Abstract
Penicillium notatum is a fungus that widely exists in the environment and is often non-pathogenic to humans. However, in immunocompromised hosts it may be recognized as a cause of systemic mycosis.
A 44-year-old man with acute myeloid leukemia (AML) was admitted to our hospital with fever and neutropenia. Due to no improvement after initial treatment, he underwent bronchoscopy. The patient was found to have P. notatum and Pneumocystis jiroveci infection, and therefore was given voriconazole, primaquine and clindamycin. The patient was successfully treated and suffered no complications.
Conclusion:
This case highlights P. notatum as a cause of infection in immunocompromised patients. To the best of our knowledge, mixed lung infection with P. notatum and P. jiroveci in a patient with AML has not been previously reported.
Keywords: Pneumocystis jiroveci, Penicillium notatum, Acute myeloid leukemia, Pulmonary
INTRODUCTION
Penicillium notatum (also known as Penicillium chrysogenum) widely exists in the environment (1). It is found in the soil, rotting vegetables or on woods (2). It has been seldom reported as a cause of human disease in immunocompetent hosts; however, infection with this microorganism seems to be more common and presents with a more severe clinical picture in immunocompromised patients (3).
Herein, we report the first known case of lung infection with P. notatum and P. jiroveci in an AML patient in Iran.
CASE SUMMARY
A 44-year-old man with AML–M3 for two months was admitted to our hospital with fever and neutropenia (absolute neutrophil count = 400 cells/mm3) starting seven days after chemotherapy. At the time of admission, the patient presented with an oral temperature of 38°C; physical examination was otherwise normal. Specimens were obtained for blood culture (B/C), urine analysis (U/A), and urine culture (U/C). Meropenem was started subsequently. Chest X-ray showed patchy consolidation in both upper lung lobes, and the patient had a provisional diagnosis of health care associated pneumonia. Parenteral vancomycin and ciprofloxacin were added to meropenem; however, P. jiroveci was also probable according to CXR. High resolution computed tomography scan (HRCT) of the lungs was performed and revealed bilateral symmetrical peribronchovascular infiltration in both upper lobes and superior segment of lower lobes in favor of P. jiroveci (Figure 1). Co-trimoxazole and hydrocortisone (due to hypoxemia, with arterial oxygen pressure of 50 mm Hg while breathing room air) were administered.
Figure 1.
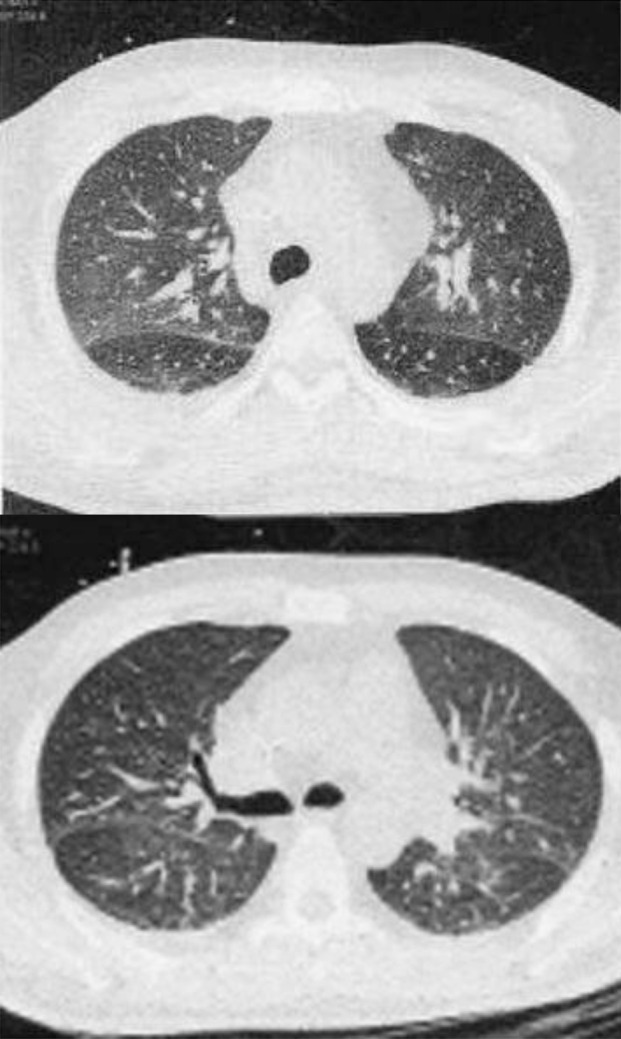
Bilateral symmetrical peribronchovascular infiltration in both upper lobes and superior segment of lower lobe.
