Abstract
The ghrelin receptor (GHSR1a) and dopamine receptor-1 (DRD1) are coexpressed in hippocampal neurons, yet ghrelin is undetectable in the hippocampus; therefore, we sought a function for apo-GHSR1a. Real-time single-molecule analysis on hippocampal neurons revealed dimerization between apo-GHSR1a and DRD1 that is enhanced by DRD1 agonism. In addition, proximity measurements support formation of preassembled apo-GHSR1a:DRD1:Gαq heteromeric complexes in hippocampal neurons. Activation by a DRD1 agonist produced non-canonical signal transduction via Gαq-PLC-IP3-Ca2+ at the expense of canonical DRD1 Gαs cAMP signaling to result in CaMKII activation, glutamate receptor exocytosis, synaptic reorganization, and expression of early markers of hippocampal synaptic plasticity. Remarkably, this pathway is blocked by genetic or pharmacological inactivation of GHSR1a. In mice, GHSR1a inactivation inhibits DRD1-mediated hippocampal behavior and memory. Our findings identify a previously unrecognized mechanism essential for DRD1 initiation of hippocampal synaptic plasticity that is dependent on GHSR1a, and independent of cAMP signaling.
INTRODUCTION
G-protein coupled receptors (GPCRs) belong to a large family of cell surface receptors that transduce their signal by activating a trimeric G-protein complex (Gαβγ). Since GPCRs are important regulators of cell signaling, developing agonists and antagonists to target GPCRs is a major focus of drug discovery. However, traditional approaches to identify drug candidates based on specificity and functional activity in “artificial” cell-based assays has failed to accurately predict clinical outcomes. GPCRs are perceived to signal as monomers, but emerging evidence also implicates signal transduction through homomers and heteromers. Heteromers may play a crucial role in allosteric interactions that occur between protomers to alter signal transduction and modify biological function. Therefore, knowing the physiological pathways regulated by GPCR heteromers, homomers and monomers in target cells is clinically relevant. Our research focuses on Class A GPCRs, and in particular the orphan growth hormone secretagogue receptor (GHSR1a) that was subsequently deorphanized by the discovery of ghrelin in stomach extracts (Howard et al., 1996; Kojima et al., 1999; Smith et al., 1997). We previously showed that GHSR1a forms heteromers with dopamine receptor-2 (DRD2) in hypothalamic neurons and that signaling through GHSR1a:DRD2 is essential for DRD2 agonist-induced suppression of food intake (Kern et al., 2012).
Paradoxically, despite expression of GHSR1a in the hippocampal structures (Guan et al., 1997), ghrelin is undetectable in the central nervous system, with the exception of trace amounts in the hypothalamus (Cowley et al., 2003; Furness et al., 2011; Grouselle et al., 2008; Sakata et al., 2009). Based on this observation, we set out to identify a function for apo-GHSR1a in the hippocampus. Using immunohistochemistry on brain sections of Ghsr-IRES-tau-GFP knock-in mice, we identified hippocampal neurons that coexpress GHSR1a and DRD1 (Jiang et al., 2006). Although DRD1 is implicated in regulating hippocampal synaptic plasticity involved in memory and learning, the mechanisms involved are incompletely understood (Hamilton et al., 2010; Rossato et al., 2009). We speculated that determining the function of apo-GHSR1a in DRD1 expressing hippocampal neurons would provide new insight into these mechanisms. Previously, studies on dopamine/DRD1 signaling in the hippocampus have focused mainly on canonical signaling, with DRD1 coupling to Gαs that enhances cAMP accumulation and activation of protein kinase A (PKA) (Abel et al., 1997; Huang and Kandel, 1995). However, DRD1 signaling through Gαq has also been described (Jin et al., 2003; Lezcano and Bergson, 2002); nevertheless, what determines Gαq over Gαs signaling, had not been elucidated.
Here, we shed light on DRD1 signaling in the hippocampus by illustrating formation of apo-GHSR1a:DRD1 heteromers that by an allosteric mechanism results in DRD1 coupling to Gαq at the expense of Gαs. Activation of apo-GHSR1a:DRD1 by a DRD1 agonist mobilizes intracellular Ca2+ ([Ca2+]i) and initiates hippocampal synaptic plasticity, independent of cAMP signaling. An essential modulatory role for apo-GHSR1a on hippocampal DRD1 signaling would not have been recognized previously, because experiments were performed in animals that express GHSR1a endogenously. By establishing that apo-GHSR1a is an essential modifier of DRD1 signaling in the hippocampus, we resolve the conundrum of hippocampal GHSR1a expression in the absence of endogenous ghrelin, and provide a mechanism for how DRD1 activation of Gαq-signaling in hippocampal neurons is regulated. While it is generally agreed that memory consolidation involves DRD1-induced PKA and cAMP signaling, our results support an essential and fundamental role for Gαq-PLC-Ca2+ signal transduction in initiating this process.
RESULTS
GHSR1a:DRD1 Heteromers in Native Hippocampal Neurons
Following confirmation of the selectivity of the DRD1 antibody (Fig. S1A), immunohistochemistry on Ghsr-IRES-tauGFP mouse brain sections revealed highest levels of GHSR1a and DRD1 coexpression in the CA3 and dentate gyrus (DG) of the hippocampal structures (Figure 1A). Hypothalamic regions also stained for GHSR1a, but lacked detectable DRD1 staining (Figure 1A).
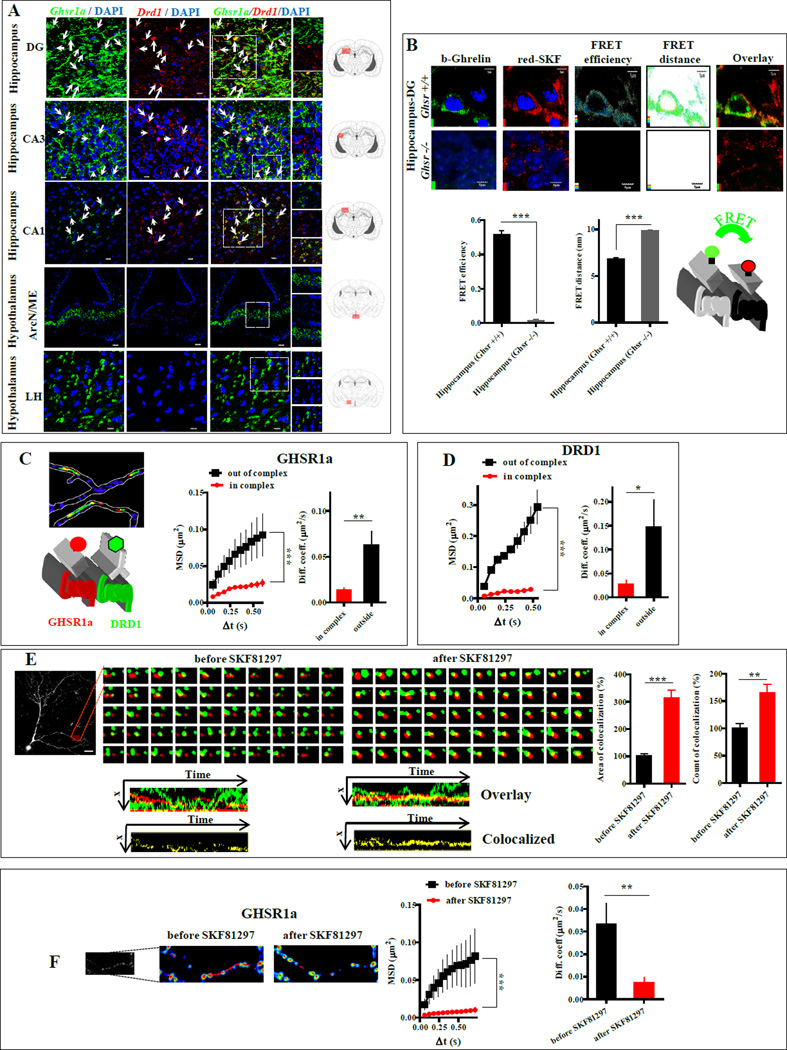
Figure 1. Co-expression of GHSR1a and DRD1 in mouse hippocampal neurons and heteromer formation measured by FRET confocal microscopy and TIRF-M.
(A) Brain sections from Ghsr-IRES-tauGFP mice stained for DRD1 (red) and GHSR1a (green). White arrows indicate individual neurons co-expressing GHSR1a and DRD1 (ArcN/ME, arcuate nucleus and median eminence; LH, lateral hypothalamus). Coexpression of GHSR1a and DRD1: 46%, DG; 17%, CA3; 10%, CA1 regions. Scale bar, 10 µm.
(B) Confocal microscopic FRET analysis of GHSR1a:DRD1 heteromers in DG, Scale bar, 5 µm.
(C and D) Quantification of trajectories of GHSR1a and DRD1 in neuron processes: GHSR1a (red), DRD1 (green), overlapping (yellow). MSD versus time, and diffusion coefficients of GHSR1a trajectories in and outside a complex with DRD1(C; ntrajectories=236), and DRD1 trajectories in and outside a complex with GHSR1a (D; ntrajectories=111).
