Abstract
Background and Aims
Previous estimates of incidence of intestinal metaplasia (IM) recurrence after achieving complete remission of IM (CRIM) through endoscopic therapy of Barrett's esophagus (BE) have varied widely. We performed a systematic review and meta-analysis of studies to estimate an accurate recurrence risk after CRIM.
Methods
We performed a systematic search of multiple literature databases through June 2015 to identify studies reporting long-term follow-up after achieving CRIM through endoscopic therapy. Pooled incidence rate (IR) of recurrent IM, dysplastic BE, and high-grade dysplasia (HGD)/esophageal adenocarcinoma (EAC) per person-year of follow-up after CRIM was estimated. Factors associated with recurrence were also assessed.
Results
We identified 41 studies that reported 795 cases of recurrence in 4443 patients over 10,427 patient-years of follow-up. This included 21 radiofrequency ablation studies that reported 603 cases of IM recurrence in 3186 patients over 5741 patient-years of follow-up. Pooled IRs of recurrent IM, dysplastic BE, and HGD/EAC after radiofrequency ablation were 9.5% (95% CI, 6.7-12.3), 2.0% (95% CI, 1.3-2.7), and 1.2% (95% CI, .8-1.6) per patient-year, respectively. When all endoscopic modalities were included, pooled IRs of recurrent IM, dysplastic BE, and HGD/EAC were 7.1% (95% CI, 5.6-8.6), 1.3% (95% CI, .8-1.7), and .8% (95% CI, .5-1.1) per patient-year, respectively. Substantial heterogeneity was noted. Increasing age and BE length were predictive of recurrence; 97% of recurrences were treated endoscopically.
Conclusions
The incidence of recurrence after achieving CRIM through endoscopic therapy was substantial. A small minority of recurrences were dysplastic BE and HGD/EAC. Hence, continued surveillance after CRIM is imperative. Additional studies with long-term follow-up are needed.
Endoscopic therapy is currently the accepted first-line treatment modality for Barrett's esophagus (BE)-related dysplasia and mucosal adenocarcinoma.1,2 Several endoscopic modalities are used in isolation or in combination for endoscopic therapy of BE, such as EMR, radiofrequency ablation (RFA), photodynamic therapy (PDT), cryotherapy, argon plasma coagulation (APC), multipolar electrocoagulation, and laser therapy.3 Endoscopic therapy with EMR followed by PDT or RFA has been shown to be effective in reducing the risk of progression to high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC).4-6
High rates of elimination of intestinal metaplasia (IM) and dysplasia have been shown in several reports from single and multicenter studies with short- and medium-term follow-up.7,8 As the benefits of initial ablative therapy are well described, attention is now focused on the durability of response to endoscopic therapy, specifically recurrence rates of IM, dysplasia, and carcinoma. Studies have varied considerably in estimates of recurrence of IM after achieving successful ablation defined as complete remission of IM (CRIM). Although some studies have reported low rates of recurrence,9-11 others have reported significantly higher rates of recurrence.12 The wide variation between studies could be because of several factors, both implicit (patient characteristics such as age, smoking status, use of potentially chemopreventive medications after CRIM) and explicit (differences in study design, follow-up duration, and surveillance protocols after CRIM). Several potential predictors of recurrence have been assessed, but only in small studies with limited power to make conclusive observations.13-15
It is important to reliably estimate the recurrence risk after successfully achieving CRIM for several reasons. First, recurrent dysplastic BE (DBE) or carcinoma is important to detect, because it may require further endoscopic therapy or esophagectomy. Second, currently, there are no consensus/guidelines on duration of follow-up and frequency of surveillance endoscopies after successfully achieving CRIM, and accurate estimates of recurrence would be helpful in determining this. Finally, the cost-effectiveness of endoscopic therapy for BE will depend on durability of CRIM and need for additional therapy of recurrent BE.
We performed a systematic review and meta-analysis of all studies that reported long-term results after achieving CRIM in BE patients using endoscopic eradication therapy to estimate an accurate recurrence risk (for IM and dysplasia). Although some techniques like PDT and APC are not currently in use, we believed it was important to include them in this review given their pioneering role in demonstrating success with endoscopic therapy and because other than RFA, level 1 evidence supporting endoscopic therapy for BE is only available for PDT.6 Also, outcomes with older modalities can serve as a useful comparator for current modalities. We also identified clinical factors associated with recurrence of IM after CRIM.
Methods
This systematic review was performed according to guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions.16 It is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.17 We followed a priori established protocol.
Search strategy
We conducted a systematic literature search of several databases from each database's inception to June 1, 2015 for relevant articles on recurrence of IM, dysplasia, or adenocarcinoma after endoscopic treatment of DBE and nondysplastic BE (NDBE). The databases included MEDLINE, EMBASE, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews. The search was restricted to the studies on human participants published in English. The search was conducted by an experienced librarian with input from the study authors (R.K., S.S., and P.G.I.). The details of the search strategy and data sources are reported in Appendix 1 (available online at www.giejournal.org).
Selection criteria
We included studies that met the following inclusion criteria: (1) reported recurrence of IM, dysplasia, and/or EAC in BE subjects (dysplastic and nondysplastic) who achieved CRIM using any endoscopic therapy and (2) reported follow-up period since CRIM in “patient-years” or reported mean/median follow-up period after CRIM and number of patients in surveillance, thereby permitting calculation of follow-up period since CRIM in “patient-years.” Recurrence was defined as the presence of IM in the esophagus and/or gastroesophageal junction (GEJ) after achieving CRIM. CRIM was defined by individual studies as biopsy samples being negative for IM on a single or 2 successive endoscopies. 12,18-20 We included all endoscopic therapeutic modalities. We excluded studies that used >1 endoscopic ablation modality, studies with mean/median follow-up <1 year after CRIM was achieved, studies with <20 subjects who achieved CRIM, studies that reported recurrence after complete remission of dysplasia instead of CRIM, studies with subjects who had previously failed endoscopic therapy, and case-control studies, letters to the editor, editorials, and review articles. Studies using a combination of 1 endoscopic ablative modality with EMR were included. When multiple publications from the same population were identified, only data from the most recent comprehensive report were included. Two of the included studies had 2 arms, 1 comparing outcomes with different endoscopic modality21 and 1 comparing outcomes in long- versus ultralongsegment BE.22 For the purpose of the review, each arm was counted as a separate study.
Data abstraction and quality assessment
After identifying relevant studies, data on study characteristics, patient characteristics, treatment characteristics, study outcomes, and risk factors for recurrence were abstracted onto a standardized form by 2 authors (R.K., K.R.). Details of data abstraction are reported in Appendix 2 (available online at www.giejournal.org).
The quality of the individual studies was independently assessed by 2 authors (RK, KR) using a scale modified from the Newcastle-Ottawa scale for cohort studies.23 This quality score consisted of 10 questions. The details of the quality scale are reported in Appendix 3 (available online at www.giejournal.org). A score of ≥7, 4 to 6.5, and <4 was considered suggestive of a high-, medium-, and low-quality study, respectively.
Outcomes assessed
The primary outcome of the review was to assess the annual incidence rate (IR) of IM recurrence after achieving CRIM using RFA given that it is the most commonly used endoscopic modality in current practice. Secondary outcomes measured included annual IR of IM recurrence after use of all endoscopic modalities and IR of recurrent DBE and HGD/EAC.
We performed preplanned subgroup analysis based on primary endoscopic modality (eg, RFA, PDT, APC), study location (eg, North America, Europe), baseline dysplasia status in pretreatment histology (NDBE vs DBE ± early neoplasia), type of publication (abstract vs full article), post-CRIM surveillance biopsy sampling protocol (inclusion vs exclusion of GEJ in surveillance biopsy specimen), and study quality (high, medium, low). In addition, we identified risk factors associated with recurrence (demographic factors such as age and sex and clinical factors such as BE length and baseline dysplasia).
Statistical analysis
For each included study we calculated the IR of recurrence based on the total number of subjects who had IM recurrence and the total follow-up duration after CRIM (either reported as person-years by study authors or estimated from mean/median follow-up of the study). Using the random-effects model described by DerSimonian and Laird,24 we calculated the pooled IR of recurrence per person-year and 95% confidence intervals (CIs).
We assessed heterogeneity between study-specific estimates using inconsistency index (I2 statistic), which estimates the proportion of total variances across studies because of heterogeneity rather than by chance. Values of <30%, 30% to 59%, 60% to 75%, and >75% were considered suggestive of low, moderate, substantial, and considerable heterogeneity, respectively.25 Once heterogeneity was noted, between-study sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates according to study characteristics (as described earlier). In this analysis, a P value for differences between subgroups of <.10 was considered statistically significant, meaning that stratifying based on those subgroups can potentially explain heterogeneity observed in the overall analysis. We assessed for publication bias qualitatively by visual inspection of funnel plot and quantitatively using Egger's regression test.26 Statistical analysis for identifying predictors of recurrence is detailed in Appendix 4 (available online at www.giejournal.org). All calculations and graphs were performed using Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ).
Results
From a total 1699 studies identified by our search strategy, 41 studies were included in the meta-analysis.7,9,12-15,18-22,27-54 Five studies4,10,11,55,56 were excluded because they had overlapping populations with already-included studies. Two studies with post-CRIM follow-up <1 year57,58 and 8 studies with <20 patients reaching CRIM59-66 were excluded. Together, the 41 studies reported a total of 795 cases of IM recurrence after CRIM in 4443 patients over 10,427 patient-years of follow-up. This included 21 RFA studies that reported 603 cases of IM recurrence in 3186 patients over 5741 patient-years of follow-up.9,12,14,15,18,21,22,27,29,30,32,39-41,43,47,49,51,53,54 Figure 1 shows the schematic diagram of study selection.
Figure 1.
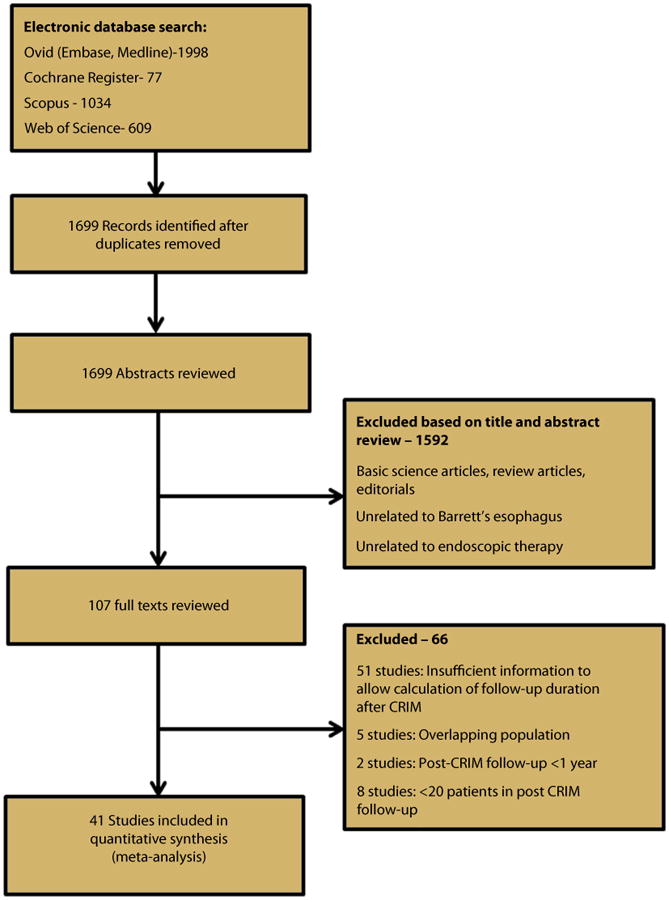
Flow sheet summarizing study identification and selection.
