Abstract
Background
Despite numerous trials assessing optimal antibiotic prophylaxis strategies for colorectal surgery, few studies have assessed real-world practice on a national scale with respect to risk of surgical site infections.
Objective
Using a large, national claims database we aimed to describe 1) current use of prophylactic antibiotics (type and duration) and 2) associations with surgical site infection after open colectomies.
Design
Retrospective study using the Premier Perspective database.
Setting
Patient hospitalizations nationwide from January 2006 to December 2013.
Patients
90,725 patients that underwent an open colectomy in 445 different hospitals.
Main Outcome Measures
Multilevel multivariable logistic regressions measured associations between surgical site infection and 1) type of antibiotic used and 2) duration (day of surgery only, day of surgery and the day after, >1 day after surgery).
Results
Overall surgical site infection prevalence was 5.2% (n=4,750). Most patients (41.8%) received cefoxitin for prophylaxis; other choices were ertapenem (18.2%), cefotetan (10.3%), metronidazole+cefazolin (9.9%), ampicillin+sulbactam (7.6%), while 12.2% received other antibiotics. Distribution of prophylaxis duration was: 51.6%, 28.5%, and 19.9% for days 0, 0+1, and 1+, respectively. Compared to cefoxitin, lower odds for surgical site infection were observed for ampicillin+sulbactam (odds ratio 0.71; 95% confidence interval 0.63–0.82), ertapenem (odds ratio 0.65; 95% confidence interval 0.58–0.71) and metronidazole+cefazolin (odds ratio 0.56; 95% confidence interval 0.49–0.64), and “other” (odds ratio 0.81; 95% confidence interval 0.73–0.90); duration was not significantly associated with altered odds for surgical site infection. Sensitivity analyses supported the main findings.
Limitations
Lack of detailed clinical information in the billing dataset used.
Conclusions
In this national study assessing real-world use of prophylactic antibiotics in open colectomies, type of antibiotic used appeared to be associated with up to 44% decreased odds for surgical site infections. While there are numerous trials on optimal prophylactic strategies, studies that particularly focus on factors that influence the choice of prophylactic antibiotic might provide insights into ways of reducing the burden of surgical site infections in colorectal surgeries.
Keywords: Antibiotic prophylaxis, Colectomy, Surgical site infection
INTRODUCTION
In addition to compromising patient safety, surgical site infections (SSI) represent a substantial burden on US healthcare costs. More than serving as a hospital quality measure—increasingly important for hospital reimbursements1—SSIs are associated with an extended length of hospital stay by 10 days, representing an additional $1.6 billion annual burden on the health care system.2–4 With the highest SSI rates, the >260,000 patients undergoing colorectal surgery each year appear at particular risk despite prophylactic antibiotics, the cornerstone of SSI prevention.5 Current guidelines recommend the use of antibiotics covering both aerobic and anaerobic bacteria, however, the relative efficacy of different regimens has yet to be established.6 Moreover, despite numerous trials assessing optimal antibiotic prophylaxis strategies for colorectal surgery, few studies have assessed real-world antibiotic prophylaxis practice on a national scale with respect to SSI risk.7,8 Notable exceptions are the Veterans Affairs Surgical Quality Improvement Program9 (n=5,750) and the Michigan Surgical Quality Collaborative5 (n=4,331) studies that compared different antibiotic prophylaxis regimens in colorectal surgery. Although these studies do demonstrate that the type of antibiotic prophylaxis matters in mitigating SSI risk after colorectal surgery, they are burdened by small and localized samples, while also lacking the most recent data.
Using a large, national claims-based database we, therefore, sought to 1) describe the real-world use of antibiotic prophylaxis (type and duration) in open colectomies, and 2) quantify the odds of developing a SSI for each prophylaxis regimen.
MATERIALS AND METHODS
Data Source and Study Design
In this retrospective cohort study we used data from the Premier Perspective database (Premier Inc., Charlotte, NC). This database contains information on patient hospitalizations nationwide from January 2006 to December 2013 and includes International Classification of Diseases-9th revision Clinical Modification (ICD-9 CM) codes, Current Procedural Terminology (CPT) codes, and billed items. These data meet the de-identification requirements as defined by the Health Insurance Portability and Accountability Act and was exempt from consent requirements of the Mount Sinai Medical Center Institutional Review Board (project HS#: 14-00647).
Study Sample
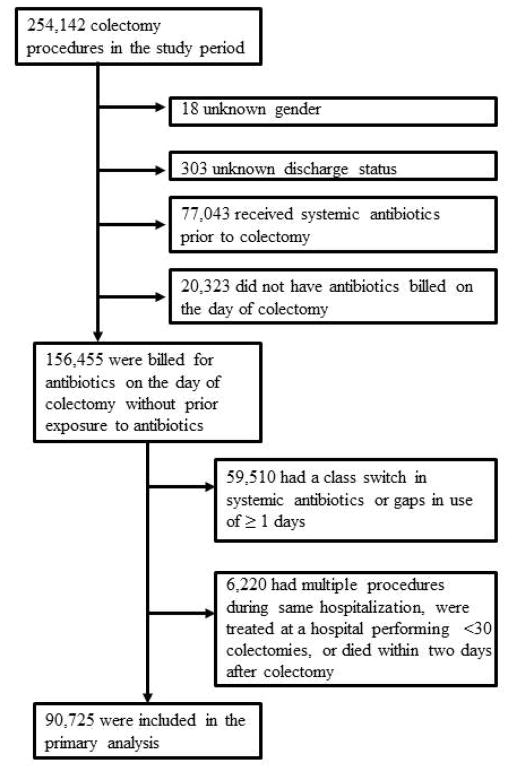
The study sample contained patients that underwent an open colectomy10 (right hemicolectomy, left hemicolectomy, resection of transverse colon, sigmoidectomy, other; indicated by ICD-9 CM procedure codes 45.7X, 45.82, 45.83). Exclusion criteria were based on previous studies.11,12 Patients were excluded (see figure 1) if they had an unknown gender or discharge type (n=321), had systemic antibiotic use prior to surgery (n=77,043), no claims for antibiotic use on the day of surgery (n=20,323), a switch in antibiotic class or gaps in antibiotic use of ≥1 days (as to distinguish between treatment and prophylaxis, n=59,510), patients who had multiple procedures during the same hospitalization (n=4,085), were treated at a hospital performing <30 colectomies (to ensure sufficient sample size per cluster) (n=1,274)13 or died within two days of surgery (n=861).
Figure 1.
