Abstract
The flavodiiron proteins (FDPs) Flv1 and Flv3 in cyanobacteria function in photoreduction of O2 to H2O, without concomitant formation of reactive oxygen species, known as the Mehler-like reaction. Both Flv1 and Flv3 are essential for growth under fluctuating light (FL) intensities, providing protection for PSI. Here we compared the global transcript profiles of the wild type (WT), Δflv1 and Δflv1/Δflv3 grown under constant light (GL) and FL. In the WT, FL induced the largest down-regulation in transcripts involved in carbon-concentrating mechanisms (CCMs), while those of the nitrogen assimilation pathways increased as compared with GL. Already under GL the Δflv1/Δflv3 double mutant demonstrated a partial down-regulation of transcripts for CCM and nitrogen metabolism, while in FL conditions the transcripts for nitrogen assimilation were strongly down-regulated. Many alterations were specific only for Δflv1/Δflv3, and not detected in Δflv1, suggesting that certain transcripts are affected primarily because of the lack of flv3. By constructing the strains overproducing solely either Flv1 or Flv3, we demonstrate that the homo-oligomers of these proteins also function in acclimation of cells to FL, by catalyzing reactions with as yet unidentified components, while the presence of both Flv1 and Flv3 is a prerequisite for the Mehler-like reaction and thus the electron transfer to O2. Considering the low expression of flv1, it is unlikely that the Flv1 homo-oligomer is present in the WT.
Keywords: fluctuating light, gene expression, Mehler-like reaction, photosynthesis, Synechocystis sp. PCC 6803
Introduction
Flavodiiron proteins (FDPs) are a large family of soluble enzymes, first found in strict or facultative anaerobes among the Bacteria, Archaea and Protozoa with a function in O2 and/or NO detoxification (Vicente et al. 2008, Wasserfallen et al. 1998). FDP-encoding genes have also been identified in certain oxygenic photosynthetic bacteria and eukaryotes (reviewed in Allahverdiyeva et al. 2015a). All FDPs consist of at least two core modules: an N-terminal metallo-β-lactamase-like domain with a diiron center and a flavodoxin-like domain with an FMN-binding site (Vicente et al. 2008, Goncalves et al. 2012). Unlike other prokaryotes, cyanobacteria have an additional NAD(P)H:flavin oxidoreductase domain at the C-termini of FDPs. The presence of a flavin module condenses a multicomponent pathway into a single protein. In other words, cyanobacterial FDPs may, in theory, be able to receive electrons directly from NAD(P)H and transfer them to the catalytic di-iron center. The genome of the cyanobacterium Synechocystis sp. PCC 6803 (hereafter Synechocystis) contains four genes encoding FDPs: sll1521 (Flv1), sll0219 (Flv2), sll0550 (Flv3) and sll0217 (Flv4).
In Synechocystis, the Flv2/Flv4 heterodimer functions in the photoprotection of PSII (Zhang et al. 2009, Zhang et al. 2012, Bersanini et al. 2014). Flv1 together with Flv3 mediates photoreduction of O2 downstream of PSI (Helman et al. 2003). In contrast to the Mehler reaction occurring in chloroplasts, the transfer of electrons to O2 via Flv1 and Flv3 happens without the formation of reactive oxygen species (ROS) and only produces water (Vicente et al. 2002, Helman et al. 2003). This reaction is referred to as a Mehler-like reaction (Allahverdiyeva et al. 2013).
Sudden short-term fluctuations in light intensity are typical in aquatic environments and are faced by most photosynthetic microorganisms. Using a unicellular, non-N2-fixing Synechocystis as well as a filamentous heterocystous N2-fixing Anabaena sp. PCC 7120 (also known as Nostoc sp. PCC 7120), we recently demonstrated that both Flv1 and Flv3 are indispensable when light intensity fluctuates during growth (Allahverdiyeva et al. 2013, reviewed in Allahverdiyeva et al. 2015b). Flv1 and Flv3 proteins, receiving electrons from downstream of PSI, function as an important electron sink during prompt over-reduction of the electron transfer chain, thus safeguarding PSI under fluctuating light.
In aquatic environments, the growth of cyanobacteria is often also restricted by the availability of inorganic carbon (Ci), which is available as HCO3− and CO2, or both, depending on the pH. Cyanobacteria have evolved a sophisticated carbon-concentrating mechanism (CCM) to avoid this problem. CCM has several components including the carboxysomes, high- and low-affinity CO2 uptake systems (NDH-1MS and NDH-1MS') and HCO3− transporters. CCM provides a high CO2 level inside the carboxysome, an intracellular microcompartment surrounded by a thick protein shell (reviewed by Badger et al. 2006, Price et al. 2008, Rae et al. 2013, Burnap et al. 2015). The CO2-fixing enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), a key component of the Calvin−Benson cycle, resides mostly inside the carboxysome. Low Ci availability negatively influences the carbon-fixing rate of Rubisco, giving advantage to its second substrate, O2. Oxygenation of ribulose-1,5-bisphosphate by Rubisco generates the toxic side product 2-phosphoglycolate (2-PG), which is detoxified by the photorespiratory pathway. Despite the efficient CCM, cyanobacteria still have the photorespiratory 2-PG metabolism (Eisenhut et al. 2008). Moreover, photorespiratory O2 uptake has been demonstrated in Synechocystis mutant cells deficient in the flv1 and flv3 genes under Ci-deprived conditions (Allahverdiyeva et al. 2011). Interestingly, the Δflv3/ΔgcvT double mutant deficient in Flv3 and the glycine decarboxylase complex (GcvT) could not be completely segregated and showed a high light (HL)-sensitive phenotype, unlike the Δflv1/ΔgcvT double mutant (Hackenberg et al. 2009). Therefore it is possible that Flv3 and the photorespiratory 2-PG metabolism co-operate during the acclimation of cells to HL by dissipating excess reducing power and without a requirement for Flv1. This was the first indication that the Flv3 protein might have an independent function in addition to the Mehler-like reaction, which is driven by Flv1 and Flv3 jointly.
Although the crucial role of the Flv1 and Flv3 proteins for the survival of cyanobacteria under fluctuating light has been demonstrated in Synechocystis and Anabaena sp. PCC7120 (Allahverdiyeva et al. 2013), the response of cyanobacterial cells to fluctuating light (FL) intensities remains elusive. In this work, we analyze long-term responses of Synechocystis WT and mutant cells lacking Flv1 and Flv3 proteins to FL by examining transcript profiles after the shift of cultures from constant growth light (GL) to FL conditions. We also demonstrate that overexpressed Flv1 and Flv3 may form a functional homodimer (or homotetramer in the case of Flv3) taking part in acclimation of cells, together with as yet to be identified components.
Results
Global analysis of gene transcripts between WT, Δflv1 and Δflv1/Δflv3 in constant growth light and under fluctuating light
Flv1 and Flv3 proteins are expressed under standard GL conditions but become crucial for cell survival only under FL intensities. Here, we first searched for genes encoding proteins or protein networks that could possibly be involved in the functional pathway of the Flv1 and Flv3 proteins and for genes whose expression is induced or repressed by FL. To achieve this, we performed a global transcriptome analysis of Synechocystis WT, Δflv1 and Δflv1/Δflv3 cells pre-grown under 3% CO2 and then shifted to ambient air for 48 h under either constant light (GL) or FL.
First, the transcriptomic analysis of WT, Δflv1 and Δflv1/Δflv3 cells cultivated in constant GL (50 μmol photons m−2 s−1) for 48 h was performed. Analyzing the data revealed genes that were up- and down-regulated both in the Δflv1 single mutant and in the Δflv1/Δflv3 double mutant compared with the WT (Supplementary Tables S1, S2). Further comparative analysis of the two sets of mutant transcriptomes was performed in order to find the differences that could be attributed to the effect of the flv3 deletion (Fig. 1). The distribution of up-regulated and down-regulated genes to different functional categories according to Cyanobase (http://genome.microbedb.jp/cyanobase) with necessary modifications is shown in Supplementary Fig. S1.
Fig. 1.
The numbers of down-regulated (A) and up-regulated (B) genes in Δflv1 and Δflv1/flv3 mutants under a constant light intensity of 50 μmol photons m−2 s−1 (GL) and under fluctuating light conditions, 20/500 μmol photons m−2 s−1 (FL) compared with the WT. The data include genes that have a log2 fold change ≥1.00 or ≤ −1.00 and P-value <0.05. The numbers inside the segments show the unique or overlapping genes in different comparisons. The numbers of differently regulated genes in each pairwise comparison are given in parentheses, and genes with known functions are indicated outside the Venn diagrams.
Analysis of the flv mutants in relation to the WT from the GL condition
The deletion of flv1 resulted in a limited set of 15 down-regulated and 49 up-regulated genes, the majority of which were categorized as ‘hypothetical’ or ‘unknown’. In contrast, the double mutant, Δflv1/Δflv3 displayed (compared with the WT cells) 94 down-regulated and 182 up-regulated genes (Fig. 1; Supplementary Tables S1, S2). Deletion of both flv1 and flv3 in the double mutant resulted in a co-ordinated down-regulation of many genes involved in the regulation of photosynthesis, respiration and CO2 assimilation; no significant changes in these categories were observed upon deletion of the single flv1 gene. Transcript levels of ssl0020 (fed1, petF) encoding the main photosynthetic ferredoxin 1 (Fed1), slr1329 (atpB), slr1330 (atpE), sll1323 (atpG) encoding ATP synthase subunits, and slr0009 (rbcL) encoding the large subunit of Rubisco were significantly down-regulated in Δflv1/Δflv3. Many genes encoding subunits of the NDH-1 complex and the structural components of carboxysomes (ccmK1, ccmK2, ccmM) showed significantly lower transcript levels compared with the WT. In line with these results, the number of carboxysomes per cell was also nearly 2-fold lower in the Δflv3 mutant compared with the WT, being 3.2 ± 1.10 and 5.8 ± 0.45, respectively. In the double mutant, the genes involved in nitrogen metabolism were also down-regulated. The sll1450 (nrtA) and sll1452 (nrtC) genes encoding ATP-dependent nitrate/nitrite transporters, slr0898 (nirA) encoding ferredoxin:nitrite oxidoreductase, and slr1756 (glnA) encoding glutamine synthetase type I demonstrated a significant decline. The transcripts corresponding to enzymes involved in ROS scavenging pathways were affected in a non-co-ordinated way. The level of slr1516, sodB, (Fe-superoxide dismutase, Fe-SOD) transcripts did not change in the single flv1 mutant, whereas in the double mutant a significant down-regulation was observed. The transcript level of sll1621 (prxII), encoding an antioxidative stress protein, type II peroxiredoxin (Kobayashi et al. 2004, Hosoya-Matsuda et al. 2005), was considerably reduced, whereas the sll0755 (tpx) encoding thioredoxin peroxidase was up-regulated in both mutants (Supplementary Tables S1, S2). Recently it was found that the sll0755 (tpx) gene did not respond to the addition of H2O2, though its transcription is regulated by HL (Stork et al. 2005), suggesting that it is subject to a different control mechanism, independent of ROS (Pérez-Pérez et al. 2009).
