Abstract
The C677T functional variant in the methylene-tetrahydrofolate reductase (MTHFR) gene results in reduced enzymatic activity and elevated blood levels of homocysteine. Plasma levels of apolipoprotein E (ApoE) are negatively correlated with cerebral amyloid burden, but plasma homocysteine concentrations are associated with increased amyloid-β (Aβ) deposition in the brain. Here, we sought to determine whether associations between low plasma ApoE levels and elevated in-vivo amyloid burden were modulated by carrying the C677T variant. We tested this hypothesis in a large sample of elderly participants from the Alzheimer’s Disease Neuroimaging Initiative. We used general linear models to examine associations between plasma homocysteine concentrations, circulating ApoE levels, cerebrospinal fluid concentrations of Aβ, and their modulation by MTHFR and ApoE genotype. Age, sex, and dementia status were included as covariates in all analyses. Higher circulating levels of ApoE predicted increased cerebrospinal fluid concentrations of Aβ, indicating lower in-vivo burden, in C-allele carriers, but not in homozygotes at the C677T variant, who showed significant elevations in plasma homocysteine levels. This modulation by the MTHFR genotype did not remain significant after controlling for ApoE genotype. In T-homozygotes who do not carry the ApoE-ε4 allele, the relationship between low plasma ApoE levels and an increased risk of dementia is likely obscured by the presence of elevated plasma homocysteine. This report suggests the value of genotyping patients at the C677T functional variant when using plasma ApoE levels as a preclinical biomarker for Alzheimer’s disease.
Keywords: amyloid-β1–42, homocysteine, methylene-tetrahydrofolate reductase, neurodegeneration biomarkers, plasma apolipoprotein E
Introduction
Methylene-tetrahydrofolate reductase (MTHFR) is involved in the conversion of the amino acid homocysteine into methionine. The C677T functional variant in the MTHFR gene (minor allele frequency=0.245), which codes for a heat-sensitive variant characterized by reduced activity of the MTHFR enzyme, leads to elevated levels of homocysteine in the blood 1. Hyperhomocysteinemia is associated with higher rates of several age-related disorders, such as cardiovascular diseases 2,3, including vascular dementia 4. We previously reported that older adults with elevated homocysteine levels had more pronounced regional brain atrophy 5 and thinner cortical gray matter 6 on MRI. We also found that the C677T variant in MTHFR was associated with smaller regional brain volumes in two independent elderly cohorts with mild cognitive impairment (MCI) 7, and most recently expanded these findings by providing evidence for these associations across the dementia spectrum of normal cognitive aging, MCI, and Alzheimer’s disease (AD) 8.
Low cerebrospinal fluid (CSF) levels of amyloid-β1–42 (Aβ1–42) indicate the sequestration of Aβ in amyloid plaques in the brain and elevated in-vivo amyloid burden 9. This plaque burden is associated with AD, vascular dementia, and other degenerative brain disorders 10, including Parkinson’s disease 11, and also correlates with higher brain atrophy rates in healthy older adults 12. CSF Aβ1–42 may serve as one preclinical – and potentially predictive – biomarker for age-related cognitive decline and accelerated brain aging.
Apolipoprotein E (ApoE) is involved in Aβ1–42 clearance 13 and plasma levels of this protein are negatively correlated with cerebral amyloid burden as measured by neuroimaging 14,15. In fact, plasma ApoE levels are being proposed as a novel preclinical biomarker for AD 16. However, as carriers of the C677T variant have increased plasma homocysteine levels 1,17 and as higher plasma homocysteine concentrations are associated with increased Aβ1–42 deposition in the brain 18,19, we hypothesized that the link between elevated plasma ApoE levels and reduced cerebral amyloid burden may not hold in carriers of this variant, with important implications for the use of plasma ApoE as a preclinical marker of neurodegeneration in these individuals.
