Abstract
Respiratory syncytial virus (RSV) infections affect millions of children and adults every year. Despite the significant disease burden, there are currently no safe and effective vaccines or therapeutics. We employed a replicon-based high throughput screen combined with live-virus triaging assays to identify three novel diversity-oriented synthesis-derived scaffolds with activity against RSV. One of these small molecules is shown to target the RSV polymerase (L protein) to inhibit viral replication and transcription; the mechanisms of action of the other small molecules are currently unknown. The compounds described herein may provide attractive inhibitors for lead optimization campaigns.
Keywords: Respiratory syncytial virus, replicon, diversity-oriented synthesis
Respiratory syncytial virus (RSV) is an enveloped, negative sense RNA virus that is a leading cause of acute respiratory infections in young children, the elderly, and immunosuppressed individuals. In the United States, recent studies estimate that approximately 2 million children under the age of 5 require medical attention annually due to RSV infection (Hall et al., 2009), with young infants at the greatest risk of hospitalization (Hall et al., 2013). Globally, RSV is thought to cause disease in approximately 30 million young children per year, with developing nations bearing the greatest burden of mortality (Nair et al., 2010). RSV infection is also a major cause of respiratory infections in adults, with more severe disease in the elderly; approximately 17,000 adults die annually in the United States as a result of RSV infection [reviewed in (Collins and Melero, 2011)]. Despite the significant disease burden, safe and effective vaccines and therapeutics are not currently available, emphasizing the need for new treatments (Collins and Melero, 2011; Simoes et al., 2015).
We recently described the development of a subgenomic replicon-based high throughput screen to identify new inhibitors of RSV (Laganas et al., 2015; Plant et al., 2015; Tiong-Yip et al., 2014). This replicon consists of the RSV genome from which the small hydrophobic (SH), glycoprotein (G) and fusion (F) genes were deleted and replaced with an antibiotic resistance selection marker (Malykhina et al., 2011). As the replicon still expresses all the proteins required for mRNA transcription and genome replication, the large polymerase subunit (L) protein, the phosphoprotein (P), the transcription elongation factor M2-1, and the nucleoprotein (N), it can be maintained in cell culture. Here we report the use of this replicon system for identification of three novel diversity-oriented synthesis-derived scaffolds, represented by BRD9101, BRD4517 and BRD3482 (Figure 1), which inhibit the replication of RSV.
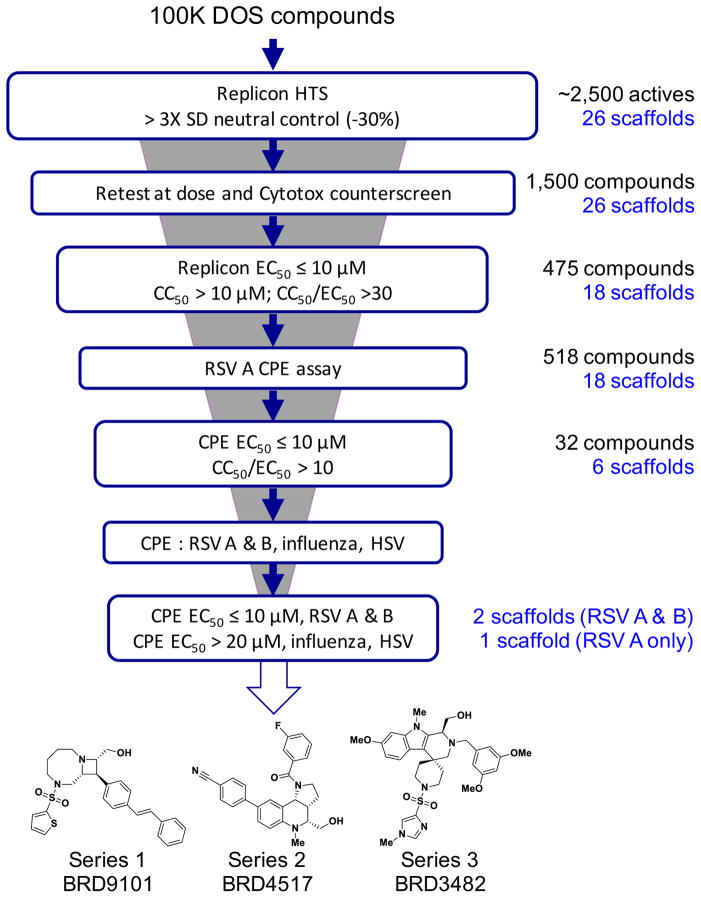
Figure 1. Screening cascade to identify DOS inhibitors of RSV replication.
The screening cascade comprised of a primary RSV replicon screen, with downstream cytotoxicity counter-screens and additional live virus assays to identify compounds of interest. Live virus assays included RSV, influenza, and HSV CPE assays. Three final scaffolds of interest were identified, two of which were active against both RSV A and B strains. The number of compounds and scaffolds progressed at each stage are indicated on the right.
A high throughput screen of the Broad Institute’s diversity-oriented synthesis (DOS) compound collection was performed with cryopreserved APC126-E cells (Tiong-Yip et al., 2014) at a single drug concentration of 10 μM, resulting in approximately 2,500 initial hits that exhibited >30% inhibition of the replicon (hit rate = 2.5%). Fig. 1 delineates the screening cascade that was used to identify validated hits. Based on cheminformatic analyses of the high throughput data using the Broad’s internal SAR analysis tools (Mulrooney et al., 2013), hits showing good activity, structurally attractive features or preliminary SAR were prioritized. For example, BRD9101 showed good inhibition within the primary screen (70% inhibition at 10 uM). Of the six stereoisomers tested, only one additional stereoisomer showed any level of inhibition within the primary screen (Figure 2). Analysis of closely related analogs identified BRD3969 and BRD65768 as having weak but significant activity against RSV, while also offering structural alternatives to the less attractive thiophene and styrene motifs (Supporting Figure 1). Additional SAR gathered from the primary screen pointed to the importance of the heteroaromatic sulfonamide off of the diazocane ring, as other sulfonamides did not show activity (Supporting Figure 1). BRD9101, BRD3969 and BRD65768 were included with 1,500 compounds that were cherry-picked for retest at dose in the replicon and cytotoxicity assays. Compounds that were active upon retest (EC50 < 10 μM) and showed little to no cellular toxicity (data not shown) were subsequently tested using a more relevant high throughput (384-well format) live virus cytopathic effect (CPE) assay followed by lower throughput (96-well format) CPE assays against RSV A2, RSV Long strain, RSV B (Washington strain), influenza, and herpes simplex virus (HSV). It should be noted that a number of compounds had no activity against the live virus, suggesting that their inhibition was replicon-specific. In addition to series 1 (exemplified by BRD9101), 2 additional series were identified that protected against RSV-induced CPE (across strains) and were inactive against influenza and HSV Type 2 (Fig. 3 and 4, data summarized in Table 1).
