patients (n = 73) | 3-y OS Rate (%) | P value | 3-y MFS Rate (%) | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 44 (60.3%) | 75.2% | 0.423 | 37.5% | 0.253 |
| Female | 29 (39.7%) | 81.7% | 46.2% | ||
| Age at diagnosis | |||||
| ≤20 | 42 (57.5%) | 63.5% | 0.515 | 59.5% | 0.438 |
| >20 | 31 (42.5%) | 74.8% | 54.8% | ||
| Tumor Location | |||||
| Femur | 29 (39.7%) | 83.2% | 0.542 | 64.3% | 0.632 |
| Tibia | 16 (21.9%) | 67.3% | 37.5% | ||
| Humerus | 11 (15.1%) | 72.5% | 54.3% | ||
| Fibula | 8 (11.0%) | 80.2% | 63.2% | ||
| Others | 9 (12.3%) | 78.5% | 56.8% | ||
| Histological classification | |||||
| Osteoblastic | 57 (78.1%) | 64.3% | 0.254 | 46.3% | 0.631 |
| Chondroblastic | 12 (16.4%) | 74.2% | 53.6% | ||
| Others | 4 (5.5%) | 81.6% | 67.2% | ||
| Metastasis | |||||
| Yes | 45 (61.6%) | 38.5% | <0.001 | 14.6% | <0.001 |
| No | 28 (38.4%) | 89.5% | 81.7% | ||
| Histological grade | |||||
| I | 19 (26.0%) | 87.9% | 0.003 | 79.3 | 0.002 |
| II | 36 (49.3%) | 62.6% | 54.2 | ||
| III | 18 (24.7%) | 50.4% | 42.3 | ||
| Enneking staging | |||||
| I | 8 (11.0%) | 100% | <0.001 | 100% | <0.001 |
| IIA | 27 (37.0%) | 84.2% | 63.2% | ||
| IIB | 32 (43.8%) | 52.4% | 46.3% | ||
| III | 6 (8.2%) | 0% | 0% | ||
| ASCL2 | |||||
| Negative | 25 (34.2%) | 87.2% | 0.006 | 85.7 | 0.004 |
| Positive | 48 (65.8%) | 61.8% | 48.7 | ||
Abbreviation: OS, Overall survival; MFS, Metastasis-free survival; ASCL2, Achaetescute-like 2.
Table 4.
Multivariate analysis of factors associated with overall survival and metastasis-free survival
| Variable | HR (95% Cl) | P value |
|---|---|---|
| OS | ||
| ASCL2 (+ vs. -) | 1.732 (1.256-3.508) | 0.033 |
| Histological grade | 1.668 (1.043-2.667) | 0.015 |
| Enneking staging | 2.963 (1.878-4.675) | <0.001 |
| MFS | ||
| ASCL2 (+ vs. -) | 2.673 (1.077-4.582) | 0.031 |
| Histological grade | 1.544 (0.994-2.399) | 0.053 |
| Enneking staging | 5.656 (1.952-3.836) | <0.001 |
Abbreviation: ASCL2, Achaetescute-like 2.
As osteosarcoma is the second highest cause of cancer-related death in children and young adolescents [10], we next analyzed the OS and MFS in patients younger than 20 years old or elder than 20 years old. Kaplan-Meier analysis showed that young patients (≤20 years old) with positive ASCL2 expression had significantly worse OS and MFS than those with negative ASCL2 expression (Figure 2B; P = 0.0021). However, it was not significantly different in the patients elder than 20 years old between positive ASCL2 expression and negative expression (Figure 2C). It suggested that ASCL2 would be more accurate biomarker for evaluating OS and MFS of the patients within children and young adolescents (≤20 years old).
ASCL2 promotes osteosarcoma cell proliferation
Since aberrant upregulation of ASCL2 was found in partial osteosarcoma tissues, we investigated the effects of ASCL2 on the proliferation of osteosarcoma cells. By CCK-8 assay, we demonstrated that ASCL2 overexpression promoted the proliferation of SaOS and MG-63 cells (Figure 3). These results suggested that a role for ASCL2 in promoting osteosarcoma cell proliferation.
Figure 3.