First B/C and U/C were negative. Due to continuous fever, B/C, U/C and CXR were repeated; serum galactomannan was measured; and amphotericin B deoxycholate was started empirically. However, after the administration of amphotericin B, the patient developed severe chills and rigors. Thus, amphotericin B was changed to caspofungin. On day nine, he complained of shortness of breath and dyspnea; he remained febrile but leukocyte count increased to 4300/mm3 with a normal differential. Due to poor response to initial treatment for P. jiroveci, we changed co-trimoxazole to primaquine and clindamycin. One day later, a serum galactomannan index (GMI) of 1.7 was reported (GMI≥0.5 is regarded as positive). Therefore, a probable diagnosis of invasive pulmonary aspergillosis (IPA) was made, and consequently caspofungin was replaced with voriconazole. Bronchoscopy, bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB) were performed; specimens were sent for tuberculosis polymerase chain reaction (PCR), P. jiroveci PCR, cytomegalovirus (CMV) PCR, herpes simplex virus PCR, galactomannan, cytology and bacterial and fungal culture. All of the above tests were negative except for the BAL sample for CMV PCR (quantitative PCR=1404 copies/mL). Because of this result and the clinical deterioration of the patient, we decided to add ganciclovir to his treatment protocol. On day 13, the fungal culture of BAL revealed Penicillium SP. This specimen was sent to the Reference Mycology Research Center and cultured on Sabouraud glucose agar (SGA), which revealed green-blue color and velvety appearance. Slide culture and lacto phenol staining were done. Penicillium notatum was identified based on slide culture morphology and colony shape (Figure 2 A,B).
Figure 2.
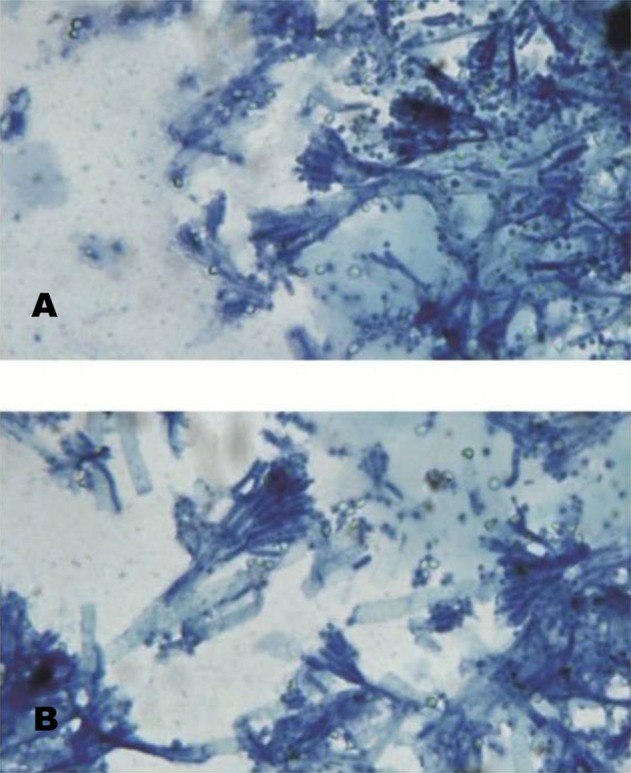
(A,B). Slide culture of P. notatum stained with lactophenol blue.
On the other hand, TBLB (methylene blue staining of lung section) revealed severe interstitial inflammatory cells, fibrin deposition and reactive pneumocytes (Figure 3A) and intra alveolar eosinophilic material (Figure 3B) suggestive of P. jiroveci; accordingly, primaquine and clindamycin were continued for 14 days. In addition, the pathology report showed angioinvasion of fungal organism.
Figure 3.
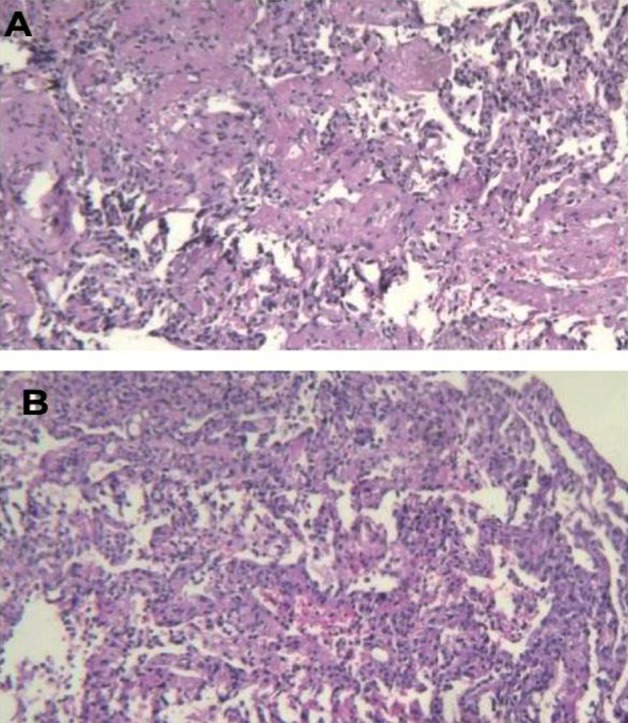
The results of histological examination of the lung specimen Showed inflammatory cells, fibrin deposition and reactive pneumocytes (3A) and intra alveolar eosinophilic material (3B).