Patient flow chart
Study Variables
The main exposures of interest were 1) the type of prophylactic antibiotic used, and 2) the duration of the use of prophylactic antibiotics. Type of prophylactic antibiotic was categorized into cefotetan, cefoxitin, ampicillin+sulbactam, ertapenem, metronidazole+cefazolin, and other (for a full list of “other” medications, see Appendix Table 1); duration of prophylaxis was categorized into day of surgery only (day 0), day of surgery and the day after (day 0+1), or >1 day after surgery (day 1+).
Patient demographic variables included age, gender, and ethnicity (White, Black, Hispanic, other). Healthcare related variables were insurance type (commercial, Medicaid, Medicare, uninsured, other), hospital location (urban, rural), hospital size (<300, 300–499, >500 beds), hospital teaching status, and the mean annual number of open colectomies performed per hospital. Procedure related variables included the indication for surgery (neoplasm, diverticular disease, inflammatory bowel disease, other), type of surgery (right hemicolectomy, left hemicolectomy, resection of transverse colon, sigmoidectomy, other), year of procedure, and length of hospitalization. Overall comorbidity burden was assessed using the Quan14 adaptation of the Charlson Comorbidity Index. In addition, other included variables deemed to influence SSI risk were obesity (ICD-9 278.0, 278.00, 278.01, 649.1, V85.3, V85.4, V85.54, 793.91) and smoking (ICD-9 305.1X, V15.82).
The main outcome of interest was the occurrence of a SSI during the index hospitalization. As different definitions of SSI have been used in previous studies, we assessed three different definitions of SSI in our study: 1) only ICD-9 codes (998.5, 998.51, 998.59, 998.13, 998.3, 998.31, 998.32, 998.83, 998.81),15 2) ICD-9 codes AND billing for a wound culture, and 3) only billing for a wound culture. We believe that the use of varying definitions will demonstrate the robustness of our main effects of interest. Unfortunately, the use of billing information does not allow us to reliably differentiate between superficial and deep wound infections; our SSI variables therefore represent a combination of both.
Statistical Analysis
First, we assessed the univariable association between type of antibiotic use and study variables using Chi-square tests and t-tests for categorical and continuous variables, respectively. We then performed multilevel multivariable logistic regressions to measure the association between type and duration of antibiotic use, and the three definitions of SSI. Models included a random intercept term that varies at the level of each hospital, accounting for correlation of patients within hospitals. The multivariable models were adjusted using all variables found significant at the P<0.15 level from the univariable tests and deemed clinically important. Adjusted odds ratio (OR), 95% confidence interval (CI), and P-value are reported. Model discrimination was evaluated using the C-statistic.
All analyses were performed in SAS v9.4 statistical software (SAS Institute, Cary, NC, USA).
RESULTS
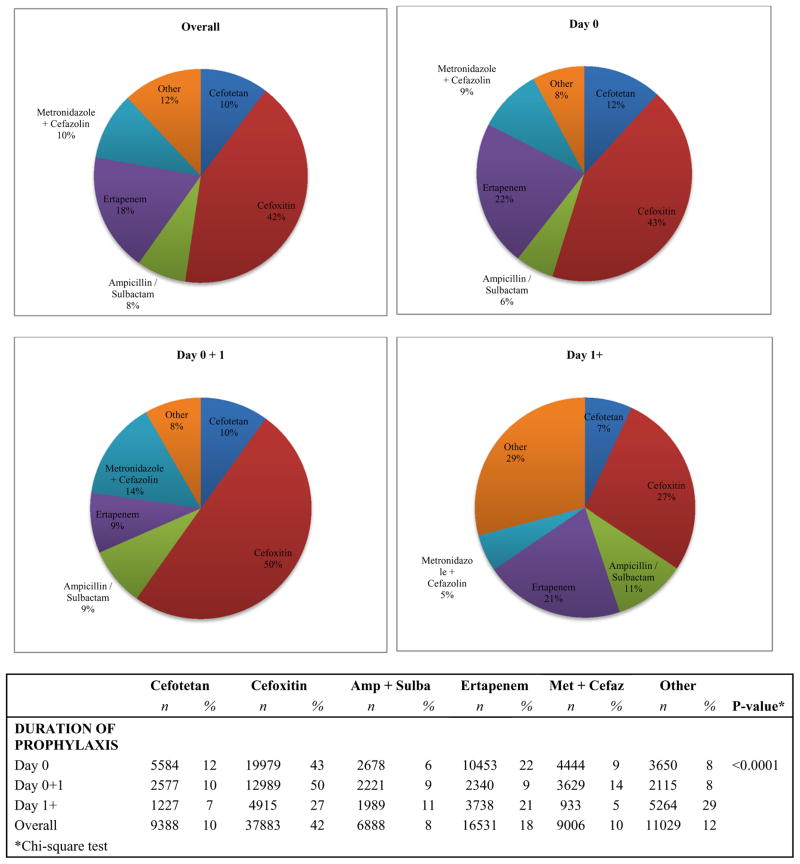
The final study cohort consisted of 90,725 patients undergoing an open colectomy at 445 hospitals between January 2006 and December 2013. Overall, 42% (n=37,883) of patients received cefoxitin as prophylaxis; this was 18% (n=16,531) for ertapenem, 10% (n=9,388) for cefotetan, 10% (n=9,006) for metronidazole+cefazolin, 8% (n=6,888) for ampicillin/sulbactam, and 12% (n=11,029) for ‘other’ antibiotics. Furthermore, 52% (n=46,788) received antibiotic prophylaxis only on the day of operation; 29% (n=25,871) received prophylaxis on the day of the operation and the following day, and 20% (n=18,066) received prophylaxis that lasted longer than the day after the operation. Figure 2 shows the overall distribution of type of antibiotic as well as sub-grouped by day 0, day 0+1, and day 1+. Appendix 1 contains a list of all antibiotics included under “other.” The most commonly used “other” medication was metronidazole + quinolone (n=8,705; 79% of all “other” medications).
Figure 2.
Distribution of type of prophylactic antibiotic used; overall and sub-grouped by day 0, day 0+1, and day
Table 1 provides a breakdown of type of prophylactic antibiotic used by patient, healthcare-related, procedure-related, comorbidity and outcome (SSI) variables. The majority of variables were univariably associated with the type of antibiotic used. Interestingly, the use of ertapenem rapidly increased from 6% of all patients in 2006 to 29% in 2013 (row percentages, not shown in table). In addition, the mean length of hospitalization was highest among patients administered ‘other’ antibiotics (8.8 days) and ampicillin+sulbactam (8.3 days), while the highest costs of hospitalization were seen for those administered "other" antibiotics ($19,916), and the highest Charlson comorbidity index was seen for those on metronidazole+cefazolin (2.51); all P<0.0001. The overall SSI prevalence—defined by only ICD-9 codes—was 5.2% (n=4,750), varying from 3.5% (metronidazole+cefazolin) to 5.9% (cefotetan). In general, estimated prevalences were lower for the other SSI definitions.