Characteristics of included studies
Table 1 describes the characteristics of the included studies. Fourteen of the 41 included studies were multicenter studies.12,21,29,34,39,41,44,47,49-51,53,54 Mean patient age at endoscopic therapy was 61.4 years, and 78.9% were men. The median of average follow-up after CRIM was 2.5 years, ranging from 1 year to 10.5 years in individual studies. Among the 41 included studies, the primary endoscopic treatment modality was RFA in 21 studies,9,12,14,15,18,21,22,27,29,30,32,39-41,43,47,49,51,53,54 APC in 7 studies,33,34,36,45,46,48,52 EMR in 7 studies,20,21,31,37,42,44,50 PDT in 2 studies,7,19 multipolar electrocoagulation in 2 studies,13,28 laser in 1 study,35 and cryotherapy in 1 study.38 Twenty-three studies were from North America,9,12,14,15,18-20,22,27,30,32,35,37-39,41-44,47,53,54 15 studies were from Europe,7,13,21,31,33,34,40,45,46,48-52 and 1 study each was from South America,28 Africa,36 and the Asia-Pacific.29 Four studies included NDBE patients only,13,28,34,52 and 16 studies included only DBE ± early neoplasia patients,7,19-21,29,31,37,39,40,42,44,49,51,53,54 with the remainder including NDBE and DBE patients.
Table 1. Characteristics of included studies.
| First author, year of publication, country | Study type, no. of center | Total no. of patients, histology included | No. reaching CRIM | No. in surveillance after CRIM | Surveillance protocol | GEJ biopsy sample in surveillance, expert GI pathologist | Follow-up after CRIM (person-years) | No. of recurrent IM and recurrent HGD/EAC | Endoscopic treatment of recurrence | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| RFA | ||||||||||
|
| ||||||||||
| Shue et al 2013 USA15 | Retrospective NR | 42; NDBE, DBE, IMC | 42 | 42 | NR | GEJ, no GI pathologist, no | 49 | Total: 11 HGD/EAC: 0 | NR | 2 |
|
| ||||||||||
| Akiyama et al 2013 USA27 | Retrospective single center | 86; NDBE, DBE, IMC | 40 | 40 | NR | GEJ, no GI pathologist, no | 80 | Total: 7 HGD/EAC: NR | NR | 2 |
|
| ||||||||||
| Phoa et al 2016 Netherlands49 | Retrospective multicenter | 132; NDBE, DBE, IMC | 115 | 115 | 6 months, then yearly | GEJ, yes GI pathologist, yes | 259 | Total: 9 HGD/EAC: 4 | 100 | 8 |
|
| ||||||||||
| Cotton et al 2015 USA32 | Retrospective single center | 198 NDBE, DBE | 198 | 198 | NR | GEJ, yes GI pathologist, yes | NR | Total: 32 HGD/EAC: 10 | NR | 7 |
|
| ||||||||||
| Strauss et al 2014 USA53 | Retrospective multicenter | 36 IMC | 27 | 27 | NR | GEJ, no GI pathologist, yes | 54 | Total: 5 HGD/EAC: NR | 66 | 4 |
|
| ||||||||||
| Wolf et al 2014 USA54 | Prospective multicenter | 127 DBE | 108 | 72 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, yes | 288 | Total: 18 HGD/EAC: 3 | 64.2 | 7.5 |
|
| ||||||||||
| Pasricha et al 2014 USA47 | Retrospective multicenter | 5521 NDBE, DBE, IMC, EAC | 3764 | 1634 | <6 months first 2 years, then yearly | GEJ, no GI pathologist, yes | 2494 | Total: 334 HGD/EAC: 28 | NR | 6.5 |
|
| ||||||||||
| Blevins et al 2014 USA18 | Retrospective single center | 338 NDBE, DBE, IMC | 158 | 158 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 411 | Total: 39 HGD/EAC: 5 | 100 | 8.5 |
|
| ||||||||||
| Haidry et al (2) 2014 UK40 | Prospective single center | 145 DBE, IMC | 94 | 94 | NR | GEJ, no GI pathologist, no | 470 | Total: 22 HGD/EAC: NR | NR | 5 |
|
| ||||||||||
| Johnson et al 2014 USA41 | Retrospective multicenter | 49 NDBE, DBE, IMC | 44 | 44 | NR | GEJ, no GI pathologist, no | 117 | Total: 12 HGD/EAC: NR | NR | 4 |
|
| ||||||||||
| Orman et al 2013 USA8 | Retrospective single center | 262 NDBE, DBE, EAC | 168 | 112 | <6 months 1st year, 6 months 2nd year, then yearly l | GEJ, yes GI pathologist, yes | 123 | Total: 8 HGD/EAC: 7 | 80 | 7 |
|
| ||||||||||
| Gupta M et al 2013 USA12 | Retrospective multicenter | 448 NDBE, DBE, EAC | 229 | 229 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 344 | Total: 37 HGD/EAC: 5 | 96 | 8.5 |
|
| ||||||||||
| Dulai et al (ULSB) 2013 USA22 | Retrospective single center | 34 NDBE, DBE, IMC | 26 | 26 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, no GI pathologist, no | 69 | Total: 6 HGD/EAC: 0 | 100 | 4 |
|
| ||||||||||
| Dulai et al (LSB) 2013 USA22 | Retrospective single center | 38 NDBE, DBE, IMC | 31 | 31 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, no GI pathologist, no | 65 | Total: 5 HGD/EAC: 0 | NR | 4 |
|
| ||||||||||
| Choi et al 2013 USA30 | Retrospective single center | 58 NDBE, DBE, IMC | 56 | 56 | NR | GEJ, no GI pathologist, no | 117 | Total: 1 HGD/EAC: 0 | 100 | 3.5 |
|
| ||||||||||
| Gupta N et al 2013 USA12 | Retrospective multicenter | 128 DBE, IMC, EAC | 128 | 128 | NR | GEJ, no GI pathologist, yes | 167 | Total: 34 HGD/EAC: 10 | 100 | 7 |
|
| ||||||||||
| Korst et al 2013 USA43 | Prospective single center | 53 NDBE, DBE, EAC | 53 | 51 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, no | 76 | Total: 11 HGD/EAC: 0 | NR | 5 |
|
| ||||||||||
| Cameron et al 2012 Australia29 | Retrospective multicenter | 114 DBE, IMC | 39 | 39 | NR | GEJ, no GI pathologist, no | 52 | Total: 4 HGD/EAC: 4 | NR | 4 |
|
| ||||||||||
| Vacarro et al 2011 USA14 | Retrospective single center | 47 NDBE, DBE, EAC | 47 | 47 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, no | 52 | Total: 15 HGD/EAC: 2 | 100 | 4.5 |
|
| ||||||||||
| van Vilsteren et al 2011 Netherlands21 | Prospective multicenter | 22 DBE | 20 | 20 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, yes | 25 | Total: 4 HGD/EAC: NR | NR | 5.5 |
|
| ||||||||||
| Pouw et al 2010 Netherlands50 | Prospective multicenter | 24 DBE | 23 | 23 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, yes | 42 | Total: 3 HGD/EAC: NR | NR | 5.5 |
|
| ||||||||||
| PDT | ||||||||||
|
| ||||||||||
| Chandra et al 2013 USA19 | Retrospective single center | 255; DBE, IMC | 194 | 194 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 436 | Total: 37 HGD/EAC: NR | NR | 5 |
|
| ||||||||||
| May et al 2002 Germany7 | Prospective single center | 115; DBE, IMC | 108 | 108 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, no | 306 | Total: 34 HGD/EAC: 34 | 100 | 5 |
|
| ||||||||||
| APC | ||||||||||
|
| ||||||||||
| Gad et al 2011 Egypt36 | Prospective single center | 73; NDBE, DBE | 69 | 69 | <6 months 1st year | GEJ, yes GI pathologist, no | 69 | Total: 0 HGD/EAC: 0 | NA | 2 |
|
| ||||||||||
| Ferraris et al 2007 Italy34 | Prospective multicenter | 96; NDBE | 94 | 94 | Yearly | GEJ, yes GI pathologist, no | 282 | Total: 17 HGD/EAC: 0 | NR | 4.5 |
|
| ||||||||||
| Mork et al 2007 Germany45 | Prospective single center | 25; NDBE, DBE | 21 | 21 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, no GI pathologist, no | 52 | Total: 14 HGD/EAC: 0 | NR | 2.5 |
|
| ||||||||||
| Pedrazzani et al 2005 Italy48 | Prospective single center | 25; NDBE, DBE | 24 | 24 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, no | 52 | Total: 1 HGD/EAC: 0 | 100 | 3.5 |
|
| ||||||||||
| Pagani et al 2003 Italy46 | Prospective single center | 94; NDBE, DBE | 68 | 43 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, no GI pathologist, no | 638 | Total: 5 HGD/EAC:0 | 100 | 2.5 |
|
| ||||||||||
| Familiari et al 2003 Italy33 | NR single center | 35; NDBE, DBE | 32 | 32 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, no | 132 | Total: 3 HGD/EAC: NR | 100 | 2 |
|
| ||||||||||
| Schulz et al 2000 Germany52 | Prospective single center | 73; NDBE | 69 | 69 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, no | 69 | Total: 0 HGD/EAC: 0 | NA | 1.5 |
|
| ||||||||||
| Cryotherapy | ||||||||||
|
| ||||||||||
| Goldberg et al 2012 USA38 | Prospective single center | 31; NDBE, DBE, IMC | 20 | 20 | NR | GEJ, yes GI pathologist, no | 80 | Total: 6 HGD/EAC: NR | NR | 3 |
|
| ||||||||||
| Multipolar electrocoagulation | ||||||||||
|
| ||||||||||
| Allison et al 2011 Venezuela28 | Prospective single center | 139; NDBE | 139 | 139 | Yearly | GEJ, yes GI pathologist, no | 1459 | Total: 7 HGD/EAC: 0 | 100 | 4.5 |
|
| ||||||||||
| Madisch et al 2005 Germany13 | Prospective single center | 73; NDBE | 69 | 66 | NR | GEJ, yes GI pathologist, no | 281 | Total: 8 HGD/EAC: 0 | 100 | 5.5 |
|
| ||||||||||
| Laser therapy | ||||||||||
|
| ||||||||||
| Fisher et al 2003 USA35 | Prospective single center | 31; NDBE, DBE | 21 | 21 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 57 | Total: 8 HGD/EAC: 1 | 87.5 | 4.0 |
|
| ||||||||||
| EMR | ||||||||||
|
| ||||||||||
| Konda et al 2014 USA42 | Retrospective single center | 107; DBE, IMC | 86 | 74 | Yearly | GEJ, yes GI pathologist, yes | 204 | Total: 21 HGD/EAC: 2 | NR | 8 |
|
| ||||||||||
| Conio et al 2014 Italy31 | Retrospective single center | 47; DBE, IMC | 37 | 37 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 57 | Total: 1 HGD/EAC: 1 | 100 | 6.5 |
|
| ||||||||||
| Gorospe et al 2011 USA20 | Retrospective single center | 47; DBE, IMC | 23 | 23 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 26 | Total: 0 HGD/EAC: 0 | NA | 5.5 |
|
| ||||||||||
| van Vilsteren et al 2011 Netherlands21 | Prospective multicenter | 25; DBE, IMC | 23 | 23 | <6 months first 2 years, then yearly | GEJ, yes GI pathologist, yes | 38 | Total: 7 HGD/EAC: NR | NR | 5.5 |
|
| ||||||||||
| Gerke et al 2011 USA37 | Retrospective single center | 41; DBE, IMC | 32 | 32 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 68 | Total: 3 HGD/EAC: 0 | 100 | 7 |
|
| ||||||||||
| Pouw et al 2010 Netherlands50 | Prospective multicenter | 169; NDBE, DBE, EAC Z | 144 | 144 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 324 | Total: 17 HGD/EAC: 0 | NR | 6.5 |
|
| ||||||||||
| Larghi et al 2007 USA44 | Prospective Multicenter | 24; DBE, IMC | 24 | 24 | <6 months 1st year, 6 months 2nd year, then yearly | GEJ, yes GI pathologist, yes | 56 | Total: 3 HGD/EAC: 1 | NR | 5.5 |
NA, Not applicable; NR, not reported; IMC, intramucosal carcinoma; RFA, radiofrequency ablation; NDBE, nondysplastic Barrett's esophagus; DBE, dysplastic Barrett's esophagus; HGD, high-grade dysplasia; EAC, esophageal adenocarcinoma; GEJ, gastroesophageal junction; IM, intestinal metaplasia; PDT, photodynamic therapy; APC, argon plasma coagulation; EMR, endoscopic mucosal resection; CRIM, complete remission of intestinal metaplasia.