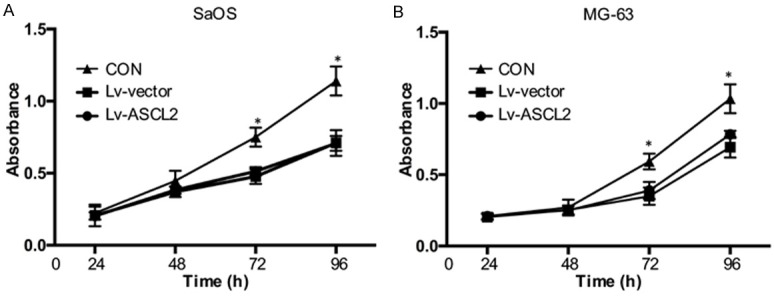
ASCL2 promotes osteosarcoma cell proliferation. SaOS (A) and MG-63 (B) cells overexpressed ASCL2 were seeded in 96-well plates, and cell proliferation was analyzed by CCK-8 reagent after 24, 48, 72 and 96 hours. The data in the figure represent the averages ± SD of triplicate samples. *p<0.05 compared with the control group based on one-way ANOVA.
ASCL2 enhances invasion and migration of human osteosarcoma cells
Migration and invasion activities are crucial prerequisites for metastatic osteosarcoma. After we found that ASCL2 expression was related with metastasis in osteosarcoma, we checked whether ASCL2 overexpression could affect the invasive and migratory activities of osteosarcoma cells in vitro. As observed in the wound healing assay, it showed a significantly promotion (Figure 4A and 4B), as also indicated by an increase in the level of MMP2 in SaOS (Figure 4C) and MG-63 (Figure 4D) cells when the cells were overexpressed of ASCL2, compared with the control cells. In Matrigel invasion assays, the average number of overexpression ASCL2 cells which were invading through the Matrigel was significantly increased than the control group (Figure 5A and 5B). Taken together, both wound healing and Matrigel invasion assays demonstrated that ASCL2 overexpression significantly increased the migration and invasion capabilities of osteosarcoma cells.
Figure 4.
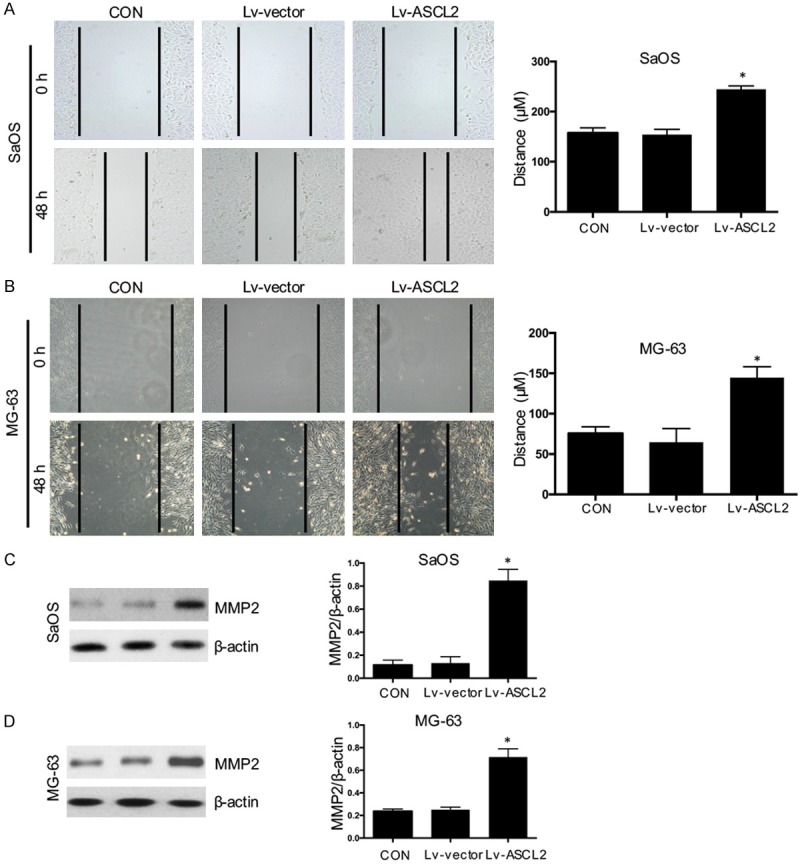
ASCL2 enhances osteosarcoma cell migration and up-regulates MMP2 expression. Cell migration in SaOS (A) and MG-63 (B) cells with ASCL2 overexpression were analyzed using wound-healing assays. The protein expression of MMP2 in SaOS (C) and MG-63 (D) cells was detected using western blot. The data in the figure represent the averages ± SD of triplicate samples. *p<0.05 compared with the control group based on one-way ANOVA.