In the Δflv1/Δflv3 double mutant, the transcript levels of the genes encoding the Flv4 and Sll0218 proteins, involved in PSII photoprotection (Zhang et al. 2009, Zhang et al. 2012, Bersanini et al. 2014), were lower than in the WT. A similar response was observed for several stress-responsive genes, slr2075, sll0416 and slr2076 encoding the GroEL/ES chaperone, and slr1963 (ocp) encoding the orange carotenoid protein, which dissipates the light absorbed by phycobilisomes via non-photochemical quenching (NPQ) (Wilson et al. 2006, Kirilovsky and Kerfeld 2012). Our results disagree with findings of Hackenberg et al. (2009) who described an increase in ocp at both transcript and protein levels in the single flv3 mutant. The discrepancy could be due to different experimental growth conditions (5% CO2 and 165 μmol photons m−2 s−1). Interestingly, among the genes belonging to the photosynthesis category, only the sll0248 transcript (isiB, encoding flavodoxin) was strongly up-regulated in Δflv1/Δflv3; this transcript was unchanged in the single Δflv1 mutant. The isiA transcription was not disturbed in the mutants. In addition, the mRNA level for sll1221 (hoxF), which encodes a diaphorase subunit of bidirectional hydrogenase, was elevated in both flv mutants.
Analysis of the flv mutants in relation to the WT from the FL 20/500 condition
Next we performed a microarray experiment with WT, Δflv1 and Δflv1/Δflv3 cells subjected to FL (20/500 μmol photons m−2 s−1) for 48 h, when low background illumination (20 μmol photons m−2 s−1) was interrupted every 5 min with 30 s periods of HL (500 μmol photons m−2 s−1). In FL 20/500, Δflv1 cells revealed 157 down-regulated and 68 up-regulated genes, whereas the Δflv1/Δflv3 double mutant demonstrated 194 down-regulated and 104 up-regulated genes, compared with the WT (Fig. 1; Supplementary Tables S3, S4).
In contrast to the GL condition, the transcript levels of many genes, particularly those involved in photosynthesis, were altered in FL 20/500 in both the Δflv1 single mutant and the Δflv1/Δflv3 double mutant. The transcript levels of genes encoding the PSII subunits (psbF, psbO and psbZ), ATP synthase subunits and the PetN subunit of the Cyt b6f complex were decreased in both mutants. Furthermore, genes encoding many proteins involved in nitrogen assimilation were distinctly down-regulated in both Δflv1 and Δflv1/Δflv3 mutants in response to FL 20/500. They include the nrtA-D operon, nirA, narB encoding ferredoxin:nitrate oxidoreductase (Rubio et al. 1996), slr0288 (glnN) encoding glutamine synthetase type III, slr0447 (urtA) coding for the urea transport system substrate-binding protein, and sll0108 (amt1) encoding high affinity ammonium/methyl ammonium permeases. Chl a biosynthesis-related genes, including chlL, chlN and chlP, were repressed in both the single and double mutants. Interestingly, we observed a down-regulation of cpcD, cpcG2 and apcC, encoding the phycobilisome linker polypeptides. However, transcript levels of the phycobilisome core subunits were not decreased; this was verified at the protein level using an ApcD antibody (Fig. 2A). From ROS scavenging enzymes, in FL 20/500 we observed an up-regulation of only slr1738 playing a regulatory role in the induction of a patent antioxidant gene, sll1621 (prxII) (Kobayashi et al. 2004).
Fig. 2.
Changes in protein (A) and transcript (B) levels after the shift from GL to FL. (A) Protein immunoblots showing the relative amounts of proteins in the WT and Δflv1/Δflv3 after a shift from constant growth light (GL) to fluctuating light FL 20/500 at pH 7.5 or FL 50/500 at pH 8.2, for 1, 3 and 7 d. Proteins were separated by SDS−PAGE, and immunoblotting was performed by using specific antibodies. (B) Transcript accumulation of flv2 upon the shift from GL to FL 20/500 (pH 7.5) analyzed with RT-qPCR. RNA was isolated after 0, 2, 24 and 48 h. The values are the mean of three biological replicates (± SD). Asterisks indicate a statistically significant difference in flv2 transcript level at each time point in FL 20/500 compared with GL in the WT and Δflv1/Δflv3, respectively.
Among genes significantly up-regulated in both Δflv1 and Δflv1/Δflv3, we discovered ssr2016 encoding Pgr5-like protein, postulated to participate in cyclic electron flow (Yeremenko et al. 2005), and the flv4-2 operon. It is important to note that at the protein level Flv4 and Flv2 apparently disappear already after 1 d of incubation under FL 20/500 in both the WT and Δflv1/Δflv3 (immunoblotting with specific antibodies is shown in Fig. 2A). To validate microarray data and clarify a possible discrepancy between transcriptome and protein levels, we performed real-time quantitative reverse transcription−PCR quantitative (RT-qPCR) analysis (Fig. 2B). As seen from the latter figure, the overall expression of flv2 was repressed upon the shift from GL to FL 20/500 in both the WT and Δflv1/Δflv3, but this effect appeared to be more dramatic in the WT. When cells, at pH 8.2, were shifted from GL to milder FL conditions (FL 50/500), the Flv4 and Flv2 proteins disappeared from the Δflv1/Δflv3 double mutant within 1 d (as was the case in FL 20/500) whereas in the WT it happened later (Fig. 2A).
Comparison of gene expression between the two flv mutants
It was evident that the Δflv1/Δflv3 double mutant had a much broader pattern of differentially regulated genes in both GL and FL 20/500 conditions compared with the single Δflv1 mutant. We searched for transcripts that specifically responded to the lack of flv3, i.e. the transcripts that showed a specific up- or down-regulation only in the Δflv1/Δflv3 mutant, not in Δflv1, independently of light regime. The transcript level of fed1 decreased both in GL (0.4-fold) and in FL 20/500 (0.3-fold); the isiB gene encoding flavodoxin was strongly (5.8 fold) up-regulated in GL in Δflv1/Δflv3 but not in Δflv1. Similarly, the transcript level of slr1516 encoding Fe-SOD decreased to 0.4-fold in GL in Δflv1/Δflv3, whereas in Δflv1 no significant decrease was observed. The slr0458 gene, encoding a 2-PG phosphatase that plays a role in 2-PG metabolism (Haimovich-Dayan et al. 2015), was up-regulated about 2-fold both in GL and in FL 20/500 in Δflv1/Δflv3, but not in Δflv1. Several genes functioning in the homeostasis of metals and transport of metals such as zinc, iron and cobalt (znuC, fhuA, exbD, exbB3, coaT, nrsB and cobN) were up-regulated solely in Δflv1/Δflv3. These results strongly imply that certain transcripts are affected primarily because of the lack of flv3.
Comparison of the WT transcriptome in constant GL and FL 20/500 conditions
To reveal further global metabolic responses to FL intensities in Synechocystis, we compared the transcriptomic data obtained from WT cells grown under FL 20/500 or in constant light (GL). We observed 49 down-regulated and 59 up-regulated genes (Table 1; Supplementary Tables S5, S6) specifically under FL conditions. In the WT, the FL 20/500 condition caused changes in expression of genes involved in regulation of photosynthesis as well as in the transport of bicarbonate and nutrients. Several genes encoding the proteins of NDH-1 complexes were drastically down-regulated, including those important for low-Ci-inducible CO2 uptake (ndhJ, ndhI, ndhC, ndhD3, ndhF3, cupA and cupS). Down-regulation of ndhD3 under fluctuating light was also confirmed at the protein level (Fig. 2A). The decreased transcript levels were observed for sbtA, encoding a high affinity sodium-dependent HCO3− transporter, the cmpA-D operon encoding BCT1, an ATP-binding cassette (ABC)-type high affinity HCO3− transporter system, and slr0006, a low-CO2-inducible gene (Carmel et al. 2013). Interestingly, flv3 was also decreased at the transcript (Table 1) and protein levels (Fig. 2A), even though Flv3 has been shown to be crucial for cell survival under FL conditions (Allahverdiyeva et al. 2013). In addition, the flv4-2 operon was significantly down-regulated. In contrast, several high-light stress-inducible genes, hliA-C, hspA, htrA and sll1862−1863, were found to be up-regulated in WT cells exposed to FL 20/500 (Table 1; Supplementary Tables S5, S6). The genes involved in nitrogen metabolism were affected in a non-co-ordinated way: the transcript level of sll0450 (norB) encoding the subunit of nitric oxide reductase was down-regulated, whereas narB and nrtC-D genes were up-regulated.
Table 1.