Quality of included studies
Table 2 summarizes the quality of the included studies. Among the RFA studies, 7 studies were deemed high quality,9,12,18,32,39,49,54 11 studies were deemed medium quality14,21,22,29,40,41,43,47,51,53 and 3 studies were deemed low quality.15,27,30 When all endoscopic modalities were included, 9 studies were deemed high quality,9,12,18,32,37,39,42,49,54 22 studies were deemed medium quality,7,13,14,19-22,28,29,31,34,35,40,41,43,44,47,50,51,53 and 10 studies were deemed low quality.15,27,30,33,36,38,45,46,48,52
Table 2. Quality of included studies.
| Author | Primary endotherapy modality |
Multi center vs single center |
Sample size |
GI pathologist |
Total follow-up |
Follow-up person- years vs mean years |
Attrition rate |
Definition of CRIM |
GEJ biopsy sample |
EMR before ablation |
Histology of recurrent BE |
Total quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shue et al15 | RFA | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 |
|
| ||||||||||||
| Akiyama J et al27 | RFA | 0 | .5 | 0 | 0 | .5 | 1 | 0 | 0 | 0 | 0 | 2 |
|
| ||||||||||||
| Phoa et al49 | RFA | 1 | 1 | 1 | 0 | .5 | 1 | .5 | 1 | 1 | 1 | 8 |
|
| ||||||||||||
| Cotton et al32 | RFA | 0 | 1 | 1 | 0 | .5 | 1 | .5 | 1 | 1 | 1 | 7 |
|
| ||||||||||||
| Strauss et al53 | RFA | 0 | 0 | 1 | 0 | .5 | 1 | .5 | 0 | 1 | 0 | 4 |
|
| ||||||||||||
| Wolf et al54 | RFA | 1 | 1 | 1 | .5 | 1 | .5 | .5 | 0 | 1 | 1 | 7.5 |
|
| ||||||||||||
| Pasricha et al47 | RFA | 1 | 1 | 1 | 0 | 1 | 0 | .5 | 0 | 1 | 1 | 6.5 |
|
| ||||||||||||
| Blevins et al18 | RFA | 1 | 1 | 1 | 0 | .5 | 1 | 1 | 1 | 1 | 1 | 8.5 |
|
| ||||||||||||
| Haidry et al40 | RFA | 0 | 1 | 0 | 1 | .5 | 1 | .5 | 0 | 1 | 0 | 5 |
|
| ||||||||||||
| Johnson et al41 | RFA | 1 | 0 | 0 | 0 | .5 | 1 | .5 | 0 | 1 | 0 | 4 |
|
| ||||||||||||
| Orman et al8 | RFA | 0 | 1 | 1 | 0 | 1 | .5 | .5 | 1 | 1 | 1 | 7 |
|
| ||||||||||||
| Gupta M et al12 | RFA | 1 | 1 | 1 | 0 | .5 | 1 | 1 | 1 | 1 | 1 | 8.5 |
|
| ||||||||||||
| Dulai et al (ULSB)22 | RFA | 0 | 0 | 0 | 0 | .5 | 1 | .5 | 0 | 1 | 1 | 4 |
|
| ||||||||||||
| Dulai et al (LSB)22 | RFA | 0 | 0 | 0 | 0 | .5 | 1 | .5 | 0 | 1 | 1 | 4 |
|
| ||||||||||||
| Choi et al30 | RFA | 0 | .5 | 0 | 0 | .5 | 0 | .5 | 0 | 1 | 1 | 3.5 |
|
| ||||||||||||
| Gupta N et al12 | RFA | 1 | 1 | 1 | 0 | .5 | 1 | .5 | 0 | 1 | 1 | 7 |
|
| ||||||||||||
| Korst et al43 | RFA | 0 | .5 | 0 | 0 | 0 | 1 | .5 | 1 | 1 | 1 | 5 |
|
| ||||||||||||
| Cameron et al29 | RFA | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 4 |
|
| ||||||||||||
| Vacarro et al14 | RFA | 0 | 0 | 0 | 0 | 0 | 1 | .5 | 1 | 1 | 1 | 4.5 |
|
| ||||||||||||
| van Vilsteren et al21 | RFA | 1 | 0 | 1 | 0 | 0 | 1 | .5 | 1 | 1 | 0 | 5.5 |
|
| ||||||||||||
| Pouw et al50 | RFA | 1 | 0 | 1 | 0 | 0 | 1 | .5 | 1 | 1 | 0 | 5.5 |
|
| ||||||||||||
| Chandra et al19 | PDT | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 5 |
|
| ||||||||||||
| May et al7 | PDT | 0 | 1 | 0 | 0 | .5 | 1 | .5 | 0 | 1 | 1 | 5 |
|
| ||||||||||||
| Gad et al36 | APC | 0 | .5 | 0 | 0 | .5 | 1 | 0 | 0 | 0 | 0 | 2 |
|
| ||||||||||||
| Ferraris et al34 | APC | 1 | .5 | 0 | .5 | 0 | 1 | .5 | 0 | 0 | 1 | 4.5 |
|
| ||||||||||||
| Mork et al45 | APC | 0 | 0 | 0 | 0 | .5 | 1 | 0 | 0 | 0 | 1 | 2.5 |
|
| ||||||||||||
| Pedrazzani et al48 | APC | 0 | 0 | 0 | 0 | .5 | 1 | 0 | 1 | 0 | 1 | 3.5 |
|
| ||||||||||||
| Pagani et al46 | APC | 0 | .5 | 0 | 0 | .5 | .5 | 0 | 0 | 0 | 1 | 2.5 |
|
| ||||||||||||
| Familiari et al33 | APC | 0 | 0 | 0 | .5 | 0 | 1 | .5 | 0 | 0 | 0 | 2 |
|
| ||||||||||||
| Schulz et al52 | APC | 0 | .5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1.5 |
|
| ||||||||||||
| Goldberg et al38 | Cryotherapy | 0 | 0 | 0 | .5 | .5 | 1 | 0 | 1 | 0 | 0 | 3 |
|
| ||||||||||||
| Allison et al39 | MPEC | 0 | 1 | 0 | 1 | .5 | 1 | 0 | 0 | 0 | 1 | 4.5 |
|
| ||||||||||||
| Madisch et al13 | MPEC | 0 | .5 | 0 | .5 | 1 | 1 | .5 | 1 | 0 | 1 | 5.5 |
|
| ||||||||||||
| Fisher et al35 | YAG | 0 | 0 | 1 | 0 | .5 | 1 | .5 | 0 | 0 | 1 | 4.0 |
|
| ||||||||||||
| Konda et al42 | EMR | 1 | 1 | 1 | 0 | .5 | 1 | .5 | 1 | 1 | 1 | 8 |
|
| ||||||||||||
| Conio et al31 | EMR | 1 | 0 | 1 | 0 | 0 | 1 | .5 | 1 | 1 | 1 | 6.5 |
|
| ||||||||||||
| Gorospe et al20 | EMR | 0 | 0 | 1 | 0 | .5 | 1 | 1 | 1 | 1 | 0 | 5.5 |
|
| ||||||||||||
| van Vilsteren et al21 | EMR | 1 | 0 | 1 | 0 | 0 | 1 | .5 | 1 | 1 | 0 | 5.5 |
|
| ||||||||||||
| Gerke et al37 | EMR | 1 | 0 | 1 | 0 | .5 | 1 | .5 | 1 | 1 | 1 | 7 |
|
| ||||||||||||
| Pouw et al50 | EMR | 0 | 1 | 1 | 0 | .5 | 1 | 0 | 1 | 1 | 1 | 6.5 |
|
| ||||||||||||
| Larghi et al44 | EMR | 0 | 0 | 1 | 0 | 0 | 1 | .5 | 1 | 1 | 1 | 5.5 |
MPEC, Multipolar electrocoagulation; RFA, radiofrequency ablation; CRIM, complete remission of intestinal metaplasia; GEJ, gastroesophageal junction; EMR, endoscopic mucosal resection; BE, Barrett's esophagus; PDT, photodynamic therapy; APC, argon plasma; coagulation.