Table 1.
Type of antibiotic used by patient, healthcare related, procedure related, comorbidity and outcome (SSI) variables.
| TYPE OF ANTIBIOTIC USED N (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefotetan | Cefoxitin | Amp+Sulba | Ertapenem | Met+Cefaz | Other | ||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | P-value** | |
| PATIENT DEMOGRAPHICS | |||||||||||||
| Mean Age* | 63.5 | 15.1 | 63.4 | 15.2 | 62.2 | 16.2 | 63.5 | 15.2 | 63.3 | 14.9 | 63.2 | 15.5 | <.0001 |
| Age category | |||||||||||||
| <45 years | 1013 | 10.8 | 4151 | 11.0 | 891 | 12.9 | 1842 | 11.1 | 999 | 11.1 | 1280 | 11.6 | <0.0001 |
| 45–54 years | 1448 | 15.4 | 6042 | 15.9 | 1167 | 16.9 | 2654 | 16.1 | 1418 | 15.7 | 1811 | 16.4 | |
| 55–64 years | 2162 | 23.0 | 8483 | 22.4 | 1541 | 22.4 | 3647 | 22.1 | 2020 | 22.4 | 2435 | 22.1 | |
| 65–74 years | 2289 | 24.4 | 9233 | 24.4 | 1561 | 22.7 | 4046 | 24.5 | 2268 | 25.2 | 2567 | 23.3 | |
| >75 years | 2476 | 26.4 | 9974 | 26.3 | 1728 | 25.1 | 4342 | 26.3 | 2301 | 25.5 | 2936 | 26.6 | |
| Gender | |||||||||||||
| Female | 4995 | 53.2 | 19980 | 52.7 | 3570 | 51.8 | 8673 | 52.5 | 4814 | 53.5 | 6737 | 61.1 | <0.0001 |
| Male | 4393 | 46.8 | 17903 | 47.3 | 3318 | 48.2 | 7858 | 47.5 | 4192 | 46.5 | 4292 | 38.9 | |
| Race | |||||||||||||
| White | 6278 | 66.9 | 27519 | 72.6 | 5094 | 74.0 | 12451 | 75.3 | 5944 | 66.0 | 8032 | 72.8 | <0.0001 |
| Black | 822 | 8.8 | 3560 | 9.4 | 790 | 11.5 | 1563 | 9.5 | 986 | 10.9 | 997 | 9.0 | |
| Hispanic | 232 | 2.5 | 965 | 2.5 | 160 | 2.3 | 285 | 1.7 | 556 | 6.2 | 309 | 2.8 | |
| Other | 2056 | 21.9 | 5839 | 15.4 | 844 | 12.3 | 2232 | 13.5 | 1520 | 16.9 | 1691 | 15.3 | |
| HEALTHCARE RELATED | |||||||||||||
| Insurance type | |||||||||||||
| Commercial | 3826 | 40.8 | 15133 | 39.9 | 2709 | 39.3 | 6243 | 37.8 | 3650 | 40.5 | 4320 | 39.2 | <0.0001 |
| Medicaid | 324 | 3.5 | 1768 | 4.7 | 418 | 6.1 | 788 | 4.8 | 449 | 5.0 | 587 | 5.3 | |
| Medicare | 4606 | 49.1 | 19087 | 50.4 | 3310 | 48.1 | 8513 | 51.5 | 4420 | 49.1 | 5533 | 50.2 | |
| Uninsured | 286 | 3.0 | 1153 | 3.0 | 258 | 3.7 | 632 | 3.8 | 277 | 3.1 | 388 | 3.5 | |
| Other | 346 | 3.7 | 742 | 2.0 | 193 | 2.8 | 355 | 2.1 | 210 | 2.3 | 201 | 1.8 | |
| Hospital location | |||||||||||||
| Rural | 849 | 9.0 | 5407 | 14.3 | 758 | 11.0 | 3355 | 20.3 | 1112 | 12.3 | 1097 | 9.9 | <0.0001 |
| Urban | 8539 | 91.0 | 32476 | 85.7 | 6130 | 89.0 | 13176 | 79.7 | 7894 | 87.7 | 9932 | 90.1 | |
| Hospital size | |||||||||||||
| <300 beds | 2941 | 31.3 | 11675 | 30.8 | 2226 | 32.3 | 6433 | 38.9 | 2430 | 27.0 | 3280 | 29.7 | <0.0001 |
| 300–499 beds | 3495 | 37.2 | 14378 | 38.0 | 2852 | 41.4 | 6263 | 37.9 | 3944 | 43.8 | 4324 | 39.2 | |
| ≥500 beds | 2952 | 31.4 | 11830 | 31.2 | 1810 | 26.3 | 3835 | 23.2 | 2632 | 29.2 | 3425 | 31.1 | |
| Hospital Teaching Status | |||||||||||||
| Non-Teaching | 5034 | 53.6 | 23023 | 60.8 | 4020 | 58.4 | 10835 | 65.5 | 5754 | 63.9 | 6705 | 60.8 | <0.0001 |
| Teaching | 4354 | 46.4 | 14860 | 39.2 | 2868 | 41.6 | 5696 | 34.5 | 3252 | 36.1 | 4324 | 39.2 | |
| Mean annual # of colectomies per hospital* | 60.76 | 30.6 | 55.17 | 28.7 | 53.96 | 25.8 | 63.31 | 14.9 | 57.19 | 25.9 | 54.76 | 27.6 | <.0001 |
| PROCEDURE RELATED | |||||||||||||
| Procedure status | |||||||||||||
| Elective | 7059 | 75.2 | 28807 | 76.0 | 4426 | 64.3 | 11159 | 67.5 | 7210 | 80.1 | 6456 | 58.5 | <.0001 |
| Emergency | 1432 | 15.3 | 5657 | 14.9 | 1749 | 25.4 | 3642 | 22.0 | 1228 | 13.6 | 3547 | 32.2 | |
| Urgent | 897 | 9.6 | 3419 | 9.0 | 713 | 10.4 | 1730 | 10.5 | 568 | 6.3 | 1026 | 9.3 | |