Selected differentially regulated genes in the WT grown in FL 20/500 compared with the WT grown in GL
| ORF | Gene | WT FL/WT GL (log2 FC) | |
|---|---|---|---|
| Nitrogen assimilation | |||
| sll1454 | narB | 1.68 | |
| sll1452 | nrtC | 1.11 | |
| sll1453 | nrtD | 1.49 | |
| sll0450 | norB | −1.82 | |
| Fatty acid metabolism | |||
| sll0262 | desD | 1.24 | |
| Photosynthesis, respiration and CO2 uptake | |||
| Flavodiiron proteins | |||
| sll0217 | flv4 | −5.93 | |
| sll0218 | sll0218 | −5.97 | |
| sll0219 | flv2 | −5.00 | |
| sll1521 | flv1 | −0.14* | |
| sll0550 | flv3 | −1.00 | |
| Adaptations | |||
| slr1964 | frp | 1.10 | |
| ssl2542 | hliA | 2.01 | |
| ssr2595 | hliB | 2.44 | |
| ssl1633 | hliC | 1.01 | |
| NDH-1 complex | |||
| sll1734 | cupA | −2.70 | |
| sll1735 | cupS | −2.13 | |
| sll1733 | ndhD3 | −3.15 | |
| sll1732 | ndhF3 | −3.40 | |
| slr1279 | ndhC | −1.49 | |
| slr2007 | ndhD5 | −1.25 | |
| sll0520 | ndhI | −1.23 | |
| slr1281 | ndhJ | −1.68 | |
| Bicarbonate transport | |||
| sll0834 | bicA | 2.18 | |
| slr0040 | cmpA | −6.53 | |
| slr0041 | cmpB | −5.62 | |
| slr0043 | cmpC | −3.58 | |
| slr0044 | cmpD | −3.55 | |
| slr1512 | sbtA | −3.46 | |
| slr1513 | sbtB | −3.66 | |
Genes were considered differentially regulated when log2 of fold change (FC) ≥1.00 or ≤ -1.00 and P-value <0.05 except that the flv1 gene is also shown (*)
Flv1 and Flv3 proteins in the flv overexpression (oe) strains
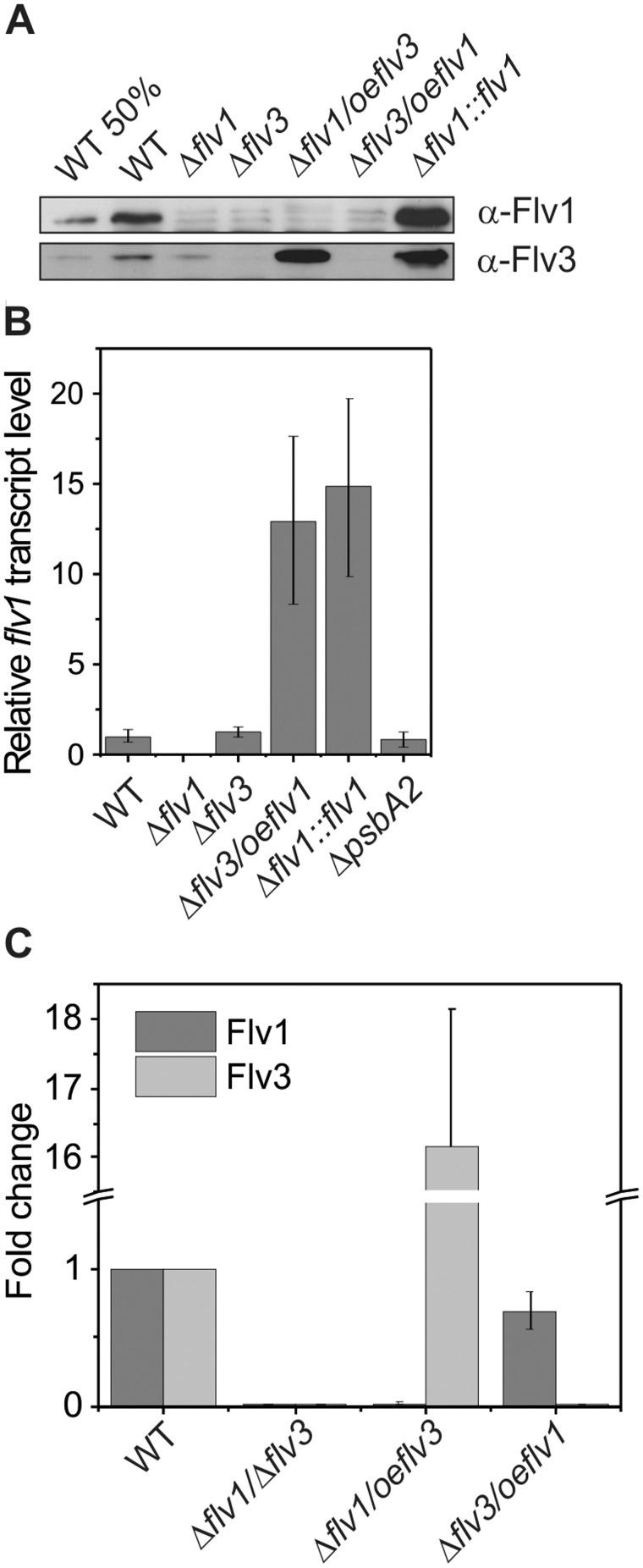
As shown in Fig. 3A, the Δflv1 mutant accumulates considerably low amount of the Flv3 protein. This has also been reported previously (Zhang et al. 2009, Allahverdiyeva et al. 2011, Allahverdiyeva et al. 2013). However, previously we failed to detect the Flv1 protein in the WT or Δflv3 mutant with immunoblotting, probably due to the low accumulation level of Flv1 and/or the low sensitivity of the Flv1 antibody employed. To overcome this problem, in this work we applied a more sensitive chemiluminescent substrate that enabled the detection of the Flv1 protein in WT cells (Fig. 3A). However, the Flv1 protein was still not detectable in Δflv3, suggesting a possible correlation between the accumulation levels of Flv1 and Flv3 proteins in cells. To eliminate the effect of the observed crosswise down-regulation of the proteins, we constructed mutants overproducing solely either Flv3 or Flv1. The proteins were expressed under the strong psbA2 promoter in a mutant background where the gene for the other FDP was deleted; thus, Δflv1/oeflv3 and Δflv3/oeflv1 strains were obtained.
Fig. 3.
Flv1 and Flv3 protein and flv1 transcript level in Flv1 and Flv3 deletion and overexpression strains. (A) Flv1 and Flv3 proteins detected with Western blotting. Proteins were separated by SDS−PAGE, and immunoblotting was performed by using specific antibodies. (B) The flv1 transcript level in strains expressing the flv1 gene under the psbA2 promoter. (C) Relative Flv1 and Flv3 protein levels detected with a quantitative mass spectrometry method, selected reaction monitoring (SRM). The values are the mean of three biological replicates (± SD).
The Δflv1/oeflv3 strain showed strong expression of the Flv3 protein, despite Flv1 being absent (Fig. 3A). However, in the Δflv3/oeflv1 strain, the expression of the Flv1 protein could still not detected by immunoblotting. In sharp contrast to this, the flv1 transcript level was strongly up-regulated compared with the control strains and Δflv3 mutant, indicating that in the Δflv3/oeflv1 overexpression mutant a proper transcription from the psbA2 promoter has been achieved (Fig. 3B). Comparable results were obtained with three independent Δflv3/oeflv1 clones (data not shown). In order to obtain more precise information about Flv1 and Flv3 protein levels, we applied a sensitive quantitative mass spectrometry method, selected reaction monitoring (SRM), to the WT and overexpression strains. The SRM experiment revealed about 16-fold expression of Flv3 in Δflv1/oeflv3 and 0.68-fold expression of Flv1 in Δflv3/oeflv1, compared with the WT (Fig. 3C). These results indicated that the expression of flv1 at the protein level is strictly dependent on the expression of Flv3. In agreement with this, in the Δflv1::flv1 complementation strain, where flv1 is expressed under control of the psbA2 promoter and the cells contain the native flv3, the Flv1 content is strongly enhanced compared with the WT (Fig. 3A; Allahverdiyeva et al. 2013).
Oligomers formed by the Flv1 and Flv3 proteins
FDPs always function as dimers or tetramers since such organization is essential for the electron transfer (Vicente et al. 2008. Goncalves et al. 2012). In vivo dimer formation has previously been demonstrated for Flv3 in Synechocystis WT and the Δflv1 mutant (Allahverdiyeva et al. 2011). In the Δflv1/oeflv3 strain, most of the overexpressed Flv3 was detected as a homodimer using Blue Native (BN)−PAGE (Fig. 4A; Supplementary Fig. S2A). However, even after application of a sensitive chemiluminescent substrate, it was not possible to detect Flv1 protein from the WT and Δflv1::flv1 samples with a specific antibody in BN−PAGE (data not shown). This could be due to fact that the antibody does not recognize the Flv1 protein in its folded state. To be able to visualize Flv1, protein complexes were separated in the first dimension with BN−PAGE, and then in the second dimension using denaturing SDS−PAGE (Fig. 4B). Immunoblots of the WT and Δflv1::flv1 samples separated by 2D-BN/SDS−PAGE demonstrated that the predominant form of Flv1 is also a dimer.
Fig. 4.
Protein complexes of Flv1 and Flv3 in Synechocystis (A, B) and Flv3 isolated from E. coli (C, D). (A) Protein complexes were separated from Synechocystis soluble fraction using 5−15% gradient BN−PAGE according to their molecular mass. (B) Two-dimensional BN−PAGE/SDS−PAGE analysis of Flv1 and Flv3 protein complexes from WT and mutant strains. Protein complexes were separated from the soluble fraction using BN−PAGE followed by separation of protein complexes in the second dimension by SDS−PAGE. Gel filtration (size exclusion) run (C) with the indicated standard peak locations and corresponding sizes in kDa, and SDS−PAGE separation (D) of purified Flv3 expressed in E. coli.