Figure 2. Series 1 compounds show stereoselective inhibition of the RSV replicon.
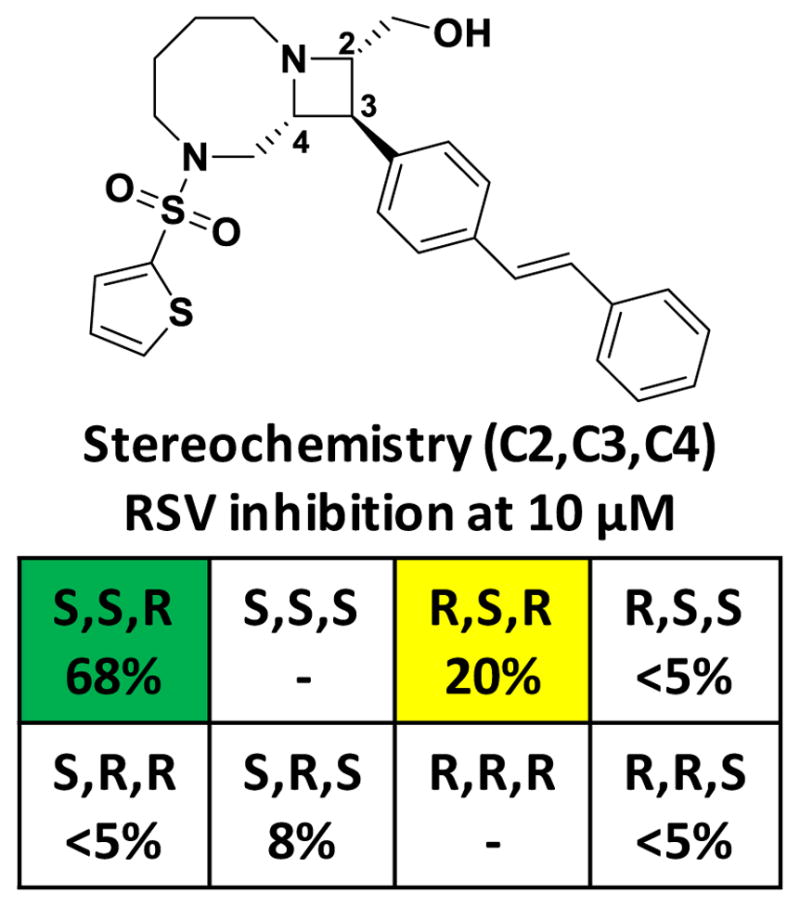
HTS data from the RSV replicon data are shown for all available stereoisomers of a representative compound from Series 1 (BRD9101). The stereochemistry of C2, C3, and C4 is indicated, along with the % inhibition of the RSV replicon; the S,S,R stereoisomer was the most potent, while the R,S,R diastereomer, differeing only in the extracyclic hydroxyl group, showed more modest inhibition. All other tested diastereomers were inactive.
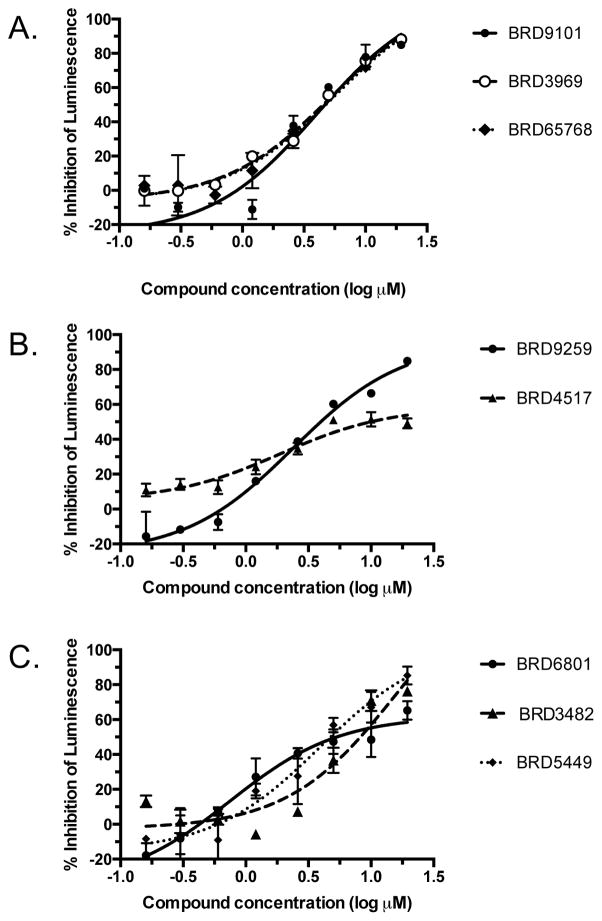
Figure 3. Hit compounds inhibit RSV replicon expression.
APC126-E cells expressing a subgenomic RSV replicon with a luciferase gene were treated with ascending doses of hit compounds for 48 hours and then assessed for total cellular luminescent signal. The selected compounds showed dose-dependent inhibition of luciferase suggesting inhibition of either replication or expression of the replicon system. Panels A, B, and C show compounds from Series 1, 2, and 3, respectively. EC50 values are provided in Table 1.