Materials and methods
We tested this hypothesis in a large sample of elderly individuals from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). ADNI participants were recruited from 58 sites across North America. The study was carried out according to the Good Clinical Practice guidelines, the Declaration of Helsinki, and US 21 CFR Part 50 (Protection of Human Subjects), and Part 56 (Institutional Review Boards). Written informed consent was obtained from all participants before protocol-specific procedures were performed. All ADNI data are publicly available at http://adni.loni.usc.edu. To avoid the known effects of population stratification on genetic analysis 20, we only included non-Hispanic White patients identified by self-report and confirmed by multidimensional scaling analysis 21. The ADNI cohort included multiple diagnostic groups: patients with AD, patients with MCI, and healthy elderly [cognitively normal (CON)] participants.
The ADNI sample was genotyped using the Illumina 610-Quad BeadChip (Illumina, San Diego, California, USA). Our analyses focused on the C677T functional variant in the MTHFR gene at the rs1801133 locus. ApoE genotyping was performed separately, on DNA samples obtained from participants’ blood, using an ApoE genotyping kit, as described in http://www.adni-info.org/scientists/ADNIStudyProcedures.aspx. Plasma homocysteine levels (pg/ml) and ApoE concentrations (mg/dl) were extracted from blood samples collected using standard venipuncture protocols. CSF samples were obtained through lumbar puncture after an overnight fast. Samples from various sites were transferred, on dry ice, to the ADNI Biomarker Core Laboratory at the University of Pennsylvania Medical Center, where Aβ1–42 concentrations were measured with a multiplex immunoassay platform under the guidance of Drs Leslie Shaw and John Trojanowski 22.
In the ADNI public database (http://www.adni.loni.usc.edu), plasma levels of homocysteine were available for 732 individuals (average age±SD=75.51±6.80 years; 436 men/296 women, including 173 AD, 355 MCI, and 204 CON); plasma ApoE levels for 517 participants (average age±SD=75.18±7.30 years; 321 men/196 women, including 110 AD, 353 MCI, and 54 CON); and CSF Aβ1–42 concentrations were accessible for 384 patients (average age±SD=75.09±7.00 years; 231 men/153 women, including 100 AD, 181 MCI, and 103 CON). We used general linear models to examine associations between plasma homocysteine concentrations, circulating ApoE levels, CSF Aβ1–42 concentrations, and their modulation by MTHFR and ApoE genotype. Age, sex, and dementia status were included as covariates in all analyses. The effect of the C677T variant on plasma homocysteine levels was additionally examined using a one-way analysis of variance with a Bonferroni post-hoc test, and the association between plasma ApoE concentrations and CSF levels of Aβ1–42 in C-allele carriers was also investigated using a two-tailed bivariate Pearson correlation. All statistical analyses were carried out in SPSS 23.0 (SPSS Inc., Chicago, Illinois, USA).
Results
As expected, the C677T variant was associated with significant elevations in plasma homocysteine levels, after controlling for age, sex, and dementia status (P<0.001, F-ratio=10.375). A one-way analysis of variance also showed significant effects of the C677T variant on plasma concentrations of homocysteine (P<0.001, F-ratio=10.705). However, a Bonferroni post-hoc test showed that levels did not differ significantly between carriers of a single T-allele and C-homozygotes. We found a significant association between higher circulating levels of ApoE and increased concentration of Aβ1–42 in the CSF, indicating a reduced in-vivo amyloid burden – in C-allele carriers, after controlling for age, sex, and dementia status (P=0.047, F-ratio=3.997). However, in T-homozygotes (who show significant elevations in plasma homocysteine levels), ApoE concentrations in the blood were not a significant predictor of CSF levels of Aβ1–42 (P=0.409, F-ratio=0.699), after controlling for age, sex, and diagnosis.
Figure 1 shows the significant positive correlation (Pearson’s r=0.289, P<0.001) between plasma ApoE concentrations and CSF levels of Aβ1–42 in C-allele carriers, which we failed to observe in T-homozygotes (Pearson’s r=−0.059, P=0.711). As the ApoE4 isotope leads to increased Aβ-peptide deposition 23 and as the ε4 allele is associated with lower plasma ApoE levels 14–16, this modulation by the MTHFR genotype did not remain significant after introducing ApoE genotype as an additional covariate in the statistical models.
Fig. 1.
