Abstract
Alzheimer disease (AD) is the most frequent cause of dementia. AD diagnosis, progression, and treatment have not been analyzed nationwide in China. The primary aim of this study was to analyze demographic and clinical characteristics related to cognitive decline in AD patients treated at outpatient clinics in China.
We performed a retrospective study of 1993 AD patients at 10 cognitive centers across 8 cities in China from March 2011 to October 2014. Of these, 891 patients were followed for more than 1 year.
The mean age at diagnosis was 72.0 ± 10.0 years (range 38–96 years), and the mean age at onset of AD was 69.8 ± 9.5 years. Most patients (65.1%) had moderate to severe symptoms at the time of diagnosis, and mean Mini-Mental State Examination at diagnosis was 15.7 ± 7.7. AD patients showed significant cognitive decline at 12 months after diagnosis. Having more than 9 years of formal education was an independent risk factor related to rapid cognitive decline [odds ratio (OR) = 1.80; 95% confidence interval (95% CI): 1.11–2.91]. Early-onset AD patients experienced more rapid cognitive decline than late-onset patients (OR = 1.83; 95% CI: 1.09–3.06).
Most AD patients in China had moderate to severe symptoms at the time of diagnosis and experienced significant cognitive decline within 1 year. Rapid cognitive decline in AD was related to having a higher educational level and younger age of onset.
Keywords: Alzheimer disease, assessment instrument, risk factors, symptom duration
1. Introduction
Alzheimer disease (AD) is a devastating neurodegenerative illness that is characterized by profound impairment of cognitive function, marked physical disability, and an enormous economic burden for the afflicted individual, caregivers, and society. AD is the most common cause of dementia, accounting for more than 65% of dementia cases in the elderly. Progressive cognitive decline is the main characteristic of AD. However, the rate of cognitive decline in AD varies considerably between individuals, with some patients showing rapid and substantial cognitive decline in a relatively short period of time and others showing little or no change over years.[1,2] Severe cognitive decline during the course of AD has been shown to be associated with a higher mortality rate.[3–5] A number of studies have investigated factors associated with rapid cognitive decline to identify patients who might benefit most from early intervention. Unfortunately, results have been heterogeneous and often contradictory. Therefore, no reliable conclusions can be drawn about factors associated with rapid cognitive decline.[6]
AD diagnosis, progression, and treatment have been analyzed in western countries, but there have been few multicenter studies in China. The primary aim of this study was to analyze the demographic and clinical characteristics that predict cognitive decline in AD patients at 10 outpatient cognitive clinics across 8 cities in China.
2. Methods
2.1. Patients
We conducted a retrospective epidemiological study. From March 2011 to October 2014, we recruited a total of 1993 Chinese patients with AD from 10 hospitals in Tianjin, Beijing, Shanghai, Nanjing, Jinan, Changsha, Yangzhou, and Chongqing, which are large cities in the northern, central, and southwestern regions of China. Other inclusion criteria were a recent diagnosis of probable or possible AD according to the criteria of the National Institute of Neurological and Communicative Diseases and Stroke, the Alzheimer's Disease and Related Disorders Association,[7] and the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision[8]; no diagnosis of major psychiatric disorders, such as major depression or anxiety, or other significant neurological diseases, such as vascular dementia or severe cerebral trauma; and no severe systemic disease in the previous 3 months. The presence of comorbid diseases, such as hypertension, diabetes, and heart disease without heart failure, was allowed, and there were no age restrictions. The exclusion criteria were not meeting the diagnostic criteria for AD; a history of drug abuse; the presence of other factors that contribute to cognitive impairment, including metabolic diseases and inflammatory conditions; neuroimaging evidence of a large cerebral infarction or a large area of cerebral hemorrhage; and severe neurologic deficits, including communication disorders and paralysis of the dominant hand. This retrospective study was approved by the Research Ethics Committees of Tianjin Huanhu hospitals, and caregivers or patients provided written informed consent before participating in the study.
Data collected from medical records included age; sex; level of education; symptom duration; body mass index (BMI); family history; history of hypertension, heart disease, stroke, and diabetes; and smoking and drinking habits. Overall, we followed a total of 891 patients from 6 hospitals every 3 months for at least 12 months (range: 12–32 months).