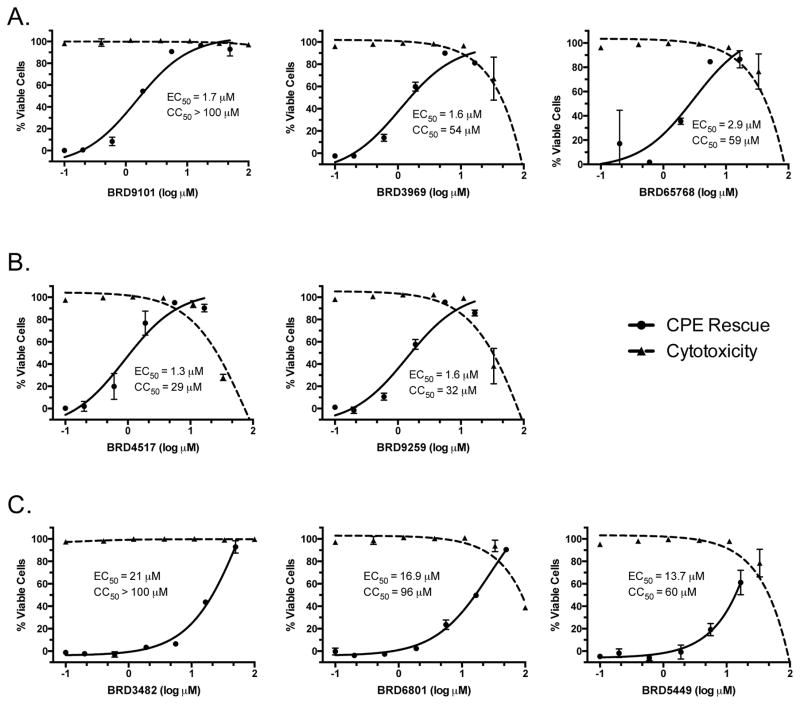
Figure 4. Hit compounds inhibit the RSV-induced CPE in a live virus infection model.
HEp2 cells were treated with ascending doses of test compounds and infected immediately thereafter with RSV A2 for five days. Parallel cytotoxicity assays were performed with uninfected cells. Relative viable cell counts were normalized to infected, untreated cells (0% viable) and uninfected, DMSO-treated cells (100% viable). The selected compounds showed dose-dependent inhibition of RSV-induced CPE with at least a ten-fold window between the antiviral effect and any observed cytotoxicity. Panels A, B, and C show compounds from Series 1, 2, and 3, respectively. EC50 and CC50 values are provided in Table 1.
Table 1. Activity of select Series 1, 2, and 3 compounds in the replicon and CPEassays.
The replicon assay was performed with APC126-E RSV replicon cells as described in the text. CPE assays were performed with Hep2 cells infected with RSV A2, RSV A Long, and RSV B Washington. Cytotoxicity was also determined in uninfected host cells. Series 1 and 2 are depicted in Panel A; Series 3 compounds are depicted in Panel B.
| A. | Series 1 Activity (μM) | Series 2 Activity (μM) | |||
|---|---|---|---|---|---|
|
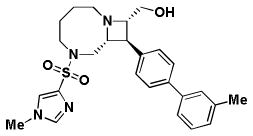
|
|
|
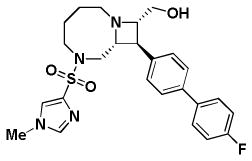
|
|
| BRD9101 | BRD3969 | BRD65768 | BRD4517 | BRD9259 | |
| Replicon EC50 | 4.3 | 4.2 | 4.3 | 10.6 | 4.4 |
| RSV A2 EC50 | 1.7/2.8* | 1.6/3.3* | 2.9/4.4* | 1.3/1.2* | 1.6/1.4* |
| RSV A2 CC50 > | 100/>100* | 54/56* | 59/64* | 29/22* | 31/60* |
| RSV Long EC50 | 1.7 | 3.9 | 7.5 | 1.6 | 1.6 |
| RSV B EC50 | > 50 | > 50 | > 50 | 5.5 | 6.8 |
| B. | Series 3 Activity (μM) | ||
|---|---|---|---|
|
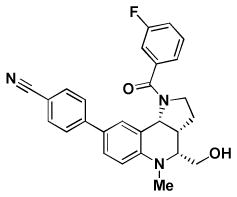
|
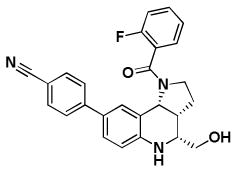
|
|
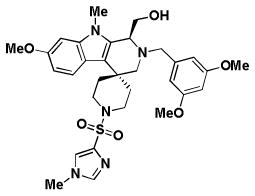
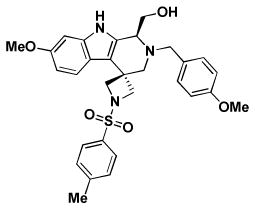
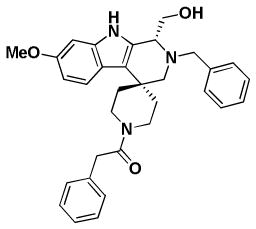
| BRD3482 | BRD6801 | BRD5449 | |
| Replicon EC50 | 7.2 | 7.3 | 4.9 |
| RSV A2 EC50 | 21/26.8* | 16.9/46.7* | 13.7/10.3* |
| RSV A2 CC50 | > 100/> 100* | 95.7/> 100* | 60/99* |
| RSV Long EC50 | 1.0 | 5.8 | 6.8 |
| RSV B EC50 | 4.7 | 11.9 | > 50 |
Activity of resynthesized compounds.
Of the three prioritized scaffolds, Series 2 showed broad activity against multiple RSV strains (RSV A & B), while Series 1 & 3 showed an activity preference towards RSV A. None of the compounds showed activity against HSV or influenza (data not shown). With the knowledge that chemistry optimization would be needed for lead development, we also considered initial SAR and in silico physicochemical properties in order to prioritize compounds. Series 2 compounds, while potent, were slightly more cytotoxic than the other compounds, and were thus deprioritized (Figure 4B). Series 3 compounds were also deprioritized due to lower initial potencies (Figure 4C). Series 1, as mentioned earlier, showed a stereochemical preference and preliminary appendage SAR from the primary screening data, while Series 2 and 3, although showing structural commonalities within each series, were less conclusive in terms of SAR, as hits within each respective series differed by stereochemistry and/or appendage groups. We feel the importance of stereochemical dependence of a chemical series on biological activity should be emphasized as it could indicate a specific interaction with the target. Finally, the compounds from Series 1, particularly BRD65768, showed good potential for further lead optimization, with good solubility, moderate microsomal and hepatic clearance, and minimal inhibition of the hERG channel (Table 2). For these reasons and in consideration of our limited resources, we chose to prioritize Series 1 for further investigation, although series 2 and 3 are still viable starting points.
Table 2. In vitro ADME/PK profiling of Series 1 and 3 compounds.