| Indication for Colectomy*** | |||||||||||||
| Neoplasm | 1958 | 20.9 | 8034 | 21.2 | 1245 | 18.1 | 3205 | 19.4 | 2110 | 23.4 | 1556 | 14.1 | <0.0001 |
| Diverticular Disease | 1183 | 12.6 | 4533 | 12.0 | 674 | 9.8 | 1938 | 11.7 | 1284 | 14.3 | 1333 | 12.1 | <0.0001 |
| Inflammatory Bowel Disease | 235 | 2.5 | 814 | 2.1 | 113 | 1.6 | 452 | 2.7 | 290 | 3.2 | 209 | 1.9 | <0.0001 |
| Type of procedure*** | |||||||||||||
| Right Hemicolectomy | 3503 | 37.3 | 14962 | 39.5 | 2657 | 38.6 | 6018 | 36.4 | 3455 | 38.4 | 3736 | 33.9 | <0.0001 |
| Left Hemicolectomy | 988 | 10.5 | 3929 | 10.4 | 682 | 9.9 | 1808 | 10.9 | 967 | 10.7 | 1140 | 10.3 | 0.18574 |
| Resection of Transverse colon | 490 | 5.2 | 2024 | 5.3 | 345 | 5.0 | 770 | 4.7 | 483 | 5.4 | 515 | 4.7 | 0.00415 |
| Sigmoidectomy | 2883 | 30.7 | 11457 | 30.2 | 2090 | 30.3 | 5235 | 31.7 | 2679 | 29.7 | 4006 | 36.3 | <0.0001 |
| Other | 1524 | 16.2 | 5511 | 14.5 | 1114 | 16.2 | 2700 | 16.3 | 1422 | 15.8 | 1632 | 14.8 | <0.0001 |
| Year of procedure | |||||||||||||
| 2006 | 1701 | 18.1 | 5966 | 15.7 | 1185 | 17.2 | 749 | 4.5 | 985 | 10.9 | 1842 | 16.7 | <0.0001 |
| 2007 | 151 | 1.6 | 7728 | 20.4 | 1286 | 18.7 | 1164 | 7.0 | 1554 | 17.3 | 1741 | 15.8 | |
| 2008 | 1465 | 15.6 | 5572 | 14.7 | 1177 | 17.1 | 2094 | 12.7 | 1428 | 15.9 | 1549 | 14.0 | |
| 2009 | 1298 | 13.8 | 3864 | 10.2 | 751 | 10.9 | 1872 | 11.3 | 904 | 10.0 | 1240 | 11.2 | |
| 2010 | 1399 | 14.9 | 3725 | 9.8 | 668 | 9.7 | 2237 | 13.5 | 1040 | 11.5 | 1183 | 10.7 | |
| 2011 | 1224 | 13.0 | 3932 | 10.4 | 688 | 10.0 | 2721 | 16.5 | 1047 | 11.6 | 1255 | 11.4 | |
| 2012 | 1182 | 12.6 | 3759 | 9.9 | 633 | 9.2 | 2919 | 17.7 | 1042 | 11.6 | 1183 | 10.7 | |
| 2013 | 968 | 10.3 | 3337 | 8.8 | 500 | 7.3 | 2775 | 16.8 | 1006 | 11.2 | 1036 | 9.4 | |
| Mean length of hospital stay* | 7.5 | 6.3 | 8.0 | 6.7 | 8.3 | 7.0 | 7.9 | 6.2 | 7.6 | 6.5 | 8.8 | 7.3 | <.0001 |
| Mean Cost of hospitalization* | 18472 | 17620 | 17415 | 18908 | 18902 | 19474 | 17840 | 16143 | 17096 | 18002 | 19916 | 20389 | <.0001 |
| COMORBIDITIES | |||||||||||||
| Mean Charlson comorbidity index | 2.46 | 3.1 | 2.47 | 3.0 | 2.31 | 3.0 | 2.34 | 3.0 | 2.51 | 3.1 | 2.12 | 2.9 | <.0001 |
| Smoking | 2483 | 26.4 | 9047 | 23.9 | 1789 | 26.0 | 4429 | 26.8 | 2062 | 22.9 | 2652 | 24.0 | <0.0001 |
| Obesity | 1060 | 11.3 | 4061 | 10.7 | 707 | 10.3 | 1924 | 11.6 | 858 | 9.5 | 1289 | 11.7 | <0.0001 |
| SURGICAL SITE INFECTIONS | |||||||||||||
| -Only ICD-9 codes | 554 | 5.9 | 2216 | 5.8 | 334 | 4.8 | 704 | 4.3 | 311 | 3.5 | 631 | 5.7 | <0.0001 |
| -ICD-9 code AND billing for wound culture | 144 | 1.5 | 457 | 1.2 | 58 | 0.8 | 115 | 0.7 | 79 | 0.9 | 115 | 1.0 | <0.0001 |
| -Only wound culture billing | 254 | 2.7 | 878 | 2.3 | 149 | 2.2 | 219 | 1.3 | 145 | 1.6 | 231 | 2.1 | <0.0001 |
Continuous variable mean and standard deviation instead of N and %, respectively
Chi-square test for categorical variables, t-test for continuous variables
Overlap between categories
After adjustment for relevant covariates (Table 2; full model results are depicted in Appendix 2), certain antibiotics remained significantly associated with lower odds for SSI: compared to cefoxitin this was true for ampicillin+sulbactam (OR 0.71 CI 0.63–0.82), ertapenem (OR 0.65 CI 0.58–0.71), and metronidazole+cefazolin (OR 0.56 CI 0.49–0.64); all P<0.05. This pattern did not change when using the varying SSI definitions. The duration of prophylaxis was not significantly associated with SSI risk. The model c-statistics varied between 0.83 and 0.91, indicating good model discrimination.
Table 2.