2.2. Assessment instruments
Primary outcomes of this study included scores on the Chinese version of the Mini-Mental State Examination (MMSE) and the activities of daily living (ADL) assessment. The secondary outcome was the score on the Montreal Cognitive Assessment - Beijing Version (MoCA-BJ). Assessments were performed at 3-month intervals during the follow-up study. The MMSE is used to evaluate orientation, registration, attention and calculation, recall, and language.[9] Scores on the MMSE reflect the number of correct items and range from 0 to 30. The MoCA-BJ is used to evaluate visuospatial executive function, naming, attention, abstraction, language, delayed memory, and orientation[10]; scores range from 0 to 30. Lower scores on the MMSE and MoCA-BJ indicate greater cognitive impairment. Scores on the ADL assessment range from 20 to 80, with higher scores indicating greater impairment.
2.3. Statistical analysis
Descriptive statistics, including frequencies of categorical variables and means and standard deviations of continuous variables, were determined for the entire group at baseline for the following variables: sex, age, marital status, level of education, BMI, past history, family history, smoking and drinking habits, physician's estimate of symptom duration, MMSE score, ADL score, and MoCA-BJ score. Demographic and clinical characteristics were analyzed using analysis of variance and the Kruskal–Wallis test for continuous variables with non-normal distribution and the Chi-square test for categorical variables, including sex; family history; histories of hypertension; diabetes; heart disease; or stroke; and smoking and drinking habits. The paired-sample t test was used to evaluate changes in the MMSE, MoCA-BJ, and ADL scores from baseline to the 6-month and 12-month follow-ups.
MMSE scores that decrease 3 or more points within 12 months from baseline indicate rapid cognitive decline.[11] Logistic regression analyses were used to explore the sociodemographic and clinical factors associated with cognitive decline. Variables with an odds ratio (OR) P value < 0.05 were included in a multivariate analysis to identify sociodemographic and clinical factors that are independently associated with cognitive decline. P values < 0.05 were considered statistically significant. All data were analyzed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY).
3. Results
3.1. Patient demographics
A total of 1993 AD patients (1142 females and 852 males) were recruited for this study. Of these, 891 patients were followed for more than 1 year. Patient demographic and clinical characteristics are summarized in Table 1.
Table 1.
Patient demographic and clinical characteristics by severity of Alzheimer disease (AD).
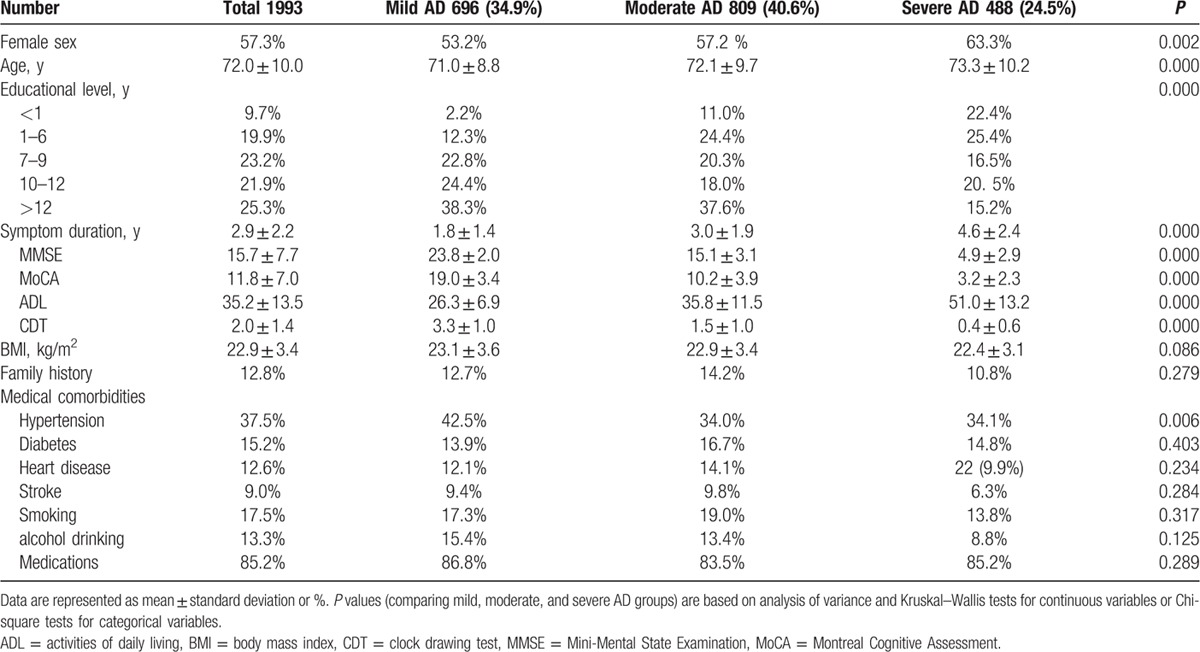
The mean age of the patients was 72.0 ± 10.0 years (range 38–96 years), and the age distribution was as follows: 12.0% were <60 years old; 9.3% were 60 to 64 years old; 14.1% were 65 to 69 years old; 19.4% were 70 to 74 years old; 22.3% were 75 to 79 years old; and 22.7% were ≥80 years old. The mean age at AD onset was 69.8 ± 9.5 years, and 25.3% of the patients were younger than 65 years at the time of onset. Of the patients with AD onset before age 65, 21.5% had a family history of dementia.