Compounds were profiled for aqueous solubility, LogD, microsomal stability (human liver microsomes, HLM), hepatic clearance (Rhep), plasma protein binding (PPB), and inhibition of the hERG channel. Compounds from Series 1 showed better solubility, LogD, and clearance than compounds from Series 3.
| Series 1 | Series 3 | |||||
|---|---|---|---|---|---|---|
| BRD9101 | BRD3969 | BRD65768 | BRD3482 | BRD6801 | BRD5449 | |
| Solubility (μM) | <1 | 49 | 121 | 22 | <1 | 16 |
| LogD | >4.8 | 3.6 | 3.3 | 2.9 | >4.3 | >4.4 |
| HLM (μL/min/mg) | 57.1 | 77.9 | 41.2 | ND | 208 | 465 |
| Rhep (μL/min/106 cells) | 67.8 | 61.7 | 23.6 | ND | 43 | 194 |
| PPB (% free) | <1 | 1.6 | 2.1 | 3.8 | <1 | <1 |
| hERG (% inh at 11 μM) | ND | 14 | 18 | ND | ND | ND |
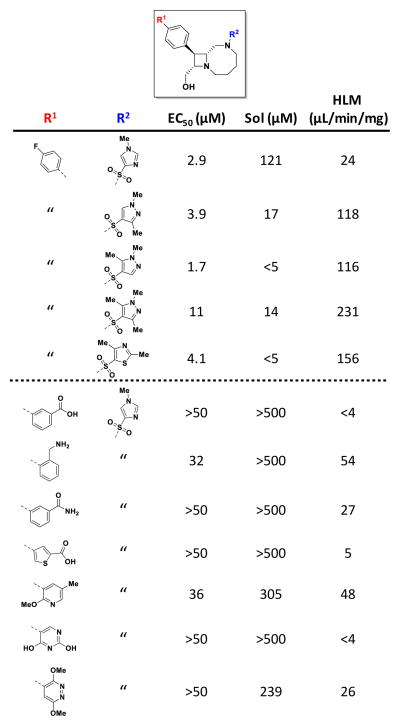
To further develop SAR within Series 1, a focused medicinal chemistry program was initiated with the goal to improve potency and physicochemical properties. Analogs were derived from two appendage groups (R1 and R2 in Figure 5), as initial SAR from the screening collection had already shown stereochemistry-dependent activity within the scaffold. Fairly conservative changes to R2, which included multiple heteroaromatic sulfonamides, including thiazoles, thiophenes and pyrazoles, were tested and showed no gains in potency (EC50 = 1–11 μM) as the SAR was relatively flat. Modifications to the biaryl group were largely not tolerated, although our efforts were solely focused on incorporating polar motifs (amines, amides, carboxylic acids and heteroaromatics) which could have affected compound permeability; the observed inactivity may have been due to poor exposure. No significant improvements in solubility or microsomal stability were attained from changes to R2; in contrast, modification of the biaryl group greatly increased solubility and microsomal stability, but this came at the expense of potency.
Figure 5. Examples of Series 1 medicinal chemistry analogs synthesized.
Approximately 28 analogs at either R1 or R2 were synthesized in an attempt to improve potency and physicochemical properties; activity against RSV in the CPE assay (EC50), solubility, and stability against human liver microsomes are indicated. Conservative changes to R2 were well tolerated, but less conservative modifications to the biaryl group resulted in a loss of activity.
In an initial effort to identify the target of Series 1 compounds, activity against RSV mutants resistant to inhibitors of the nucleoprotein (N) and polymerase (L) was assessed in CPE assays. The N mutant was resistant to RSV604, a benzodiazepine that inhibits viral replication (Chapman et al., 2007), and contains mutations mapped to the N protein, which encapsidates the RNA genome and is essential for replication and transcription. The L mutant virus was resistant to YM53403, and contains a single substitution (Y1631H) within the L polymerase gene (Sudo et al., 2005). None of the Series 1 compounds showed a significant shift in activity to the N mutant virus in CPE assays. In contrast, BRD9101 showed a greater than 15-fold shift in activity against the L-mutant strain, with BRD3969 and BRD65768 showing greater than 6-fold and 3-fold shifts in activity, respectively (Table 3). The activity shifts are comparable to the shift in activity seen with YM53403 (>19X shift, data not shown), indicating that Series 1 compounds target the RSV polymerase to inhibit replication.
Table 3.
Preliminary mechanism of action studies suggest that compounds from Series 1 target the RSV polymerase. Activity of select compounds against RSV strains containing mutations rendering them resistant to inhibitors of the viral polymerase (L protein, Sudo et al., 2005) or the nucleocapsid protein (N, Chapman et al., 2007) was assessed in CPE assays. Compounds from Series 1 showed a shift in activity against the L mutant virus but no significant shift in activity against the N mutant virus.