Thus the experiments with 2D-BN/SDS−PAGE confirmed that Flv1 can form a dimer and Flv3 can also organize into a homodimer in Synechocystis. However, the BN approach failed to distinguish between the Flv1/Flv3 heterodimer and possible homodimeric forms in the WT since the molecular masses of Flv1 and Flv3 are very similar (66.4 and 63.7 kDa, respectively). To solve this problem and to separate proteins not only by size but also according to their isoelectric point (pI), we attempted to use Native−PAGE (Supplementary Fig. S2B). The theoretical pI values for Flv1 and Flv3 are 6.04 and 5.54, respectively. Unfortunately, the resolution of the 2D-Native/SDS−PAGE was not adequate to make definite conclusions. To exclude an involvement of Flv2 in oligomer formation with the Flv3 protein, we also probed our samples with the Flv2-specific antibody. Flv2 could be detected from the soluble fraction of cells only if the isolation buffer contained no bivalent metal cations, and the dimer complex containing Flv2 was different in size from the Flv3-containing complex (Supplementary Fig. S2A).
Considering that in anaerobic prokaryotes and protozoa the active form of FDPs can also be a homotetramer (Vicente et al. 2008), we decided to study further the Flv3 protein complexes by expressing His-tagged Flv3 heterologously in Escherichia coli. Gel filtration elution profiles of the purified Flv3 clearly showed three fractions corresponding to the sizes of monomers, dimers and tetramers (Fig. 4C). The purity of the gel filtration and the correct size of Flv3 were verified with SDS−PAGE (Fig. 4D). The detection of two oligomeric fractions confirmed that cyanobacterial FDPs can also organize a tetramer; however, this has to be verified in vivo in Synechocystis.
Growth phenotype of the Flv1 and Flv3 overexpression strains
The growth phenotypes of the Δflv1/oeflv3 and Δflv3/oeflv1 overexpression strains were examined using Δflv1 and Δflv3 deletion mutants and a ΔpsbA2 mutant containing a disrupted psbA2 gene (Zhang et al. 2012) as controls alongside the WT (Fig. 5). Under the FL 20/500 μmol photons m−2 s−1 light regime (pH 7.5), neither Δflv1 nor Δflv3 was able to grow (Fig. 5A), which agrees with our previous findings (Allahverdiyeva et al. 2013). Importantly, the Δflv3/oeflv1 and Δflv1/oeflv3 strains demonstrated improved growth compared with the corresponding deletion mutants. However, the overexpression of either Flv1 or Flv3 could not completely rescue the growth phenotype. In contrast, the complementation strain Δflv1::flv1 grew as fast as the WT. Although the growth of the overexpression strains was somewhat improved compared with the deletion mutants, they still showed reduced Chl a levels compared with the WT. This was evident from the whole-cell absorption spectra peak at around 680 nm (corresponding to Chl a), whereas a peak at 625 nm, corresponding to phycocyanin, was unaltered in all mutant cells (Fig. 5B).
Fig. 5.
Growth (A, C, D) and spectrum (B) of the Flv1 and Flv3 deletion and overexpression strains. (A) Growth under a FL 20/500 μmol photons m−2 s−1 light regime in pH 7.5. (B) In vivo room temperature absorption spectrum measured from cells grown for 4 d under a FL 20/500 μmol photons m−2 s−1 light regime in pH 7.5. Spectra were normalized at 750 nm. Growth of the Flv1 and Flv3 deletion and overexpression strains under a FL 50/500 μmol photons m−2 s−1 light regime in BG-11 with pH 7.5 (C) and pH 8.2 (D). Asterisks indicate significant difference in growth between Δflv1/oeflv3 and Δflv3/oeflv1 mutants and Δflv1 and Δflv3 mutants.
Next, the growth of the mutants was monitored under milder fluctuating light conditions, FL 50/500, when background light intensity of 50 μmol photons m−2 s−1 was interrupted every 5 min with 500 μmol photons m−2 s−1 for 30 s. Surprisingly, in FL 50/500 (pH 7.5), the deletion and overexpression cells grew as well as the WT (Fig. 5C). The same light regime (FL 50/500) at pH 8.2 resulted in significantly slower growth for the deletion mutants (Fig. 5D) as we have reported earlier (Allahverdiyeva et al. 2013). Both the Δflv3/oeflv1 and Δflv1/oeflv3 strains again demonstrated enhancement in the growth at FL50/500 and pH 8.2 compared with the deletion mutants.
Gas exchange and photosynthetic electron transfer properties of the overexpression strains
To determine possible modifications in the functional status of the photosynthetic apparatus behind the observed improvement in the growth of Δflv3/oeflv1 and Δflv1/oeflv3 strains in FL 20/500, we evaluated the PSI status by monitoring P700 using Dual-PAM. It has previously been shown that PSI is the main target of damage in Δflv1/Δflv3 cells grown under FL conditions (Allahverdiyeva et al. 2013). The overexpression strains showed slightly but significantly higher levels of the maximum oxidizable amount of P700 (Pm), compared with the double Δflv1/Δflv3 mutant (Fig. 6A). In line with this, the amount of PsaB, the reaction center protein of PSI, was strongly decreased in the Δflv1/Δflv3 mutant (Fig. 6B; Allahverdiyeva et al. 2013), whereas overexpression strains and WT cells demonstrated similar amounts of PsaB (Fig. 6B). There was no significant difference in the maximum photochemical efficiency of PSII (Supplementary Fig. S3) or in the level of the PSII reaction center protein D1 between the WT and the studied mutants (Fig. 6B).
Fig. 6.
The maximum oxidizable amount of P700 (Pm) of the WT and mutant cells (A) and protein immunoblots showing the relative amounts of photosynthetic proteins in the WT and mutant cells (B). Cells were grown for 4 d under a FL 20/500 μmol photons m−2 s−1 light regime in BG-11 with pH 7.5. [The values are the mean of three biological replicates (± SD).]
To determine whether the homo-oligomers of the Flv1 or Flv3 proteins are able to perform light-induced O2 uptake in the overexpression strains, we monitored real-time gas exchange with membrane inlet mass spectrometry (MIMS) using the 18O2 isotope. Both the overexpression strains and deletion mutants, which were grown under the FL 20/500 regime (pH 7.5) and adjusted to equal Chl a concentration, demonstrated lower dark O2 uptake rates compared with the WT (Fig. 7; Supplementary Table S7). Application of strong HL (500 μmol photons m−2 s−1) stimulated a prominent Flv1/Flv3-mediated O2 photoreduction in the WT, which was missing in the Δflv1/Δflv3 deletion mutant (Fig. 7A, B; Helman et al. 2003, Allahverdiyeva et al. 2011). Both Δflv1/oeflv3 and Δflv3/oeflv1 overexpression strains showed, similarly to the double deletion mutant, a lack of strong O2 photoreduction under the HL conditions (Fig. 7C, D; Supplementary Table S7). However, in contrast to strongly impaired gross and net O2 evolution in the deletion mutant occurring due to a malfunction of PSI and blocked linear electron transfer (Allahverdiyeva et al. 2013), both overexpression strains demonstrated only about 11–13% lower net O2 evolution rates compared with the WT (Fig. 7; Supplementary Table S7).
Fig. 7.
Mass spectrometric simultaneous monitoring of O2 and CO2 exchange in cultures of WT (A) and mutant strains (B−D). Cells were grown for 5 d under the FL 20/500 light regime in BG-11 (supplemented with Na2CO3) with pH 7.5. After a 4 min dark period, actinic white light at an intensity of 500 mol photons m−2 s−1 was applied for 5 min. O2 consumption was calculated from 18O2/16O2 exchange measurements.
In order to study CO2 assimilation in the overexpression strains, we monitored with MIMS the level of free CO2 in samples. Fig. 7A shows the typical curve of changes in CO2 levels consisting of several phases in the medium with Synechocystis cells upon application of a strong light (Badger at al. 1994, Allahverdiyeva et al. 2011). A strong decline in CO2 level observed during the first minute of illumination is usually ascribed to the function of the CCM in cyanobacteria (Badger et al. 1994). This phase, by inducing disequilibrium in the medium between dissolved CO2 and HCO3−, gives a rise to the second phase where regeneration of CO2 due to chemical conversion of HCO3− to CO2 is observed. Finally the third phase demonstrates CO2 uptake by the Calvin−Benson cycle. In line with previous results, all three phases were strongly inhibited in the Δflv1/Δflv3 mutant grown under FL 20/500 (Fig 7; Allahverdiyeva et al. 2013). It is important to note that in both overexpression mutants the first phase, attributed to the function of the CCM, was slow and less pronounced than in the WT, while the subsequent second phase was missing. However, overexpression strains showed only about a 28−30% decrease in CO2 fixation under HL as compared with the WT (Fig. 7).
Discussion
Acclimation of WT Synechocystis cells to low Ci is impaired under fluctuating light
Our results explicitly show that WT Synechocystis responds to FL conditions by down-regulating the genes encoding subunits of the NDH-1 complex and carbon uptake systems, and by up-regulating the genes involved in nitrogen metabolism (summarized in Fig. 8). This does not follow a typical response pattern of the cells in low Ci acclimation (Eisenhut et al. 2007). From the microarray results, it was not possible to evaluate changes in the NDH-1L complex participating in cyclic electron transport and respiration (Battchikova et al. 2011). However, here we show that the transcripts from genes encoding the subunits specific to CO2 uptake (ndhD3, ndhF3, cupA and cupS) are strongly down-regulated in FL compared with GL (Fig. 8; Table 1). In accordance with these results, the transcripts for other NDH-1 subunits (NdhC, NdhJ and NdhI) also show down-regulation, but to a lesser extent.
Fig. 8.
A scheme of transcriptional responses of Synechocystis sp. PCC 6803 WT and the Δflv1/Δflv3 mutant strain in growth light (GL) and fluctuating light (FL). Differentially expressed genes or operons functioning in the carbon-concentrating mechanism (CCM), nitrogen metabolism and flavodiiron protein (FDP) related processes are depicted. The direction of the arrow indicates up- or down-regulation of transcripts from the gene groups indicated, and the boldness of the arrow describes the extent of regulation. WT in GL served as a control for WT in FL and for Δflv1/Δflv3 in GL, while the transcript profile of WT in FL served as a control for differential gene expression of Δflv1/Δflv3 in FL.