Most of the patients had received formal education: 19.9% completed primary school (6 years), 23.2% completed secondary school (9 years), 21.9% completed high school (12 years), and 25.3% completed more than 12 years education. Only 9.7% of the patients were illiterate. The average BMI was 22.9 kg/m2. According to Chinese BMI guidelines, 8.5% of the patients were classified as underweight, 54.1% as healthy weight, 27.8% as overweight, and 9.7% as obese.
The mean MMSE score at diagnosis was 15.7 ± 7.7. On the basis of MMSE scores and the National Institute for Health and Clinical Excellence guidelines,[12] we classified patients as having mild AD [MMSE 21–26; n = 696 (34.9%)], moderate AD [MMSE 10–20; n = 809 (40.6%)], or severe AD [MMSE <10; n = 488 (24.5%)]. We observed no significant differences among baseline characteristics in any of the AD groups, including BMI; family history; histories of diabetes, heart disease, or stroke; and smoking and alcohol drinking. We observed significant differences with respect to sex (P = 0.002), age (P < 0.001), educational level (P < 0.001), and history of hypertension (P = 0.006) among the AD groups. In the mild AD group, only 2.2% were illiterate, and 38.3% completed university; in the severe AD group, 22.4% were illiterate, and 15.2% completed university. As expected, symptom duration was also significantly different among the AD groups (P < 0.001) with longer symptom duration in more severe AD: 1.8 ± 1.4 years in mild AD, 3.0 ± 1.9 years in moderate AD, and 4.6 ± 2.4 years in severe AD.
3.2. Relevance of anti-dementia drug selection
All patients in this study received their first AD diagnosis in the hospital and did not receive any treatment to improve cognition before their diagnosis. Patients (14.8%) were considered untreated if they received AD treatment for less than 3 months or received no AD treatment after diagnosis. The decision not to receive treatment was influenced by economic burden, family dispute/conflict, and fear of adverse drug reactions. Treated patients received 1 of 5 anti-dementia drug treatments: donepezil (43.7%), memantine (13.4%), combination of donepezil and memantine (13.0%), exelon (10.4%), or combination of exelon and memantine (4.7%).
3.3. Follow-up evaluations
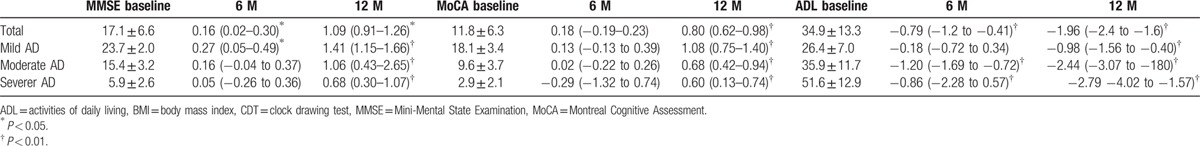
Of the 1152 AD patients in the study across 6 centers, 891 patients completed more than 1 year of follow-up. Of the 261 patients who were lost to follow-up, 11 died and 250 could not be located, had moved, were too ill to be examined, or refused to be followed. Changes in assessment instrument scores during the 12-month follow-up period are presented in Table 2. AD patients showed stable cognitive status on the MoCA-BJ and a slight cognitive decline on the MMSE at the 6-month follow-up, then significant cognitive decline on the MMSE at the 12-month follow-up. ADL scores increased (worsened) significantly from baseline to the 6- and 12-month follow-ups in the whole population, as well as in moderate and severe AD patients. In the mild AD group, ADL scores were stable at the 6-month follow-up, but increased at the 12-month follow-up.
Table 2.