(E) Two color single molecule TIRF-M images of interactions of GHSR1a with DRD1 in dendritic processes of hippocampal neurons before and after SKF81297 (1µM) treatment: GHSR1a (red), DRD1 (green). Kymographs images of co-localization (yellow); quantitative analysis of the interactions measured by area of co-localization and counts of co-localization.
(F) Individual GHSR1a trajectories by TIRF-M before and after SKF81297 treatment: MSD vs time, diffusion coefficients.
Data represent the mean ± SEM of at least three experiments (*, p<0.05 versus control; **, p<0.01 versus control; ***, p<0.001 versus control).
To test for formation of GHSR1a:DRD1 heteromers in hippocampal neurons we employed confocal FRET microscopy on hippocampal brain slices. Biotin-ghrelin labeled with FITC-avidin (green) was used to detect GHSR1a, and a fluorescently labeled DRD1 antagonist (red-SKF83566) used for DRD1. We validated the utility of fluorescently labeled ligands for FRET and binding specificity of each fluorophore (Figure S1B,C,D). FRET confocal microscopy and image analysis showed GHSR1a and DRD1 in close proximity (6.8±0.1 nm) with a FRET efficiency of 0.51±0.02, consistent with formation of GHSR1a:DRD1 heteromers (Figure 1B). Parallel incubations using hippocampal slices from Ghsr−/− mice illustrated red-SKF83566 labeling of DRD1 receptors without green fluorescence labeling (Figure 1B).
GPCRs are recruited and dynamically assembled on the cell membrane (Kasai and Kusumi, 2014). To monitor real-time dynamics of DRD1 and GHSR1a interactions in primary hippocampal neuronal cultures, we performed two color single-molecule analyses using total-internal reflection fluorescence microscopy (TIRF-M). Qdot565-avidin-labeled biotinylated-ghrelin was used to detect GHSR1a, and Qdot655-labeled DRD1 monoclonal antibody for DRD1. GHSR1a and DRD1 exhibited mobility and co-localization in the membrane (Figure S1C). Analysis of the individual trajectories of GHSR1a and DRD1 molecules illustrated significantly slower diffusion within, than outside the complex (Figure 1C,D). Treatment with a DRD1-specific agonist (SKF81297) increased co-localization compared to vehicle treatment (312±31.2% vs 100±9%, p<0.001; Figure 1E), and augmented the number of co-localization events (163.8±17.1% vs 100±8.5%, p<0.01; Figure 1E). By measuring diffusion of the individual GHSR1a molecules, we showed that SKF81297 significantly increased the confinement of GHSR1a in a complex with DRD1, as indicated by a reduction in the steady state of the mean square displacement (MSD) curve (p<0.001; Figure 1F). The diffusion coefficient of mobile receptors was higher before SKF81297 treatment (0.033±0.009), than after treatment (0.0072±0.002; p<0.01; Figure 1F); hence, single-molecule analysis revealed that a DRD1 agonist enhances formation of GHSR1a:DRD1 heteromeric complexes on mouse hippocampal neurons.
DRD1 Agonist Activation of GHSR1a:DRD1 Induces Ca2+ Transients via Gαq at the Expense of Gαs Coupling in Hippocampal Neurons
Having demonstrated close proximity and dynamic interactions of GHSR1a and DRD1 in hippocampal neurons, we asked whether these interactions modified canonical DRD1 signal transduction. To test for mobilization of [Ca2+]i, we transduced primary cultures of mouse hippocampal pyramidal neurons with lentivirus expressing the genetically encoded Ca2+ sensor GCaMP3. Treatment with SKF81297 dose-dependently induced rapid Ca2+ transients (EC50= 50.37± 0.12 nM; Figure 2A). Post treatment with ghrelin induced Ca2+ transient in the same neurons that responded to SKF81297 (Figure 2A), which confirmed coexpression of GHSR1a and DRD1. To test for GHSR1a dependence, we compared the effects of SKF81297 in organotypic hippocampal slices from Ghsr +/+ and Ghsr −/− mice expressing GCaMP3. Ca2+ transients were induced by SKF81297 in slices from Ghsr +/+ mice, but not in slices from Ghsr −/− mice; thus, Ca2+ mobilization is dependent on interactions between DRD1 and GHSR1a (Figure 2B, p<0.001). A characteristic property of GPCR heteromers is the ability of an antagonist of one protomer to modify signaling of its protomer partner (Smith and Milligan, 2010). Pretreating organotypic hippocampal slices from Ghsr+/+ mice with the GHSR1a neutral antagonist, JMV2959, blocked SKF81297-induced Ca2+ mobilization (p<0.05; Figure 2C), which is consistent with modification of allosteric interactions between GHSR1a and DRD1 in the GHSR1a:DRD1 heteromeric complex.
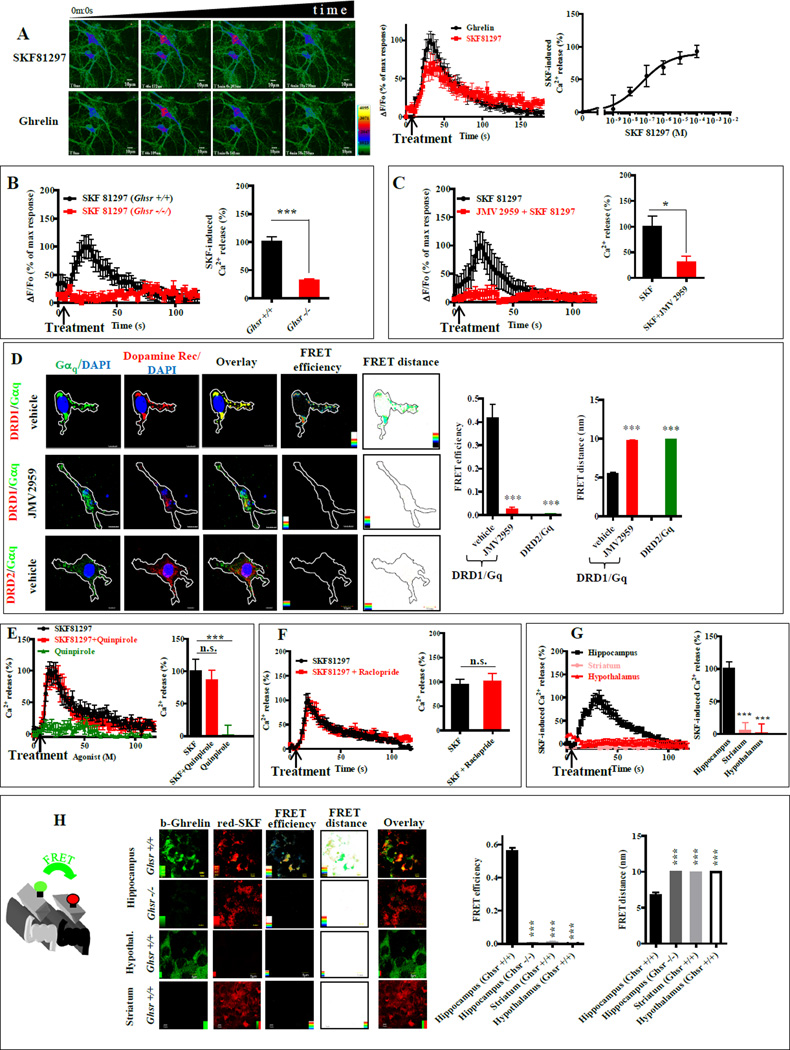
Figure 2. In hippocampal neurons, DRD1 agonist-induced [Ca2+]i mobilization is dependent on GHSR1a, but not on DRD2.
(A) SKF81297 (10 µM, upper row) produced a transient Ca2+ response in hippocampal neurons; after removing SKF81297 the same cells responded to ghrelin (100 nM, lower row). SKF81297 induced dose-dependent [Ca2+]i release (right). Scale bar, 10 µm.
(B) SKF81297-induced [Ca2+]i mobilization in hippocampal organotypic slices from Ghsr+/+ and Ghsr −/− mice (left).
(C) JMV2959 (10 µM) inhibits SKF81297 (10 µM) induced [Ca2+]i mobilization in hippocampal organotypic slices.
(D) Hippocampal neurons treated with vehicle (upper) or JMV2959 (middle); Gαq (green) and DRD1 (red) images analyzed by FRET microscopy. Lower row: DRD2 (red), Gαq (green) quantitative analysis of FRET efficiency and FRET distance (right). Scale bar, 10 µm.
(E and F) [Ca2+]i mobilization in hippocampal organotypic slices in the presence of SKF81297 (10µM), SKF81297 (10µM) + Quinpirole (10µM) and Quinpirole (10µM) (E); or SKF81297 (10µM) and SKF81297 (10µM) + Raclopride (10µM) (F).
(G) SKF81297-induced (10 µM) [Ca2+]i mobilization in neurons from hippocampus, hypothalamus and striatum (left).
(H) FRET confocal microscopy images on organotypic brain slices from hippocampus, hypothalamus and striatum of Ghsr+/+ mice; slices from Ghsr−/− as control. GHSR1a (green), DRD1 (red); Scale bar, 5 µm.