After eight days of receiving voriconazole, the patient’s fever subsided and his dyspnea improved. This course of treatment was continued during the next 12 weeks. He is currently asymptomatic and repeated CXR revealed a regression of infiltration.
DISCUSSION
P. notatum is a blue-green mold commonly found in mild climates and semi-tropical areas. It can be found on stale bread, fruits and nuts (4, 5). It often grows rapidly on fungal culture media (6). Penicillium is mostly found in clinical laboratories contaminating the cultures (7), but it has been rarely linked etiologically to invasive infection especially in immunocompromised hosts (3).
P. notatum infection is often difficult to diagnose because of its rarity and non-specific clinical and imaging patterns (6). Infections caused by Penicillium species may be mistaken for aspergillosis due to similar hyaline septate hyphae on microscopic examination (8) and can be positive for galactomannan (9).
In our opinion, although this patient had P. jiroveci pneumonia suggested by the lung tissue biopsy, P. notatum was the major pathogen for the following reasons: no resolution of clinical symptoms after primary antibiotic therapy and treatment of P. jiroveci; positive serum galactomannan without evidence of IPA, positive fungal culture of BAL specimen for P. notatum, dyspnea improvement and fever defervescence after the administration of voriconazole.
Multiple antifungal agents such as amphotericin B, itraconazole, voriconazole, caspofungin and flucytosine have been used for variable periods of time in order to treat P. notatum infection; however, due to the extreme rarity of infections with P. notatum, antifungal susceptibility profiles of this species have not yet been determined (2,6).
Based on the patient’s clinical responses, the length of antifungal treatment is recommended to be between two-12 weeks (9).
CONCLUSION
In conclusion, this was a unique case of mixed lung infection with P. jiroveci and P. notatum in a patient with AML, which according to our knowledge has not been formerly reported. No response to caspofungin and efficient treatment with voriconazole were the other prominent features in this case.
Although human infections with P. notatum are quite rare, clinicians should know that isolation of this pathogen in immunocompromised host might indicate the presence of serious fungal infection.
Acknowledgements
We are deeply indebted to Dr. Shidfar for identification of P. notatum and Dr. Bateni for pathology report and photographs.
Footnotes
Conflict of interest
Authors have no conflicts of interest to declare.
REFERENCES
- 1. Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 2013; 175 (1–2): 57– 67. [DOI] [PubMed] [Google Scholar]
- 2. Kantarcioğlu AS, Apaydin H, Yücel A, de Hoog GS, Samson RA, Vural M, et al. Central nervous system infection due to Penicillium chrysogenum. Mycoses 2004; 47 (5–6): 242– 8. [DOI] [PubMed] [Google Scholar]
- 3. D'Antonio D, Violante B, Farina C, Sacco R, Angelucci D, Masciulli M, et al. Necrotizing pneumonia caused by Penicillium chrysogenum. J Clin Microbiol 1997; 35 (12): 3335– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen HD, Lin WL, Tam MF, Wang SR, Tzean SS, Huang MH, et al. Characterization of allergens from Penicillium oxalicum and P. notatum by immunoblotting and N-terminal amino acid sequence analysis. Clin Exp Allergy 1999; 29 (5): 642– 51. [DOI] [PubMed] [Google Scholar]
- 5. Barcus AL, Burdette SD, Herchline TE. Intestinal invasion and disseminated disease associated with Penicillium chrysogenum. Ann Clin Microbiol Antimicrob 2005; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok T, Koehler AP, Yu MY, Ellis DH, Johnson PJ, Wickham NW. Fatal Penicillium citrinum pneumonia with pericarditis in a patient with acute leukemia. J Clin Microbiol 1997; 35 (10): 2654– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyratzopoulos G, Ellis M, Nerringer R, Denning DW. Invasive infection due to penicillium species other than P. marneffei. J Infect 2002; 45 (3): 184– 95. [DOI] [PubMed] [Google Scholar]
- 8. Huang YT, Hung CC, Liao CH, Sun HY, Chang SC, Chen YC. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. J Clin Microbiol 2007; 45 (9): 2858– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cormier Y, Israël-Assayag E, Bédard G, Duchaine C. Hypersensitivity pneumonitis in peat moss processing plant workers. Am J Respir Crit Care Med 1998; 158 (2): 412– 7. [DOI] [PubMed] [Google Scholar]