Moreover, the expression of the high-affinity bicarbonate transporter genes cmpA-D and sbtA was strongly decreased, whereas the transcript level of bicA, a low-affinity bicarbonate transporter gene, was up-regulated in FL 20/500 compared with GL. In Synechocystis, bicA appears to be constitutively transcribed, whereas both sbtA and cmpA-D are up-regulated when the cells are exposed to limiting Ci levels (Wang et al. 2004, Burnap et al. 2013). Therefore, it is conceivable that WT Synechocystis cells exposed to FL fail to trigger changes in gene expression activating a low Ci acclimation mechanism. In good agreement with this, the transcription of flv2, flv3 and flv4 genes, which usually show up-regulation under low Ci conditions (Zhang et al. 2009), was also significantly lowered under FL.
It is known that linear electron transfer is necessary for the expression of low Ci-inducible genes (Hihara et al. 2003, McGinn et al. 2004). We assume that periodic application of HL pulses over the background light impairs low Ci acclimation in Synechocystis by disturbing stable electron flow, which in turn might create specific redox poise triggers and aberrant signals. The exact mechanism, or carrier, of the redox signal that inhibits such acclimation remains to be identified. The Ci uptake components involved in the CCM pathways also function in the alleviation of excess electrons induced under stress conditions (Tchernov et al. 2003, Xu et al. 2008). Considering their strong down-regulation in the WT under FL (Fig. 8), we assume that the Synechocystis cells try to use the nitrogen assimilation pathway as an alternative electron sink downstream of PSI. The narB gene, coding for ferredoxin-nitrate reductase, which is significantly up-regulated under FL 20/500, uses reduced Fed as a physiological electron donor (Flores and Herrero 2005) and nitrate/nitrite reductases are considered as an important electron sink in relieving the over-reduction of electron transfer components (Klotz et al. 2015, Gutthann et al. 2007).
Inactivation of flv1 and flv3 genes modulates gene expression in many ways
The Δflv1/Δflv3 double mutant acclimated to constant light (GL) demonstrates a decrease in transcripts of genes encoding several subunits of NDH-1 (Fig.1A). However, different from the WT response to FL, deletion of both flv genes did not disturb transcription of bicarbonate transporters, but affected components of carboxysomes, resulting in decreased carboxysome numbers per cell. Eisenhut et al. (2007) demonstrated that different components of the cyanobacterial CCM are distinctly regulated. It is important to note that a partial suppression of CCM in the double mutant occurs along with the down-regulation of ccmR, a negative regulator of CCM (Klähn et al. 2015, reviewed in Burnap et al. 2015).
Further, and in contrast to the WT response under FL, transcript levels of the genes involved in nitrogen assimilation were co-ordinately down-regulated in Δflv1/Δflv3, but not in Δflv1, under GL. Interestingly, we detected significant up-regulation of flavodoxin (isiB) together with the down-regulation of some photosynthetic components (e.g. fed1 and rbcL). It has been shown previously that flavodoxin can efficiently replace Fed in catalyzing electron transfer from PSI to NADP+ photoreduction (reviewed in Sétif 2001). A significant increase in the transcript abundance of hoxF in the Δflv1/Δflv3 mutant might suggest a stimulation of an alternative electron transfer pathway involving flavodoxin and bidirectional hydrogenase (Gutekunst et al. 2014), to compensate for the lack of FDPs in dissipation of excess electrons. Moreover, it is known that besides flavodoxin and the main Fed, Fed1, Synechocystis possesses eight more Feds (reviewed in Cassier-Chauvat and Chauvat 2014). Such a diversity of analogous proteins clearly suggests specialization of their functions, e.g. in distribution of electrons for different metabolic processes.
The transcriptomic results clearly demonstrate that under GL the cells do not respond strongly to the lack of the flv1 gene, indicating that most of the alterations in transcript abundance of the genes observed in the double mutant are due to the lack of flv3. The fact that the Flv1 protein is expressed only in the presence of Flv3 might be explained by post-transcriptional control of flv1 that could involve regulatory RNAs. Indeed, by employing a differential RNA-sequencing approach, Mitschke et al. (2011) showed the presence of an internally located cis-encoded asRNA in the flv1 open reading frame (ORF) that is probably up-regulated in the absence of flv3, leading to degradation of flv1 transcripts. Moreover, as seen from the overexpression mutants, the presence of Flv3 affects the expression of Flv1 at the protein level (Fig. 4A). Even in the case of expression under the control of a strong promoter, the amount of the Flv1 protein did not exceed the WT level, conceivably because of an altered amount of the Flv3 protein. This finding implies that Flv1 homodimers also cannot function in the absence of corresponding amounts of Flv3; its excess might even have toxic effects on the viability of Synechocystis.
In fluctuating light conditions, both Δflv1 and Δflv1/Δflv3 mutants demonstrated strong alterations in transcripts compared with the WT, mostly the down-regulation of genes related to photosynthesis, nitrogen assimilation, energy metabolism and chaperons. In contrast to the GL condition, where the Δflv1/Δflv3 double mutant (but not Δflv1) showed suppression of many genes encoding the subunits of the NDH-1 complexes, including the CO2 uptake system, under FL 20/500 only the transcript levels of bicarbonate transporters were affected. Interestingly, the down-regulation of BCT1 (cmpA and cmpD) was detected only in the single Δflv1 mutant, and in these cells suppression of sbtA and sbtB was also more pronounced.
Moreover, under mild fluctuating light conditions (FL 50/500) at pH 7.5, Δflv1 and Δflv3 do not show growth impairment (Fig. 5C); this is despite a retarded growth of both the single and double mutants at pH 8.2 (Fig. 5D; Allahverdiyeva et al. 2013). The inhibition of bicarbonate transporters observed in the present microarray analysis could explain this phenomenon. In the mutant grown under FL 50/500 in the media at pH 7.5, where the Ci source shifts more to CO2, NDH-1 complexes involved in CO2 uptake are regulated as in the WT, and thus seem to be enough to satisfy the growth demands. In contrast, in media of pH 8.2, HCO3− transporters play a more important role (Shibata et al. 2002) and their more pronounced down-regulation in the mutant lacking flv1 and flv3 results in impaired cell growth.
It has previously been proposed that 2-PG, produced by the oxygenation activity of Rubisco, serves as a co-inducer in the activation of the cmp operon by Cmp-R (Nishimura et al. 2008). Recent results described by Haimovich-Dayan et al. (2015) demonstrated that the overexpression of a putative 2-PG phosphatase, slr0458, resulted in reduced levels of 2-PG in Synechocystis leading to a slow acclimation to low CO2 levels. In our microarray data, slr0458 was up-regulated only in the double Δflv1/Δflv3 mutant (but not in Δflv1), providing compelling evidence for a possible involvement of 2-PG phosphatase in impaired Ci acclimation of Δflv1/Δflv3 under both GL and FL 20/500 conditions. This is in line with the observation by Hackenberg et al. (2009) that photorespiratory 2-PG metabolism co-operates with Flv3, but not with Flv1. Moreover, application of the bacterial adenylate cyclase two-hybrid (BACTH) system demonstrated that Flv3 can interact with Fed9 (slr2059), a component of the ferredoxin−glutaredoxin−thioredoxin cross-talk pathway operating in stress tolerance (Cassier-Chauvat and Chauvat 2014), and, therefore, could play a role in distribution of electrons under stress conditions. Among the alternative routes of photosynthetic electron flow, pgr5 was clearly up-regulated in both mutants, suggesting a possible enhancement of cyclic electron flow (Yeremenko et al. 2005) as a regulatory pathway.
The Flv1 and Flv3 homo-oligomers cannot function in O2 photoreduction, but can contribute to acclimation of cells to FL
Biochemical analysis confirmed the presence of a 120 kDa protein complex in the BN−PAGE from the soluble protein fraction of WT Synechocystis cells, which strongly supports the homodimer formation of Flv3 (Fig. 4B; Allahverdiyeva et al. 2011). Likewise, the in vitro activity assay with recombinant Synechocystis Flv3 protein suggested that the Flv3 protein can function independently of Flv1, most probably as a homodimer (Vicente et al. 2002) or homotetramer (Fig. 4C). However, functional data obtained with the single Δflv1 and Δflv3 mutants thus far strongly suggested that the presence of both proteins is necessary for the Mehler-like reaction (Helman et al. 2003, Helman et al. 2005, Allahverdiyeva et al. 2011, Allahverdiyeva et al. 2013). The findings that in the absence of the Flv1 protein the accumulation of the Flv3 protein is strongly down-regulated (Fig. 3A; Allahverdiyeva et al. 2011), and that in the absence of the Flv3 protein an overexpression of Flv1 is not possible (Fig. 3A, C), also support the hypothesis that Flv1 and Flv3 function in a co-ordinated manner. To date, however, a clear biochemical demonstration of formation of the Flv1−Flv3 heterodimer is still missing. It is also noticeable from immunoblot results that in the Δflv1::flv1 mutant, where clear overexpression of Flv1 is visible by SDS−PAGE (Fig. 3A; Allahverdiyeva et al. 2013), the amount of the dimer complex detected with the Flv3 antibody is not higher than the WT level (Fig. 4A). These data suggest that ‘extra’ Flv1 does not contribute to the heterodimer formation. Considering that His-tagged Flv3 expressed in E. coli can form a tetramer (Fig. 4C), we cannot exclude the possibility of Flv1 and Flv3 also form heterotetramers with 1 : 3 or 1 : 1 ratios in vivo. Indeed, it is possible that the tetramer form is lost during the Synechocystis protein isolation procedure, preventing its detection with BN−PAGE. Finally, considering the low transcript abundance of flv1 (Zhang et al. 2009) and low accumulation of the Flv1 protein in the WT, the possibility of Flv1 acting as an auxiliary protein for the organization of the Flv3 homodimer or homotetramer functioning in O2 photoreduction in vivo cannot yet be excluded.