Recurrence of IM: RFA studies
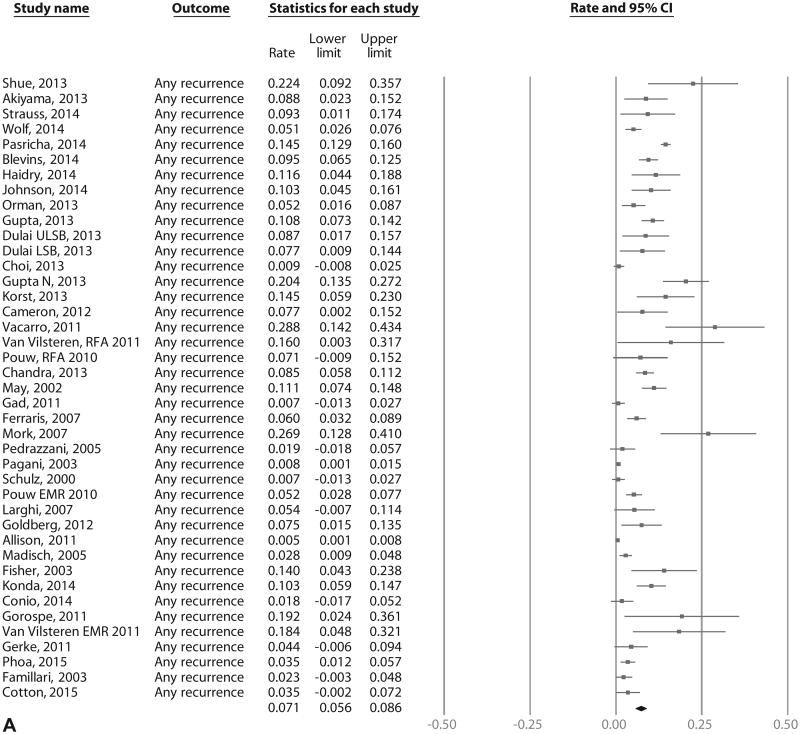
On meta-analysis of 21 RFA studies (603 cases of recurrence in 3186 patients over 5741 patient-years of follow-up), the pooled incidence of IM recurrence (with or without dysplasia/EAC) was 9.5% per patient-year (95% CI, 6.7-12.3), with rates in individual studies ranging from .9% to 28.8% (Fig. 2A). Substantial heterogeneity (I2 = 90%) was seen in the analysis. On meta-analysis of the 15 RFA studies that reported histology of recurrent IM,9,12,14,15,18,22,29,30,32,39,43,47,49,54 the pooled incidence of DBE was 2.0% per patient-year (95% CI, 1.3-2.7) (Fig. 2B) and of HGD/EAC was 1.2% per patient-year (95% CI, .8-1.6) (Fig. 2C). Only 4.6% of patients with recurrence needed surgical treatment in 11 studies where data were available, whereas the rest were treated endoscopically.9,12,14,18,22,29,30,39,49,53
Figure 2.
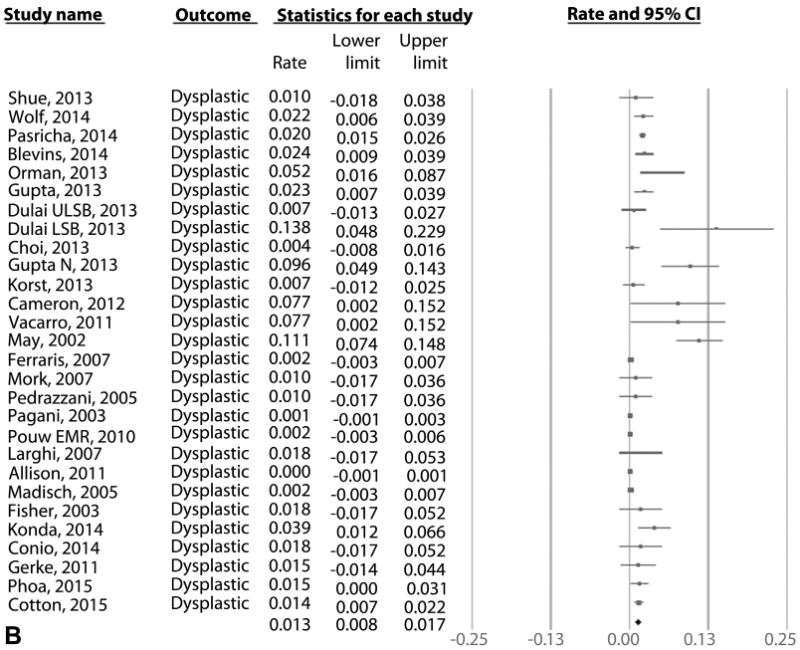
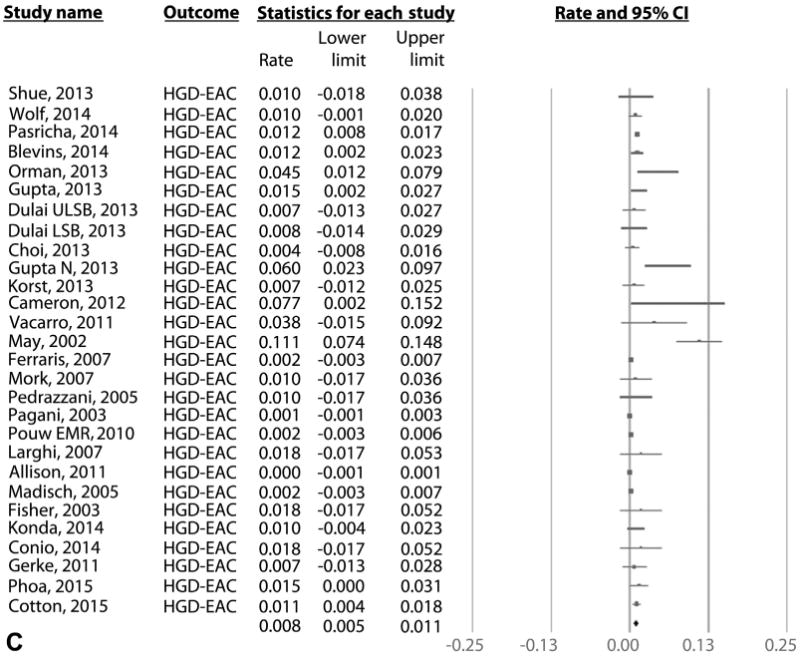
A, Incidence of recurrent IM after achieving CRIM using any endoscopic modality in patients with BE. B, Incidence of recurrent DBE after achieving CRIM using any endoscopic modality in patients with BE. C, Incidence of recurrent HGD/EAC after achieving CRIM using any endoscopic modality in patients with BE. IM, intestinal metaplasia; CRIM, complete remission of intestinal metaplasia; BE, Barrett's esophagus; HGD/EAC, high-grade dysplasia/esophageal adenocarcinoma.
Subgroup analysis: RFA studies
Several subgroup analyses were performed to explore reasons for heterogeneity (Table 3). Recurrence rates in the RFA + EMR studies (19 studies, IR, 9.2% per patient-year) were numerically lower than RFA alone studies (2 studies, IR, 14.3% per patient-year) without statistical significance (P = .46). Recurrence rates in European RFA studies (4 studies, IR, 7.5% per patient-year) and North American RFA studies (16 studies, IR, 10.0% per patient-year) were statistically similar (P = .67).
Table 3. Incidence of IM recurrence after CRIM with RFA.
| Subgroup | Number of studies | Recurrence rate % per patient-year (95% CI) |
|---|---|---|
| Endoscopic modality (P = .46) | ||
|
| ||
| RFA15,27 | 2 | 14.3 (11.4-27.5) |
|
| ||
| RFA + EMR9,12,14,18,21,22,29,30,32,39-41,43,47,49,51,53,54 | 19 | 9.2 (6.3-12.1) |
|
| ||
| Location of study (P = .67) | ||
|
| ||
| North America9,12,14,15,18,22,27,30,32,39,41,43,47,53,54 | 16 | 10.0 (6.7-13.4) |
|
| ||
| Europe21,40,49,51 | 4 | 7.5 (2.2-12.8) |
|
| ||
| Asia-Pacific | 1 | 7.7 (.2-15.2) |
|
| ||
| Publication type (P = .97) | ||
|
| ||
| Full text9,12,14,21,22,32,43,47,49,51,53 | 12 | 9.4 (5.8-13.1) |
|
| ||
| Abstract15,18,27,29,30,39-41,54 | 9 | 9.6 (5.5-13.6) |
|
| ||
| Inclusion of GEJ biopsy sample in post-CRIM surveillance (P = .52) | ||
|
| ||
| Yes9,12,14,18,21,32,43,49,51 | 9 | 8.3% (5.1-11.5) |
|
| ||
| No15,22,27,29,30,39-41,47,53,54 | 12 | 10.1% (5.7-14.4) |
|
| ||
| Study quality (P = .16) | ||
|
| ||
| High9,12,18,32,39,49,54 | 7 | 7.5% (4.5-10.6) |
|
| ||
| Medium14,21,22,29,40,41,43,47,51,53 | 11 | 11.5% (8.8-14.1) |
|
| ||
| Low15,27,30 | 3 | 8.8% (0-18.6) |
IM, intestinal metaplasia; CRIM, complete remission of intestinal metaplasia; RFA, radiofrequency ablation; EMR, endoscopic mucosal resection; GEJ, gastroesophageal junction.
Recurrence rates were statistically similar between subgroups based on type of publication (abstract vs full article), post-CRIM surveillance biopsy sampling protocol (inclusion vs exclusion of GEJ in surveillance biopsy sample), and study quality (high, medium, low). Subgroup analysis based on baseline dysplasia status was not performed in RFA studies because none of the included RFA studies had a study population of only NDBE subjects. However, on restricting analysis to the 7 RFA studies 21,29,39,40,51,53,54 that had an exclusive study population of DBE ± early neoplasia subjects, pooled IRs of recurrent IM, DBE, and HGD/EAC recurrence rates were 10.3% (95% CI, 5.7-15.0), 6.0% (95% CI, .5-11.6), and 4.1% (95% CI, .0-8.5) per patient-year, respectively. These recurrence rates were statistically similar to the overall recurrence rates in RFA studies.