Mean changes in Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and activities of daily living (ADL) scores in Alzheimer disease (AD) patients at 6 and 12 months of follow-up.
3.4. Risk factors related to cognitive decline
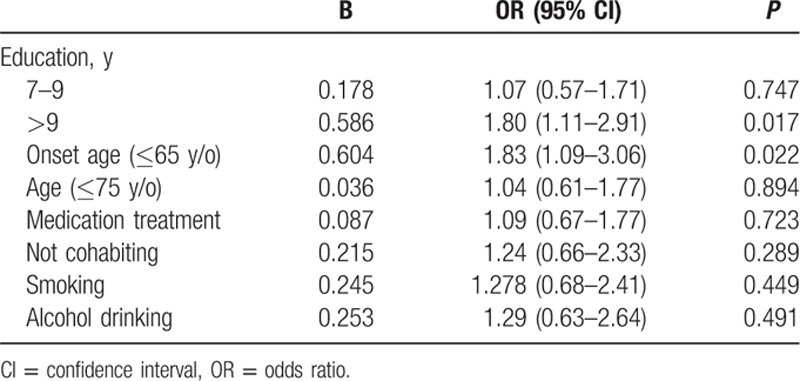
Of the 891 AD patients who were followed for more than 1 year (range, 12–36 months), 767 had mild or moderate AD (MMSE ≥10 at baseline) and were used to analyze risk factors for rapid cognitive decline. Table 3 summarizes the demographic and clinical characteristics of these 767 mild and moderate patients. Table 4 summarizes ORs for rapid cognitive decline, defined as a decrease of ≥3 MMSE points in 1 year. Rapid cognitive decline was associated with educational level, AD onset age, marital status, smoking, and alcohol drinking.
Table 3.
Patient demographic and clinical characteristics of mild and moderate Alzheimer disease (AD) with more than 12 months of follow-up.
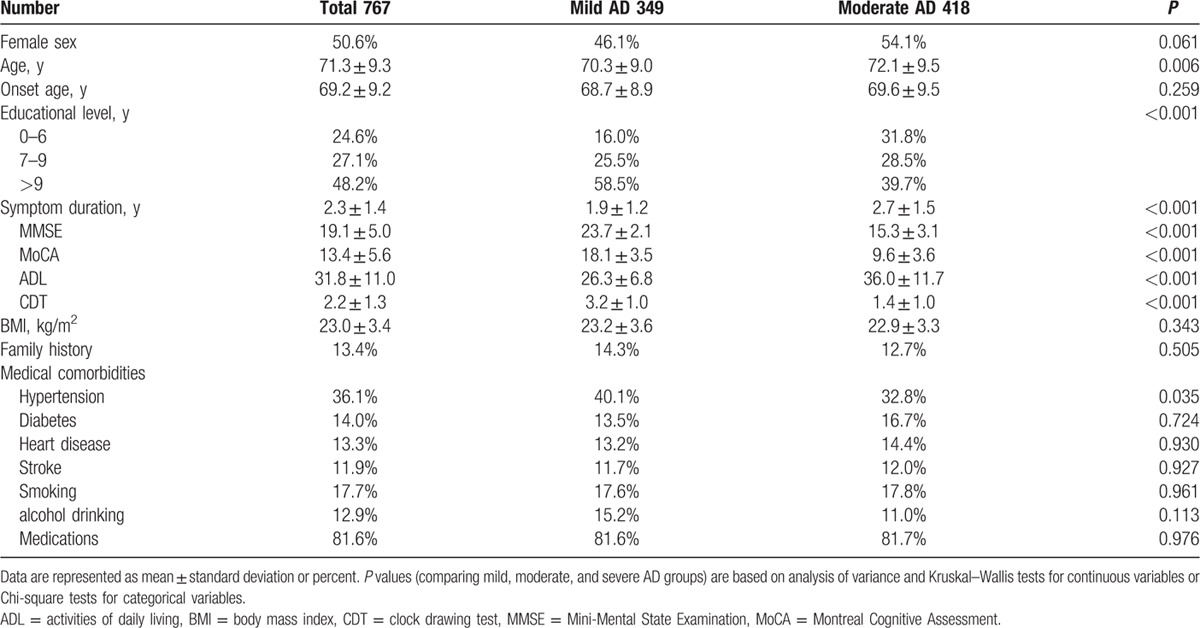
Table 4.
Univariate analysis of variables significantly associated with rapid cognitive decline in Alzheimer disease (AD) patients.