This work demonstrates that the excess formation of solely the Flv3 homodimer in the Δflv1/oeflv3 strain can significantly improve, but by no means fully complement, the growth phenotype under FL (Fig. 5A). Conversely, even though the sole expression of the Flv1 protein in the Δflv3/oeflv1 strain was less efficient and the Flv1 homodimer was not detected biochemically, this mutant also demonstrated an improved growth phenotype similar to the Δflv1/oeflv3 strain. Hence it is clear that Flv1 is also forming a functional homodimer. Importantly, the improvement of the FL growth phenotype of the double mutant Δflv1/Δflv3 by overexpression of only one Flv protein (Flv1 or Flv3) in the absence of another is not due to restoring the Mehler-like reaction, since neither the Δflv1/oeflv3 nor the Δflv3/oeflv1 strain was capable of performing light-induced O2 uptake (Fig. 7; Supplementary Table S7). Unlike the Δflv1/Δflv3 deletion mutant which shows a strong inhibition of net photosynthesis and CO2 uptake due to PSI damage occurring under FL, both overexpression strains showed an increase in the maximum oxidizable amount of P700, Pm (Fig. 6A), and accumulation of PsaB, the PSI reaction center protein (Fig. 6B). Consequently, net photosynthesis and CO2 uptake were also significantly improved, being nearly comparable with those of the WT (Fig. 7; Supplementary Table S7). Recovery of CO2 uptake and net photosynthesis, but the absence of a detectable light-induced O2 uptake in Δflv1/oeflv3 and Δflv3/oeflv1 strains strongly suggest that Flv3 and Flv1 homodimers are involved in alternative electron transfer pathways other than Mehler-like O2 photoreduction.
Nevertheless, considering the low expression of flv1, we assume that all Flv1 protein is engaged with Flv3; therefore, it is unlikely that the Flv1 homo-oligomer is present in the WT. In line with this, deletion of the flv1 gene modified the transcriptome of the cells less strongly compared with the double mutant. Moreover, even though the Δflv1::flv1 complementation mutant showed a higher expression of Flv1 than the WT, the growth was not improved under FL (Fig. 5). Hasunuma et al. (2014) recently reported a better growth phenotype and an increase in O2 evolution in a mutant overexpressing the flv3 gene and possessing native flv1. Contrary to the authors’ suggestion of increased activity of the Mehler-like reaction, which was not experimentally proven, it is conceivable, based on the results reported here, that the production of ATP for the observed increase in intracellular glycogen resulted from the formation and function of the Flv3 homo-oligomers, leading to improved photosynthetic electron flow.
Taken together, we conclude that both Flv1 and Flv3 proteins are necessary for the Mehler-like reaction, probably by organizing as a hetero-oligomer. Additionally, homo-oligomers of Flv3 are involved in alternative electron transfer pathway(s), where the terminal acceptor is not O2, but still remains to be identified. Therefore, Flv3 protein is a more important player than the Flv1 protein in the systems-wide acclimation of Synechocystis cells to stress.
Materials and Methods
Synechocystis strains and growth conditions
The Synechocystis sp. PCC 6803 glucose-tolerant strain was used as the WT. The Δflv1, Δflv3 single and Δflv1/Δflv3 double mutants have been described by Helman et al. (2003) and Allahverdiyeva et al. (2011), respectively. The constructions of the Δflv1::flv1 complementation and ΔpsbA2 control strain were described by Allahverdiyeva et al. (2013) and Zhang et al. (2012), respectively. The Δflv3/oeflv1 strain was obtained using the same procedure applied for the Δflv1::flv1 mutant, but the transformation host was the Δflv3 strain and the antibiotic cassette for spectinomycin resistance was replaced with a kanamycin cassette. For constructing the Δflv1/oeflv3 strain, the Δflv1 mutant was transformed with a plasmid containing the flv3 ORF under the control of the psbA2 promoter, and the construct was integrated into the chromosome by disrupting the psbA2 gene. Transformants were selected on agar plates supplemented with the appropriate antibiotics.
The WT and the mutant strains were cultivated in growth chambers (AlgaeTron AG 130-ECO; PSI) equipped with LED white light at 30°C in BG-11 medium supplemented with 20 mM HEPES-NaOH (pH 7.5) or 10 mM TES-KOH (pH 8.2) if specifically mentioned. Liquid cultures were shaken at 150 r.p.m. to ensure continuous gas exchange and kept at high CO2 (air enriched with 3% CO2; HC) or at low CO2 (ambient air with 0.039% CO2; LC). Cultures used in experiments were not supplemented with antibiotics, and sodium carbonate was omitted from the BG-11 ingredients under LC. Absence of contamination was checked by spreading liquid culture on LB and R2A agar plates. Continuous growth light GL (photosynthetic photon flux density of 50 μmol photons m−2 s−1), fluctuating light FL 20/500 (20 μmol photons m−2 s−1 background light interrupted with 500 μmol photons m−2 s−1 for 30 s every 5 min) or FL 50/500 (50 μmol photons m−2 −1 background light interrupted with 500 μmol photons m−2 s−1 for 30 s every 5 min) was applied. For growth curves, physiological experiments and protein isolation, cells were pre-cultivated under LC and GL conditions, adjusted to OD750 = 0.1–0.4 and then grown under LC and GL or LC and FL. For physiological measurements, the cells were harvested at the logarithmic phase (OD750 between 0.4 and 1.0), resuspended in fresh BG-11 medium and adjusted to a Chl a concentration of 10−15 μg ml−1.
Isolation of RNA and DNA microarray analysis
Total RNA was isolated from exponentially growing cultures from three independent biological replicates, and RNA concentration and purity were measured as described previously (Mustila et al. 2014).
For transcription profiling, Synechocystis WT, Δflv1 and Δflv1/Δflv3 strains were pre-cultivated in constant growth light (GL, 50 μmol photons m−2 s−1) and 3% CO2 before shifting to experimental conditions. Cells were subjected to either constant growth light (GL, 50 μmol photons m−2 s−1) or fluctuating light (FL 20/500, 20 μmol photons m−2 s−1 background light interrupted with 500 μmol photons m−2 s−1 for 30 s every 5 min) at ambient air (LC) for 48 h before harvesting. The starting OD750 used for the experimental cultures was OD750 = 0.1 and 0.4 in GL and FL, respectively, to obtain enough cells.
DNA microarray experiments were performed using an Agilent 8×15K custom cyanobacterium Synechocystis sp. PCC 6803 array at The Finnish DNA Microarray and Sequencing Centre (Turku, Finland). RNA hybridization, data collection and normalization were performed as described previously (Mustila et al. 2014). A gene was considered up-regulated if log2 of the fold change (FC) was ≥1 and down-regulated if FC was ≤ −1, and the results were statistically significant (P < 0.05). Gene annotation was performed according to Cyanobase (http://genome.microbedb.jp/cyanobase/Synechocystis).
Real-time quantitative PCR
First strand cDNA synthesis and RT-qPCR was performed using a Bio-Rad IQ5 system as described in Mustila et al. (2014). Three genes were used as reference genes: rnpB, rimM and cysK, the two latter being selected based on DNA microarray results (non-responsive to any treatment in any of the used strains). For the rnpB gene, the primers described in Pollari et al. (2009) were used. The forward and reverse primers for reference gene rimM (slr0808, 16S rRNA processing protein) were 5'-GGGAATTCCACGTCACTGAT-3' and 5'-GGGACGAGCACTTCTTTGTC-3' and for cysK (slr1842, cysteine synthase) were 5'-ACCCCAAAATAGCCCAGTCT-3' and 5'-GAGCTGCTCCAGTGGAAATC-3'. The forward and reverse primers for flv1 were 5'-GGCGGATGCTGAAGTCAATA-3' and 5'-ACTTGGTCACCACAAACGTG-3' and for flv2 were 5'-GATCGCCCAAGGATTAGTCA-3' and 5'-CAAAGACTCCGGCCAATTTA-3'.
Protein isolation, electrophoresis and immunodetection
Total cell extracts and the soluble fractions of Synechocystis cells were isolated as described by Zhang et al. (2009), except that the resuspension buffer was supplemented with a protease inhibitor cocktail tablet (Roche).
SDS−PAGE was applied to analyze denatured samples. Protein samples were solubilized in Laemmli sample buffer (Laemmli 1970) containing 138 mM Tris−HCl (pH 6.8), 6 M urea, 22.2% (v/v) glycerol, 4.3% (w/v) SDS and 5% (v/v) 2-mercaptoethanol for 2 h. Proteins were separated by 10% (w/v) SDS−PAGE containing 6 M urea. The proteins were transferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore) and analyzed with protein-specific antibodies. Horseradish peroxidase (HRP) chemiluminescent substrate WesternBright™ ECL-spray (Advansta) was used to visualize Flv1, and Amersham ECL Western Blotting Detection Reagent (GE Healthcare) was used with the other antibodies.
Soluble protein complexes were analyzed by BN−PAGE. The BN gel was prepared as described by Kügler et al. (1997) with modifications from Herranen et al. (2004), and the Hoefer SE 250 running system was used. Samples were diluted in 4× BN buffer containing 100 mM BisTris, pH 7.0, 80% (w/v) glycerol and 40 mM pefabloc. Prior to loading, samples were supplemented with a one-tenth volume of sample buffer containing 100 mM BisTris−HCl (pH 7.0), 500 mM ACA, 30%(w/v) sucrose and 50 mg ml − 1 Serva Blue G. Samples were loaded on an equal protein basis of 90 μg per well in a 5−15% acrylamide gradient gel.
For two-dimensional separation, the strips from the first dimension BN−PAGE were excised and proteins were denatured by gentle shaking in Laemmli sample buffer for 30 min at 20°C. After solubilization, the strips were transferred to the top of the 12% (w/v) SDS−PAGE gel containing 6 M urea and sealed with 0.5% agarose in SDS−PAGE running buffer (25 mM Tris base, 190 mM glycine and 0.1% SDS) followed by the separation of the protein subunits of the complexes.
In vivo absorption spectra
In vivo absorption spectra were measured using an Infinite 200 PRO multiplate reader (Tecan) from 400 to 750 nm with the cell OD750 adjusted to 0.6.
Transmission electron microscopy
Transmission electron microscopy was performed as described previously (Orf et al. 2016). Ultrathin sections were analyzed with Zeiss EM 902 and Zeiss Libra 120 electron microscopes (Carl Zeiss NTS GmbH). The number of carboxysome was counted in at least five representative cells of the WT and the Δflv3 mutant sampled from LC conditions.