Recurrence of IM: all endoscopic modalities
On meta-analysis of 41 studies (795 cases of IM recurrence over 10,427 patient-years of follow-up), the pooled incidence of IM recurrence (with or without dysplasia/EAC) was 7.1% per patient-year (95% CI, 5.6-8.6), with rates in individual studies ranging from .07% to 28.8% (Fig. 3A). Substantial heterogeneity (I2 = 93%) was seen in the analysis. On meta-analysis of the 28 studies that reported histology of recurrence,7,9,12-15,18,22,28-32,34,35,37,39,42-50,54 the pooled incidence of DBE was 1.3% per patient-year (95% CI, .8-1.7) (Fig. 3B) and of HGD/EAC was .8% per patient-year (95% CI, .5-1.1) (Fig. 3C). Only 3.4% of recurrences needed surgical treatment in 20 studies where data were available, whereas the rest were treated endoscopically.7,9,12-14,18,22,28-31,33,35,37,39,46,48,49,53 In the 17 studies that reported if recurrences where endoscopically visible,9,13,21,28,31-35,37,43-45,49-51 only 58% of recurrences were endoscopically visible. The remaining 42% of recurrences were noted in biopsy specimens from normal-appearing mucosa. In the 17 studies that reported location of recurrence,12-14,18,21,22,31-34,37,38,43,44,50,51 43% of recurrences occurred in tubular esophagus, 55% of recurrences occurred in the GEJ, and 2% occurred in tubular esophagus and GEJ.
Figure 3.
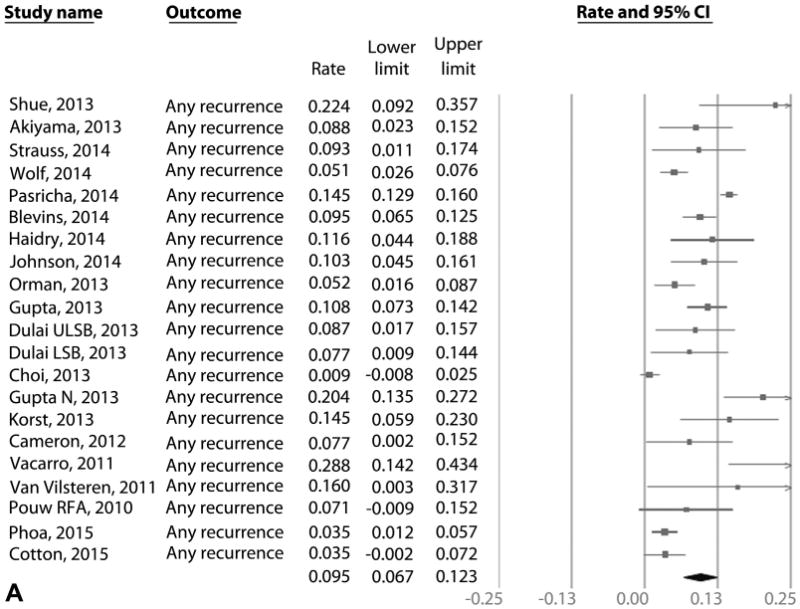
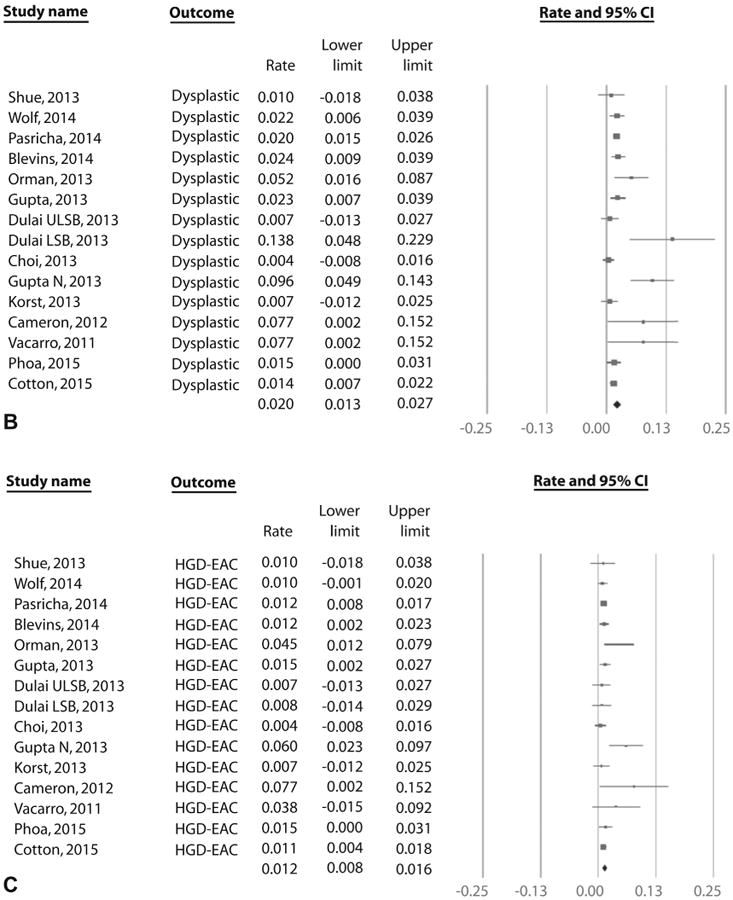
A, Incidence of recurrent IM after achieving CRIM using RFA in patients with BE. B, Incidence of recurrent DBE after achieving CRIM using RFA in patients with BE. C, Incidence of recurrent HGD/EAC after achieving CRIM using RFA in patients with BE. IM, intestinal metaplasia; CRIM, complete remission of intestinal metaplasia; RFA, radiofrequency ablation; Barrett's esophagus; DBE, dysplastic Barrett's esophagus; HGD/EAC, high-grade dysplasia/esophageal adenocarcinoma.
Subgroup analysis: all endoscopic modalities
Table 4 describes the subgroup analysis of studies including all endoscopic modalities. Considerable differences were observed in the risk of recurrence based on primary endoscopic eradication modality, with RFA studies reporting higher rates of recurrence than APC studies. The IM recurrence rates associated with 2 commonly used modalities, RFA (21 studies, IR, 9.5% per patient-year) and EMR (7 studies, IR, 6.3% per patient-year), were statistically similar (P = .16). The recurrence rate in studies using current modalities (ie, RFA, EMR, and cryotherapy) was significantly higher than studies using historical modalities (ie, PDT, APC, multipolar electrocoagulation, and laser): 9.2%, 29 studies vs 3.8%, 12 studies (P < .01).
Table 4. Incidence of IM recurrence after CRIM with all endoscopic modalities.
| Subgroup | Number of studies | Recurrence rate % per patient-year (95% CI) |
|---|---|---|
| Endoscopic modality (P < .01) | ||
|
| ||
| RFA9,12,14,15,18,21,22,27,29,30,32,39-41,43,47,49,51,53,54 | 21 | 9.5 (6.7-12.3) |
|
| ||
| APC33,34,36,45,46,48,52 | 7 | 2.3 (.5-4.1) |
|
| ||
| PDT7,19 | 2 | 9.5 (7.0-12.0) |
|
| ||
| EMR20,21,31,37,42,44,50 | 7 | 6.3 (3.2-9.4) |
|
| ||
| MPEC13,28 | 2 | 1.5 (0-3.7) |
|
| ||
| Cryotherapy38 | 1 | 7.5 (1.5-13.5) |
|
| ||
| Laser35 | 1 | 14 (4.3-23.8) |
|
| ||
| Age of modality | ||
|
| ||
| Current modalities (RFA, EMR, and cryotherapy) | 29 | 9.2 (6.8-11.6) |
|
| ||
| Historical modalities (PDT, APC, MPEC, and laser) | 12 | 3.8 (2.4-5.2) |
|
| ||
| Location of study (P < .01) | ||
|
| ||
| North America9,12,14,15,18-20,22,27,30,32,35,37-39,41-44,47,53,54 | 23 | 9.5 (7.0-12.1) |
|
| ||
| Europe7,13,21,31,33,34,40,45,46,48-52 | 15 | 4.6 (2.8-6.5) |
|
| ||
| Asia-Pacific29 | 1 | 7.7 (.2-15.2) |
|
| ||
| Africa36 | 1 | .7 (.0-2.7) |
|
| ||
| South America28 | 1 | .5 (.1-.8) |
|
| ||
| Baseline dysplasia status (P < .01) | ||
|
| ||
| NDBE13,28,34,52 | 4 | 2.2 (.1-4.3) |
|
| ||
| DBE ± early neoplasia7,19-21,29,31,37,39,40,42,44,49,51,53,54 | 16 | 8.8 (6.3-11.4) |
|
| ||
| Publication type (P = .29) | ||
|
| ||
| Full text7,9,12-14,21,22,28,31-35,37,42-53 | 28 | 6.6 (4.8-8.4) |
|
| ||
| Abstract15,18-20,27,29,30,36,38-41,54 | 13 | 8.5 (5.4-11.5) |
|
| ||
| Inclusion of GEJ biopsy samples in post-CRIM surveillance (P = .64) | ||
|
| ||
| Yes9,12-14,18,20,21,31,32,37,38,42-44,48-51 | 19 | 6.6 (4.7-8.4) |
|
| ||
| No7,15,19,22,27-30,33-36,39-41,45-47,52-54 | 22 | 7.2 (5.2-9.3) |
|
| ||
| Study quality (P < .01) | ||
|
| ||
| High9,12,18,32,37,39,42,49,54 | 9 | 7.5 (4.9-10.1) |
|
| ||
| Medium7,13,14,19-22,28,29,31,34,35,40,41,43,44,47,50,51,53 | 22 | 9.1 (6.0-12.2) |
|
| ||
| Low15,27,30,33,36,38,45,46,48,52 | 10 | 2.5 (0.8-4.1) |
IM, intestinal metaplasia; CRIM, complete remission of intestinal metaplasia; RFA, radiofrequency ablation; APC, argon plasma coagulation; PDT, photodynamic therapy; EMR, endoscopic mucosal resection; NDBE, nondysplastic Barrett's esophagus; DBE, dysplastic Barrett's esophagus; GEJ, gastroesophageal junction.
Recurrence rates in European studies (15 studies, IR, 4.6% per patient-year) were lower than North American studies (23 studies, IR, 9.5% per patient-year) (P < .01). Recurrence rates in studies with NDBE patients (4 studies; IR, 2.2% per patient-year) were lower than studies with DBE patients (16 studies, IR, 8.8% per patient-year) (P < .01).
The recurrence rates observed in high-quality studies (9 studies, IR 7.5% per patient-year) were statistically similar to recurrence rates in medium-quality studies (22 studies, IR 9.1% per patient-year) (P = .66) but were higher than recurrence rates in low-quality studies (10 studies, IR 2.5% per patient-year) (P < .01). Recurrence rates were statistically similar between subgroups based on type of publication (abstract vs full article) and post-CRIM surveillance biopsy sampling protocol (inclusion vs exclusion of GEJ in surveillance biopsy).