Multivariable associations between type of antibiotic / duration of prophylaxis and three definitions of SSI.
| Only ICD-9 codes | ICD-9 codes AND billing for wound culture | Only wound culture billing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR* | 95% CI | OR* | 95% CI | OR* | 95% CI | ||||
| TYPE OF ANTIBIOTIC USED | |||||||||
| Reference = Cefoxitin | |||||||||
| Cefotetan | 0.99 | 0.89 | 1.11 | 0.97 | 0.74 | 1.28 | 0.93 | 0.76 | 1.15 |
| Ampicillin/Sulba ctam | 0.71** | 0.63 | 0.82 | 0.52** | 0.37 | 0.73 | 0.72** | 0.57 | 0.90 |
| Ertapenem | 0.65** | 0.58 | 0.71 | 0.55** | 0.42 | 0.71 | 0.53** | 0.43 | 0.64 |
| Metronidazole/Cefazolin | 0.56** | 0.49 | 0.64 | 0.68** | 0.49 | 0.94 | 0.57** | 0.44 | 0.73 |
| Other | 0.81** | 0.73 | 0.90 | 0.72** | 0.56 | 0.92 | 0.69** | 0.58 | 0.83 |
| DURATION OF PROPHYLAXIS | |||||||||
| Reference = Day 0 | |||||||||
| Day 0+1 | 1.05 | 0.96 | 1.14 | 0.98 | 0.81 | 1.18 | 1.01 | 0.96 | 1.26 |
| Day 1+ | 0.93 | 0.86 | 1.01 | 0.90 | 0.76 | 1.07 | 0.90 | 0.80 | 1.03 |
OR odds ratio / CI confidence interval; models adjusted for: age, gender, race, insurance type, hospital geographic location, hospital teaching status, hospital size, indication for colectomy, type of procedure, year of procedure, length of hospital stay, comorbidity burden, smoking status, obesity, and emergent/urgent procedure
P<0.05
DISCUSSION
Although several others have assessed differences between antibiotic prophylaxis regimens in open colectomies,7 to our knowledge this is the largest nationwide study representing real-world clinical practice among 90,725 patients from 445 US hospitals over eight years. We demonstrated significant variations in antibiotic prophylaxis practices for open colectomies; while cefoxitin was most commonly used (42%) the role of ertapenem increased from 6% in 2006 to 29% in 2013 (of all prophylactics used). Moreover, the type of antibiotic used appeared to be associated with SSI risk: with lower (compared to cefoxitin) odds seen for ampicillin+sulbactam (29% decreased odds), ertapenem (35% decreased odds), and metronidazole+cefazolin (44% decreased odds). These effects remained with varying definitions of SSI. Interestingly, the duration of prophylaxis was not significantly associated with SSI risk.
Previous attempts to describe antibiotic prophylaxis choice and SSI rates suffer from small population size or diverse study design, and the optimal regimen of antibiotic prophylaxis for open colectomies has yet to be established.8 The most commonly used prophylactic (cefoxitin) in our study differs from what was found in the only other similarly large description of clinical practice in colectomies using Medicare data in 2005. Using a random sample of 5,279 Medicare inpatients undergoing colon surgery, Bratzler et al. found the most commonly used prophylactic to be cefotetan (52.8%), with only 30.7% receiving cefoxitin.16 Similarly, using data from the Michigan Surgical Quality Collaborative (n=3,002) Hendren et al. also found variations in antibiotic choice with cefoxitin used in 20.0% while metronidazole+cefazolin and ertapenem were used in 18.5% and 16.9%, respectively.17 In addition to this variation, we also demonstrated an increasing use of ertapenem. This may be due to an increasing number of studies in which ertapenem compares favorably to other antibiotics in SSI prevention.5,10,18,19 One of the main drivers in particular may have been the highly publicized 2006 trial by Itani et al. which showed ertapenem to be more effective than cefotetan in the prevention of SSIs in patients undergoing elective open colorectal surgery.10 In addition, ertapenem covers both anaerobes and aerobes found in bowel lumen, has a relatively long half-life (preventing the need for a second administration), and a similar safety profile compared to other prophylaxis choices.10,20,21
Examining real-world clinical practice, we also observed a deviation from recommended antibiotic prophylaxis guidelines. According to the 2013 Surgical Infection Society Guidelines, antibiotic prophylaxis should be “continued for no more than 24 hours and can typically be stopped when the procedure is completed.19” We found that 48% of patients received prophylactics beyond the day of surgery, meaning in only 52% of cases prophylaxis lasted for just the day of surgery, as recommended by current guidelines. This is higher than the 41% of patients discontinuing prophylaxis within 24 hours of the end of surgery found in the 2005 Medicare study.16
The overall SSI rate was 5.2% in our study, lower than the rates found in other studies and most probably an underestimation as rates have been shown to vary from 5% up to 30%.8,10,22,23 Important factors affecting this variation are differences in clinical case definition,24 differences in definition based on billing information (in our study we varied between ICD-9 codes and billing for wound cultures), and follow-up time.25,26 Indeed, 50% of SSI cases have been shown to occur after discharge26 and, thus, were not captured in our data. Importantly, however, this underestimation is likely to be distributed equally among all types of antibiotic prophylaxis. To our knowledge, there is no literature suggesting differing rates of post-discharge incidence of SSI by antibiotic choice. Therefore, although absolute risk of SSI may be underestimated, we expect the comparison of relative odds of SSI to still be valid.
Interestingly, we found that antibiotic choice (after adjustment for relevant covariates) was significantly associated with SSI risk, with lower odds consistently seen for ampicillin+sulbactam, ertapenem, and metronidazole+cefazolin. This is comparable to results found in a study using data from the Michigan Surgical Quality Collaborative including 4,331 patients undergoing a colectomy in twenty-four different Michigan hospitals between 2008 and 2010. Here, the authors compared SSI rates between Surgical Care Improvement Project (SCIP) recommended antibiotic prophylactic choices to those that were non-SCIP-compliant.5 The authors found that SCIP compliant antibiotics (ciprofloxacin+metronidazole, metronidazole+cefazolin, ertapenem) were associated with decreased odds of developing a SSI. The favorable comparison of metronidazole+cefazolin and ertapenem against cefotetan and cefoxitin is confirmed by the findings in the present study. Moreover, among 5,750 veterans undergoing a colectomy at 112 different VA hospitals between 2005 and 2009 metronidazole+cefazolin again appeared to be associated with the lowest SSI rates while second-generation cephalosporins accounted for the majority of antibiotic prophylaxis.9 Although we do not know the reasons for antibiotic choices in our study, one important factor contributing to differences in effectiveness of antibiotic prophylaxis might be an emerging resistance of common antibiotics used in prophylaxis to Bacteroides fragilis, the most commonly isolated organism in these SSIs.27 In particular, Bacteroides resistance to clindamycin has been found to range from 20% to 60%, while increased resistance has also been found in cefoxitin, cefotetan, and ampicillin+sulbactam.9,28
While a significant proportion of patients in our study received antibiotic prophylaxis beyond the day of surgery (48%), duration of prophylaxis was not associated with SSI risk, thus supporting the rationale behind current guidelines.7,19 Between 1978 and 2011, thirty-three studies examined the association between duration of prophylaxis and SSI; combined, their data showed no association between duration of antibiotic prophylaxis and SSI risks.7 It is unclear what is behind the observed extended use of prophylactic antibiotics; qualitative studies geared towards this question will shed some light on this intriguing issue and thus provide targets for intervention.