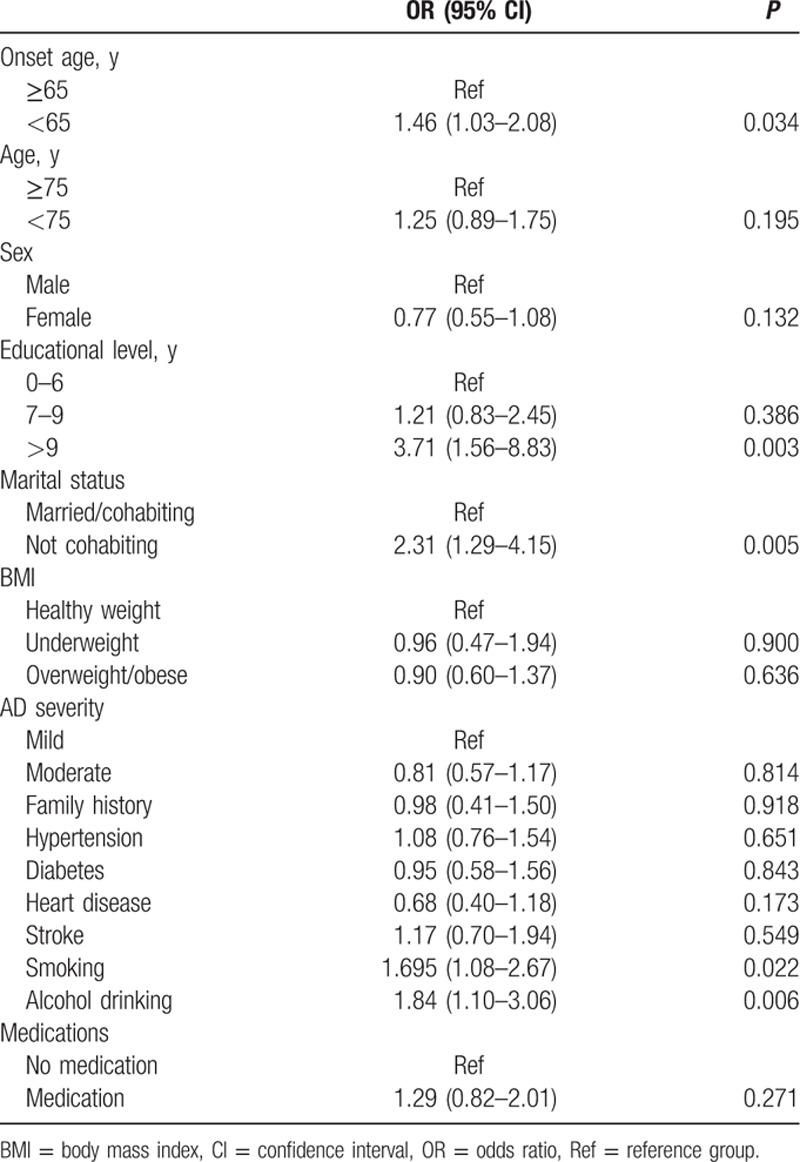
In multivariate analysis for adjusted ORs, having more than 9 years of formal education (adjusted OR = 1.80; 95% CI: 1.11–2.91) and onset age younger than 65 years (adjusted OR = 1.83; 95% CI: 1.09–3.06) remained independent risk factors for cognitive decline (Table 5). However, not cohabiting, smoker, alcohol drinking were no longer significant risk factors.
Table 5.
Multivariate analysis of variables significantly associated with rapid cognitive decline in Alzheimer disease (AD) patients.
4. Discussion
Mortality has been declining among the old, causing an increase in the proportion of older individuals in China and worldwide, especially in major urban areas. In 2006, a study of 4 major cities in China reported an AD prevalence of 4.8%.[13] In 2012, research from a rural area in Tianjin reported an AD prevalence of 5.4%.[14] However, recent multicenter research has indicated that AD prevalence is underestimated in low-income and middle-income countries, including China, and new estimates of AD prevalence in China are 6.5% in rural areas and 7.6% in urban areas.[15] In western countries, the prevalence of AD increases with age, reaching approximately 30% by 80 years of age. The incidence of AD may be lower in men and in persons of African and Asian descent.[16] In our study, 12% of AD patients were younger than 60 years, and the percentage of patients with AD increased by 4.8%, 5.3%, and 3.9% every 5 years between the ages of 60 and 80 years. More than half (57.3%) of our AD patients were women, and 63.3% of patients with severe AD were women.