Additional subgroup analysis based on definition of CRIM (negative biopsy samples from single endoscopy versus 2 successive endoscopies), inclusion of cardia in surveillance biopsy samples (inclusion vs exclusion of cardia), and the biopsy sampling protocol (4-quadrant biopsy samples every 1 to 2 cm vs biopsy samples from GEJ and visible lesions) did not reveal a statistically significant difference in recurrence rates. However, the analysis was limited by the fact that only 4 studies used the latter definition of CRIM,12,18-20 2 studies reported biopsy sampling cardia,32,42 and 3 studies used the latter biopsy sampling protocol.13,14,37
Publication bias
Based on visual inspection of the funnel plot (Fig. 4) as well as quantitative measurement using Egger's test, there was evidence of publication bias (P < .01). Given considerable heterogeneity observed in the analysis, the assessment of publication bias should be interpreted with caution.
Figure 4.
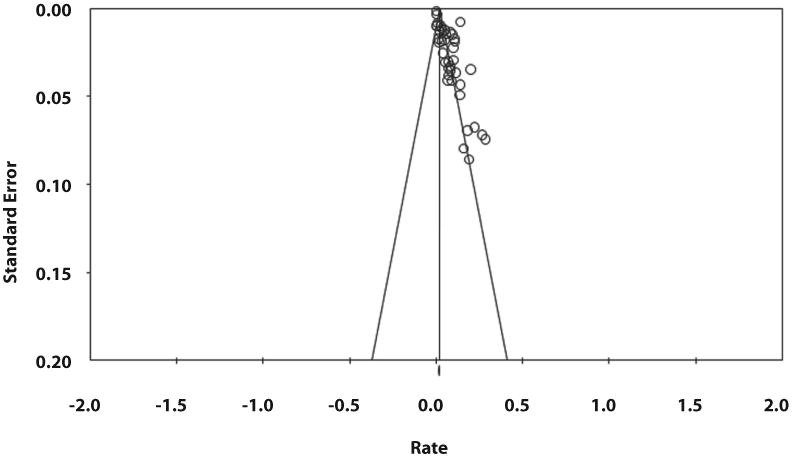
Funnel plot assessing publication bias in primary analysis.
Predictors of recurrence
Only 10 studies reported predictors of recurrence.9,12-15,27,34,39,47,54 Increasing age (4 studies, odds ratio, 1.02; 95% CI, 1.01-1.03) and BE length (4 studies, odds ratio, 1.10; 95% CI, 1.05-1.15) were predictive of recurrence (Table 5). Male sex (5 studies, odds ratio, 1.12; 95% CI, .85-1.47) and baseline dysplasia grade (4 studies, odds ratio, 1.03, 95% CI, .63-1.70) were not statistically significant predictors. However, these estimates are limited by the small number of studies providing relevant data.
Table 5. Predictors of IM recurrence after CRIM.
| Predictors | Number of studies | Odds ratio (95% CI) |
|---|---|---|
| Age12,27,34,47 | 4 | 1.02 (1.01-1.03) |
|
| ||
| Sex12,27,34,47,54 | 5 | 1.12 (.85-1.47) |
|
| ||
| BE length (per cm)12,27,34,47 | 4 | 1.10 (1.05-1.15) |
|
| ||
| Baseline dysplasia12,27,47,54 | 4 | 1.03 (.63-1.70) |
IM, intestinal metaplasia; CRIM, complete remission of intestinal metaplasia; BE, Barrett's dysplasia.
Discussion
Endoscopic therapy is an established treatment for BE-related dysplasia and mucosal adenocarcinoma. Systematic reviews have reported a high efficacy and low adverse event rate with endoscopic therapy.8,67 However, currently, there is no reliable estimate of recurrence risk after successfully achieving CRIM. In this systematic review and meta-analysis of 21 RFA studies, the estimated annual incidence of IM recurrence after CRIM was considerable at 9.5%. Annual recurrence rates of DBE and HGD/EAC (in the 15 RFA studies that reported histology of recurrence) were 2.0 % and 1.2%, respectively. When “all” endoscopic modalities were included in the meta-analysis (41 studies), the estimated annual incidence of recurrent IM was also considerable at 7.1%. Annual recurrence rates of DBE and HGD/EAC (in the 28 studies that reported histology of recurrence) were 1.3% and 0.8%, respectively. Most recurrences (97%) were amenable to endoscopic therapy without the need for esophagectomy.
Several GI society guidelines recommend endoscopic therapy as a treatment for BE with HGD and early EAC. Two recent studies supported consideration of endoscopic therapy for BE with low-grade dysplasia as well.5,68 Hence, the use of endoscopic therapy for treatment of BE is expected to increase in the near future. This makes the type of data in the current study attempting to reliably assess the long-term durability of CRIM essential for physicians and patients in weighing the benefits and risks of ablative therapy. To our knowledge, Orman et al's8 systematic review on durability of CRIM is the only other study that addressed this question. This review was restricted to RFA studies, and the meta-analysis included a total of 5 studies on durability. The current review was not restricted to a single endoscopic modality and included a total of 41 studies with 21 detailing results after RFA. Although the value of including historical modalities is questioned, we believed it to be important because level 1 evidence supporting endoscopic therapy for BE is available only for PDT other than RFA.6 Additionally, older modalities such as PDT provided crucial information on the comparability of outcomes in subjects treated endoscopically and surgically. The inclusion of multiple endoscopic modalities also allowed us to compare the relative long-term durability of CRIM across different endoscopic modalities. The previously published systematic review estimated the proportion of patients with recurrent IM after successful RFA therapy and did not calculate the incidence of recurrence per patient-year of follow-up. In the current review we chose “incidence of recurrence per patient-year” over “proportion of patients who recurred” because the latter is more susceptible to variation depending of follow-up duration.
Another highlight of the review is the use of strict inclusion and exclusion criteria that we developed a priori. To be included, the studies had to report details that allowed calculation of follow-up patient-years with CRIM as the starting point. Studies with follow-up duration < 1 year were excluded because our objective was to assess long-term durability. We also developed a detailed quality scoring scale with 10 different variables to identify high-quality studies.
Recurrence risk after endoscopic therapy
Focusing on the currently used modalities, the recurrence rate with RFA + EMR (9.2%) was numerically lower than RFA alone (14.3%) but without statistical significance. The recurrence rates in RFA studies (9.5%) were numerically higher but statistically similar to studies using EMR only (6.3%). The recurrence rates were higher in RFA studies (9.5%) compared with APC studies (2.9%). Both RFA and APC are thermal ablation techniques. No randomized control trials have directly compared the treatment outcomes with RFA and APC. In current practice, RFA is preferred over APC for BE treatment for the ease of ablating longer segments and stronger level 1 evidence of efficacy and safety.
In subgroup analyses of RFA studies, there were no differences in recurrence rates based on study location or study quality. Unlike the RFA studies, the subgroup analysis of “all” modalities revealed significant differences in recurrence rates based on study location and study quality. The lower recurrence rates in European studies compared with North American studies (4.6% vs 9.5%, P < .01) may be explained by the fact that 6 of the 7 APC studies included in the review were from Europe and none was from North America. Similarly, the lower recurrence rates in low-quality studies compared with high-quality studies (2.5% vs 7.5%, P < .01) and historical modalities' studies compared with current modalities' studies (3.8% vs 9.2%, P < .01) may be explained by the fact that 6 of the 10 low-quality and 7 of the 12 historical modalities' studies were APC studies.
None of the RFA studies included in the review had an exclusive study population of NDBE patients, which limited our ability to analyze the impact of baseline dysplasia status on recurrence after successful RFA therapy. However, subgroup analysis of “all” modalities revealed lower recurrence rates in studies with NDBE patients than studies with DBE patients (1.7% vs 7.6%, P < .01). Currently, there is debate on whether the presence of dysplasia in pretreatment histology influences recurrence risk after achieving CRIM. Several studies have investigated the association between baseline dysplasia and recurrence risk without conclusive results.9,12,27,47,54 Our results provide indirect evidence to support the hypothesis that recurrence rates may be higher in those with DBE at baseline.
Predictors of recurrence
Increasing age and BE length were found to predict recurrence. A longer preablation BE segment likely reflects a higher biologic propensity to redevelop BE, likely through more severe gastroesophageal reflux and other mechanisms such as genetic predisposition or risk factors such as obesity. Our estimates of association need to be interpreted with caution, because several studies that reported nonsignificant associations did not report the actual hazard/odds ratio, leading to their exclusion. It is interesting to note that in our analysis of predictors of recurrence, baseline dysplasia status was not significantly associated with risk of recurrence of IM, but this was reported only in 4 studies12,27,47,54 and is likely related to reporting bias in individual studies.
Limitations
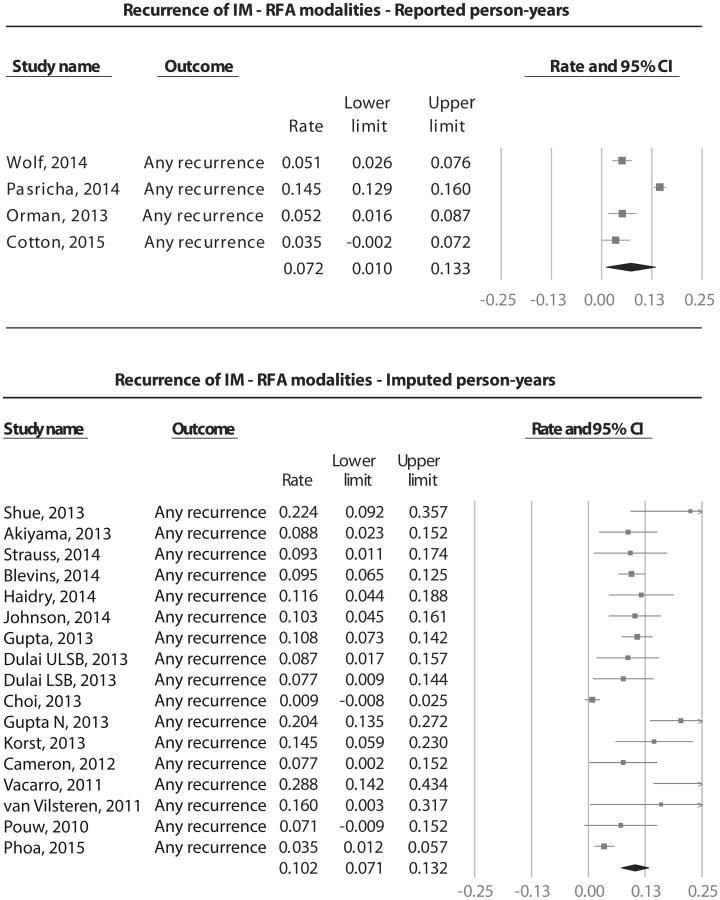
The current systematic review has several potential limitations. Substantial heterogeneity was noted in assessment of recurrence risk with all endoscopic modalities. At a conceptual level, heterogeneity could be because of various factors, both implicit (patient characteristics such as age, smoking status, use of potentially chemopreventive medications after CRIM, etc) and explicit (differences in study design, follow-up duration, and biopsy sampling protocols after CRIM). We tried to minimize conceptual heterogeneity by using strict inclusion and exclusion criteria in study design. We also performed preplanned subgroup analyses to assess stability of association and explore sources of heterogeneity and observed that heterogeneity could be partially explained based on modality of endoscopic therapy, study location, baseline dysplasia status, and study quality. Regardless, the presence of considerable heterogeneity for most of the analyses does decrease the confidence in a single summary estimate of recurrence risk and decreases the rating of overall quality of evidence. Second, we found evidence of publication bias, but it should be interpreted with caution given the high heterogeneity. Third, most of the included studies did not directly report follow-up periods as patient-years, and hence it was imputed. However, there was no statistically significant difference in recurrence rates between RFA studies that reported follow-up in patient-years and studies in which it was imputed (7.2% [4 studies] vs 10.2% [17 studies], P = .39) (Appendix 5, available online at www.giejournal.org). The same was true for studies of “all” endoscopic modalities (6.9% [5 studies] vs 6.7% [36 studies], P = .89). Finally, in our attempt to quantify risk factors associated with recurrence of IM, there was significant concern for selective reporting bias with only a few studies consistently reporting on plausible factors.