Data represent the mean ± SEM of at least three independent experiments in duplicate (*, p<0.05 versus control; ***, p<0.001 versus control).
We next asked whether SKF81297-induced [Ca2+]i mobilization was mediated by DRD1:Gαq coupling through a preassembled GHSR1a:DRD1:Gαq complex. Gαq and DRD1 showed punctate co-localization in neuritic processes indicating formation of signalosomes (Figure 2D). FRET confocal microscopy showed Gαq and DRD1 in close proximity (5.45±0.2 nm; FRET efficiency, 0.416±0.06; Figure 2D), consistent GHSR1a:DRD1:Gαq preassembly in hippocampal neurons. In contrast to DRD1:Gαq, the FRET distance (9.98±0.035 nm) and FRET efficiency (0.005±0.02; Figure 2D) measured for DRD2 and Gαq in hippocampal neurons is inconsistent with their close proximity.
In the striatum, DRD2 and DRD1 are reported to form heteromers that couple to Gαq; concomitant agonist activation of DRD2 and DRD1 mobilized Ca2+, which was blocked by a DRD2 antagonist (Hasbi et al., 2009). While proximity measurements did not support DRD2:Gαq interactions in hippocampal neurons (Figure 2D), the possibility remained that DRD1 agonist-induced [Ca2+]i mobilization was explained by DRD1 coupling to Gαq in a DRD2:DRD1 complex rather than GHSR1a:DRD1. To address this possibility, we measured [Ca2+]i release after treatment with pharmacologic agents: DRD1 agonist, SKF81297; DRD2 agonist, quinpirole; DRD2 antagonist, raclopride. Quinpirole neither mobilized, nor enhanced SKF81297-induced [Ca2+]i mobilization (Figure 2E), and raclopride did not attenuate SKF81297-induced Ca2+ release (Figure 2F); thus, signaling through a DRD2:DRD1:Gαq complex does not explain SKF81297-induced Ca2+ release in hippocampal neurons. In addition, SKF81297 did not mobilize Ca2 in hypothalamic neurons or in striatal neurons (Figure 2G). Further supporting hippocampal specificity, confocal FRET microscopy showed no evidence of GHSR1a:DRD1 heteromers in hypothalamic or striatal neurons (Figure 2H).
To test whether SKF81297 activation of [Ca2+]i release through GHSR1a:DRD1 was mediated by PLC, we monitored, in real-time, the localization of phosphatidylinositol 4,5-bisphosphate (PIP2). Primary hippocampal neurons from Ghsr+/+ and Ghsr−/− mice were transduced with lentivirus encoding the PIP2 biosensor, GFP-PH (Stauffer et al., 1998). In the basal state, GFP-PH was confined to the plasma membrane, indicating that the GHSR1a:DRD1 complex was not constitutively active. Treatment with SKF81297 rapidly induced cytoplasmic accumulation of GFP-PH in the soma and in neuritic processes of hippocampal neurons from Ghsr+/+, but not in neurons from Ghsr−/− mice (Figure 3A); thus, translocation of PIP2 is dependent on GHSR1a. Pretreatment of hippocampal neurons with the PLC inhibitor, U73122, inhibited DRD1 agonist-induced Ca2+ mobilization, further supporting dependence on PLC (p<0.001; Figure 3B). To determine whether Gαq coupling and PLC activation occurred at the expense of canonical DRD1 Gαs coupling, membranes isolated from the hippocampus of Ghsr+/+ and Ghsr−/− mice were treated with SKF81297 or vehicle, and cAMP production measured. Hippocampal membranes from Ghsr+/+ mice produced markedly less cAMP in response to SKF81297 compared to those from Ghsr −/− mice (Figure 3C). Therefore, the presence of GHSR1a on the membranes reduces DRD1 coupling to Gαs in favor of Gαq, suggesting an equilibrium between GHSR1a:DRD1 and DRD1:DRD1 on the hippocampal neuron to allow signaling through Gαq and Gαs, respectively.
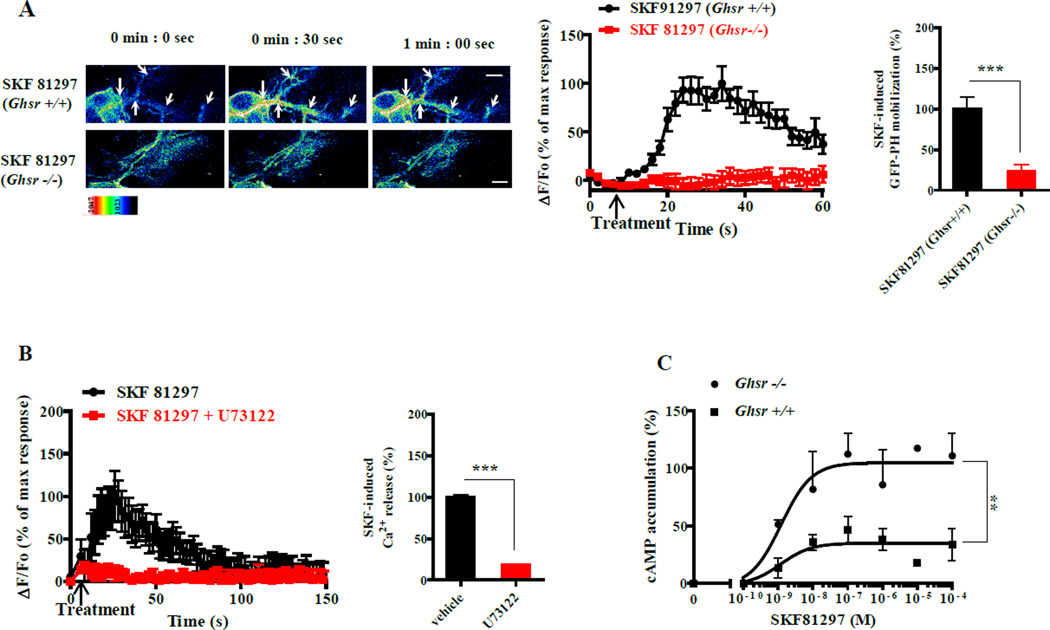
Figure 3. In hippocampal neurons, GHSR1a modifies DRD1 canonical signaling to induce [Ca2+]i release via Gαq at the expense of Gαs coupling.
(A) SKF81297 (10 µM) induces translocation of the PLC sensor (GFP-PH) to cytoplasm in neurons of Ghsr+/+ mice (upper row), but not neurons of Ghsr−/− mice (lower row); Scale bar, 5 µm.
(B) PLC inhibitor (U73122, 10 µM) blocks SKF81297 (10 µM) -induced [Ca2+]i release in hippocampal neurons.
(C) Dose-dependent effects of SKF81297 treatment on cAMP accumulation is lower in hippocampal membranes from Ghsr+/+ (■) than in membranes from Ghsr−/− (●) mice.
Data represent the mean ± SEM of at least three independent experiments (**, p<0.01 versus control; ***, p<0.001 versus control).
DRD1 agonist-induced [Ca2+]i Mobilization Correlates With GHSR1a:DRD1 Heteromer Formation
To further characterize interactions between apo-GHSR1a and DRD1, and to determine whether GHSR1a heteromerization and dopamine activation of non-canonical DRD1 signaling is conserved across species, we expressed human GHSR1a and DRD1 in the human embryonic kidney (HEK293) cell line. We monitored heteromerization by time-resolved (Tr)-FRET using GHSR1a and DRD1 with a SNAP- or CLIP- tagged N-terminus, using methods described previously (Kern et al, 2012). Tr-FRET receptor titration assays and competition assays supported the formation of GHSR1a and DRD1 homomers and heteromers on the plasma membrane (Figure S2A,B). As additional proof of heteromer formation, BRET saturation, type-2 BRET, and microscopic BRET assays were conducted. The results ruled out random stochastic interactions between apo-GHSR1a and DRD1 and confirmed formation of GHSR1a:DRD1 heteromers (Figure S2C,D,E).