| BRD9101 | BRD3969 | BRD65768 | |
|---|---|---|---|
| RSV A2 EC50 | 1.7 μM | 3.9 μM | 6.1 μM |
| N Mutant (fold shift) | 7.8 μM (4.6x) | 6.9 μM (1.8x) | 13.1 μM (2x) |
| L Mutant (fold shift) | > 25 μM (>19x) | > 25 μM (>6x) | > 25 μM (>4x) |
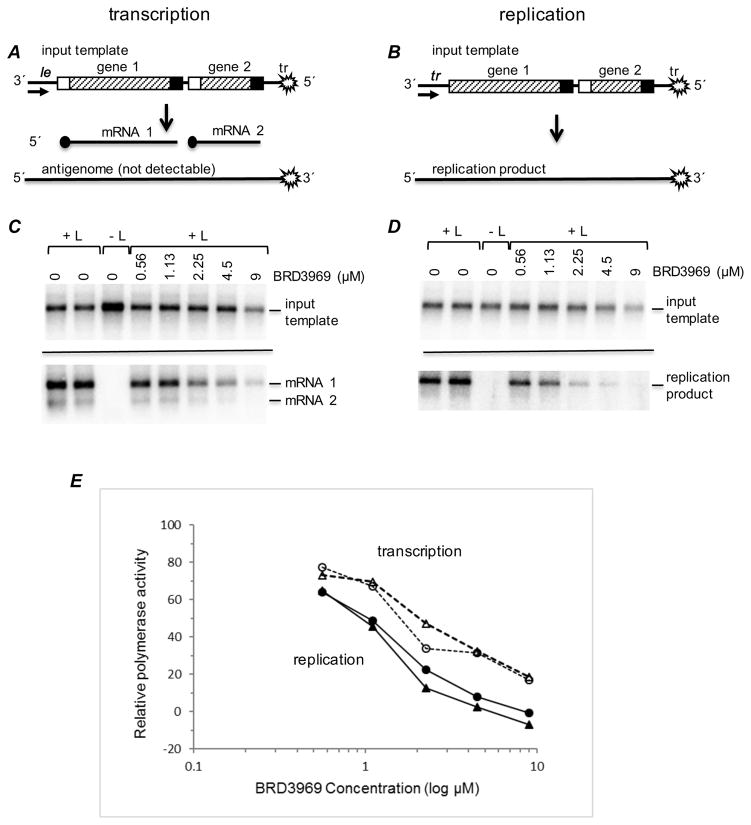
The RSV polymerase performs multiple activities during infection. During transcription, it synthesizes subgenomic mRNAs and catalyzes guanosine cap addition and cap methylation. During genome replication, it synthesizes a full-length encapsidated complement of the genome, the antigenome, which in turn acts as a template for genome synthesis. All these activities are necessary for replicon gene expression in the screen described above. To gain insight into which of these polymerase activities was inhibited by the Series 1 compounds, the representative compound BRD3969 was tested in a transient minigenome assay (Grosfeld et al, 1995). The minigenome assay differs from the replicon assay described above in two key ways. First, the only RSV proteins that are involved are those required for transcription and replication: N, P, M2-1 and L proteins. Second, the minigenome can be engineered so that it expresses non-specific mRNAs, and RNA replication is limited to the synthesis of the complementary strand. This means that, unlike the replicon assay, the efficiency of minigenome transcription and RNA replication are uncoupled from one another, allowing them to be analyzed as independent activities. The minigenome assay was used to determine if the compound inhibited a step specific to transcription, or replication, or if it inhibited both. Two minigenome templates were used, one optimized for measuring mRNA transcription, and the other for measuring RNA replication, as described previously (Noton et al, 2015) (Fig. 6A and B, respectively). RSV polymerase activity on the minigenome templates was reconstituted intracellularly by transient transfection, as described previously (Fearns et al, 1997). Total intracellular RNA was isolated and analyzed by Northern blot using probes that recognized either the input minigenome template (generated by T7 RNA polymerase) as a control (Fig. 6 C and D, upper panels), or the RNA products generated by the RSV polymerase (Fig. 6C and D, lower panels). Compound BRD3969 was added to the cell medium immediately following transfection and maintained at the concentrations indicated until cells were harvested. As shown in Fig. 6C–E, BRD3969 caused a dose-dependent reduction in RNA synthesis by the RSV polymerase. Accumulation of mRNA and replication products were inhibited to the same extent, indicating that BRD3969 inhibited a step common to transcription and replication. At high concentrations, the compound also inhibited T7 RNA polymerase, most likely due to non-specific cytotoxic effects (Fig. 6C and D, upper panels).
Figure 6. BRD3969 inhibits the mRNA transcription and RNA replication activities of the RSV polymerase.
A and B) Schematic diagrams of the minigenomes used to measure RSV transcription and replication, respectively. The transcription minigenome in (A) contains two reporter genes, CAT 1 and CAT 2 (each gene contains a different portion of the CAT open reading frame sequence) each flanked with RSV gene start and gene end sequences, and the 3′ and 5′ ends of the minigenome contain the 44 nt le and 155 nt tr sequences, respectively. The trailer region contained a C-to-G substitution at position 2 relative to the 5′ end, to inactivate the tr promoter that would typically be present at the 3′ end of the replication product. The replication minigenome (B) is similar to the one described above, except that in this minigenome, all transcription signals from the 3′ end, including the le and first gene start signal, were removed and replaced with nucleotides 1–36 of the tr promoter. The trailer region at the 5′ end of the minigenome contained a deletion of the 5′ terminal 22 nucleotides to avoid terminal complementarity and to inactivate the tr promoter that would typically be present at the 3′ end of the replication product. (C and D) Effect of varying concentrations of BRD3969 on the synthesis of minigenome templates by T7 RNA polymerase, and transcription and replication products by RSV polymerase, as determined by Northern blot analysis. The upper panels show input minigenome template, and the lower panel of (C) shows CAT 1 and CAT 2 mRNAs, whereas the lower panel of (D) shows the replicative RNA. (E) Quantification of the CAT 1 mRNA and replication RNA. The quantified RNA was normalized to the level of input template for that particular transfection, and the amounts of RNA generated by the RSV polymerase in the presence of compound were expressed relative to the mean levels of RNA generated from the minigenome in the absence of compound. The graph shows data from two independent experiments, with the levels of transcription product shown as dotted lines and the levels of replication product shown as solid lines.
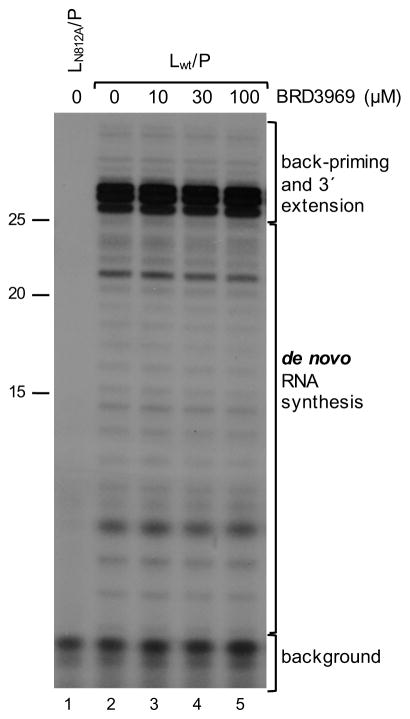
Given that BRD3969 inhibited both transcription and replication, possible points of inhibition were the RNA synthesis initiation and elongation activities of the polymerase. These possibilities were investigated by testing BRD3969 in an in vitro RNA synthesis assay (Noton et al., 2015). Purified recombinant RSV polymerase (L/P complex) was incubated in a transcription buffer containing an oligonucleotide RNA template, consisting of nucleotides 1–25 of the RSV tr promoter sequence, NTPs and a trace amount of [α-32P]-GTP. Radioactive products were analyzed by denaturing gel electrophoresis and autoradiography. The relative abundance of de novo RNA synthesis products (≤ 25 nt in length) synthesized in the presence of BRD3969 at concentrations up to 100 μM were indistinguishable from those synthesized in the presence of the DMSO control (Figure 7, compare lanes 2–5), demonstrating that BRD3969 does not inhibit the ability of the polymerase to synthesize RNA. Further increasing inhibitor concentrations to 1000 μM also had no effect on either RSV RNA synthesis initiation or elongation (data not shown). In addition to de novo RNA synthesis from the promoter, RSV RdRp has also been shown to support formation of a secondary loop structure at the 3′ end of the promoter, to which one to three nucleotides may be added, and occasionally elongated further (Noton et al., 2012; Noton et al., 2014), yielding prominent bands of 26 to 28 nucleotides as well as longer, less-abundant products (Figure 7, Lane 2). BRD3969 also had no effect on this RSV polymerase activity (Figure 7, Lanes 2–5).