Conclusions
The incidence of recurrence after achieving CRIM through endoscopic therapy was substantial. Although only a small proportion of recurrences were dysplastic, HGD, or EAC, the risk was not negligible. Increasing age and BE length might have a role in predicting recurrence. Based on current results, it is imperative that patients who successfully achieved CRIM should continue to stay on lifelong surveillance. Reassuringly, most recurrences could be treated endoscopically without need for esophagectomy. Further prospective studies with standardized protocols and long-term follow-up are needed to accurately estimate the recurrence risk after BE endotherapy.
Acknowledgments
We sincerely thank Mr. Larry Prokop, Medical Librarian at the Mayo Clinic Library, for helping in the literature search for this systematic review and meta-analysis.
Abbreviations
- APC
argon plasma coagulation
- BE
Barrett's esophagus
- CRIM
complete remission of intestinal metaplasia
- DBE
dysplastic Barrett's esophagus
- EAC
esophageal adenocarcinoma
- GEJ
gastroesophageal junction
- HGD
high-grade dysplasia
- IM
intestinal metaplasia
- IR
incidence rate
- NDBE
nondysplastic Barrett's esophagus
- PDT
photodynamic therapy
- RFA
radiofrequency ablation
Appendix 1. Summary of search strategy
A systematic literature search of several databases from each database's inception to June 1, 2015 for relevant articles on recurrence of IM, dysplasia, or adenocarcinoma after endoscopic treatment of DBE and NDBE was conducted. The databases included MEDLINE, EMBASE, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews. The search was restricted to the studies on human participants published in English. The search was conducted by an experienced librarian with input from the study authors (RK, SS, PGI). The search was performed using a combination of keywords and medical subject heading terms, including “Barrett's (o)esophagus,” “dysplasia,”“low-grade dysplasia,” “high-grade dysplasia,” “intramucosal carcinoma,” AND “endoscopic therapy,” “endoscopic resection,” “endoscopic mucosal resection,” “ablation,” “photodynamic therapy,” “radiofrequency ablation,”“cryotherapy,” “laser,” “Nd-YAG,” “KTP,” “multipolar electrocoagulation,” and “argon plasma coagulation.” Two authors (RK, KR) independently reviewed the title and abstract of the identified studies to exclude studies that were not pertinent to the research question, based on prespecified inclusion and exclusion criteria (see below). The full text of the remaining articles was examined to determine if they were relevant to the research question. Any discrepancy in article selection was resolved by consensus and in discussion with an additional coauthor (PGI). Next, a manual search of bibliographies of the selected articles and review articles on the topic was performed for additional articles. Finally, we manually searched conference proceedings from major gastroenterology meetings for additional abstracts on the topic. In case of missing information, we attempted to contact the study authors with specific questions regarding their studies.
Appendix 2. Summary of data abstraction
After identifying relevant studies, data on study characteristics (design, location, number of centers, enrollment time, number of patients undergoing endoscopic therapy, reaching CRIM, and in surveillance after CRIM), patient characteristics (age, sex, race, smoking status, body mass index, proton pump inhibitor use, presence of baseline dysplasia, and BE segment length), treatment characteristics (type of endoscopic modality, number of endoscopic modalities [endoscopic ablation alone vs endoscopic ablation + EMR], and definition of CRIM), outcome assessment (number of patients who recurred after achieving CRIM, post-CRIM follow-up duration, histologic grade of recurrent BE, and treatment [endoscopic vs surgical] of recurrence), covariates (post-CRIM surveillance intervals, inclusion of gastric cardia in surveillance biopsy sampling protocol, and availability of expert GI pathologist), and risk factors for recurrence (all reported associations from univariate/multivariate analysis, regardless of statistical significance) were abstracted onto a standardized form by 2 authors (RK, KR).
Appendix 3. Study Quality Assessment Scale
| 1. Representative of the average BE subject in the community | |
| 1 point | Multicenter study |
| 0 points | Single center |
| 2. Large cohort size | |
| 1 point | Cohort size > 100 patients |
| .5 points | Cohort size between 50 and 100 patients |
| 0 points | Cohort size < 50 patients |
| 3. Definite histologic confirmation of recurrent BE | |
| 1 point | Histology reviewed by GI pathologist |
| 0 points | Histology reviewed only by community pathologist/not reported |
| 4. Adequate follow-up of cohort after CRIM for the outcome to occur | |
| 1 point | Mean follow-up of entire cohort > 5 years |
| .5 points | Mean follow-up 3-5 years |
| 0 points | Mean follow-up of cohort 1-3 years |
| 5. Reporting of duration of follow-up of patients after CRIM | |
| 1 point | Reported in study in total person-years after CRIM |
| .5 points | Reported as mean follow-up years after CRIM |
| 0 points | Reported as median follow-up years after CRIM |
| 6. Attrition rate in follow-up after CRIM | |
| 1 point | 80% of cohort followed-up |
| .5 points | 60%-80% of cohort followed-up |
| 0 points | 60% of cohort followed-up |
| 7. Definition of CRIM | |
| 1 point | ≥2 endoscopies with biopsy specimen showing CRIM |
| .5 points | 1 endoscopy with biopsy specimen showing CRIM |
| 0 points | Not reported |
| 8. Inclusion of biopsy sample from GEJ as part of surveillance protocol | |
| 1 point | Biopsy specimens were obtained from GEJ and esophagus |
| .5 points | Biopsy specimens were obtained from esophagus only |
| 0 points | Not reported |
| 9. EMR done before ablation in dysplastic subjects | |
| 1 point | EMR was done before ablation |
| 0 points | EMR was not done before ablation |
| 10. Reporting histology of recurrent BE | |
| 1 point | Histology of recurrent BE was reported |
| 0 points | Histology recurrent BE was not reported |
BE, Barrett's esophagus; CRIM, complete remission of intestinal metaplasia; GEJ, gastroesophageal junction; EMR, endoscopic mucosal resection.
Appendix 4. Statistical analysis: meta-analysis of predictors of recurrence
To identify risk factors associated with recurrence of IM, we performed a meta-analysis of reported demographic and clinical factors associated with recurrent IM, if reported in ≥2 studies. We preferentially used adjusted estimates for the pooled analysis; however, if adjusted estimates were not reported, we used results from univariate analysis pooling. When studies reported exposure grouped into categories (such as for body mass, BE length, etc.) to provide a dose-specific odds ratio (using the lowest category as referent category), we transformed this into a risk estimate per unit exposure (for example, per unit body massindex, per cm of BE length, etc.), using linear trend meta-analytic statistical methodology. Briefly, we assigned the midpoint of the cut-points of the class as the dose value. For studies with open-ended categories, we used the lowest and highest reported exposure category from the study to calculate the midpoint. We then calculated the odds ratio for that range of exposure category (subtracting the midpoints from the highest risk category with the lowest-risk category) to estimate a per-unit odds ratio, after log-transformation. This methodology assumes a linear relationship between exposure and logarithm of the odds ratio.
Appendix 5
Footnotes
DISCLOSURE: The following authors disclosed financial relationships relevant to this publication: K. Wang: Research grant recipient from Nine Point Medical and CSA Medical; P. Iyer: Research grant recipient from Intromedic and Exact Sciences. All other authors disclosed no financial relationships relevant to this publication.