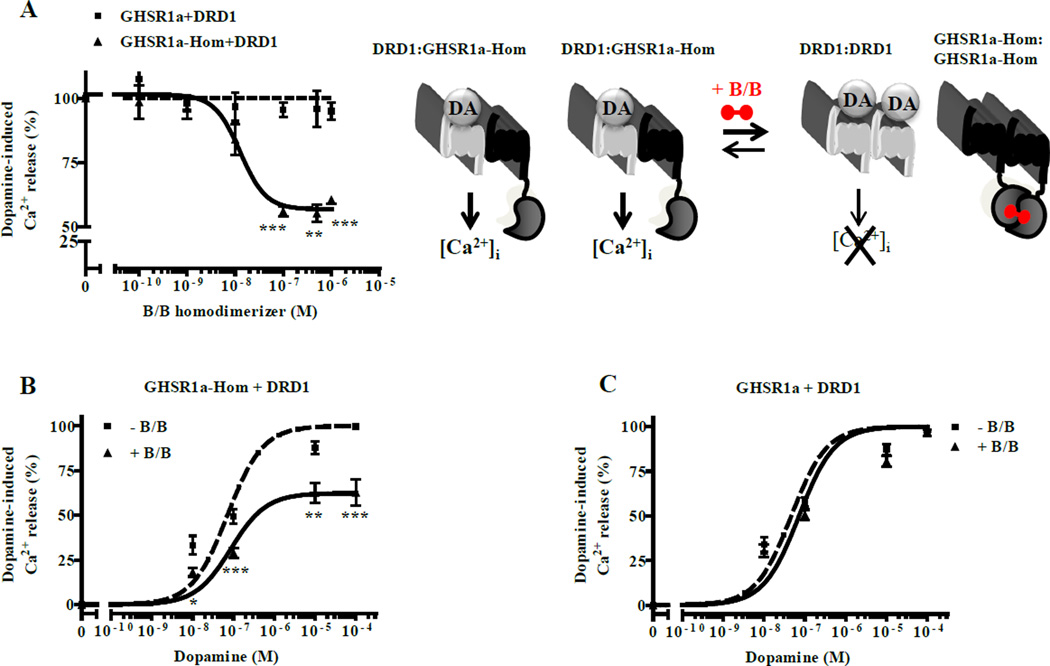
We employed HEK293-AEQ cells that stably express the bioluminescence Ca2+ sensor aequorin to monitor [Ca2+]i mobilization. Dopamine and SKF81297 dose-dependently induced Ca2+ mobilization with dependence on apo-GHSR1a, which was blocked by the DRD1 antagonist, SCH23390, but not by the DRD2 antagonist, raclopride (Figure S3A,B,C). In control experiments, Ca2+ release was not detected when GHSR1a or DRD1 were coexpressed with other Gαs or Gαq coupled GPCRs (Figure S3D), which was consistent with their failure to form heteromers (Figure S2B). Collectively, these results show that dopamine-induced [Ca2+]i mobilization correlates with GHSR1a:DRD1 heteromer formation rather than cross-talk between Gαq and Gαs signaling. To quantify the correlation between GHSR1a:DRD1 formation and [Ca2+]i release, we constructed GHSR1a fusion proteins containing inducible homodimerization domains at the C-terminus (GHSR1a-Hom). The cross linking agent, B/B, dose-dependently reduced dopamine-induced [Ca2+]i mobilization by 40–50% in HEK293-AEQ cells expressing GHSR1a-Hom + DRD1, compared to cells expressing GHSR1a + DRD1 (Figure 4A,B,). B/B itself did not attenuate Ca2+ release, or cell surface expression of either GHSR1a-Hom or DRD1 (Figures 4C and S3E,F,G). TIRF-M analyses showed B/B treatment enhanced GHSR1a-Hom homodimerization by ~50%, thereby reducing the concentration of GHSR1a-Hom monomers available for forming heteromers (Figure S3H). The resulting reduction in the concentration of GHSR1a-Hom:DRD1 heteromers correlated with dopamine-induced [Ca2+]i mobilization (Figure 4B).
Figure 4. Dopamine-induced [Ca2+]i mobilization correlates with formation of GHSR1a:DRD1 heteromers.
(A) Dopamine-induced [Ca2+]i mobilization measured in HEK-AEQ cells expressing DRD1 with either GHSR1a (■) or GHSR1a-Hom (▲) preincubated with increasing concentrations of homodimerization inducer (B/B).
(B and C) Dose-dependent dopamine-induced [Ca2+]i release in the absence (■) or presence (▲) of B/B in cells coexpressing GHSR1a-Hom and DRD1 (B) or in cells coexpressing GHSR1a and DRD1 (C).
Data represent the mean ± SEM of at least three independent experiments (*, p<0.05 versus control; **, p<0.01 versus control; ***, p<0.001 versus control).
Additional experiments tested whether dopamine-induced Ca2+ release involved cross-talk between Gαs and Gαq within the heteromeric complex, or involved GHSR1a constitutive activity. We found that dopamine-induced [Ca2+]i mobilization by GHSR1a:DRD1was not dependent on signaling through Gαs, AC-PKA, Gαi/o-Gβγ, or PKC (Figure S4A,B,C,D,E). Coexpressing DRD1 with GHSR1a mutants lacking constitutive activity (F279L and A204E) resulted in dopamine-induced Ca2+ release (Figure S4F); hence, non-canonical DRD1 signaling is independent of GHSR1a constitutive activity. The observed 30–40% reduction in the Ca2+ response is likely explained by the lower affinity of the mutants for forming heteromers with DRD1 (Figure S4G).
To test for formation of an apo-GHSR1a:DRD1:Gαq complex in HEK293 cells we combined bimolecular fluorescence complementation (BiFC) and BRET analysis (Galés et al., 2006). Co-expression of split YFP-tagged GHSR1a and DRD1 (GHSR1a-YFP-C and DRD1-YFP-N) produced YFP fluorescence localized on the plasma membrane, confirming formation of GHSR1a:DRD1 heteromers (Figure S5A). Expression of Gαq-Rluc8 with the split YFP-tagged GHSR1a and DRD1 produced a robust BRET signal, consistent with Gαq in a preassembled complex with GHSR1a:DRD1, which was not observed in control experiments (Gαq-Rluc coexpressed with DRD1-YFP-N + DRD1-YFP-C or empty vector) (Figure S5A). Agonist treatment markedly reduced the BRET signal associated with the GHSR1a-YFP-C:DRD1-YFP-N:Gαq-Rluc8 complex, illustrating agonist-induced dissociation of Gαq (Figure S5A). To assess direct coupling to the Gαqβγ trimer, Gαq-Rluc8 and split Venus-Gβ1 and -Gγ2 proteins were coexpressed with GHSR1a and DRD1 (Figure S5B). The BRET signal was attenuated by SKF81297 treatment (p<0.001; Figure S5B, middle panel), consistent with DRD1 pre-coupling to Gαq and agonist-induced dissociation of the heterotrimeric G-protein complex. Pretreatment with JMV2959, dose-dependently inhibited the SKF81297-induced decrease in BRET (IC50 = 477 ± 45 nM; Figure S5B, right panel); hence, GHSR1a is intimately involved in DRD1:Gαq coupling. Dopamine-induced cAMP accumulation was reduced when DRD1 was expressed with GHSR1a, indicating GHSR1a promotes DRD1 coupling to Gαq at the expense of Gαs coupling (Figure S5C).
To determine whether GHSR1a antagonists inhibit dopamine-induced Ca2+ mobilization by interfering with allosteric interactions between GHSR1a and DRD1, three structurally distinct GHSR1a neutral antagonists (JMV2959, JMV3002, and BIM-28163) equipotent in inhibiting ghrelin activation of GHSR1a were tested (Figure S5D). The antagonists had no effect on DRD1-induced cAMP accumulation (Figure S5E). JMV2959 was a full antagonist of dopamine-induced [Ca2+]i release, while JMV3002 and BIM were partial antagonists (Figure S5F). BRET titration employing Nluc-GHSR1a and SNAP-DRD1 in the presence or absence of each GHSR1a antagonist was performed. JMV2959 did not affect the BRET50, but significantly changed the BRETmax (BRETmax control, 0.0038±0.0008 and BRETmax, JMV2959, 0.0017±0.0005; p<0.05; Figure S5G), indicating JMV2959 modifies allosteric interactions between GHSR1a and DRD1 (Hamdan et al., 2006). Furthermore, in agreement with their inhibitory effects on dopamine-induced [Ca2+]i mobilization (Figure S5F), JMV3002 and BIM-28163 were less active than JMV2959 in the BRET assay (Figure S5H). These results support an allosteric mechanism for GHSR1a dependent non-canonical DRD1 signaling.
Although the collective data supported formation of a preassembled apo-GHSR1a:DRD1:Gαq complex, and indicated dopamine-induced [Ca2+]i release is mediated via DRD1:Gαq, an alternative explanation is that dopamine binding to DRD1 modifies the conformation of DRD1 that in turn allosterically induces apo-GHSR1a:Gαq coupling. To test this possibility, we generated a GHSR1a mutant where residues in the third intracellular loop necessary for Gαq coupling were inactivated by alanine substitution. A(237–244)-GHSR1a did not couple to Gαq, was refractory to activation by ghrelin, and ELISA assays confirmed expression on the cell surface (Figure S6A,B,C,D); Tr-FRET assays illustrared heteromer formation with DRD1 (Figure S6E,F). Despite the inability of A(237–244)-GHSR1a to couple to Gαq, dopamine treatment of A(237–244)-GHSR1a:DRD1 heteromers dose-dependently induced Ca2+ transients, confirming direct coupling of DRD1 to Gαq. However, the amplitude of the Ca2+ response was ~50% that of WT-GHSR1a:DRD1 (Figure S6G), suggesting that signaling via WT-GHSR1a:DRD1 heteromers is mediated by a combination of DRD1:Gαq and apo-GHSR1a:Gαq coupling (Figure S6H).