Progressive cognitive decline is a fundamental characteristic of AD.[17] In this retrospective study, the mean time from onset of symptoms to diagnosis was 2.9 years, and most patients were diagnosed with moderate (40.6%) or severe (24.5%) AD. Only 34.9% were diagnosed with mild AD. Similarly, a previous multicenter study in China reported a mean time from onset to diagnosis of 1 year, and 67% of patients were diagnosed with moderate to severe AD.[18] These results indicate that most patients with mild AD in China either do not recognize their cognitive decline or choose not to see a doctor in the initial stages of the disease. This may be another reason for underestimation of AD prevalence in China.
Treatments used by patients in our retrospective study included donepezil (43.7%), memantine (13.4%), exelon (10.4%), and combinations of 2 of these medications (17.4%). A small proportion (14.8%) of patients did not receive any anti-dementia treatment. On the basis of MMSE and MoCA-BJ scores, AD patients showed stable cognitive status after 6 months of follow-up, but significant cognitive decline by 12 months of follow-up. These results are consistent with previous studies of treatment with acetylcholinesterase inhibitors.[19–21] ADL scores increased (worsened) significantly from baseline to the 6 and 12-month follow-ups. These findings indicate that progressive cognitive decline is a fundamental characteristic of AD that occurs even with treatment. The multivariate analysis also showed that medication could not improve the rapid cognitive decline among our patients. However, this study was not a case–control study designed to assess efficacy of interventions, and other factors were not controlled. More research is needed to better identify AD patients at an increased risk of rapid cognitive decline and to assess the effect of pharmacological interventions in this population.
In our study, only 9.7% of AD patients were illiterate, and 25.3% had more than 12 years of formal education. In contrast, epidemiology studies in mainland China[13,22] found 36.1% and 30.1% of AD patients to be illiterate and only 11.5% and 9.2% to have more than 12 years formal education. On the basis of the dementia prevalence in these studies, there should be a greater number of dementia patients in China without formal education than with more than 12 years of formal education. We hypothesize that patients with more education are more aware of their cognitive decline and more willing to seek diagnosis and treatment.
Several studies have addressed the role of education on progression of AD. In our study, having more than 9 years of formal education was an independent risk factor predicting cognitive decline in AD patients. These results are consistent with other studies. In a longitudinal cohort of 154 newly diagnosed AD patients, Musicco et al[23] demonstrated that AD patients with at least 9 years of formal education had a 2-fold risk of rapid cognitive decline. Many other studies have found similar results,[24–29] leading to the hypothesis that education and occupational attainment provide a sort of “cognitive reserve,” protecting individuals from the early clinical manifestations of AD, followed by more rapid deterioration as the disease progresses. Imaging studies have suggested that given comparable clinical severity of dementia, AD pathology is more advanced in patients with higher educational and occupational attainment.[30–32] Another study showed AD patients with higher education have more rapid cortical atrophy.[33] When more educated patients were aware of their cognitive decline, they were classified clinically as mild or moderate stage AD, but their pathological changes were already advanced. This suggests that there is a tipping point when cognitive reserve can no longer mask the underlying AD pathology, and cognitive abilities decline more rapidly.
In our study, early-onset AD patients were more likely to show rapid cognitive decline than late-onset patients. A study by Ito et al[34] showed that younger subjects had a more rapid decline in cognitive function than older subjects. In a longitudinal study of 1062 patients with possible or probable AD, O’ Hara et al[35] found that being ≤75 years old at the clinic visit was associated with a greater risk for rapid decline. Many other papers have replicated these findings.[27,36–40] This observation suggests that early-onset AD, which includes more cases of familial AD, may have a worse prognosis than sporadic cases. Of the patients with AD onset before age 65, 21.5% had a family history of dementia. However, all of the patients were from different families, and only 1 was suspected to have familial AD (PSEN1 mutation) based on an onset age of 43 and having a parent with dementia. Genotyping was only performed on a subset of these patients with AD onset before age 65. Thus, other forms of familial AD could not be excluded.
This multicenter retrospective study had some limitations. First, not all centers completed the 3-month interview follow-up, and 12-month follow-up data were therefore only available for 891 patients. Fortunately, this was a sufficient sample size to analyze risk factors. The short follow-up period of 12 months was second limitation; a longer study might reveal additional risk factors related to cognitive decline in AD patients. Another limitation was the selection bias inherent to a retrospective study; it was not possible to match cognitive scores across different educational levels. Although we analyzed risk factors in mild and moderate AD groups, the mild AD group had a higher educational level than the moderate group, and there was no association of AD severity with rapid cognitive decline. We will continue to follow these patients in future research with a special focus on patients with mild and moderate AD. Finally, a more detailed longitudinal study of disease course and outcomes beyond cognitive function and ADLs would be informative, but this was outside the scope of the current study.
