Abstract
Background:
Malnutrition is very common among chronically hospitalized patients, especially those in the intensive care unit (ICU). Identifying the patients at risk and providing suitable nutritional support can prevent and/or overcome malnutrition in them. Total parenteral nutrition (TPN) and partial parenteral nutrition (PPN) are two common routes to deliver nutrition to hospitalized patients. We conducted a multicenter, prospective double blind randomized controlled trial to evaluate the benefits and compare their adverse effects of each method.
Materials and Methods:
97 patients were enrolled and divided into two groups based on the inclusion criteria. Serum protein, serum albumin, serum transferrin, and total lymphocyte count were measured on days 7 and 14.
Results:
We did not find any statistically significant differences in clinical status or laboratory values between the two groups but there were significant improvements in measured lab values between days 7 and 14 (p<0.005) indicating improved nutritional status in each groups.
Conclusion:
This study shows that both TPN and PPN can be used safely in chronic ICU patients to provide nutritional support and prevent catabolic state among chronic critically ill patients. We need to develop precise selection criteria in order to choose the patients who would benefit the most from TPN and PPN. In addition, appropriate laboratory markers are needed to monitor the metabolic requirements of the patients and assess their progress.
Keywords: Total parenteral nutrition, peripheral parenteral nutrition, critical illness, chronic critical illness, intensive care unit (ICU)
INTRODUCTION
Malnutrition is very common among chronically hospitalized patients and it may be more severe in chronic critically ill patients in the ICU, in whom oral intake may be reduced or even impossible. Based on recent studies as much as 40% of all critically ill adult patients are seriously malnourished upon admission, and up to two thirds of the patients experience deterioration of their nutritional status during their ICU stay (1). Acute illness further increases metabolic rate and impairs utilization of nutritional substrates (2).
Patients with chronic pulmonary diseases, particularly ventilator dependent patients, are at increased risk of developing malnutrition due to underestimation of their nutritional needs and delayed initiation of appropriate nutritional support by their physicians (3).
Several other factors such as hepatic and respiratory insufficiency, increased metabolic rate, negative nitrogen balance, weight loss, muscle atrophy, and impaired gastrointestinal function may occur during their hospital stay and may be due to stress response and/or hormonal changes (4).
Appropriate medical treatments, as well as adequate nutrition are required to overcome the patients’ underlying illness. Based on the patients’ ability and their general conditions there are three different ethods to provide nutrition, including enteral nutrition, TPN, and PPN. Enteral feeding may not be possible in some patients due to technical problems (i.e. access) and/or the severity of their illness. On the other hand TPN and PPN have potential benefits and disadvantages (5, 6). TPN is an accepted and approved method for balancing caloric and nutritional needs in chronically hospitalized patients. However, the combination of intravenous nutrition and enteral feeding may be beneficial and have fewer side effects (7, 8).
There are several clinical and laboratory markers for evaluating nutritional status. Clinical markers include body weight and arm circumference, and laboratory markers consist of serum albumin, transferrin, total protein, and total lymphocyte count. These markers should not be interpreted independently and should always be considered together in order to accurately assess the patients’ nutritional status (7).
The study aimed to evaluate the benefits and compare their adverse effects of each method in ICU patients with chronic respiratory diseases.
MATERIALS AND METHODS
We conducted a prospective double blinded randomized controlled trial to evaluate the superiority of TPN and PPN and compare their adverse effects in ICU patients with chronic respiratory diseases. From 2012 to 2014, 97 patients (49 males, 48 females) with chronic ventilator dependence in three university ICUs were randomly were randomly divided into two groups. Chronic ventilator dependence was defined as requiring ventilator support for more than ten days and failure to wean during the next seven days. Informed consent was obtained from the patients or their next of keen and was approved by each hospital’s ethics committee and the study was registered in the Iranian registry of clinical trials: http://www.irct.ir.
Every patient received a central venous catheter via internal jugular vein, a Foley catheter, and a naso-, or orogastric tube. Patients did not receive albumin supplements. Individual data such as age, sex, nutritional method, and GCS were recorded. Harris-Benedict formula was used in order to estimate the basal energy requirements(this method is used to estimate an individual’s basal metabolic rate (BMR) and daily kilocalorie requirement) and Clifton formula was used to calculate caloric requirements (9, 10).
In group A patients TPN was started after achieving hemodynamic stability. The TPN solution consisted of amino acids, 10% dextrose, intralipid, vitamins, and minerals and included 40% fat, 42% carbohydrates, and 18% proteins. Total caloric and nutritional needs were calculated for each patient by our nutritionist.