DRD1 Agonist-Induced Activation of Synaptic Plasticity Markers in Hippocampal Neurons is Dependent on apo-GHSR1a
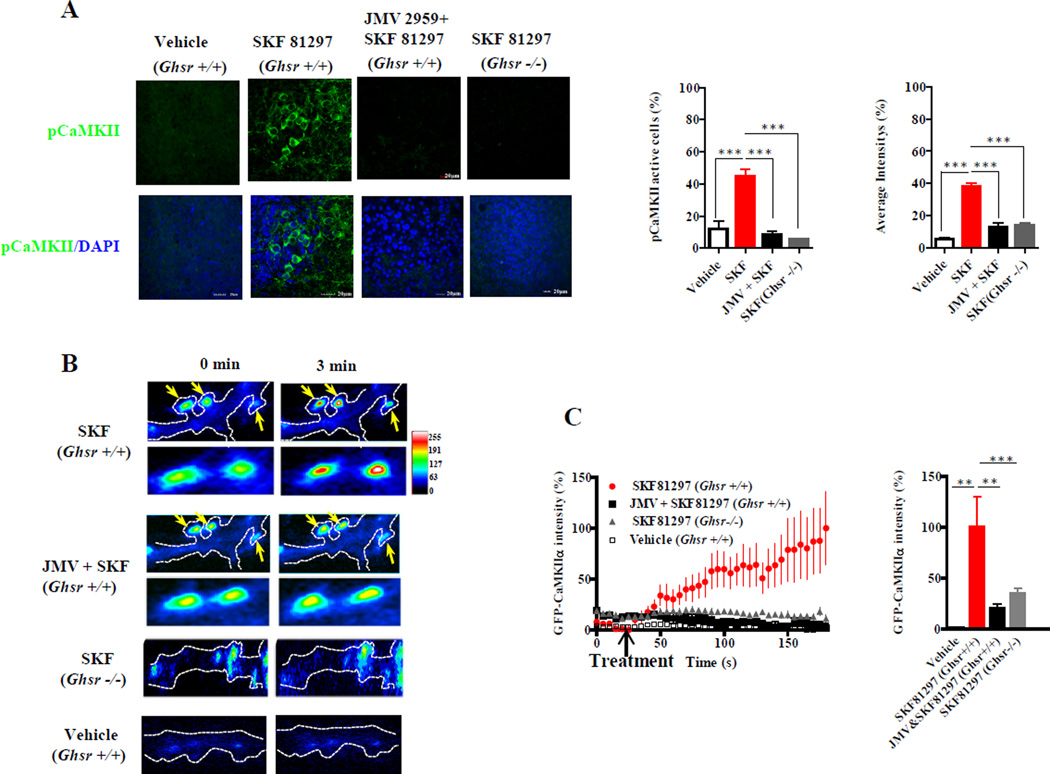
Phosphorylation of CaMKII is involved in activation of synaptic plasticity and is regulated by Ca2+; therefore, we asked if apo-GHSR1a was essential for DRD1 agonist activation of CaMKII. When we treated organotypic hippocampal slices from Ghsr+/+ mice with SKF81297 the number of pCaMKII positive neurons was markedly enhanced (44.56±4.3% vs vehicle 11.94±4.9%, p<0.001; Figure 5A), as was the intensity of staining (37.8±2.2% vs vehicle 5.4±1%, p<0.001; Figure 5A). Pretreatment with JMV2959 inhibited the effect of SKF81297. Also, hippocampal slices from Ghsr−/− mice were refractory to SKF81297-induced CaMKII phosphorylation (Figure 5A), further illustrating dependence on GHSR1a. Synaptic activity results in pCaMKII translocation from the dendritic cytoplasm to the synapse. Time-lapse imaging of hippocampal neurons from Ghsr+/+ mice expressing GFP-CaMKIIα cDNA showed that SKF81297-induced accumulation of GFP-CaMKII at synapses (Figure 5B); this phenomenon was markedly attenuated in hippocampal neurons from Ghsr−/− mice (100±30.5% vs 34.78±4.8%, p<0.01; Figure 5C). Pretreating Ghsr+/+ neurons with JMV2959 inhibited SKF81297-induced CaMKII translocation (20±4.9%, p<0.01; Figure 5C). Hence, DRD1 agonist-mediated CaMKII activation and translocation is dependent on GHSR1a. Synaptic localization of translocated GFP-CaMKIIα was confirmed using the post-synaptic marker PSD95-DsRed (Figure S7A).
Figure 5. DRD1 agonist-induced CaMKII activation in hippocampal neurons is dependent upon GHSR1a:DRD1 interactions.
(A) Hippocampal slices from Ghsr+/+ and Ghsr−/− mice treated with SKF81297 (10 µM) ± JMV2959 illustrating phosphorylation of CaMKII (left) and quantitative analysis (right panels).
(B and C) Representative TIRF-M images (B) and time lapse plot (C; left graph) with quantitative analysis (C; right graph) from neurons of Ghsr+/+ or Ghsr−/− mice expressing GFP-CaMKII α following treatment with SKF81297± JMV2959.
Data represent the mean ± SEM of at least three independent experiments: **, p<0.01 versus control; ***, p<0.001 versus control.
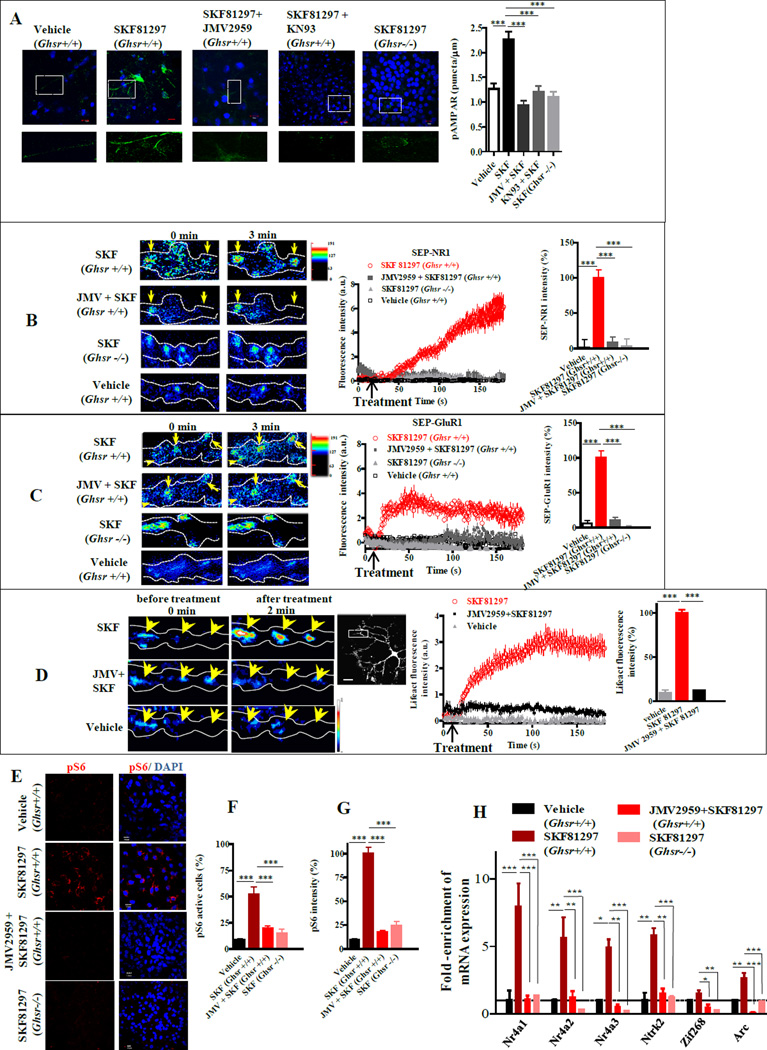
Synaptic plasticity and hippocampal memory formation are regulated by glutamate receptors. Phosphorylation of the GluR1 subunit of AMPAR at Ser831 by CaMKII is critical for AMPAR function and trafficking (Barria et al., 1997). SKF81297 increased Ser831 phosphorylation in organotypic hippocampal slices from Ghsr+/+ mice that was inhibited by pretreatment with either the CaMKII inhibitor (KN93), or JMV2959 (p<0.001; Figure 6A). In Ghsr−/− hippocampal slices, SKF81297 activation of AMPAR was markedly lower, illustrating dependence on GHSR1a (p<0.001; Figure 6A). To determine whether exocytosis of glutamate receptors in hippocampal neurons was dependent on GHSR1a:DRD1 signaling, we employed pHluorin-tagged NR1 and GluR1 subunits (SEP-NR1 and SEP-GluR1). When hippocampal neurons from Ghsr+/+ expressing SEP-NR1 or SEP-GluR1 were treated with SKF81297 significant increases in fluorescence intensity were observed, indicating exocytosis of glutamate receptors at synapses (Figure 6B,C); similarly treated neurons from Ghsr−/− mice were unresponsive. Pretreatment of Ghsr+/+ mouse neurons with JMV2959, inhibited SKF81297-induced exocytosis of NR1 and GluR1 (p<0.001; Figure 6B,C), confirming that DRD1 agonist-induced exocytosis of glutamate receptors is dependent on interactions between GHSR1a and DRD1. Lifeact was used to measure actin accumulation (Riedl et al., 2008); SKF81297 treatment enhanced actin accumulation in hippocampal neurons, which was significantly reduced by JMV2959 pretreatment, indicating that SKF81297-mediated synaptic reorganization is dependent on GHSR1a (p <0.001; Figure 6D).