This retrospective study analyzed the diagnosis, clinical characteristics, and medical treatment of AD in 8 large cities in China. Approximately two-thirds of AD patients displayed moderate or severe symptoms at the time of initial diagnosis by neurologists. Cognition, assessed by MMSE and MoCA-BJ scores, was stable at 6 months after diagnosis, but declined by 12 months. Functionality, assessed by ADL, also declined significantly by 12 months. Onset age was ≤65 years and having more than 9 years of formal education were independent risk factors predicting rapid cognitive decline in AD patients.
Footnotes
Abbreviations: AD = Alzheimer disease, ADL = activities of daily living, BMI = body mass index, CDT = clock drawing test, CI = confidence interval, MMSE = Mini-Mental State Examination, MoCA-BJ = Montreal Cognitive Assessment - Beijing Version, OR = odds ratio.
Authorship: Dantao Peng, Zhihong Shi, Jun Xu, Lu Shen, and Shifu Xiao contributed equally to this work.
Funding: The study was supported by Tianjin Science and Technology Support Programs (grant numbers: 12ZCZDSY02900 and 13ZCZDSY01600), Science and Technology Project of Tianjin Municipal Health Bureau (grant number: 2014KR10), and the Tianjin Natural Science Foundation (grant number: 13JCYBJC21300).
The authors declare that they have no conflicts of interest. All authors had full access to study data, and the corresponding author had final responsibility for the decision to submit the manuscript for publication. The funding agency had no involvement in study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1.Capitani E, Cazzaniga R, Francescani A, Spinnler H. Cognitive deterioration in Alzheimer's disease: is the early course predictive of the later stages? Neurol Sci 2004; 25:198–204. [DOI] [PubMed] [Google Scholar]
- 2.Doody RS, Massman P, Dunn K. A method for estimating progression rates in Alzheimer's disease. Arch Neurol 2001; 58:449–454. [DOI] [PubMed] [Google Scholar]
- 3.Schupf N, Tang MX, Albert SM, et al. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology 2005; 65:1218–1226. [DOI] [PubMed] [Google Scholar]
- 4.Schupf N, Costa R, Tang MX, et al. Preservation of cognitive and functional ability as markers of longevity. Neurobiol Aging 2004; 25:1231–1240. [DOI] [PubMed] [Google Scholar]
- 5.Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol 2000; 151:676–688. [DOI] [PubMed] [Google Scholar]
- 6.Sona A, Ellis KA, Ames D. Rapid cognitive decline in Alzheimer's disease: a literature review. Int Rev Psychiatry 2013; 25:650–658. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34:939–944. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; ed 4, Text Revision. 2000; Washington: American Psychiatric Association, 157–158. [Google Scholar]
- 9.Dick JP, Guiloff RJ, Stewart A, et al. Mini-mental state examination in neurological patients. J Neurol Neurosurg Psychiatry 1984; 47:496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 2012; 12:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carcaillon L, Peres K, Pere JJ, et al. Fast cognitive decline at the time of dementia diagnosis: a major prognostic factor for survival in the community. Dement Geriatr Cogn Disord 2007; 24:138–145. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Health and Clinical Excellence. Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer's Disease. NICE Technology Appraisal Guidance 217. 2009. London: NICE; 2011. [Google Scholar]
- 13.Zhang ZX, Zahner GE, Román GC, et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 2005; 62:447–453. [DOI] [PubMed] [Google Scholar]
- 14.Ji Y, Shi Z, Zhang Y, et al. Prevalence of dementia and main subtypes in rural northern China. Dement Geriatr Cogn Disord 2015; 39:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llibre Rodriguez JJ, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet 2008; 372:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke 2004; 35:2620–2622. [DOI] [PubMed] [Google Scholar]
- 17.Gelb DJ. Measurement of progression in Alzheimer's disease: a clinician's perspective. Stat Med 2000; 19:1393–1400. [DOI] [PubMed] [Google Scholar]