Figure 7. BRD3969 does not inhibit the RNA synthesis activity of the RSV polymerase in vitro.
Purified L-P complexes containing wt L protein (lanes 2–5) or an L protein variant (lane 1) were added to reaction mixtures containing either DMSO or varying concentrations of BRD3969 (serially diluted in DMSO) as indicated. The labeled products were separated by denaturing gel electrophoresis and visualized by autoradiography. Bands ≤ 25 nt in length represent products of de novo synthesis, bands of ≥26 nt in length represent products of back-priming and 3′ extension. The sizes of the RNA products were determined by comparison to an RNA ladder generated by alkali hydrolysis of a 32P end-labeled RNA oligonucleotide representing the anticipated RNA products. The positions of the 15, 20 and 25 markers are shown.
The mutation conferring resistance to YM53403 has been shown to also confer resistance to a related analog, AZ-27 (Noton et al, 2015). The partial resistance of this mutant to BRD3969 in the CPE assay (Table 3) suggests that this inhibitor may share the same target with YM53403 and AZ-27. However, previous studies have shown that AZ-27 inhibits initiation of RNA synthesis at the promoter in vitro, albeit at approximately 10-fold higher concentrations than in the minigenome assays, whereas BRD3969 had no discernible effect at a concentration 100-fold above its cellular EC50. Taken together the data presented here suggest that BRD3969 affects an RSV polymerase activity that is common to both mRNA transcription and RNA replication, but that does not come into play in the in vitro RNA synthesis assay. An important difference between the minigenome and in vitro assays is that in the cell based minigenome assays, the template is encapsidated by N-protein while in the in vitro assay the template is naked RNA. It is conceivable that BRD3969 impacts a critical step in the interaction of the L/P polymerase complex with the encapsidated template that is required for the active site of the polymerase to gain access to the otherwise inaccessible template RNA. Unfortunately, as yet it is not possible to reconstitute the N-RNA template in vitro, or to reproducibly obtain sufficient purified N-RNA from RSV infected cells, to test this hypothesis directly. However, this would certainly be of interest for future studies when these techniques have been further developed.
In conclusion, this report confirms the previous results of Laganas et al (2015) that the RSV replicon system is a robust method for the identification of novel inhibitors for RSV. When combined with follow-up live virus CPE assays, we identified three novel diversity-oriented synthesis-derived scaffolds, which bear no resemblance to known RSV inhibitors including YM-53403, that inhibit the replication of RSV, but not influenza or HSV. Series 1 compounds were shown to inhibit the viral transcription and replication, likely via their inhibition of the L polymerase. Diversity-oriented synthesis-derived molecules have proven to be a rich source for diverse antimicrobial inhibitors (Comer et al, 2014, Heidebrecht et al, 2012, Dandapani et al, 2014). Here we show that the Broad’s DOS libraries may also be a source for new antiviral agents, having described three unique scaffolds as potential starting points for novel therapies for RSV infections.
Methods and Materials
Cells lines and viruses
The APC126-E replicon cell line (Tiong-Yip et al, 2014) was used under license from Apath LLC. Frozen aliquots of the cell line were obtained from the AstraZeneca Alderley Park team. For live virus assays, strains RSV A2, RSV A-Long, RSV B-Washington, influenza, and herpes simplex virus were used. RSV A2 (VR-1540), RSV A-Long (BR-26), RSV B-Washington (ATCC, VR-1580), and HSV-2 (VR-540) were obtained from the American Type Culture Collection (ATCC). Influenza strain B/Harbin/7/94 was a gift from the World Health Organization Reference Library (Melbourne, Australia).
High throughput replicon screen
The replicon HTS and cytotoxicity counter-screens were performed essentially as described in Tiong-Yip et al (2014), with the exception that compounds were added to wells containing the APC126-E cells by a pin tool transfer (HighRes Biosolutions). This assay was previously validated by Tiong-Yip et al (2014) and Xiong et al (2013), with Compound 1 exhibiting an EC50 = 0.31 μM (Xiong et al) and YM53403 and ribavirin exhibiting EC50s of 0.63 μM and 6.28 μM, respectively (Tiong-Yip et al). In-plate negative (DMSO) and positive [YM-53403 analog, compound 1, (Xiong et al., 2013)] controls were included on all plates for quality control and normalization. Raw screening data was normalized in Genedata Assay Analyzer (Genedata AG), and dose response results were analyzed in Condoseo (Genedata AG).
RSV CPE assays
High throughput CPE assays with RSV A2 were performed using Hep2 cells seeded at a density of 1,800 cells per well into 384 well plates the day before treatment and infection. On the day of infection, compounds were introduced by acoustic dispensing (Echo, LabCyte); the cells were subsequently infected with RSV A2 at a multiplicity of infection (MOI) of 0.5 infectious units/cell. Plates were incubated at 37°C, 5% CO2 for 5 days; the proportion of viable cells was determined using the Cell Counting Kit-8 CCK-8 (Dojindo), which produces an orange formazan product in the presence of living cells. Compound-related cytotoxicity was assessed in the same manner in uninfected cells. Secondary CPE assays with RSV A2, RSV Long, RSV Washington, influenza, and HSV were performed essentially as described above, except that assays were performed in 96-well plates.
Compound synthesis
Compounds from Series 1 (Lowe et al., 2012) and Series 2 (Gerard et al., 2012) were prepared following the synthetic routes previously described and analytical characterization data was consistent with the described compounds. Compounds from Series 3 were synthesized using methodology described previously (Klausen and Jacobsen, 2009).