Figure 6. DRD1 activation of neuronal plasticity markers in hippocampal neurons is dependent on GHSR1a:DRD1 interactions.
(A) Phosphorylation of AMPAR (p831-GluR1) in organotypic hippocampal slices from Ghsr+/+ mice treated with vehicle, SKF81297 (10 µM), JMV2959 (10 µM) + SKF81297 (10 µM) and CaMKII inhibitor (KN93) + SKF81297 (10 µM); control, slices from Ghsr−/− mice treated with SKF81297 (10 µM) (left); and quantitative analyses (right). Scale bar, 10 µm.
(B and C) SKF81297-induced exocytosis of NR1or GluR1 detected by TIRF-M. Hippocampal neurons from Ghsr+/+ and Ghsr−/− mice expressing SEP-NR1 (B) or SEP-GluR1 (C) treated with SKF81297 (10 µM) and ± JMV2959 (10 µM); representative images before and after treatment (left); time lapse plot and quantitative analysis (right).
(D) Representative images of hippocampal neuronal processes expressing mCherry-Lifeact, following treatment with SKF81297 (10 µM) ± JMV2959 (10 µM) (left); time lapse plot of (middle), quantitative analysis (right); scale bar, 20 µm.
(E) Activation of hippocampal neurons in organotypic slices measured by pS6 immunofluorescence (red); nuclei (blue); scale bar, 10 µm.
(F and G) Quantitative analysis according to number of pS6 positive cells (F) or to pS6 immunofluorescence intensity (G).
(H) Gene expression of early markers associated with initiation of neuronal plasticity in hippocampal organotypic slices treated with vehicle or SKF81297 (10 µM) ± JMV2959 (10 µM).
Data represent the mean ± SEM of at least three independent experiments (*, p<0.05 versus control; **, p<0.01 versus control; ***, p<0.001 versus control).
If DRD1 agonist activation of neuronal plasticity in hippocampal neurons is dependent on GHSR1a, transcription of genes involved in initiation of neuronal plasticity should also be dependent on GHSR1a. Neuronal activity frequently correlates with signaling pathways that induce phosphorylation of ribosomal protein S6. To enrich for mRNAs expressed in response to SKF81297 activation of hippocampal neurons, we applied ribosome capture and to enhance the dynamic range of enrichment employed pS6-244 selective immunoprecipitation (Knight et al., 2012). SKF81297 markedly increased pS6-244 immunofluorescent cells in hippocampal slices from Ghsr+/+ mice relative to vehicle treatment (52.43±7 % vs, 8.84±1.18% p<0.001, Figure 6E,F); fluorescence intensity was also enhanced (100±6.85% vs 9.57±1%, p<0.001, Figure 6E,G). JMV2959 pretreatment markedly lowered SKF81297-induced S6-244 phosphorylation in Ghsr+/+ slices; slices from Ghsr−/− mice were refractory to SKF81297 treatment (p<0.001; Figure 6E,F,G). Treatment with 2-aminoethoxydiphenyl borate (2-APB) inhibited SKF81297-induced S6-244 phosphorylation (Figure S7B), indicating activation is dependent on release of [Ca2+]i. Analysis of mRNA eluted from immunoprecipitated pS6-244 positive ribosomes from SKF81297 treated Ghsr+/+ hippocampal slices showed enriched expression of genes associated with initiation of synaptic plasticity: Nr4a1, Nr4a2, Nr4a3, Ntrk2, Arc and Zif268 (Figure 6H). Pretreatment with JMV2959 inhibited SKF81297-induced expression of these genes (Figure 6H). In addition, enhanced expression of these genes was not observed when slices from Ghsr−/− mice were treated with SKF81297 (Figure 6H). These data illustrate that DRD1-induced initiation of hippocampal synaptic plasticity is GHSR1a-dependent, and further support an important functional role for apo-GHSR1a:DRD1 heteromers.
Dependence on Hippocampal GHSR1a for DRD1 Regulated Behaviors
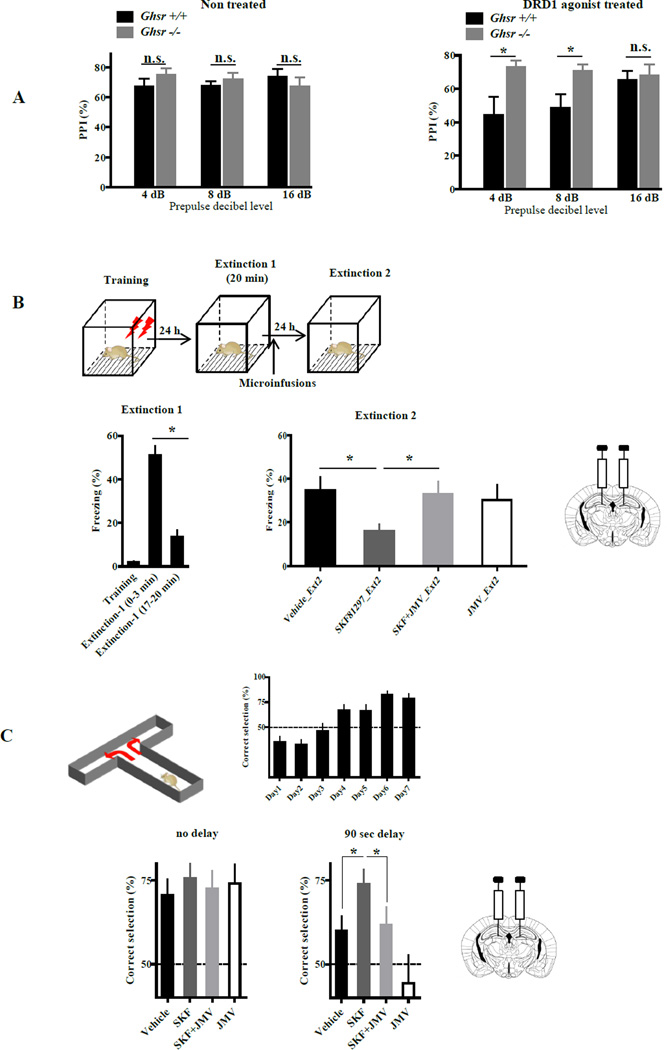
We next examined the biological relevance of apo-GHSR1a-dependent DRD1 signaling in the hippocampus by measuring DRD1 agonist-induced behavioral responses when GHSR1a was either genetically or pharmacologically inactivated. PPI is a measure of sensorimotor gating that determines how well an animal can integrate and inhibit sensory information (Mansbach et al., 1988). Although the nucleus accumbens was once viewed as the primary structure involved in dopamine-induced interference with PPI, subsequent studies with DRD1 selective agonists and antagonists applied systemically or directly to the hippocampus, showed that disruption of PPI involved DRD1 agonist action on the dorsal hippocampus (Ellenbroek et al., 2002). Given these findings, we tested whether DRD1-induced interference with PPI was dependent upon GHSR1a by comparing PPI in DRD1agonist treated Ghsr+/+ and Ghsr−/− mice. In the absence of the DRD1 agonist, both genotypes produced identical PPI responses (Figure 7A). When mice were injected with the DRD1 agonist, PPI was disrupted in Ghsr+/+ mice, but not in Ghsr−/− mice (Figure 7A); therefore, DRD1-induced interference with PPI is dependent upon GHSR1a.
Figure 7. DRD1-agonist specific behaviors dependent on GHSR1a:DRD1 interactions.
(A) Ghsr +/+ and Ghsr −/− mice exhibit a normal PPI response in absence of DRD1 agonist (left). DRD1 agonist treatment interferes with PPI in Ghsr +/+ mice, but Ghsr −/− mice are resistant (right) (n=8–12 animals per group).
(B) Bilateral infusion of SKF81297 (SKF) into the DG induces extinction of contextual fear conditioning that is blocked by coinfusion of JMV2959 (JMV); (7–10 animals per group).
(C) Alternating T-maze test of working memory. Bilateral infusion of SKF81297 (SKF) into the DG enhances of working memory that is blocked by coinfusion of JMV2959 (JMV); (9–10 animals/group).
Data represent the mean ± SEM (*, p<0.05 versus control).
Coexpression of GHSR1a and DRD1 is most abundant in the DG of the hippocampal structures and neuronal activity that influences synaptic potentiation and memory is propagated through the DG. Our ex vivo experiments showed that DRD1 agonist induced biochemical changes associated with initiation of hippocampal synaptic plasticity are blocked by JMV2959. Therefore, we selected behavioral tests of memory associated with activation of synaptic plasticity that are enhanced by hippocampal DRD1 agonism, and asked if enhanced performance was inhibited by JMV2959.