Fluorescence and P700 measurement
A pulse amplitude modulated fluorometer Dual-PAM-100 (Walz) was used to monitor Chl a fluorescence and P700 oxido-reduction in intact cells. Saturating pulses (5,000 μmol photons m−2 s−1, 300 ms) were applied to close all PSII centers transiently in order to measure Fm'. Far-red light (75 W m−2) for 10 s and a saturating pulse were applied for determination of the Pm value.
Membrane inlet mass spectrometry (MIMS)
Online measurements of gas exchange were monitored using a mass spectrometer (model Prima PRO, Thermo Scientific). The membrane inlet system, consisting of a modified DW1 oxygen electrode chamber (Hansatech Instruments Ltd.) water-jacketed thermoregulated at 30°C, was attached to the vacuum line of a mass spectrometer through a thin gas-permeable PTFE membrane (0.0125 mm) sealing the bottom of the chamber. 18O2 (isotope purity >98%; CK Gas Products Ltd.) tracing was used to discriminate O2 uptake and O2 production by PSII. 16O2 (m/z 32), 18O2 (m/z 36) and CO2 (m/z 44) were recorded with a time resolution of around 4 s. Samples were evenly mixed by constant stirring using a cross-shaped magnetic stirrer. A 2 ml aliquot of a cell suspension (10 μg ml−1 Chl) was placed in the measuring chamber and carbon concentration was saturated by adding bicarbonate (1.3 mM final concentration). A similar abundance of isotopic oxygen species available in the chamber was reached before the experimental run. After a 4 min dark period, the cell suspension was illuminated with light (500 μmol photons m−2 s−1) using a 150 W halogen illuminator (model Fiber-Lite DC950H, Dolan Jenner) for 5 min. Gas exchange kinetics and rates were determined according to Beckmann et al. (2009).
Quantitative SRM-based proteomics assay
The analyzed samples were prepared according to the protocol described in Vuorijoki et al. (2016). The proteins extracted from Synechocystis cells were reduced in 5 mM dithiotreitol (DTT; Sigma), alkylated with 10 mM iodoacetamide (IAA; Sigma) and precipitated with 1 : 5 (v/v) of 50% acetone/50% ethanol overnight at −20°C. The pellets were solubilized in extraction buffer and trypsin added at a ratio of 1 : 100 enzyme to protein. An additional portion of trypsin was added after 4 h when the pellet was solubilized. After overnight incubation with trypsin, the peptide mixture was further desalted and injected into the LC-MS (liquid chromatograph−mass spectrometer) in biological triplicate. The data were analyzed in Skyline (3.1.0.7382) (MacLean et al. 2010) software, and statistics were performed in the MSstats (3.1.4) package (Choi et al. 2014a, Choi et al. 2014b) implemented in Skyline.
Peptides were measured using the nanoLC system (EasyNanoLC 1000, Thermo Fisher Scientific) coupled to a triple quadrupole mass spectrometer (TSQ Vantage, Thermo Scientific) equipped with a nanoelectrospray source. Samples were loaded onto a pre-column (75 μm×2 cm, 3 μm 120 Å C18, Reprosil-Pur, Dr. Maisch GmbH), then eluted and separated on an analytical column (75 μm×15 cm, 3 μm 120 Å C18, Reprosil-Pur, Dr. Maisch GmbH) using a 60 min non-linear gradient at a flow rate of 300 nl min−1 (5−20% B in 35 min; 20–35% B in 50 min; B = acetonitrile : water, 98 : 5). The transitions were measured with a 2.0 s cycle time, 0.7 unit resolution for both quadrupoles (Q1 and Q3) and the isolation width of 0.002 m/z. The mass spectrometer operated in SRM positive mode with a spray voltage of 1,600 V, 270 °C capillary temperature, collision gas pressure of 1.2 mTorr argon in Q2 and tuned at the S-lens value.
Procedure for Flv3 purification
His-tagged Flv3 was produced in E. coli BL21 (DE3) cells. The cells were grown at 37 °C in a 1 liter flask containing 700 ml of LB supplemented with 10 μM FeSO4. An aliquot of 700 μl of benzyl alcohol was added at a cell density of A600 = 0.5, and the incubation temperature was dropped to room temperature. After30 min, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a concentration of 25 μM to induce protein production. Production was continued for 4 h with mild shaking, after which the cells were harvested (4,000×g, 10 min).
All purification steps were performed at temperatures under 10°C. The cells were resuspended in 40 ml of extraction buffer (20 mM Tris−HCl pH 7.5, 20 mM NaCl, 10 mM imidazole, 10% glycerol) and broken using a cell disruptor. The supernatant was separated (38,000×g, 1 h), and mixed with 1 ml of HisPur™ cobalt resin (ThermoFisher Scientific). After 30 min of incubation with shaking, the resin was packed into an empty gravity flow column. The resin was washed with 10 ml of extraction buffer, and bound protein was eluted with 5 ml of elution buffer (20 mM Tris−HCl pH 7.5, 20 mM NaCl, 150 mM imidazole, 10% glycerol). The purity of the eluted protein was checked with SDS−PAGE.
For size exclusion chromatography, a HiLoad 16/600 Superdex 200 pg column (GE Healthcare) was used with a 0.25 ml min−1 flow rate. The buffer used in this step consisted of 20 mM Tris−HCl pH 7.5, 20 mM NaCl and 5% glycerol. Elution of the protein was recorded by measuring absorbance at 280 nm. The column was calibrated with protein standards from the Gel Filtration HMW Calibration Kit (GE Healthcare).
Supplementary data
Supplementary data are available at PCP online.
Acknowledgements
We thank the Finnish DNA Microarray and Sequencing Centre (Turku, Finland), and the Biocenter Finland, its Turku Proteomics facility. Janne Isojärvi is acknowledged for assistance with microarray analyses, Duncan Fitzpatrick for assistance with MIMS measurements, and Dr. Marcus Frank, Ute Schulz and Gerhard Fulda (Medical Biology and Electron Microscopy Centre, University Medicine Rostock) are acknowledged for assistance with electron microscopy. We thank Dr. Maria Ermakova for critical reading of the manuscript.
Funding
This research was financially supported by the Academy of Finland projects [271832 and 273870]; the FinSynBio program [272424]; the Finnish Doctoral Programme in Computational Chemistry and Molecular Spectroscopy (LasKeMo) [to H.M.]; the Kone foundation [to Y.A.].
Disclosures
The authors have no conflicts of interest to declare.
Glossary
Abbreviations
- BN–PAGE
Blue Native–PAGE
- CCM
carbon-concentrating mechanism
- FDP
Flavodiiron protein
- Fed
ferredoxin
- FL
fluctuating light
- GL
growth light
- HC
high CO2
- HL
high light
- LC
low CO2
- MIMS
membrane inlet mass spectrometry
- NDH-1
type 1 NAD(P)H dehydrogenase
- ORF
open reading frame
- 2-PG
2-phosphoglycolate
- ROS
reactive oxygen species
- RT-qPCR
real-time quantitative reverse transcription–PCR
- SRM
selected reaction monitoring
- WT
wild type
References
- Allahverdiyeva Y., Ermakova M., Eisenhut M., Zhang P., Richaud P., Hagemann M., et al. (2011) Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J. Biol. Chem. 286: 24007–24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y., Isojärvi J., Zhang P., Aro E.M. (2015a) Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life 5: 716–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y., Mustila H., Ermakova M., Bersanini L., Richaud P., Ajlani G., et al. (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA 110: 4111–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y., Suorsa M., Tikkanen M., Aro E.M. (2015b) Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 66: 2427–2436. [DOI] [PubMed] [Google Scholar]
- Badger M.R., Palmqvist K., Yu J.W. (1994) Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol. Plant. 90: 529–536. [Google Scholar]
- Badger M.R., Price G.D., Long B.M., Woodger F.J. (2006) The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J. Exp. Bot. 57: 249–265. [DOI] [PubMed] [Google Scholar]
- Battchikova N., Eisenhut M., Aro E.M. (2011) Cyanobacterial NDH-1 complexes: novel insights and remaining puzzles. Biochim. Biophys. Acta 1807: 935–944. [DOI] [PubMed] [Google Scholar]
- Beckmann K., Messinger J., Badger M.R., Wydrzynski T., Hillier W. (2009) On-line mass spectrometry: membrane inlet sampling. Photosynth. Res. 102: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersanini L., Battchikova N., Jokel M., Rehman A., Vass I., Allahverdiyeva Y., et al. (2014) Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol.164: 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnap R.L., Hagemann M., Kaplan A. (2015) Regulation of CO2 concentrating mechanism in cyanobacteria. Life 5: 348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnap R.L., Nambudiri R., Holland S. (2013) Regulation of the carbon-concentrating mechanism in the cyanobacterium Synechocystis sp. PCC6803 in response to changing light intensity and inorganic carbon availability. Photosynth. Res. 118: 115–124. [DOI] [PubMed] [Google Scholar]
- Carmel D., Dahlström K.M., Holmström M., Allahverdiyeva Y., Battchikova N., Aro E.M., et al. (2013) Structural model, physiology and regulation of Slr0006 in Synechocystis PCC 6803. Arch. Microbiol. 195: 727–736. [DOI] [PubMed] [Google Scholar]
- Cassier-Chauvat C., Chauvat F. (2014) Function and regulation of ferredoxins in the cyanobacterium, Synechocystis PCC6803: recent advances. Life 4: 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M., Chang C.Y., Clough T., Broudy D., Killeen T., MacLean B., et al. (2014a) MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30: 2524–2526. [DOI] [PubMed] [Google Scholar]
- Choi M., Chang C., Vitek O. (2014b) MSstats: Protein Significance Analysis in DDA, SRM and DIA for Label-free or Label-based Proteomics Experiments. R package version 2.6.0.