Figure 5.
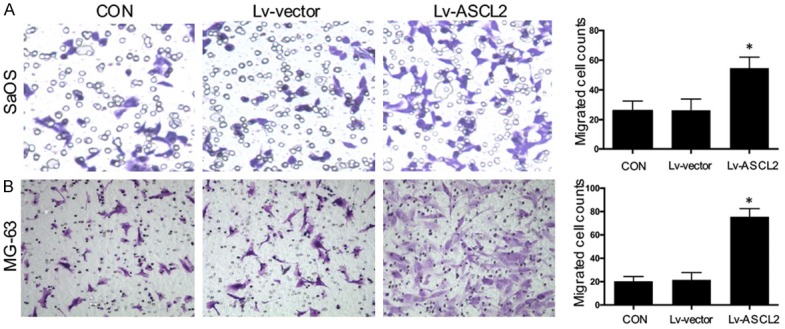
ASCL2 promotes osteosarcoma cell invasion. Cell invasion in SaOS (A) and MG-63 (B) cells with ASCL2 overexpression were analyzed using Transwell Matrigel invasion assays. The data in the figure represent the averages ± SD of triplicate samples. *p<0.05 compared with the control group based on one-way ANOVA.
Discussion
Osteosarcoma is the most frequent primary malignant bone tumor and is a highly aggressive tumor that metastasizes primarily to the lungs [11]. Although the combined treatment (neoadjuvant chemotherapy, surgery, and adjuvant chemotherapy) was used in osteosarcoma patients, the prognosis for patients with metastatic osteosarcoma remains poor with a 5-year survival rate at only 10 to 20%, despite treatment modalities have been improved over the past decades [12-16]. Thus new prognosis biomarkers have been expected, but no remarkable advances have been made in the prognostic prediction and treatment of osteosarcoma. Therefore, the study of biomarkers for osteosarcoma metastasis is important for improving the survival of patients. Here, we reported that ASCL2 was expressed at a higher level in human osteosarcoma patients with metastasis compared with non-metastatic patients and that the positive ASCL2 expression in osteosarcoma tissues was significantly correlated with metastatic features and with shorter OS and MFS.
Recent studies have shown that aberrant expression of ASCL2 plays crucial roles in tumor progression and metastasis. Zhou et al. showed that ASCL2 could promote the CXCR4 expression in colorectal cancer, which was important in tumor malignancy and metastasis [17]. In colorectal cancer, inhibition of ASCL2 down-regulated miRNA-302b expression and up-regulated the miRNA-200 cluster, leading to the reversal of the epithelial-mesenchymal transition [7,8]. In order to further explicate the relationship between ASCL2 expression and metastasis in osteosarcoma, the functional tests, including cell growth and migration with ASCL2 overexpression, were performed as well. Our results showed that ASCL2 overexpression significantly promoted proliferation and enhanced migration and invasion of osteosarcoma cells. Since migratory and invasive abilities are essential features for initiating metastatic or recurrent processes for malignant tumors, inhibiting migratory and invasive abilities by targeting ASCL2 would presumably decrease local recurrence or metastasis, thereby improving survival of osteosarcoma patients.
Patients outcome of osteosarcoma varies greatly depending on patients age, histological classification, and Enneking stage. In our study, we showed that the positive ASCL2 group had significantly less survival rates for OS and MFS than did the negative ASCL2 group of osteosarcoma patients. Especially, it is significantly worse in the patients younger than 20 years old. However, it is not significant association between ASCL2 and the OS or the MFS in the patients elder than 20 years old. It suggested that ASCL2 would be more accurate biomarker for evaluating OS and MFS in the subgroups of patients within children and young adolescents (≤20 years old).
In conclusion, to the best of our knowledge, this is the first report that describes ASCL2 expression in osteosarcoma and its associations with this tumor metastasis and prognosis. Our results showed that ASCL2 expression is correlated with osteosarcoma outcome. This study demonstrates that ASCL2 can serve as an independent and significant prognostic factor of OS and MFS, especially when the patients are children and young adolescents (≤20 years old). It also has been documented that overexpression of ASCL2 could promote migration and invasion in osteosarcoma cells. Further studies are needed for the underlying molecular mechanism that ASCL2 contributes to migration and invasion of osteosarcoma cells. Our study highlights the potential role for ASCL2 as a promising therapy target for metastatic osteosarcoma patients in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81472076, 81271982, 81301944 and 81401801).