In group B patients PPN was started if gastric secretions were less than 100 ml during 2 consecutive hours and bowel sounds were present. Total energy requirements for each patient were calculated and total caloric intake was divided equally between enteral and parenteral nutrition. Parenteral solutions were prepared in a similar fashion to those in group A and the enteral nutrition formula was prepared such that it contained one Kcal per ml. Each 2000 ml of the enteral nutrition formula contained 100 grams of tomatoes, 100 grams of yogurt, 60 gram of beef, 70 grams of soy, 20 grams of sugar, 40 grams of oil, and 60 grams of rice powder. The osmolarity of the solution was 440 mosm/kg. Enteral nutrition was delivered every 4 hours by an experienced nurse, starting with a 100 ml bolus and increasing by 100 ml until reaching the daily goal of ? If gastric residual volume was higher than 100 ml before the next bolus, we did not increase the amount of the next bolus.
The quality and quantity of parenteral solutions were similar in both groups. In both groups nutrition was provided at least for 2 weeks and during this period blood samples were drawn everyday at 7 and 9 AM. Serum total protein (STP), serum albumin (SA), serum transferrin (ST) and total lymphocyte count (TLC) were measured for each patient on days 7 and 14 of their ICU stay. SP, SA and ST were calculated by technician RA-1000 device and TLC was measured by a cell counter. Height (in cm) was measured on the day of admission and all patients were weighed on the 1st, 7th, and the 14th day of their ICU stay. We used two sample T-test and Mann-Whitney U-test. P<0.01 was set as statistically significant.
RESULTS
A total of 97 patients (49 in group A and 48 in group B, mean age ± SD: 50.9 ± 7.5 years) were included in this study. Baseline characteristics of both groups are provided in Table 1.
Table 1.
Individual characristics of two groups
| Baseline characteristic | Group A | Group B | p value |
|---|---|---|---|
| Number | 49 | 48 | – |
| Gender, male (%) | 21 (43%) | 28 (58%) | 0.15 |
| Age ± SD (y) | 51.33 ± 7.26 | 50.58 ± 7.82 | 0.62 |
| Weight (kg) | 71.80 ± 11.52 | 70.38 ± 11.65 | 0.54 |
| Height (cm) | 166.7 ± 10.1 | 169.0 ± 9.3 | 0.24 |
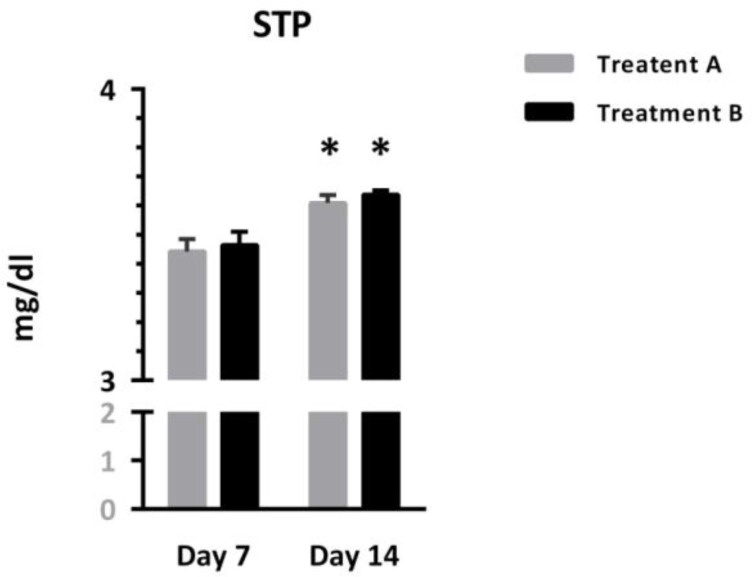
As shown in figure 1, STP was comparable between both patients treated with different protocols, but showed significant increases over time [Interaction: F (1, 95) = 0.02, p = 0.88; Time: F (1, 95) = 40.98, p < 0.0001. Treatment: F (1, 95) = 0.37, p = 0.54]. Asterisks represent significant differences from respective values on day 7.
Figure 1.