- Eisenhut M., von Wobeser E.A., Jonas L., Schubert H., Ibelings B.W., Bauwe H, et al. (2007) Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant. Physiol. 144: 1946–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M., Ruth W., Haimovich M., Bauwe H., Kaplan A., Hagemann M. (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc. Natl. Acad. Sci. USA 105: 17199–17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E., Herrero A. (2005) Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 33: 164–167. [DOI] [PubMed] [Google Scholar]
- Goncalves V.L., Vicente J.B., Saraiva L.M., Teixeira M. (2012) Flavodiiron proteins and their role in cyanobacteria In Bioenergetic Process of Cyanobacteria. Edited by Peschek G.A., Obinger C., Renger G. pp. 631–653. Springer, Berlin. [Google Scholar]
- Gutekunst K., Chen X., Schreiber K., Kaspar U., Makam S., Appel J. (2014) The bidirectional NiFe-hydrogenase in Synechocystis sp. PCC 6803 is reduced by flavodoxin and ferredoxin and is essential under mixotrophic, nitrate-limiting conditions. J. Biol. Chem. 289: 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutthann F., Egert M., Marques A., Appel J. (2007). Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentrations in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1767: 161–169. [DOI] [PubMed] [Google Scholar]
- Hackenberg C., Engelhardt A., Matthijs H.C., Wittink F., Bauwe H., Kaplan A., et al. (2009) Photorespiratory 2-phosphoglycolate metabolism and photoreduction of O2 cooperate in high-light acclimation of Synechocystis sp. strain PCC 6803. Planta 230: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich-Dayan M., Lieman-Hurwitz J., Orf I., Hagemann M., Kaplan A. (2015) Does 2-phosphoglycolate serve as an internal signal molecule of inorganic carbon deprivation in the cyanobacterium Synechocystis sp. PCC 6803? Environ. Microbiol. 17: 1794–1804. [DOI] [PubMed] [Google Scholar]
- Hasunuma T., Matsuda M., Senga Y., Aikawa S., Toyoshima M., Shimakawa G., et al. (2014) Overexpression of flv3 improves photosynthesis in the cyanobacterium Synechocystis sp. PCC6803 by enhancement of alternative electron flow. Biotechnol. Biofuels. 7: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman Y., Tchermov D., Reinhold L., Shibata M., Ogawa M., Schwarz R., et al. (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr. Biol. 13: 230–235. [DOI] [PubMed] [Google Scholar]
- Helman Y., Barkan E., Eisenstadt D., Luz B., Kaplan A. (2005) Fractionation of the three stable oxygen isotopes by oxygen-producing and oxygen-consuming reactions in photosynthetic organisms. Plant Physiol. 138: 2292–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranen M., Battchikova N., Zhang P., Graf A., Sirpiö S., Paakkarinen V., et al. (2004) Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803. Plant Physiol. 134: 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara Y., Sonoike K., Kanehisa M., Ikeuchi M. (2003) DNA microarray analysis of redox-responsive genes in the genome of the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 185: 1719–17125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya-Matsuda N., Motohashi K., Yoshimura H., Nozaki A., Inoue K., Ohmori M., Hisabori T. (2005) Anti-oxidative stress system in cyanobacteria. Significance of type II peroxiredoxin and the role of 1-Cys peroxiredoxin in Synechocystis sp. strain PCC 6803. J. Biol. Chem. 280: 840–846. [DOI] [PubMed] [Google Scholar]
- Kirilovsky D., Kerfeld C.A. (2012) The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim. Biophys. Acta 1817: 158–166. [DOI] [PubMed] [Google Scholar]
- Klotz L.O., Sánchez-Ramos C., Prieto-Arroyo I., Urbánek P., Steinbrenner H., Monsalve M. (2015) Redox regulation of FoxO transcription factors. Redox Biol. 6: 51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klähn S., Orf I., Schwarz D., Matthiessen J.K., Kopka J., Hess W.R., et al. (2015). Integrated transcriptomic and metabolomic characterization of the low-carbon response using an ndhR mutant of Synechocystis sp. PCC 6803. Plant Physiol. 169: 1540–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Ishizuka T., Katayama M., Kanehisa M., Bhattacharyya-Pakrasi M., Pakrasi H.B., et al. (2004) Response to oxidative stress involves a novel peroxiredoxin gene in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 45: 290–299. [DOI] [PubMed] [Google Scholar]
- Kügler M., Jänsch L., Kruft V., Schmitz U.K., Braun H.P. (1997) Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth. Res. 53: 35–44. [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- MacLean B., Tomazela D.M., Shulman N., Chambers M., Finney G.L., Frewen B., et al. (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26: 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn P.J., Price G.D., Badger M.R. (2004) High light enhances the expression of low-CO2-inducible transctipts involved in the CO2-concentrating mechanism in Synechocystis sp. PCC6803. Plant Cell Environ. 27: 615–626. [Google Scholar]
- Mitschke J., Georg J., Scholz I., Sharma C.M., Dienst D., Bantscheff J., et al. (2011) An experimentally anchored map of transcriptional start sites in the model cyanobacterium Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 108: 2124–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustila H., Allahverdiyeva Y., Isojärvi J., Aro E.M., Eisenhut M. (2014) The bacterial-type [4Fe–4S] ferredoxin 7 has a regulatory function under photooxidative stress conditions in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1837: 1293–1304. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Takahashi Y., Yamaguchi O., Suzuki H., Maeda S., Omata T. (2008) Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol. Microbiol. 68: 98–109. [DOI] [PubMed] [Google Scholar]
- Orf I., Klähn S., Schwarz D., Frank M., Hess W.R., Hagemann M., et al. (2015) Integrated analysis of engineered carbon limitation in a quadruple CO2/HCO3– uptake mutant of Synechocystis sp. PCC 6803. Plant Physiol. 169: 1787–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez M.E., Mata-Cabana A., Sánchez-Riego A.M., Lindahl M., Florencio F.J. (2009) A comprehensive analysis of the peroxiredoxin reduction system in the cyanobacterium Synechocystis sp. strain PCC 6803 reveals that all five peroxiredoxins are thioredoxin dependent. J. Bacteriol. 191: 7477–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollari M., Ruotsalainen V., Rantamäki S., Tyystjärvi E., Tyystjärvi T. (2009) Simultaneous inactivation of sigma factors B and D interferes with light acclimation of the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 191: 3992–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.D., Badger M.R., Woodger F.J., Long B.M. (2008). Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59: 1441–1461. [DOI] [PubMed] [Google Scholar]
- Rae B.D., Long B.M., Whitehead L.F., Förster B., Badger M.R., Price G.D. (2013) Cyanobacterial carboxysomes: microcompartments that facilitate CO2 fixation. J. Mol. Microbiol. Biotechnol. 23: 300–307. [DOI] [PubMed] [Google Scholar]
- Rubio L.M., Herrero A., Flores E. (1996) A cyanobacterial narB gene encodes a ferredoxin-dependent nitrate reductase. Plant Mol. Biol. 30: 845–850. [DOI] [PubMed] [Google Scholar]
- Sétif P. (2001) Ferredoxin and flavodoxin reduction by photosystem I. Biochim. Biophys. Acta 1507: 161–179. [DOI] [PubMed] [Google Scholar]
- Shibata M., Ohkaqa H., Katoh H., Shimoyama M., Ogawa T. (2002) Two CO2 uptake systems in cyanobacteria: four systems for inorganic carbon acquisition in Synechocystis sp strain PCC6803. Funct. Plant Biol. 29: 123–129. [DOI] [PubMed] [Google Scholar]
- Stork T., Michel K.P., Pistorius E.K., Dietz K.J. (2005) Bioinformatic analysis of the genomes of the cyanobacteria Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942 for the presence of peroxiredoxins and their transcript regulation under stress. J. Exp. Bot. 56: 3193–3206. [DOI] [PubMed] [Google Scholar]
- Tchernov D., Silverman J., Luz B., Reinhold L., Kaplan A. (2003) Massive light-dependent cycling of inorganic carbon between oxygenic photosynthetic microorganisms and their surroundings. Photosynth. Res. 77: 95–103. [DOI] [PubMed] [Google Scholar]
- Vicente J.B., Gomes C.M., Wasserfallen A., Teixeira M. (2002) Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem. Biophys. Res. Commun. 294: 82–87. [DOI] [PubMed] [Google Scholar]
- Vicente J.B., Justino M.C., Gonçalves V.L., Saraiva L.M., Teixeira M. (2008) Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 437: 21–45. [DOI] [PubMed] [Google Scholar]
- Vuorijoki L., Isojärvi J., Kallio P., Kouvonen P., Aro E.M., Corthals G.L., et al. (2016) Development of a quantitative SRM-based proteomics method to study iron metabolism of Synechocystis sp. PCC 6803. J. Proteome. Res. 15: 266–279. [DOI] [PubMed] [Google Scholar]
- Wang H.L., Postier B.L., Burnap R.L. (2004) Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 279: 5739–5751. [DOI] [PubMed] [Google Scholar]
- Wasserfallen A., Ragettli S., Jouanneau Y., Leisinger T. (1998) A family of flavoproteins in the domains Archaea and Bacteria. Eur. J. Biochem. 254: 325–332. [DOI] [PubMed] [Google Scholar]
- Wilson A., Ajlani G., Verbavatz J.M., Vass I., Kerfeld C.A., Kirilovsky D. (2006) A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 18: 992–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Bernat G., Singh A., Mi H., Rögner M., Pakrasi H.B., et al. (2008) Properties of mutants of Synechocystis sp. strain PCC 6803 lacking inorganic carbon sequestration systems. Plant Cell Physiol. 49: 1672–1677. [DOI] [PubMed] [Google Scholar]
- Yeremenko N., Jeanjean R., Prommeenate P., Krasikov V., Nixon P.J., Vermaas W.F., et al. (2005) Open reading frame ssr2016 is required for antimycin A-sensitive photosystem I-driven cyclic electron flow in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 46: 1433–1436. [DOI] [PubMed] [Google Scholar]
- Zhang P., Allahverdiyeva Y., Eisenhut M., Aro E.M. (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS One 4: e5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Eisenhut M., Brandt A.M., Carmel D., Silén H.M., Vass I., et al. (2012) Operon flv4–flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24: 1952–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.