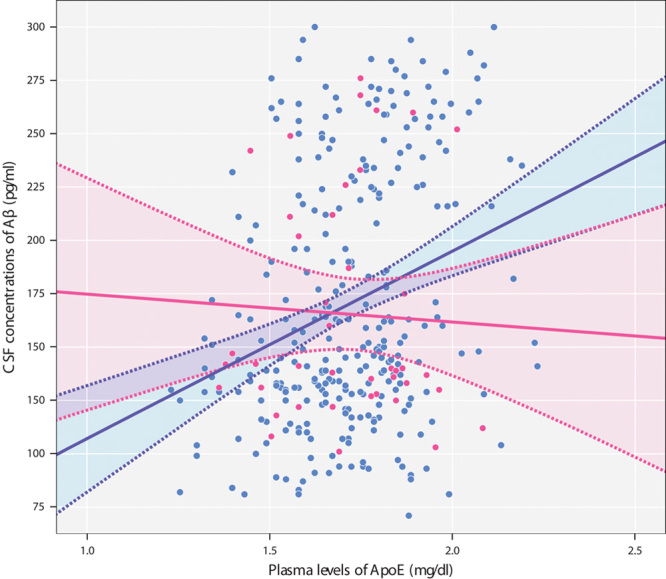
Higher plasma ApoE levels are associated with increased CSF Aβ1–42 concentrations in C-allele carriers (blue), but not in T-homozygotes (pink). x-axis: plasma ApoE levels; y-axis: CSF levels of Aβ1–42. Each dot represents a single patient's plasma and CSF concentrations of these biomarkers. Solid lines indicate regression lines. Dotted lines represent 95% confidence intervals for the mean. Aβ1–42, amyloid-β1–42; ApoE, apolipoprotein E; CSF, cerebrospinal fluid.
Conclusion
This study is the first to show that the C677T variant in MTHFR modulates associations between blood-based and CSF biomarkers of neurodegeneration. Low levels of ApoE, a plasma protein involved in Aβ1–42 clearance 13, are associated with higher amyloid burden in the brain 14,15, and with an increased risk of dementia, independent of ApoE genotype 14–16. Here, higher plasma ApoE levels predicted increased concentrations of Aβ1–42 in the CSF, indicating decreased in-vivo amyloid burden 9, in C-allele carriers, but not in individuals with the TT genotype. The modulation of the relationship between plasma ApoE and CSF Aβ1–42 by MTHFR genotype did not remain significant after controlling for ApoE genotype, suggesting that, although plasma homocysteine promotes amyloid deposition in the brain 18,19, its contribution toward cerebral amyloid burden may be weaker than that of the ApoE4 isotope, which leads to a reduction in the Aβ1–42 clearance rate 13.
Consistent with published findings 1,17, we found that T-homozygotes showed significant elevations in plasma homocysteine levels. As elevated plasma homocysteine concentrations are associated with increased Aβ1–42 deposition in the brain 18,19, it is possible that high homocysteine may offset the protective effects of elevated plasma ApoE levels in homozygotes at the C677T variant (who showed no correlation between plasma ApoE and CSF concentrations of Aβ1–42). Although genome-wide association studies provide no evidence that the C677T variant is an independent risk factor for AD, homocysteine itself is clearly associated with some of the hallmarks of dementia, including cerebral amyloid aggregation 19. Consequently, the relationship between low plasma ApoE levels and increased risk of AD may be obscured by the presence of elevated plasma homocysteine in T-homozygotes at this functional variant.
Our finding that the C677T functional variant in MTHFR affects the relationship between low plasma ApoE and increased amyloid deposition in the aging human brain is descriptive in nature and future investigations will need to clarify the precise biological mechanisms underlying these observations. Nonetheless, our results have important practical and clinical implications as plasma levels of ApoE are being proposed as a promising new and easily accessible preclinical biomarker for AD 16. In T-homozygotes who do not carry the ApoE-ε4 allele, high plasma ApoE may not be an accurate predictor of low cerebral amyloid burden. This report therefore highlights the importance of genotyping patients at the C677T variant in MTHFR when using plasma ApoE levels as a circulatory biomarker for degenerative brain disorders.
Acknowledgements
F.F.R. was supported, in part, by a research award from the Turken Foundation. This work was also supported by National Institutes of Health grants (R01 MH097268, R01 AG040060) to P.M.T. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development LLC; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace Inc.; Merck & Co. Inc.; Meso Scale Diagnostics LLC; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization for ADNI is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Institute for Neuroimaging and Informatics at the University of Southern California.