Comparison of serum total protein (STP) between two groups
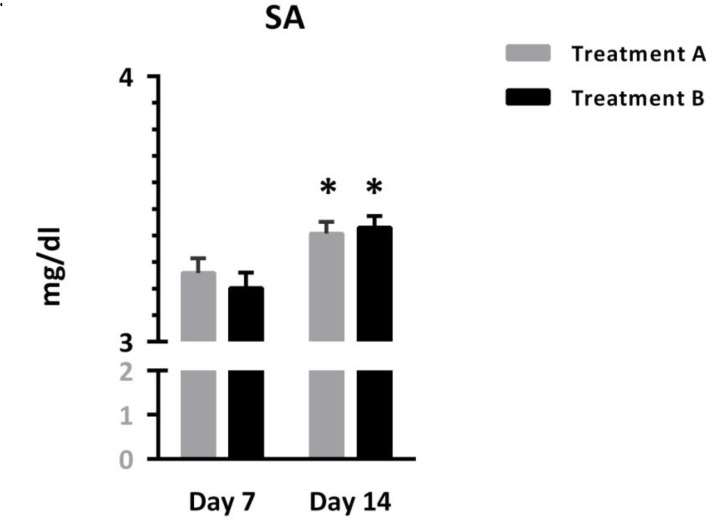
Figure 2 depicts SA as it was comparable in patients treated with different protocols, but showed significant increases over the time [Interaction: F (1, 95) = 1.87, p=0.17; Time: F (1, 95) = 41.57, p < 0.0001, Treatment: F(1, 95) = 0.07, p = 0.78]. Asterisks represent significant differences relative to respective values on day 7
Figure 2.
Comparison of serum albumin between two groups
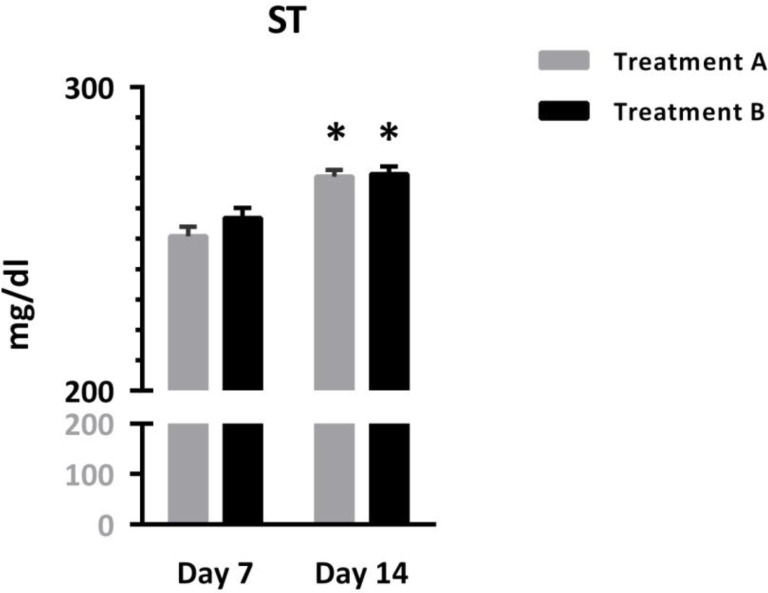
Figure 3 shows that ST was comparable in patients treated with different protocols, but increased significantly over time [Interaction: F (1, 95) = 1.67, p = 0.19; Time: F (1, 95) = 71.55, p < 0.0001, Treatment: F (1, 95) = 0.98, p = 0.32]. Asterisks represent significant differences from respective values on day 7.
Figure 3.
Comparison of serum transferin between two groups
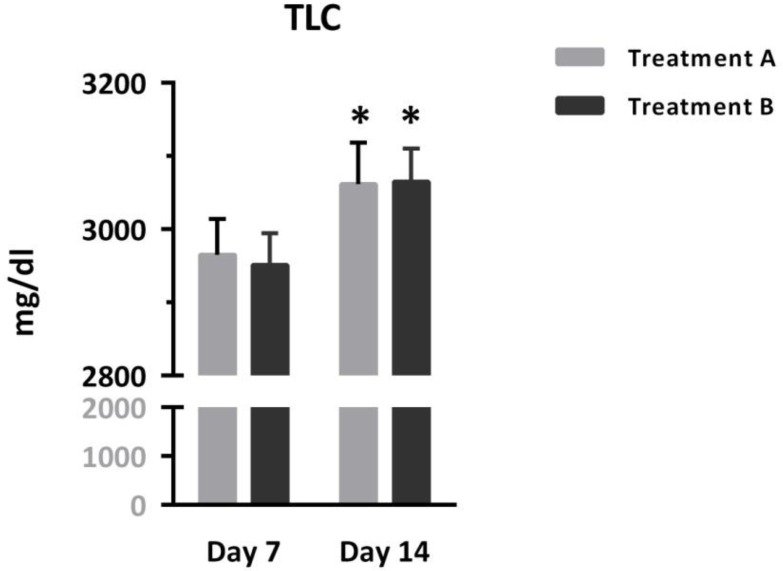
Figure 4 shows that TLC was comparable in patients treated with different protocols, but increased significantly over time [Interaction: F (1, 95) = 0.20, p = 0.65; Time: F (1, 95) = 32.05, p < 0.0001, Treatment: F (1, 95) = 0.00, p = 0.93]. Asterisks represent significant differences from respective values on day 7.