DRD1 signaling in the DG is implicated in contextual fear conditioning (CFC) (Sariñana et al., 2014), and agonist activation of DRD1 in the dorsal hippocampus results in consolidation of CFC extinction (Fiorenza et al., 2012). Therefore, we asked whether DRD1-mediated extinction of CFC was dependent on GHSR1a. Mice were trained in the CFC apparatus and then placed in their home cage. The mice were returned to the apparatus 24 h later and Extinction-1 phase (20 min) measured (Figure 7B). The freezing response of the mice declined as a function of time (compare 0–3 min with 17–20 min, Figure 7B). Immediately after Extinction-1, mice received intra-DG infusions of vehicle, SKF81297, SKF81297 + JMV2959, or JMV2959 alone (Figure S7C). Twenty-four hours later, Extinction-2 was performed. Mice treated with SKF81297 exhibited a significant decrease in freezing behavior compared to the vehicle treated group, consistent with DRD1 agonist-induced consolidation of extinction memory (Figure 7B). In mice coadministered JMV2959 and SKF81297, the freezing response was equal to that in vehicle-treated controls; hence, JMV2959 inhibits SKF81297-induced consolidation of extinction (Figure 7B), illustrating dependence on allosteric interactions between GHSR1a and DRD1.
To determine if allosteric interactions between GHSR1a and DRD1 in the DG are important for DRD1 mediated enhancement of working memory, we utilized the T-maze alternation test (Deacon and Rawlins, 2006). Alternation is viewed as an excellent test for determining hippocampal function in mice, and for mice is a more robust test than the Morris water maze (Deacon and Rawlins, 2006). Mice were trained to learn a food pellet is placed in alternate arms of the T-maze. Training is considered complete when the mice make the correct choice 75% of the time (Fig. 7C). On the test day, mice received intra-DG infusion of vehicle, SKF81297, SKF81297 + JMV2959, or JMV2959 alone. The mice were retested without delay and then after a 90 sec delay. In contrast to vehicle infusion, mice infused with SKF81297 continued to make 75% correct choices following the 90 sec delay consistent with enhanced working memory (p<0.05; Figure 7C). Coadministration of JMV2959 with SKF81297 blocked the beneficial effect of SKF81297 on working memory (p<0.05; Figure 7C). Infusion of JMV2959 alone had no effect on immediate performance, but performance was impaired following a 90 sec delay and inferior to that of vehicle infused mice (Fig. 7C), indicating that JMV2959 inhibited endogenous dopamine/DRD1 signaling. These results provide additional evidence for the biological significance of allosteric interactions between GHSR1a and DRD1 in the DG.
DISCUSSION
Our objective was to determine the functional role of the ghrelin receptor (GHSR1a) in the hippocampus where endogenous ghrelin is undetectable (Banks et al., 2002; Furness et al., 2011; Grouselle et al., 2008; Sakata et al., 2009). The absence of endogenous ghrelin in the mouse brain is supported by pharmacokinetic studies following i.v. administration of mouse 131I-ghrelin (Banks et al., 2002). When administered systemically to mice, ghrelin binding in the CNS is confined to hypothalamic neurons (Schaeffer et al., 2013); c-Fos is activated in the arcuate nucleus, paraventricular nucleus (PVN) and lateral hypothalamus (LH) (Pirnik et al., 2011). Infusing ghrelin directly into the LH releases orexin, and orexin acts on ventral tegmental area (VTA) neurons causing dopamine release (Cone et al., 2014). Dopaminergic neurons in the VTA innervate the hippocampus, implicating a VTA-hippocampal loop that regulates hippocampal plasticity (Gasbarri et al., 1994; Lisman and Grace, 2005; Swanson, 1982). These data argue that the effects of pharmacological doses of ghrelin on the hippocampus that have been proposed to be a direct effect on synaptic plasticity are instead indirect and mediated by dopamine. Physiologically, the absence of endogenous ghrelin in the hippocampus allows apo-GHSR1a to modify dopamine signaling locally through apo-GHSR1a:DRD1 heteromer formation, which initiates synaptic plasticity through DRD1 coupling to Gαq.
Intriguingly, dynamic studies in hippocampal neurons show DRD1 agonism enhances interactions between DRD1 and GHSR1a. DRD1 agonism also regulates the dynamics of DRD1 and NMDA receptor interactions in synapses (Ladepeche et al., 2013). Studies in wild-type rodents that express GHSR1a endogenously demonstrated that DRD1 regulates synaptic plasticity and LTP by enhancing NMDA responses. DRD1 and NMDAR interact through intracellular signaling involving heteromerization (Cepeda and Levine, 2006). DRD1 agonism enhances NMDA responses in the hippocampus, cortex and striatum through signaling cascades involving release of [Ca2+]i and activation of CaMKII, PKC and PKA (Cepeda and Levine, 2006). Our results support this mechanism, and further suggest dopamine activation of GHSR1a:DRD1 as an initiation step for enhancing NMDA currents. Indeed, dependence on GHSR1a for DRD1-induced exocytosis of NR1 supports GHSR1a:DRD1 heteromers as enhancers of the NMDA response. In hippocampal neurons, dissociation of DRD1:NR1 complexes upon DRD1 activation facilitates CaMKII activity (Nai et al., 2010), which is consistent with the idea that dissociation of the DRD1:NR1 heteromer could shift stoichiometry towards an increase in GHSR1a:DRD1 heteromers, and as a consequence increase dopamine activation of CaMKII as observed here. Clearly, additional studies are needed to elucidate the role of GHSR1a:DRD1 in DRD1 regulation of NMDA signaling and relationships to DRD1:NR1 complex formation.
Elucidating the mechanism of signaling through GHSR1a:DRD1 hippocampal neurons revealed preassembly of an apo-GHSR1a:DRD1:Gαq macro-complex. Proximity measurements and biochemical studies implicate a mechanism where dopamine-induced [Ca2+]i mobilization is mediated by direct coupling of DRD1 to Gαq. DRD1:Gαq coupling was confirmed by substituting WT-GHSR1a for a GHSR1a mutant where sites for Gαq coupling were inactivated and showing that dopamine activation of the mutant-GHSR1a:DRD1 heteromer mobilized [Ca2+]i. Nevertheless, compared to WT-GHSR1a:DRD1 the magnitude of dopamine-induced Ca2+ release was reduced by ~50% suggesting that with WT-GHSR1a:DRD1, both DRD1:Gαq and apo-GHSR1a:Gαq coupling was involved. What is the stoichiometry? In lipid nanodiscs apo-GHSR1a homomers assemble asymmetrically with only one protomer actively coupling to Gαq, while the other protomer is inactive and in the case of an apo-GHSR1a heteromer, apo-GHSR1a is not actively coupled to Gαq (Mary et al., 2013). Based on these findings, we speculate that dopamine-induced [Ca2+]i release is mediated through an asymmetric heterotetramer (Gαq:GHSR1a:DRD1:GHSR1a:DRD1:Gαq) where asymmetry allows just one GHSR1a and one DRD1 molecule to actively couple to Gαq. Gαq coupling and signaling through PLC is fundamentally important for DRD1 agonist activation of Ca2+ transients and induction of hippocampal synaptic plasticity. When GHSR1a is inactivated, DRD1 couples to Gαs and DRD1 agonism augments cAMP accumulation, but synaptic plasticity is not initiated. We conclude that Gαq coupling via GHSR1a:DRD1 is essential for initiating synaptic plasticity and priming hippocampal neurons for subsequent events involving cAMP signaling and protein synthesis-dependent long-term memory formation (Abel et al., 1997). Do Gαq and Gαs sequentially and/or synergistically regulate downstream pathways that lead to memory formation? By dissociating DRD1-induced Gαq-PLC from Gαs-PKA signaling we provide a strategy for dissecting these mechanisms.
Since GHSR1a:DRD1 heteromers are abundantly expressed in the DG, the gateway for regulating hippocampal function, we asked whether dependence on GHSR1a for activating hippocampal synaptic plasticity ex vivo, translates to performance in vivo. In the DG, contextual memory is dependent on DRD1 (Sariñana et al., 2014). We showed that direct infusion of a DRD1 agonist into the DG of WT mice augments extinction of contextual fear conditioning was blocked by coinfusion of the GHSR1a antagonist JMV2959. Similarly, DRD1 agonist infusion into the DG improved working memory that was also blocked by JMV2959 coinfusion. The results of these behavioral tests show that DRD1 agonist-induced behaviors are dependent on interactions between DRD1 and GHSR1a in the DG, confirming the biological relevance of conclusions derived from ex vivo experiments in hippocampal neurons.
In the dorsal hippocampus, DRD1 plays a role in integrating and filtering sensory information (Ellenbroek et al., 2002). Impairments in this process have been linked to neuropsychiatric disorders, which can be assessed in rodents and humans by measuring PPI of the acoustic startle response. Peripheral administration of a DRD1 agonist to WT mice caused disruption of PPI, but Ghsr null mice were resistant. Hence, by selectively blocking dopamine signaling in GHSR1a:DRD1 expressing neurons, GHSR1a antagonists may have utility in treating psychoses, such as schizophrenia. As a further illustration of dependence on GHSR1a for sensorimotor gating, a recent report showed that disruption of PPI in rats by the noncompetitive inhibitor of the NMDA receptor phencyclidine was blocked by JMV2959 (Engel et al., 2015).