Representative structural characterization data for Series 1 – BRD3969. 1H NMR (300 MHZ, CD3OD) δ 7.75 (s, 1H), 7.68 (s, 1H), 7.58 (d, 2H, J = 8.2 Hz), 7.44-7.33 (m, 4H), 7.29 (d, 1H, J = 7.5 Hz), 7.15 (d, 1H, J = 7.5 Hz), 3.88 (dd, 1H, J = 2.8, 13.9 Hz), 3.76 (s, 3H), 3.92-3.56 (m, 5H), 3.40-3.20 (m, 3H), 3.18-3.08 (m, 2H), 2.98-2.68 (br m, 2H), 2.40 (s, 3H), 2.10-1.95 (m, 1H), 1.93-1.79 (m, 1H), 1.78-1.60 (m, 2H). LRMS (ES+) calc’d for C27H34N3O3S [M + H]+ 481.2, found 481.1
In vitro drug metabolism and pharmacokinetics (DMPK) analyses
DMPK screening methods were employed to determine the solubility, Log D, protein binding, hERG inhibition and microsomal or hepatic stability of the selected compounds at 1 or 10 μM concentrations. Drug concentrations in the various assays were determined by LC-MS/MS. Methods for LogD, microsomal clearance, and plasma protein binding are described in Basarab et al (Basarab et al., 2013); the method for hERG inhibition screening is described in Bridgland-Taylor et al (Bridgland-Taylor et al., 2006).
Minigenome assay
Minigenomes for measuring RSV transcription or RNA replication have been described previously (Noton et al, 2015). HEp-2 cells were grown to nearconfluence on 12-well plates and transfected with T7 expression plasmid expressing the minigenome RNA (0.1 μg) and plasmids pTM1 N (0.2 μg), pTM1 P (0.1 μg), pTM1 M2-1 (0.05 μg) and pTM1 L (0.05 μg) with Lipofectin (Invitrogen) according to the manufacturer’s instructions. Cells were concurrently infected with MVA-T7 which provided T7 RNA polymerase to drive plasmid expression (Wyatt et al, 1995). Immediately after adding the transfection mix to cells, BRD3969, serially diluted in DMSO, was added at the concentrations indicated such that the concentration of DMSO was constant in all wells. After 18 h the transfection mix was replaced by complete medium. Cells were harvested approximately 40 h post-transfection and total intracellular RNA was isolated using an RNEasy kit (Qiagen) according to the manufacturer’s instructions.
Northern Blot analysis
Northern blot transfer, probe preparation and probe hybridization were performed as described previously (Fearns et al, 1997). Negative- and positive-sense 32P-labeled, CAT-specific riboprobes were generated using T7 RNA polymerase as described previously (Fearns et al, 1997). Blots were quantified by Phosphorimage analysis using a Personal Molecular Imager and Quantity One imaging software (BioRad).
In vitro RNA synthesis
The RSV L/P complex was purified as described previously (Noton et al., 2012). Reaction mixtures contained 2 μM of a template spanning nucleotides 1–25 of the RSV tr promoter sequence (Dharmacon); 50 mM Tris-HCl, pH 7.4; 8 mM MgCl2; 5 mM DTT; 10% glycerol; ATP, CTP, UTP and GTP each at 250 μM with 10 μCi of [α-32P]GTP. BRD3969, serially diluted in DMSO, was added to reactions to final concentrations indicated such that all reactions received an equal amount of DMSO. Reactions were incubated at 30ºC, and 200 ng L protein (in complex with P protein) was added, such that the final reaction volume was 50 μl. Reactions were allowed to proceed at 30ºC for 2 h. The RSV polymerase was inactivated by heating to 90ºC for 3 min. After adding 50 μl of stop buffer (deionized formamide containing 20 mM EDTA, bromophenol blue, and xylene cyanol), samples were heated to 95ºC for 5 min, briefly cooled on ice and analyzed by electrophoresis on a 20% polyacrylamide gel containing 7M urea in Tris-borate-EDTA buffer. RNA products were analyzed by autoradiography.
Supplementary Material
High throughput screening data identified additional active compounds (R1 across horizontal axis, R2 on vertical axis). Analysis of the primary high throughput screening data (RSV replicon assay) identified multiple compounds with statistically significant activity against RSV (yellow highlight) while offering structural alternatives to the less attractive thiophene and styrene motifs in BRD9101 (highlighted in green). Duvall et al.
Highlights.
A high throughput replicon-based screen was combined with live virus triaging assays to identify inhibitors of RSV.
Several novel diversity-oriented synthesis-derived chemical scaffolds that inhibit RSV were identified.
These compounds may provide attractive starting points for lead optimization campaigns for RSV therapeutics.
Acknowledgments
We thank the Broad Institute’s Informatics, Analytical, and Compound Management teams for their support of this project, and AstraZeneca’s Discovery Science HTS team and Choi Lai Tiong-Yip for reagents and technical support. We thank Drs. Peter Collins and Bernard Moss for providing minigenome components and MVA-T7, respectively. High throughput RSV CPE studies were performed by WuXi AppTec; lower throughput RSV A, RSV B, influenza, HPV, and mutant RSV CPE studies were performed by Biota Pharmaceuticals (Melbourne, Australia). Minigenome and in vitro RNA synthesis assays were performed with funding from NIAID R01AI113321 to RF. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References (new references highlighted)
- Baell JB. Screening-Based Translation of Public Research Encounters Painful Problems. ACS medicinal chemistry letters. 2015;6:229–234. doi: 10.1021/acsmedchemlett.5b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarab GS, Manchester JI, Bist S, Boriack-Sjodin PA, Dangel B, Illingworth R, Sherer BA, Sriram S, Uria-Nickelsen M, Eakin AE. Fragment-to-hit-to- lead discovery of a novel pyridylurea scaffold of ATP competitive dual targeting type II topoisomerase inhibiting antibacterial agents. Journal of medicinal chemistry. 2013;56:8712–8735. doi: 10.1021/jm401208b. [DOI] [PubMed] [Google Scholar]
- Bridgland-Taylor MH, Hargreaves AC, Easter A, Orme A, Henthorn DC, Ding M, Davis AM, Small BG, Heapy CG, Abi-Gerges N, et al. Optimisation and validation of a medium-throughput electrophysiology-based hERG assay using IonWorks HT. Journal of pharmacological and toxicological methods. 2006;54:189–199. doi: 10.1016/j.vascn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Chapman J, Abbott E, Alber DG, Baxter RC, Bithell SK, Henderson EA, Carter MC, Chambers P, Chubb A, Cockerill GS, et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrobial agents and chemotherapy. 2007;51:3346–3353. doi: 10.1128/AAC.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus research. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol. 2013;372:3–38. doi: 10.1007/978-3-642-38919-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer E, Duvall JR, duPont Lee Mt. Utilizing diversity-oriented synthesis in antimicrobial drug discovery. Future medicinal chemistry. 2014;6:1927–1942. doi: 10.4155/fmc.14.111. [DOI] [PubMed] [Google Scholar]