Figure 4.
Comparison of serum total lymphocyte count (TLC) between two groups
A two-way repeated measured ANOVA was applied to evaluate the effect of the measured variables of each treatment paradigm, time, and their interaction. No significant difference was found in the STP, SA, ST, and TLC between the two treatment groups. However, all of these variables showed significant increases on day 14 compared to their values on day 7. In addition, no significant interaction was detected between time and treatment, indicating that none of the treatment protocols had a significant effect on the measured variables at a specific time point. Table 2 summarizes the early results of this study.
Table 2.
Summary of data analysis in two groups
| STP | ||
| Interaction | F (1, 95) = 0.02070 | P = 0.8859 |
| Time | F (1, 95) = 40.98 | P < 0.0001 |
| Treatment | F (1, 95) = 0.3737 | P = 0.5425 |
| SA | ||
| Interaction | F (1, 95) = 1.874 | P = 0.1743 |
| Time | F (1, 95) = 41.57 | P < 0.0001 |
| Treatment | F (1, 95) = 0.07246 | P = 0.7884 |
| ST | ||
| Interaction | F (1, 95) = 1.679 | P = 0.1982 |
| Time | F (1, 95) = 71.55 | P < 0.0001 |
| Treatment | F (1, 95) = 0.9811 | P = 0.3245 |
| TLC | ||
| Interaction | F (1, 95) = 0.2047 | P = 0.6519 |
| Time | F (1, 95) = 32.05 | P < 0.0001 |
| Treatment | F (1, 95) = 0.006659 | P = 0.9351 |
DISCUSSION
Patients with chronic illness, especially those in the ICU may develop a catabolic state, which increases the risk of malnutrition, multiple organ dysfunction, and worsens outcomes. There are some clinical and laboratory parameters that are used to assess the nutritional status of patients. STP, SA, ST, and TLC are commonly used for this purpose (11). TPN and PPN have been previously shown to improve the nutritional status of critically ill patients. However, they both have potential side effects. Therefore, our goal was to find the nutritional method with less side effects in these patients. Schloerb et al. have previously reported the patterns and problems of TPN use in adult critically ill patients in the US (12). Our results confirm improved caloric and protein intake, based on laboratory values, in both groups. In 2003, Datta et al. reported a steady level of serum protein in PPN (13). Also Lapp et al. reported that TPN inhibited the transferrin decrement in patients undergoing spinal fusion (14). Griffiths found that PPN would have fewer complications in these serum elements and was better preserved (15). Borzotta et al. found that serum albumin was identical in both enteral and partial parenteral nutrition groups (16). These studies show the importance of laboratory evaluation in assessing nutritional needs as well as preventing catabolic state. In our study we show benefits of these measurement both in comparing PPN and TPN, as well as reaching a good nutritional state in both groups.
Mokhalalaty et al. found that non-standard solutions are associated with greater risk of infections (17). Therefore standard formula for both TPN and PPN are strongly advised. In our study we use standard formulas in both groups.
Total lymphocyte count is routinely used to assess immunological status. However, it has been used in some studies to assess nutritional status, severe physiological stress, corticosteroid therapy and hematological disorders (18, 19).
While there are no statistically significant differences between the two groups, we observed increases in all the measured parameters on day 14 relative to day 7, which shows a significant progress in nutritional status in both groups. Therefore we found both TPN and PPN effective for providing nutritional support in chronically ill patients in the ICU. As each method has potential side effects, future studies are required to better determine the best nutritional route in chronically critically ill patients who require ventilator support. Additionally, it is clear that standard formulas should be used in order to provide adequate nutrition support to patients.
Serum total protein can be used to asses nutritional status of the patients and detect the presence of catabolic state. In 1996, Miles et. al, noticed that proteins should be included for calorie measure and precise metabolic evaluation. (20)
CONCLUSION
This study shows that both PPN and TPN can be used safely to provide nutrition for chronically ventilator dependent patients in the ICU and preventing catabolic state among them. Following precise studies in the future can clear the value of each method as well as probable side effects.