- 18.Peng D, Yu P. Survey of Alzheimer disease treatment status and the effect of donepezil [Chinese]. Chinese J Geriatr 2010; 29:691–693. [Google Scholar]
- 19.Molinuevo JL, Berthier ML, Rami L. Donepezil provides greater benefits in mild compared to moderate Alzheimer's disease: implications for early diagnosis and treatment. Arch Gerontol Geriatr 2011; 52:18–22. [DOI] [PubMed] [Google Scholar]
- 20.Perera G, Khondoker M, Broadbent M, et al. Factors associated with response to acetylcholinesterase inhibition in dementia: a cohort study from a secondary mental health care case register in London. PLoS One 2014; 9:e109484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behl P, Edwards JD, Kiss A, et al. Treatment effects in multiple cognitive domains in Alzheimer's disease: a two-year cohort study. Alzheimers Res Ther 2014; 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement 2014; 10:1–9. [DOI] [PubMed] [Google Scholar]
- 23.Musicco M, Salamone G, Caltagirone C, et al. Neuropsychological predictors of rapidly progressing patients with Alzheimer's disease. Dement Geriatr Cogn Disord 2010; 30:219–228. [DOI] [PubMed] [Google Scholar]
- 24.Roselli F, Tartaglione B, Federico F, et al. Rate of MMSE score change in Alzheimer's disease: influence of education and vascular risk factors. Clin Neurol Neurosurg 2009; 111:327–330. [DOI] [PubMed] [Google Scholar]
- 25.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rate of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry 2006; 77:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 1999; 53:1942–1947. [DOI] [PubMed] [Google Scholar]
- 27.Teri L, McCurry SM, Edland SD, et al. Cognitive decline in Alzheimer's disease: a longitudinal investigation of risk factors for accelerated decline. J Gerontol A Biol Sci Med Sci 1995; 50:M49–M55. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Li Y, Aggarwal N, et al. Education and the course of cognitive decline in Alzheimer disease. Neurology 2004; 63:1198–1202. [DOI] [PubMed] [Google Scholar]
- 29.Rasmusson DX, Carson KA, Brookmeyer R, et al. Predicting rate of cognitive decline in probable Alzheimer's disease. Brain Cogn 1996; 31:133–147. [DOI] [PubMed] [Google Scholar]
- 30.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol 1992; 32:371–375. [DOI] [PubMed] [Google Scholar]
- 31.Stern Y, Alexander GE, Prohovnik I, et al. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology 1995; 45:55–60. [DOI] [PubMed] [Google Scholar]
- 32.Alexander GE, Furey ML, Grady CL, et al. Association of premorbid function with cerebral metabolism in Alzheimer's disease: implications for the reserve hypothesis. Am J Psychiatry 1997; 154:165–172. [DOI] [PubMed] [Google Scholar]
- 33.Cho H, Jeon S, Kim C, et al. Higher education affects accelerated cortical thinning in Alzheimer's disease: a 5-year preliminary longitudinal study. Int Psychogeriatr 2015; 27:111–120. [DOI] [PubMed] [Google Scholar]
- 34.Ito K, Corrigan B, Zhao Q, et al. Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement 2010; 1:10. [DOI] [PubMed] [Google Scholar]
- 35.O’ Hara R, Thompson J, Kraemer H, et al. Which Alzheimer patients are at risk for rapid cognitive decline? J Geriatr Psychiatry Neurol 2002; 15:233–238. [DOI] [PubMed] [Google Scholar]
- 36.Ho GJ, Hansen L, Alford M, et al. Age at onset is associated with disease severity in Lewy body variant and Alzheimer's disease. Neuroreport 2002; 13:1825–1828. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs D, Sano M, Marder K, et al. Age at onset on Alzheimer's disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology 1990; 40:8–14. [DOI] [PubMed] [Google Scholar]
- 38.Lucca U, Comelli M, Tettamanti M, et al. Rate of progression and prognostic factors in Alzheimer's disease: a prospective study. J Am Geriatr Soc 1993; 41:45–49. [DOI] [PubMed] [Google Scholar]
- 39.Neumann PJ, Araki S, Arcelus A, et al. Measuring Alzheimer's disease progression with transition probabilities. Estimates from CERAD. Neurology 2001; 57:957–964. [DOI] [PubMed] [Google Scholar]
- 40.Sluimer JD, Vrenken H, Blankenstein M, et al. Whole-brain atrophy rate in Alzheimer disease: identifying fast progressors. Neurology 2008; 70:1836–1841. [DOI] [PubMed] [Google Scholar]