- Dandapani S, Germain AR, Jewett I, le Quement S, Marie JC, Muncipinto G, Duvall JR, Carmody LC, Perez JR, Engel JC, et al. Diversity-oriented synthesis yields a new drug lead for treatment of chagas disease. ACS Med Chem Lett. 2014;5:149–153. doi: 10.1021/ml400403u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearns R, Peeples ME, Collins PL. Increased expression of the N protein of respiratory syncytial virus stimulates minigenome replication but does not alter the balance between the synthesis of mRNA and antigenome. Virology. 1997;23:188–201. doi: 10.1006/viro.1997.8734. [DOI] [PubMed] [Google Scholar]
- Fearns R, Peeples ME, Collins PL. Mapping the transcription and replication promoters of respiratory syncytial virus. J Virol. 2002;76:1663–167. doi: 10.1128/JVI.76.4.1663-1672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosfeld H, Hill MG, Collins PL. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins; transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J Virol. 1995;69:5677–86. doi: 10.1128/jvi.69.9.5677-5686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard B, O’Shea MW, Donckele E, Kesavan S, Akella LB, Xu H, Jacobsen EN, Marcaurelle LA. Application of a catalytic asymmetric Povarov reaction using chiral ureas to the synthesis of a tetrahydroquinoline library. ACS combinatorial science. 2012;14:621–630. doi: 10.1021/co300098v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. The New England journal of medicine. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidebrecht RW, Jr, Mulrooney C, Austin CP, Barker RH, Jr, Beaudoin JA, Cheng KC, Comer E, Dandapani S, Dick J, Duvall JR, et al. Diversity- Oriented Synthesis Yields a Novel Lead for the Treatment of Malaria. ACS medicinal chemistry letters. 2012;3:112–117. doi: 10.1021/ml200244k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen RS, Jacobsen EN. Weak Bronsted acid-thiourea co-catalysis: enantioselective, catalytic protio-Pictet-Spengler reactions. Organic letters. 2009;11:887–890. doi: 10.1021/ol802887h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganas VA, Dunn EF, McLaughlin RE, Tiong-Yip CL, Yuzhakov O, Isabella VM, Hill P, Yu Q. Characterization of novel respiratory syncytial virus inhibitors identified by high throughput screen. Antiviral Research. 2015;115:71–74. doi: 10.1016/j.antiviral.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Lowe JT, Lee MDt, Akella LB, Davoine E, Donckele EJ, Durak L, Duvall JR, Gerard B, Holson EB, Joliton A, et al. Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. The Journal of organic chemistry. 2012;77:7187–7211. doi: 10.1021/jo300974j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhina O, Yednak MA, Collins PL, Olivo PD, Peeples ME. A respiratory syncytial virus replicon that is noncytotoxic and capable of long-term foreign gene expression. Journal of Virology. 2011;85:4792–4801. doi: 10.1128/JVI.02399-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney CA, Lahr DL, Quintin MJ, Youngsaye W, Moccia D, Asiedu JK, Mulligan EL, Akella LB, Marcaurelle LA, Montgomery P, et al. An informatic pipeline for managing high-throughput screening experiments and analyzing data from stereochemically diverse libraries. Journal of computer-aided molecular design. 2013;27:455–468. doi: 10.1007/s10822-013-9641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noton SL, Deflube LR, Tremaglio CZ, Fearns R. The respiratory syncytial virus polymerase has multiple RNA synthesis activities at the promoter. PLoS Pathog. 2012;8:e1002980. doi: 10.1371/journal.ppat.1002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noton SL, Aljabr W, Hiscox JA, Matthews DA, Fearns R. Factors affecting de novo RNA synthesis and back-priming by the respiratory syncytial virus polymerase. Virology. 2014;462–463:318–327. doi: 10.1016/j.virol.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noton SL, Nagendra K, Dunn EF, Mawhorter ME, Yu Q, Fearns R. Respiratory Syncytial Virus Inhibitor Az-27 Differentially Inhibits Different Polymerase Activities at the Promoter. Journal of Virology. 2015;89:7786–7798. doi: 10.1128/JVI.00530-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant H, Stacey C, Tiong-Yip CL, Walsh J, Yu Q, Rich K. High- Throughput Hit Screening Cascade to Identify Respiratory Syncytial Virus (RSV) Inhibitors. Journal of Biomolecular Screening. 2015;20:597–605. doi: 10.1177/1087057115569428. [DOI] [PubMed] [Google Scholar]
- Simoes EA, DeVincenzo JP, Boeckh M, Bont L, Crowe JE, Jr, Griffiths P, Hayden FG, Hodinka RL, Smyth RL, Spencer K, et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. The Journal of Infectious Diseases. 2015;211(Suppl 1):S1–S20. doi: 10.1093/infdis/jiu828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K, Miyazaki Y, Kojima N, Kobayashi M, Suzuki H, Shintani M, Shimizu Y. YM-53403, a unique anti-respiratory syncytial virus agent with a novel mechanism of action. Antiviral Research. 2005;65:125–131. doi: 10.1016/j.antiviral.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tiong-Yip CL, Plant H, Sharpe P, Fan J, Rich K, Gorseth E, Yu Q. Development of a high-throughput replicon assay for the identification of respiratory syncytial virus inhibitors. Antiviral research. 2014;101:75–81. doi: 10.1016/j.antiviral.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Wyatt LS, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]
- Xiong H, Foulk M, Aschenbrenner L, Fan J, Tiong-Yip CL, Johnson KD, Moustakas D, Fleming PR, Brown DG, Zhang M, et al. Discovery of a potent respiratory syncytial virus RNA polymerase inhibitor. Bioorganic & medicinal chemistry letters. 2013;23:6789–6793. doi: 10.1016/j.bmcl.2013.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High throughput screening data identified additional active compounds (R1 across horizontal axis, R2 on vertical axis). Analysis of the primary high throughput screening data (RSV replicon assay) identified multiple compounds with statistically significant activity against RSV (yellow highlight) while offering structural alternatives to the less attractive thiophene and styrene motifs in BRD9101 (highlighted in green). Duvall et al.