REFERENCES
- 1. McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ 1994; 308 (6934): 945– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cerra FB, Benitez MR, Blackburn GL, Irwin RS, Jeejeebhoy K, Katz DP, et al. Applied nutrition in ICU patients. A consensus statement of the American College of Chest Physicians. Chest 1997; 111 (3): 769– 78. [DOI] [PubMed] [Google Scholar]
- 3. Mault J, ICU Nutritional Study Group Energy balance and outcome in critically ill patients: results of a multi-center, prospective, randomized trial. InScientific Abstracts 2000. ( Vol. 35). [Google Scholar]
- 4. Wilson RF, Tyburski JG. Metabolic responses and nutritional therapy in patients with severe head injuries. J Head Trauma Rehabil 1998; 13 (1): 11– 27. [DOI] [PubMed] [Google Scholar]
- 5. Mooney JF., 3rd. Perioperative enteric nutritional supplementation in pediatric patients with neuromuscular scoliosis. J South Orthop Assoc 2000; 9 (3): 202– 6. [PubMed] [Google Scholar]
- 6. Donaldson J, Borzatta MA, Matossian D. Nutrition strategies in neurotrauma. Crit Care Nurs Clin North Am 2000; 12 (4): 465– 75. [PubMed] [Google Scholar]
- 7. Satinský I, Mitták M, Foltys A, Kretek J, Dostalík J. Comparison various types of artificial nutrition on postoperative complications after major surgery. Rozhl Chir 2005; 84 (3): 134– 41. [PubMed] [Google Scholar]
- 8. Scolapio JS. A review of the trends in the use of enteral and parenteral nutrition support. J Clin Gastroenterol 2004; 38 (5): 403– 7. [DOI] [PubMed] [Google Scholar]
- 9. Harris JA, Benedict FG. Standard basal metabolism constants for physiologists and clinicians: a biometric study of basal metabolism in man. Washington, DC: Carnegie Institution of Washington; 1919. [Google Scholar]
- 10. Clifton GL, Robertson CS, Choi SC. Assessment of nutritional requirements of head-injured patients. J Neurosurg 1986; 64 (6): 895– 901. [DOI] [PubMed] [Google Scholar]
- 11. Stene John K., Thomas C. Nutiritional aspects: Miller's Anesthesia. 6th ed. CN: Churchill Livingstone; 2005. P. 2887– 2922. [Google Scholar]
- 12. Schloerb PR, Henning JF. Patterns and problems of adult total parenteral nutrition use in US academic medical centers. Arch Surg 1998; 133 (1): 7– 12. [DOI] [PubMed] [Google Scholar]
- 13. Datta G, Gnanalingham KK, van Dellen J, O'Neill K. The role of parenteral nutrition as a supplement to enteral nutrition in patients with severe brain injury. Br J Neurosurg 2003; 17 (5): 432– 6. [DOI] [PubMed] [Google Scholar]
- 14. Lapp MA, Bridwell KH, Lenke LG, Baldus C, Blanke K, Iffrig TM. Prospective randomization of parenteral hyperalimentation for long fusions with spinal deformity: its effect on complications and recovery from postoperative malnutrition. Spine (Phila Pa 1976) 2001; 26(7): 809– 17; discussion 817. [DOI] [PubMed] [Google Scholar]
- 15. Griffiths RD. Which critically ill patient should receive TPN.
- 16. Borzotta AP, Pennings J, Papasadero B, Paxton J, Mardesic S, Borzotta R, et al. Enteral versus parenteral nutrition after severe closed head injury. J Trauma 1994; 37 (3): 459– 68. [DOI] [PubMed] [Google Scholar]
- 17. Mokhalalati JK, Druyan ME, Shott SB, Comer GM. Microbial, nutritional and physical quality of commercial and hospital prepared tube feedings in Saudi Arabia. Saudi Med J 2004; 25 (3): 331– 41. [PubMed] [Google Scholar]
- 18. Weigley ES, Mueller DH, Robinson CH. Robinson's basic nutrition and diet therapy. Merrill 1997. [Google Scholar]
- 19. Evans C. The nutritional status of Asian end stage renal failure patients: a cross sectional study. EDTNA ERCA J 1998; 24 (3): 33– 5. [PubMed] [Google Scholar]
- 20. Miles JM, Klein JA. Should protein be included in calorie calculations for a TPN prescription? Point--counterpoint. Nutr Clin Pract 1996; 11 (5): 204– 6. [DOI] [PubMed] [Google Scholar]