Disclosure of conflict of interest
None.
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles AS, Hartmann O. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome--the French pediatric experience. Cancer. 2005;104:1100–1109. doi: 10.1002/cncr.21263. [DOI] [PubMed] [Google Scholar]
- 3.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Jubb AM, Chalasani S, Frantz GD, Smits R, Grabsch HI, Kavi V, Maughan NJ, Hillan KJ, Quirke P, Koeppen H. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene. 2006;25:3445–3457. doi: 10.1038/sj.onc.1209382. [DOI] [PubMed] [Google Scholar]
- 5.Jubb AM, Hoeflich KP, Haverty PM, Wang J, Koeppen H. Ascl2 and 11p15.5 amplification in colorectal cancer. Gut. 2011;60:1606–1607. doi: 10.1136/gut.2010.231746. author reply 1607. [DOI] [PubMed] [Google Scholar]
- 6.Stange DE, Engel F, Longerich T, Koo BK, Koch M, Delhomme N, Aigner M, Toedt G, Schirmacher P, Lichter P, Weitz J, Radlwimmer B. Expression of an ASCL2 related stem cell signature and IGF2 in colorectal cancer liver metastases with 11p15.5 gain. Gut. 2010;59:1236–1244. doi: 10.1136/gut.2009.195701. [DOI] [PubMed] [Google Scholar]
- 7.Zhu R, Yang Y, Tian Y, Bai J, Zhang X, Li X, Peng Z, He Y, Chen L, Pan Q, Fang D, Chen W, Qian C, Bian X, Wang R. Ascl2 knockdown results in tumor growth arrest by miRNA-302b-related inhibition of colon cancer progenitor cells. PLoS One. 2012;7:e32170. doi: 10.1371/journal.pone.0032170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Y, Pan Q, Shang Y, Zhu R, Ye J, Liu Y, Zhong X, Li S, He Y, Chen L, Zhao J, Chen W, Peng Z, Wang R. MicroRNA-200 (miR-200) cluster regulation by achaete scute-like 2 (Ascl2): impact on the epithelial-mesenchymal transition in colon cancer cells. J Biol Chem. 2014;289:36101–36115. doi: 10.1074/jbc.M114.598383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu XG, Chen L, Wang QL, Zhao XL, Tan J, Cui YH, Liu XD, Zhang X, Bian XW. Elevated expression of ASCL2 is an independent prognostic indicator in lung squamous cell carcinoma. J Clin Pathol. 2016;69:313–318. doi: 10.1136/jclinpath-2015-203025. [DOI] [PubMed] [Google Scholar]
- 10.Collins M, Wilhelm M, Conyers R, Herschtal A, Whelan J, Bielack S, Kager L, Kuhne T, Sydes M, Gelderblom H, Ferrari S, Picci P, Smeland S, Eriksson M, Petrilli AS, Bleyer A, Thomas DM. Benefits and adverse events in younger versus older patients receiving neoadjuvant chemotherapy for osteosarcoma: findings from a meta-analysis. J. Clin. Oncol. 2013;31:2303–2312. doi: 10.1200/JCO.2012.43.8598. [DOI] [PubMed] [Google Scholar]
- 11.Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 12.Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- 13.Ta HT, Dass CR, Choong PF, Dunstan DE. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247–263. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 14.Cho Y, Jung GH, Chung SH, Kim JY, Choi Y, Kim JD. Long-term survivals of stage IIb osteosarcoma: a 20-year experience in a single institution. Clin Orthop Surg. 2011;3:48–54. doi: 10.4055/cios.2011.3.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamoureux F, Trichet V, Chipoy C, Blanchard F, Gouin F, Redini F. Recent advances in the management of osteosarcoma and forthcoming therapeutic strategies. Expert Rev Anticancer Ther. 2007;7:169–181. doi: 10.1586/14737140.7.2.169. [DOI] [PubMed] [Google Scholar]
- 16.Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137:89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhou ZH, Rao J, Yang J, Wu F, Tan J, Xu SL, Ding Y, Zhan N, Hu XG, Cui YH, Zhang X, Dong W, Liu XD, Bian XW. SEMA3F prevents metastasis of colorectal cancer by PI3K-AKT-dependent down-regulation of the ASCL2-CXCR4 axis. J Pathol. 2015;236:467–478. doi: 10.1002/path.4541. [DOI] [PubMed] [Google Scholar]