Next to replication and validation of our study results using other data sources, we feel that the current study hints towards potentials for improvement in the perioperative care for these patients. This opportunity pertains particularly to the most commonly used prophylactic (cefotetan) being associated with the highest odds for SSIs, and 48% of patients receiving antibiotic prophylaxis beyond the day of surgery, despite this practice not showing altered odds for SSI risk. Despite general consensus on the importance of SSI prevention in hospital policies, there is a lack of studies looking into so-called ‘return on investment’ calculations from the hospital perspective. Using data from the Johns Hopkins Health System for a variety of surgeries, Shepard et al. found the change in annual profit due to SSIs was around $650,000—a conservative measure given their methodology—thus illustrating the financial incentive for hospitals to reduce SSIs.29 The results from our study suggest that even relatively straightforward alterations in antibiotic prophylaxis strategies may yield substantial patient safety and economic benefits. Future studies should explore the full scope of benefits and harms while also evaluating implementation strategies and their costs.
The main limitation of our study is the lack of detailed clinical information in the billing dataset used; data are collected for the purpose of billing, not specific research questions. Therefore, important information such as reasons behind antibiotic choice and duration of prophylaxis, SSI pathogens, type of SSI (deep or superficial), or other interventions that might influence SSI risk (e.g. mechanical bowel preparation with or without oral antibiotics) cannot be taken into account, and, thus, residual confounding cannot be ruled out. However, while there is evidence on specifically the benefits of the latter on SSI risk, some reports suggest a decrease in the use of oral antibiotics: 30 92% of surgeons reported using oral antibiotics in 199231 while this was only 36% in 2010.32 Additionally, information from billing databases does not necessary reflect what is actually administered to the patient as there might be a mismatch between the two. Given the de-identified nature of the dataset auditing was not possible. However, we expect the effect of the mismatch to be minimal as this should be unrelated to SSI risk and type/duration antibiotics. Another limitation—partly due to the lack of clinical information—pertains to the definition of SSI from billing data for the inpatient period only. This appears particularly important as a substantial proportion (almost fifty percent) of infections after colorectal surgery occur after hospital discharge.26 This limitation would theoretically only affect our results when the occurrence of SSI post-discharge is dependent on choice and duration of antibiotic prophylaxis. Although this assumption might be debatable we feel confident in our findings as they confirm those found in previous studies. Our study might also have been burdened by differential definitions of SSI. While we have tried to account for this by assessing the effects of antibiotic choice and duration on three different SSI definitions, the issue of differences in clinical interpretation persists. Hedrick et al. recently showed that there is a poor agreement between the SSI definition put forward by the Centers for Disease Control and Prevention and a more objective SSI scoring system. While this is an important issue for particularly the validity of SSI reporting, we feel that for the current study the comparison between differences in clinical interpretation and differential (billing) definitions is independent of antibiotic choice, and therefore reducing its effect on the current findings. Also, while the rate of laparoscopic colectomy is increasing, our results only apply to those undergoing open colectomies.33 We specifically only studied patients undergoing open colectomies as their SSI risk is higher and therefore the effect of antibiotic practices is expected to be more profound.34 As an increasing number of patients is undergoing laparoscopic surgery it would indeed be of interest to assess antibiotic practices in this group in future studies. Adding to the issue of generalizability is the use of multiple exclusion criteria. These were used to minimize confounding (next to covariate adjustment), however, with the risk of compromising generalizability. This indeed is an inevitable limitation of retrospective studies. The explicit statement of our exclusion criteria should ensure the correct interpretation of our results.
In conclusion, in this large, nationwide study, we found significant variations in antibiotic prophylaxis practices for open colectomies, with cefoxitin the most commonly used, and a rapid increase of ertapenem usage. The type of prophylactic antibiotic used appeared to be associated with SSI risk, with 29% to 44% lower odds seen for ampicillin+sulbactam, ertapenem, and metronidazole+cefazolin as compared to the most commonly administered antibiotic, cefoxitin. These effects persisted with varying definitions of SSI. Lending support to current guidelines, the duration of prophylaxis was not significantly associated with SSI risk.
Acknowledgments
Disclaimer: Drs. Mazumdar and Poeran are partially funded by the NCI Cancer Center Support Grant P30 CA196521-01.
APPENDIX I
Table 1.
List of “Other” antibiotics
| Medication | n |
|---|---|
| Metronidazole + Cefuroxime | 83 |
| Metronidazole + Ceftriaxone | 639 |
| Clindamycin + Aminoglycoside | 764 |
| Clindamycin + Quinolone | 441 |
| Clindamycin + Aztreonam | 164 |
| Metronidazole + Aminoglycoside | 233 |
| Metronidazole + Quinolone | 8705 |
Table 2.