In conclusion, we show apo-GHSR1a is fundamentally important for dopamine/DRD1-induced initiation of hippocampal synaptic plasticity and formation of hippocampal memory. All previously reported studies that elucidated mechanisms of dopamine regulation of hippocampal function were conducted in rodents expressing GHSR1a endogenously; therefore, the critical role of apo-GHSR1a would have been overlooked. The results we describe, combined with conclusions from our previous work showing a functional role for apo-GHSR1a:DRD2 heteromers in native hypothalamic neurons (Kern et al., 2012), illustrate a previously unrecognized, but critical role for apo-GHSR1a as a modulator of dopamine signaling. Of significance towards CNS drug discovery, the GHSR1a antagonists tested allosterically inhibit dopamine-induced Ca2+ mobilization through apo-GHSR1a:DRD1. Based on this mechanism, different structural classes of GHSR1a antagonists have the potential to enhance, rather than inhibit, dopamine signaling. This concept has profound therapeutic implications because it allows selective pharmacological fine-tuning of dopamine signaling in subsets of neurons that express GHSR1a:DRD2 or GHSR1a:DRD1, without affecting neurons expressing DRD2 or DRD1 alone. Broader application of this concept involves identifying neutral molecules that target the unique structural interface formed by GPCR protomers in a GPCR heteromeric complex. Finally, our results reinforce the importance of knowing the GPCR composition of the clinically important native target cell when developing pharmacologic agents, because the presence or absence of a potential GPCR protomer partner markedly affects biological responses. Indeed, antagonists, agonists and biased agonists for GPCRs have the potential to modify signal transduction, depending on whether signaling proceeds via GPCR monomers, homomers or heteromers.
EXPERIMENTAL PROCEDURES
Hippocampal Primary Cell Isolation and Organotypic Brain Slice Preparation
Primary cells were isolated from hippocampi of P1–P3 mice. Organotypic brain slices (300 µm) were prepared from hippocampi of postnatal day 5–7 mice and cultured on membrane inserts (see Supplemental Experimental Procedures for more details).
Intracellular Ca2+ Mobilization, Inducible Homomerization and cAMP accumulation
In hippocampal primary cells and organotypic brain slices, Ca2+ was detected using GCaMP3. For the inducible homomerization assay, Ca2+ was detected in HEK-AEQ cells (Kern et al., 2012) in the absence or presence of homodimerizer (B/B; Clontech). cAMP production in hippocampal brain membranes was measured with LANCE Ultra cAMP assay (Perkin Elmer) (see Supplemental Experimental Procedures for more details).
Microscopic TIRF Measurements
TIRF imaging was performed using Olympus FluoView 1000 equipped with commercially available objective-based TIRF (Olympus); see Supplemental Experimental Procedures for more details.
Microscopic FRET Analysis of Hippocampal Neurons and Brain Slices
Gαq proximity and GHSR1a:DRD1 heteromers in brain slices were detected by FRET microscopy, using Olympus FluoView 1000 (see Supplemental Experimental Procedures for more details).
Immunofluorescence Microscopy of Mouse Brain Sections and Organotypic Brain Slices
GHSR1a and DRD1 immunofluorescence was performed on brain sections from adult male Ghsr-IRES-tauGFP mice as described previously (Kern et al., 2012) with modifications. After treatments, organotypic brain slices were processed for immunofluorescence staining. See more details in Supplemental Experimental Procedures.
Ribosome Immunoprecipitation, Purification of mRNA and Quantitative PCR
Ribosomal pS6 immunoprecipitation, mRNA purification and qRT-PCR was performed on hippocampal organotypic slices as previously described (Knight et al., 2012) (see Supplemental Experimental Procedures for more details).
Behavioral Tests
PPI was performed as described previously (Geyer and Dulawa, 2003), CFC was performed as described (Fiorenza et al., 2012) and the delayed alternation T-maze task was performed as described (Deacon and Rawlins, 2006); with modifications (see Supplemental Experimental Procedures for more details).
Data Analysis
Values are given as means ± SEM and obtained from the number of separate experiments indicated. Comparisons between the different groups were made using Student’s t test or one-way ANOVA test. Data were analyzed using GraphPad Instat software, and a difference of p<0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors thank Bryan Wharram and Dr. Sukhvir Mahal (TSRI) for assistance in managing the mouse colonies; and Dr. Cristina Grande (TSRI) for help with intracranial drug delivery. We thank Æterna Zentaris Inc (Germany) for providing JMV2959; IPSEN (Milford, MA, USA) for BIM-28163; and Dr. Jeffry M. Friedman (Rockefeller University, NY) for 3P peptide used in pS6 experiments. We thank Dr. Courtney Miller and Dr. Sukhvir Mahal (TSRI) for helpful comments on the manuscript. This work was supported by NIH grant R01AG019230 to R.G.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
A.K. and R.G.S. conceived and designed the study. A.K. performed most of the experiments and data analysis. M.M. contributed to design and performance of mice cannulation and the CFC behavioral experiment. C.U. contributed to organotypic brain slice preparation. R.A-Z. and A.F.B. designed, performed and analyzed the PPI behavioral experiment. A.K. and R.G.S. wrote the manuscript with input from all authors.
REFERENCES
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;333:pe20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin Acts as an Interface between Physiological State and Phasic Dopamine Signaling. J Neurosci. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Lubbers LJ, Cools AR. The role of hippocampal dopamine receptors in prepulse inhibition. Eur J Neurosci. 2002;15:1237–1243. doi: 10.1046/j.1460-9568.2002.01948.x. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E, Svensson L, Smith RG, Egecioglu E. Blockade of growth hormone secretagogue receptor 1A signaling by JMV 2959 attenuates the NMDAR antagonist, phencyclidine-induced impairments in prepulse inhibition. Psychopharmacology (Berl) 2015;232:4285–4292. doi: 10.1007/s00213-015-4054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232:210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Furness JB, Hunne B, Matsuda N, Yin L, Russo D, Kato I, Fujimiya M, Patterson M, McLeod J, Andrews ZB, et al. Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience. 2011;193:1–9. doi: 10.1016/j.neuroscience.2011.07.063. [DOI] [PubMed] [Google Scholar]
- Galés C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2003;Chapter 8(Unit 8.17) doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- Grouselle D, Chaillou E, Caraty A, Bluet-Pajot M, Zizzari P, Tillet Y, Epelbaum J. Pulsatile cerebrospinal fluid and plasma ghrelin in relation to growth hormone secretion and food intake in the sheep. J Neuroendocrinol. 2008;20:1138–1146. doi: 10.1111/j.1365-2826.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Percherancier Y, Breton B, Bouvier M. Monitoring protein-protein interactions in living cells by bioluminescence resonance energy transfer (BRET) Current Protocols in Neuroscience. 2006;5:523. doi: 10.1002/0471142301.ns0523s34. [DOI] [PubMed] [Google Scholar]
- Hamilton TJ, Wheatley BM, Sinclair DB, Bachmann M, Larkum ME, Colmers WF. Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc Natl Acad Sci U S A. 2010;107:18185–18190. doi: 10.1073/pnas.1011558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, George SR. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Kasai RS, Kusumi A. Single-molecule imaging revealed dynamic GPCR dimerization. Curr Opin Cell Biol. 2014;27C:78–86. doi: 10.1016/j.ceb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW, Emiliano A, Ekstrand MI, Friedman JM. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151:1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Ladepeche L, Dupuis JP, Bouchet D, Doudnikoff E, Yang L, Campagne Y, Bézard E, Hosy E, Groc L. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc Natl Acad Sci U S A. 2013;110:18005–18010. doi: 10.1073/pnas.1310145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezcano N, Bergson C. D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol. 2002;87:2167–2175. doi: 10.1152/jn.00541.2001. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Mary S, Fehrentz JA, Damian M, Gaibelet G, Orcel H, Verdié P, Mouillac B, Martinez J, Marie J, Banères JL. Heterodimerization with Its splice variant blocks the ghrelin receptor 1a in a non-signaling conformation: a study with a purified heterodimer assembled into lipid discs. J Biol Chem. 2013;288:24656–24665. doi: 10.1074/jbc.M113.453423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Li S, Wang SH, Liu J, Lee FJ, Frankland PW, Liu F. Uncoupling the D1-N-methyl-D-aspartate (NMDA) receptor complex promotes NMDA-dependent long-term potentiation and working memory. Biol Psychiatry. 2010;67:246–254. doi: 10.1016/j.biopsych.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Bundziková J, Holubová M, Pýchová M, Fehrentz JA, Martinez J, Zelezná B, Maletínská L, Kiss A. Ghrelin agonists impact on Fos protein expression in brain areas related to food intake regulation in male C57BL/6 mice. Neurochem Int. 2011;59:889–895. doi: 10.1016/j.neuint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, et al. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariñana J, Kitamura T, Künzler P, Sultzman L, Tonegawa S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc Natl Acad Sci U S A. 2014;111:8245–8250. doi: 10.1073/pnas.1407395111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdié P, Bourrier E, Dehouck B, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A. 2013;110:1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt MJ, Jr, Fisher MH, Nargund RP, Patchett AA. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.