References
- 1.Evans JA, Early DS, Fukami N, et al. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–94. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett's esophagus. Gastroenterology. 2011;140:e18–52. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blevins CH, Iyer PG. Endoscopic therapy for Barrett's oesophagus. Best Pract Res Clin Gastroenterol. 2015;29:167–77. doi: 10.1016/j.bpg.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 5.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 6.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 7.May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol. 2002;14:1085–91. doi: 10.1097/00042737-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett's Esophagus: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245–55. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orman ES, Kim HP, Bulsiewicz WJ, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett's esophagus with radiofrequency ablation. Am J Gastroenterol. 2013;108:187–95. doi: 10.1038/ajg.2012.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phoa KN, Pouw RE, Van Vilsteren FG, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145:96–104. doi: 10.1053/j.gastro.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 11.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011;141:460–8. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett's esophagus: results from a US Multicenter Consortium. Gastroenterology. 2013;145:79–86. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madisch A, Miehlke S, Bayerdorffer E, et al. Long-term follow-up after complete ablation of Barrett's esophagus with argon plasma coagulation. World J Gastroenterol. 2005;11:1182–6. doi: 10.3748/wjg.v11.i8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett's esophagus with radiofrequency ablation. Dig Dis Sci. 2011;56:1996–2000. doi: 10.1007/s10620-011-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shue P, Kataria R, Pathikonda M, et al. Factors associated with recurrence of Barrett's esophagus after completion of radiofrequency ablation. Gastroenterology. 2013;144:S-697. [Google Scholar]
- 16.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0. The Cochrane Collaboration. 2011;5 [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Blevins C, Gorospe EC, Devanna S, et al. Outcomes of recurrent intestinal metaplasia following successful endotherapy of Barrett's esophagus associated dysplasia [abstract] Gastrointest Endosc. 2014;79:AB396. [Google Scholar]
- 19.Chandra S, Gorospe EC, Leggett CL, et al. Durability of photodynamic therapy for barrett's dysplasia: a single center 20-year experience. Gastroenterology. 2013;144:S-692. [Google Scholar]
- 20.Gorospe E, Tian J, Dunagan K, et al. Can a single EMR cure Barrett's dysplasia? Complete remission after a single endoscopic mucosal resection with negative margins 2011 ACG Presidential Poster. Am J Gastroenterol. 2011;106:S12–3. [Google Scholar]
- 21.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–73. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 22.Dulai PS, Pohl H, Levenick JM, et al. Radiofrequency ablation for long- and ultralong-segment Barrett's esophagus: a comparative long-term follow-up study. Gastrointest Endosc. 2013;77:534–41. doi: 10.1016/j.gie.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Metaanalysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Easterbrook PJ, Gopalan R, Berlin J, et al. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama J, Roorda AK, Marcus SN, et al. Erosive esophagitis is a major predictor for recurrence of barrett's esophagus after successful radiofrequency ablation. Gastroenterology. 2013;144:S-692. [Google Scholar]
- 28.Allison H, Banchs MA, Bonis PA, et al. Long-term remission of nondysplastic Barrett's esophagus after multipolar electrocoagulation ablation: report of 139 patients with 10 years of follow-up. Gastrointest Endosc. 2011;73:651–8. doi: 10.1016/j.gie.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 29.Cameron G, Jayasekera C, Williams R, et al. Victorian Barrett's experience: outcomes of patients undergoing combination endoscopic therapy for dysplastic Barrett's oesophagus. J Gastroenterol Hepatol. 2012;27:53–7. [Google Scholar]
- 30.Choi KD. Clinical outcomes of patients with Barrett's esophagus treated with radiofrequency ablation using only the focal device. Am J Gastroenterol. 2013;108:S4. [Google Scholar]
- 31.Conio M, Fisher DA, Blanchi S, et al. One-step circumferential endoscopic mucosal cap resection of Barrett's esophagus with early neoplasia. Clin Res Hepatol Gastroenterol. 2014;38:81–91. doi: 10.1016/j.clinre.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Cotton CC, Wolf WA, Pasricha S, et al. Recurrent intestinal metaplasia after radiofrequency ablation for Barrett's esophagus: endoscopic findings and anatomic location. Gastrointest Endosc. 2015;81:1362–9. doi: 10.1016/j.gie.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Familiari L, Scaffidi M, Bonica M, et al. Endoscopic treatment of Barrett's epithelium with argon plasma coagulation. Long-term follow-up. Min Gastroenterol Dietol. 2003;49:63–70. [PubMed] [Google Scholar]
- 34.Ferraris R, Fracchia M, Foti M, et al. Barrett's oesophagus: long-term follow-up after complete ablation with argon plasma coagulation and the factors that determine its recurrence. Aliment Pharmacol Therap. 2007;25:835–40. doi: 10.1111/j.1365-2036.2007.03251.x. [DOI] [PubMed] [Google Scholar]
- 35.Fisher RS, Bromer MQ, Thomas RM, et al. Predictors of recurrent specialized intestinal metaplasia after complete laser ablation. Am J Gastroenterol. 2003;98:1945–51. doi: 10.1111/j.1572-0241.2003.07628.x. [DOI] [PubMed] [Google Scholar]
- 36.Gad YZ, Zeid AA. The role of argon plasma coagulation in the management of Barrett's esophagus: a single-center experience. Gastrointest Cancer Targets Ther. 2011;1:21–6. [Google Scholar]
- 37.Gerke H, Siddiqui J, Nasr I, et al. Efficacy and safety of EMR to completely remove Barrett's esophagus: experience in 41 patients. Gastrointest Endosc. 2011;74:761–71. doi: 10.1016/j.gie.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg ME, Horwhat D, Cash BD. Long-term analysis of outcomes associated with endoscopic spray cryotherapy for Barrett's esophagus with and without dysplasia [anstract] Gastrointest Endosc. 2012;75:AB463. [Google Scholar]
- 39.Gupta N, Wani S, Hollander TG, et al. Recurrence of disease after endoscopic eradication therapy (EET) for Barrett's esophagus (BE) with high grade dysplasia (HGD) and early cancer (EC) Gastroenterology. 2012;142:S-750–1. [Google Scholar]
- 40.Haidry RJ, Banks MR, Gupta A, et al. Five year outcomes for patients undergoing endoscopic therapy for Barrett's related neoplasia from the United Kingdom's largest single centre experience [abstract] Gastrointest Endosc. 2014;79:AB497. [Google Scholar]
- 41.Johnson CS, Louie BE, Wille A, et al. The durability of endoscopic therapy for treatment of Barrett's metaplasia, dysplasia, and mucosal cancer after nissen fundoplication. J Gastrointest Surg. 2015;19:799–805. doi: 10.1007/s11605-015-2783-6. [DOI] [PubMed] [Google Scholar]
- 42.Konda VJ, Ruiz MGH, Koons A, et al. Complete endoscopic mucosal resection is effective and durable treatment for Barrett's-associated neoplasia. Clin Gastroenterol Hepatol. 2014;12:2002–10. doi: 10.1016/j.cgh.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Korst RJ, Santana-Joseph S, Rutledge JR, et al. Patterns of recurrent and persistent intestinal metaplasia after successful radiofrequency ablation of Barrett's esophagus. J Thorac Cardiovasc Surg. 2013;145:1529–34. doi: 10.1016/j.jtcvs.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Larghi A, Lightdale C, Ross A, et al. Long-term follow-up of complete Barrett's eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy. 2007;39:1086–91. doi: 10.1055/s-2007-966788. [DOI] [PubMed] [Google Scholar]
- 45.Mörk H, Al-Taie O, Berlin F, et al. High recurrence rate of Barrett's epithelium during long-term follow-up after argon plasma coagulation. Scand J Gastroenterol. 2007;42:23–7. doi: 10.1080/00365520600825125. [DOI] [PubMed] [Google Scholar]
- 46.Pagani M, Granelli P, Chella B, et al. Barrett's esophagus: combined treatment using argon plasma coagulation and laparoscopic antireflux surgery. Dis Esoph. 2003;16:279–83. doi: 10.1111/j.1442-2050.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 47.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett's esophagus. Clin Gastroenterol Hepatol. 2014;12:1840–7. doi: 10.1016/j.cgh.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedrazzani C, Catalano F, Festini M, et al. Endoscopic ablation of Barrett's esophagus using high power setting argon plasma coagulation: a prospective study. World J Gastroenterol. 2005;11:1872–5. doi: 10.3748/wjg.v11.i12.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of a European multicentre study (EURO-II) Gut. 2016;65:555–62. doi: 10.1136/gutjnl-2015-309298. [DOI] [PubMed] [Google Scholar]
- 50.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169–77. doi: 10.1136/gut.2010.210229. [DOI] [PubMed] [Google Scholar]
- 51.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for Barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–9. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Schulz H, Miehlke S, Antos D, et al. Ablation of Barrett's epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc. 2000;51:659–63. [PubMed] [Google Scholar]
- 53.Strauss AC, Agoston AT, Dulai PS, et al. Radiofrequency ablation for Barrett's-associated intramucosal carcinoma: a multi-center follow-up study. Surg Endosc. 2014;28:3366–72. doi: 10.1007/s00464-014-3629-0. [DOI] [PubMed] [Google Scholar]
- 54.Wolf WA, Overholt BF, Li N, et al. Durability of radiofrequency ablation (RFA) in Barrett's esophagus with dysplasia: the AIM Dysplasia Trial at five years. Gastroenterology. 2014;5:S-131. [Google Scholar]
- 55.Tian J, Gorospe E, Sun G, et al. What should be the goal for Barrett's ablation: is elimination of dysplasia enough? Am J Gastroenterol. 2011;106:S26. [Google Scholar]
- 56.Chandra S, Gorospe EC, Leggett CL, et al. Durability of radiofrequency ablation for Barrett's esophagus: a single center 10-year experience. Gastroenterology. 2013;5:S-685. [Google Scholar]
- 57.Gondrie J, Pouw R, Sondermeijer C, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370–9. doi: 10.1055/s-2007-995589. [DOI] [PubMed] [Google Scholar]
- 58.Pereira-Lima JC, Busnello JV, Saul C, et al. High power setting argon plasma coagulation for the eradication of Barrett's esophagus. Am J Gastroenterol. 2000;95:1661–8. doi: 10.1111/j.1572-0241.2000.02197.x. [DOI] [PubMed] [Google Scholar]
- 59.Herrero LA, van Vilsteren FG, Pouw RE, et al. Endoscopic radiofrequency ablation combined with endoscopic resection for early neoplasia in Barrett's esophagus longer than 10 cm. Gastrointest Endosc. 2011;73:682–90. doi: 10.1016/j.gie.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Gondrie J, Pouw R, Sondermeijer C, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359–69. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 61.Yassin B, Kazemi S, Kalaghchi B. Results of one year follow-up of RF ablation of dysplastic Barrett's esophagus in community setting. Am J Gastroenterol. 2012;107:S39–40. [Google Scholar]
- 62.Pinotti A, Cecconello I, Sakai P, et al. Endoscopic ablation of Barrett's esophagus using argon plasma coagulation: a prospective study after fundoplication. Dis Esoph. 2004;17:243–6. doi: 10.1111/j.1442-2050.2004.00415.x. [DOI] [PubMed] [Google Scholar]
- 63.Van Laethem JL, Cremer M, Peny MO, et al. Eradication of Barrett's mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut. 1998;43:747–51. doi: 10.1136/gut.43.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonavina L, Ceriani C, Carazzone A, et al. Endoscopic laser ablation of nondysplastic Barrett's epithelium: is it worthwhile? J Gastrointest Surg. 1999;3:194–9. doi: 10.1016/s1091-255x(99)80033-x. [DOI] [PubMed] [Google Scholar]
- 65.Bowers S, Mattear S, Waring P, et al. KTP laser ablation of Barrett's esophagus after anti-reflux surgery results in long-term loss of intestinal metaplasia. Surg Endosc Other Intervent Techn. 2003;17:49–54. doi: 10.1007/s00464-001-8155-1. [DOI] [PubMed] [Google Scholar]
- 66.Sharma P, Bhattacharyya A, Garewal HS, et al. Durability of new squamous epithelium after endoscopic reversal of Barrett's esophagus. Gastrointest Endosc. 1999;50:159–64. doi: 10.1016/s0016-5107(99)70218-x. [DOI] [PubMed] [Google Scholar]
- 67.Almond L, Hodson J, Barr H. Meta-analysis of endoscopic therapy for low-grade dysplasiain Barrett's oesophagus. Br J Surg. 2014;101:1187–95. doi: 10.1002/bjs.9573. [DOI] [PubMed] [Google Scholar]
- 68.Small AJ, Araujo JL, Leggett CL, et al. Radiofrequency ablation is associated with decreased neoplastic progression in patients with Barrett's esophagus and confirmed low-grade dysplasia. Gastroenterology. 2015;149:567–76. doi: 10.1053/j.gastro.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]