SSI rates by year
| SSI Definition | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|---|---|
| ICD 9 + wound culture | 0.9% | 0.9% | 1.0% | 1.1% | 0.9% | 1.0% | 1.1% | 0.9% |
| ICD 9 | 4.8% | 4.7% | 5.0% | 4.9% | 4.7% | 4.7% | 4.9% | 4.5% |
| Wound Culture | 1.8% | 2.0% | 1.9% | 1.8% | 1.9% | 1.8% | 1.9% | 1.9% |
APPENDIX II
Full multivariable model results
| Only ICD-9 codes | ICD-9 codes AND billing for wound culture | Only wound culture billing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| TYPE OF ANTIBIOTIC USED (Reference = Cefoxitin) | |||||||||
| Cefotetan | 0.99 | 0.89 | 1.11 | 0.97 | 0.74 | 1.28 | 0.93 | 0.76 | 1.15 |
| Ampicillin/Sulbactam | 0.71 | 0.63 | 0.82 | 0.52 | 0.37 | 0.73 | 0.72 | 0.57 | 0.90 |
| Ertapenem | 0.65 | 0.58 | 0.71 | 0.55 | 0.42 | 0.71 | 0.53 | 0.43 | 0.64 |
| Metronidazole/Cefazolin | 0.56 | 0.49 | 0.64 | 0.68 | 0.49 | 0.94 | 0.57 | 0.44 | 0.73 |
| Other | 0.81 | 0.73 | 0.90 | 0.72 | 0.56 | 0.92 | 0.69 | 0.58 | 0.83 |
| DURATION OF PROPHYLAXIS (Reference = Day 0) | |||||||||
| Day 0+1 | 1.05 | 0.96 | 1.14 | 0.98 | 0.81 | 1.18 | 1.01 | 0.96 | 1.26 |
| Day 1+ | 0.93 | 0.86 | 1.01 | 0.90 | 0.76 | 1.07 | 0.90 | 0.80 | 1.03 |
| PATIENT DEMOGRAPHICS | |||||||||
| Age (continuous) | 1.00 | 0.99 | 1.00 | 1.00 | 0.99 | 1.01 | 1.00 | 0.99 | 1.00 |
| Gender (Reference = Male) | |||||||||
| Female | 0.80 | 0.75 | 0.85 | 0.80 | 0.70 | 0.91 | 0.76 | 0.69 | 0.84 |
| Race (Reference = White) | |||||||||
| Black | 0.79 | 0.70 | 0.88 | 0.84 | 0.64 | 1.09 | 0.92 | 0.76 | 1.11 |
| Hispanic | 1.08 | 0.89 | 1.32 | 1.02 | 0.63 | 1.66 | 1.29 | 0.93 | 1.80 |
| Other | 1.03 | 0.94 | 1.13 | 1.19 | 0.95 | 1.48 | 1.20 | 1.01 | 1.41 |
| HEALTHCARE RELATED | |||||||||
| Insurance type (Reference = Commercial) | |||||||||
| Medicaid | 1.00 | 0.87 | 1.15 | 1.19 | 0.90 | 1.59 | 0.99 | 0.79 | 1.23 |
| Medicare | 0.88 | 0.88 | 0.96 | 0.84 | 0.69 | 1.01 | 0.89 | 0.78 | 1.03 |
| Uninsured | 0.94 | 0.94 | 1.12 | 0.78 | 0.51 | 1.18 | 1.06 | 0.81 | 1.39 |
| Other | 1.03 | 1.03 | 1.26 | 1.04 | 0.64 | 1.67 | 0.96 | 0.67 | 1.39 |
| Hospital location (Reference = Urban) | |||||||||
| Rural | 1.08 | 0.97 | 1.20 | 0.76 | 0.42 | 1.37 | 0.67 | 0.35 | 1.25 |
| Hospital size (continuous) | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 | 1.01 | 0.99 | 0.98 | 1.00 |
| Hospital Teaching Status (Reference = Teaching) | |||||||||
| Non-Teaching | 0.88 | 0.81 | 0.95 | 1.23 | 0.73 | 2.08 | 1.01 | 0.58 | 1.79 |
| PROCEDURE RELATED | |||||||||
| Procedure status (Reference = Elective) | |||||||||
| Emergency | 0.95 | 0.87 | 1.03 | 1.21 | 1.01 | 1.45 | 1.28 | 1.12 | 1.46 |
| Urgent | 0.88 | 0.79 | 0.99 | 1.10 | 0.85 | 1.43 | 1.17 | 0.96 | 1.41 |
| Indication for Colectomy | |||||||||
| Neoplasm | 1.59 | 1.05 | 2.42 | 2.43 | 1.20 | 4.94 | 1.89 | 1.04 | 3.45 |
| Diverticular Disease | 2.18 | 1.43 | 3.32 | 3.76 | 1.83 | 7.71 | 2.46 | 1.34 | 4.51 |
| Inflammatory Bowel Disease | 2.07 | 1.36 | 3.13 | 4.08 | 2.06 | 8.11 | 3.00 | 1.67 | 5.39 |
| Type of procedure | |||||||||
| Right Hemicolectomy | 0.75 | 0.69 | 0.83 | 0.79 | 0.65 | 0.96 | 0.77 | 0.67 | 0.89 |
| Sigmoidectomy | 0.96 | 0.88 | 1.06 | 0.89 | 0.72 | 1.09 | 0.91 | 0.78 | 1.05 |
| Other | 0.95 | 0.85 | 1.06 | 1.14 | 0.92 | 1.43 | 0.99 | 0.84 | 1.17 |
| Year of procedure (Reference = 2006) | |||||||||
| 2007 | 0.91 | 0.80 | 1.03 | 0.90 | 0.68 | 1.19 | 0.93 | 0.76 | 1.13 |
| 2008 | 1.02 | 0.90 | 1.16 | 1.10 | 0.84 | 1.44 | 0.92 | 0.75 | 1.12 |
| 2009 | 1.23 | 1.08 | 1.39 | 1.31 | 1.00 | 1.73 | 1.05 | 0.85 | 1.28 |
| 2010 | 1.13 | 0.99 | 1.28 | 1.01 | 0.75 | 1.34 | 1.02 | 0.83 | 1.25 |
| 2011 | 1.05 | 0.93 | 1.20 | 1.07 | 0.81 | 1.43 | 0.93 | 0.75 | 1.15 |
| 2012 | 1.20 | 1.06 | 1.36 | 1.19 | 0.90 | 1.59 | 1.04 | 0.85 | 1.29 |
| 2013 | 1.18 | 1.04 | 1.35 | 1.12 | 0.83 | 1.51 | 1.11 | 0.90 | 1.37 |
| Mean length of hospital stay | 1.12 | 1.12 | 1.12 | 1.07 | 1.07 | 1.08 | 1.09 | 1.09 | 1.09 |
| COMORBIDITIES | |||||||||
| Mean Charlson comorbidity index | 1.00 | 0.99 | 1.01 | 1.03 | 1.00 | 1.05 | 1.01 | 0.99 | 1.03 |
| Smoking | 1.17 | 1.09 | 1.25 | 1.14 | 0.98 | 1.33 | 1.19 | 1.06 | 1.33 |
| Obesity | 1.73 | 1.59 | 1.88 | 1.56 | 1.29 | 1.89 | 1.36 | 1.18 | 1.58 |
Footnotes
Contributors Statement: All authors were involved in attaining data from Premier Perspective Inc. and were involved in designing the study. Nicole Zubizarreta and Isaac Wasserman analyzed data under guidance of Jashvant Poeran and Madhu Mazumdar. All authors contributed to the interpretation of the results, reviewed and approved the final document, and take responsibility for the content of the manuscript. Jashvant Poeran and Nicole Zubizarreta take responsibility for the completeness of the data and the accuracy of the analysis. Jashvant Poeran and Madhu Mazumdar are the study guarantors.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) authors have no support from companies for the submitted work; (2) all authors have no relationships with companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) all authors have no non-financial interests that may be relevant to the submitted work.