Data used in preparing this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://www.adni.loni.usc.edu). As such, the investigators within the ADNI contributed toward the design and implementation of ADNI and/or provided data, but did not participate in analysis or writing of this report. A complete listing of ADNI investigators may be found at: http://www.adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10:111–113. [DOI] [PubMed] [Google Scholar]
- 2.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost 1999; 81:165–176. [PubMed] [Google Scholar]
- 3.Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: causal relationship and potential mechanisms. Biofactors 2009; 35:120–129. [DOI] [PubMed] [Google Scholar]
- 4.Hainsworth AH, Yeo NE, Weekman EM, Wilcock DM. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 2016; 1862:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopalan P, Hua X, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Homocysteine effects on brain volumes mapped in 732 elderly individuals. Neuroreport 2011; 22:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen SK, Rajagopalan P, Joshi SH, Toga AW, Thompson PM. Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer’s Disease Neuroimaging Initiative. Neurobiol Aging 2015; 36 (Suppl 1):S203–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopalan P, Jahanshad N, Stein JL, Hua X, Madsen SK, Kohannim O, et al. Common folate gene variant, MTHFR C677T, is associated with brain structure in two independent cohorts of people with mild cognitive impairment. Neuroimage Clin 2012; 1:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussotte FF, Hua X, deZubicaray GI, McMahon KL, Wright MJ, Thompson PM. Alterations in regional brain volumes in carriers of the C677T mutation in MTHFR: interactions with circulating vitamin B12 levels. 12th International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD). Nice, France; 18–22 March 2015.
- 9.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006; 59:512–519. [DOI] [PubMed] [Google Scholar]
- 10.Sancesario GM, Bernardini S. How many biomarkers to discriminate neurodegenerative dementia? Crit Rev Clin Lab Sci 2015; 52:314–326. [DOI] [PubMed] [Google Scholar]
- 11.Berlyand Y, Weintraub D, Xie SX, Mellis IA, Doshi J, Rick J, et al. An Alzheimer’s disease-derived biomarker signature identifies Parkinson’s disease patients with dementia. PLoS One 2016; 11:e0147319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schott JM, Bartlett JW, Fox NC, Barnes J. Alzheimer’s Disease Neuroimaging Initiative I. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1-42. Ann Neurol 2010; 68:825–834. [DOI] [PubMed] [Google Scholar]
- 13.Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for impaired amyloid β clearance in Alzheimer’s disease. Alzheimers Res Ther 2013; 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta VB, Laws SM, Villemagne VL, Ames D, Bush AI, Ellis KA, et al. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology 2011; 76:1091–1098. [DOI] [PubMed] [Google Scholar]
- 15.Gupta VB, Wilson AC, Burnham S, Hone E, Pedrini S, Laws SM, et al. Follow-up plasma apolipoprotein E levels in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL) cohort. Alzheimers Res Ther 2015; 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol 2015; 77:301–311. [DOI] [PubMed] [Google Scholar]
- 17.Hustad S, Midttun Ø, Schneede J, Vollset SE, Grotmol T, Ueland PM. The methylenetetrahydrofolate reductase 677C→T polymorphism as a modulator of a B vitamin network with major effects on homocysteine metabolism. Am J Hum Genet 2007; 80:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JG, Chu J, Barrero C, Merali S, Praticò D. Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann Neurol 2014; 75:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamat PK, Vacek JC, Kalani A, Tyagi N. Homocysteine induced cerebrovascular dysfunction: a link to Alzheimer’s disease etiology. Open Neurol J 2015; 9:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lander ES, Schork NJ. Genetic dissection of complex traits. Science 1994; 265:2037–2048. [DOI] [PubMed] [Google Scholar]
- 21.Stein JL, Hua X, Morra JH, Lee S, Hibar DP, Ho AJ, et al. Genome-wide analysis reveals novel genes influencing temporal lobe structure with relevance to neurodegeneration in Alzheimer’s disease. Neuroimage 2010; 51:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, et al. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement 2010; 6:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA 1993; 90:9649–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]