References
- 1.Kao LS, Ghaferi AA, Ko CY, Dimick JB. Reliability of superficial surgical site infections as a hospital quality measure. J Am Coll Surg. 2011;213:231–235. doi: 10.1016/j.jamcollsurg.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–397. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Kerwel TG, Leichtle SW, Asgeirsson T, Hendren SK, Cleary RK, Luchtefeld MA. Risk factors for readmission after elective colectomy: postoperative complications are more important than patient and operative factors. Dis Colon Rectum. 2014;57:98–104. doi: 10.1097/DCR.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 4.Ho VP, Stein SL, Trencheva K, et al. Differing risk factors for incisional and organ/space surgical site infections following abdominal colorectal surgery. Dis Colon Rectum. 2011;54:818–825. doi: 10.1007/DCR.0b013e3182138d47. [DOI] [PubMed] [Google Scholar]
- 5.Hendren S, Fritze D, Banerjee M, et al. Antibiotic choice is independently associated with risk of surgical site infection after colectomy: a population-based cohort study. Ann Surg. 2013;257:469–475. doi: 10.1097/SLA.0b013e31826c4009. [DOI] [PubMed] [Google Scholar]
- 6.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 7.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2014;5:Cd001181. doi: 10.1002/14651858.CD001181.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song F, Glenny AM. Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomized controlled trials. Br J Surg. 1998;85:1232–1241. doi: 10.1046/j.1365-2168.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 9.Deierhoi RJ, Dawes LG, Vick C, Itani KM, Hawn MT. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763–769. doi: 10.1016/j.jamcollsurg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Itani KM, Wilson SE, Awad SS, Jensen EH, Finn TS, Abramson MA. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med. 2006;355:2640–2651. doi: 10.1056/NEJMoa054408. [DOI] [PubMed] [Google Scholar]
- 11.Calvert JK, Holt SK, Mossanen M, et al. Use and outcomes of extended antibiotic prophylaxis in urological cancer surgery. J Urol. 2014;192:425–429. doi: 10.1016/j.juro.2014.02.096. [DOI] [PubMed] [Google Scholar]
- 12.Bateman BT, Rassen JA, Schneeweiss S, et al. Adjuvant vancomycin for antibiotic prophylaxis and risk of Clostridium difficile infection after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2013;146:472–478. doi: 10.1016/j.jtcvs.2013.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Poeran J, Yeo H, Rasul R, Opperer M, Memtsoudis SG, Mazumdar M. Anesthesia type and perioperative outcome: open colectomies in the United States. J Surg Res. 2015;193:684–692. doi: 10.1016/j.jss.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 16.Bratzler DW, Houck PM, Richards C, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140:174–182. doi: 10.1001/archsurg.140.2.174. [DOI] [PubMed] [Google Scholar]
- 17.Hendren S, Englesbe MJ, Brooks L, Kubus J, Yin H, Campbell DA., Jr Prophylactic antibiotic practices for colectomy in Michigan. Am J Surg. 2011;201:290–293. doi: 10.1016/j.amjsurg.2010.08.024. discussion 293–294. [DOI] [PubMed] [Google Scholar]
- 18.Eagye KJ, Nicolau DP. Selection of prophylactic antimicrobial agent may affect incidence of infection in small bowel and colorectal surgery. Surg Infect. 2011;12:451–457. doi: 10.1089/sur.2010.108. [DOI] [PubMed] [Google Scholar]
- 19.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 20.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. quiz 133–134; discussion 196. [PubMed] [Google Scholar]
- 21.Gorbach SL. Antimicrobial prophylaxis for appendectomy and colorectal surgery. Rev Infect Dis. 1991;13(Suppl 10):S815–820. doi: 10.1093/clinids/13.supplement_10.s815. [DOI] [PubMed] [Google Scholar]
- 22.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single–center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234:181–189. doi: 10.1097/00000658-200108000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedrick TL, Harrigan AM, Sawyer RG, et al. Defining Surgical Site Infection in Colorectal Surgery: An Objective Analysis Using Serial Photographic Documentation. Dis Colon Rectum. 2015;58:1070–1077. doi: 10.1097/DCR.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 25.Hubner M, Diana M, Zanetti G, Eisenring MC, Demartines N, Troillet N. Surgical site infections in colon surgery: the patient, the procedure, the hospital, and the surgeon. Arch Surg. 2011;146:1240–1245. doi: 10.1001/archsurg.2011.176. [DOI] [PubMed] [Google Scholar]
- 26.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg. 2004;239:599–605. doi: 10.1097/01.sla.0000124292.21605.99. discussion 605–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols RL. Prophylaxis for intraabdominal surgery. Rev Infect Dis. 1984;6(Suppl 1):S276–282. doi: 10.1093/clinids/6.supplement_1.s276. [DOI] [PubMed] [Google Scholar]
- 28.Liu CY, Huang YT, Liao CH, Yen LC, Lin HY, Hsueh PR. Increasing trends in antimicrobial resistance among clinically important anaerobes and Bacteroides fragilis isolates causing nosocomial infections: emerging resistance to carbapenems. Antimicrob Agents Chemoter. 2008;52:3161–3168. doi: 10.1128/AAC.00355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepard J, Ward W, Milstone A, et al. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148:907–914. doi: 10.1001/jamasurg.2013.2246. [DOI] [PubMed] [Google Scholar]
- 30.Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262:416–425. doi: 10.1097/SLA.0000000000001416. discussion 423–415. [DOI] [PubMed] [Google Scholar]
- 31.Solla JA, Rothenberger DA. Preoperative bowel preparation. A survey of colon and rectal surgeons. Dis Colon Rectum. 1990;33:154–159. doi: 10.1007/BF02055549. [DOI] [PubMed] [Google Scholar]
- 32.Markell KW, Hunt BM, Charron PD, et al. Prophylaxis and management of wound infections after elective colorectal surgery: a survey of the American Society of Colon and Rectal Surgeons membership. J Gastrointest Surg. 2010;14:1090–1098. doi: 10.1007/s11605-010-1218-7. [DOI] [PubMed] [Google Scholar]
- 33.Bardakcioglu O, Khan A, Aldridge C, Chen J. Growth of laparoscopic colectomy in the United States: analysis of regional and socioeconomic factors over time. Ann Surg. 2013;258:270–274. doi: 10.1097/SLA.0b013e31828faa66. [DOI] [PubMed] [Google Scholar]
- 34.Kang CY, Chaudhry OO, Halabi WJ, et al. Outcomes of laparoscopic colorectal surgery: data from the Nationwide Inpatient Sample 2009. Am J Surg. 2012;204:952–957. doi: 10.1016/j.amjsurg.2012.07.031. [DOI] [PubMed] [Google Scholar]