Abstract
Susceptibility-weighted MRI (SWI) is sensitive to T2∗ effects and mineralization.
We investigated differences in the extrapyramidal brain structures on SWI between Parkinson disease (PD) and postural instability gait disorder (PIGD) patients and correlated the SWI values with the degree of gait dysfunction.
Forty patients diagnosed with PD and PIGD underwent 3 Tesla magnetic resonance imaging (MRI) brain study. An SWI sequence (TE/TR/FA 20/33/15) was used. Ten regions of interest were placed in the midbrain and basal ganglia by 2 independent raters blinded to subject data and quantitatively evaluated.
The inter-rater reliability between the raters was excellent (interclass correlation coefficient >0.8). The SWI intensity values in all regions were on average lower in PIGD than in PD patients, with the lowest results found in globus pallidus.
Multivariate analysis showed a lower SWI hypointensity in the putamen and globus pallidus in PIGD compared with PD patients, with a similar trend for the other basal ganglia nuclei. Pearson correlation analysis showed a statistically significant positive correlation between SWI putaminal hypointensity and the Tinetti total score (r = 0.39, P = 0.01) in both PD and PIGD.
SWI putaminal hypointensity may be a useful imaging marker in prospective evaluation for clinical progression for Parkinsonian disorders.
Keywords: imaging, Parkinsonian disorders, susceptibility-weighted MRI
1. Introduction
Parkinson disease (PD) is 1 of the most common neurodegenerative disorders, characterized by rest tremor, bradykinesia, rigidity, and postural instability. In addition to the loss and degeneration of the dopamine-containing neurons in the substantia nigra (SN),[1] Parkinsonian disorders have been strongly associated with increased iron content in the basal ganglia. Although it is still unknown if the increase in iron content is primary or secondary to the disease, brain structures with naturally higher iron content, namely SN, red nucleus (RN), putamen (PUT), globus pallidus (GP), and caudate nucleus (CA), seem to be more vulnerable to iron deposition in neurodegenerative disorders such as PD.[2–4]
Postural instability and gait disorder (PIGD), a subtype of PD, is associated with a rapid rate of disease progression and a highly increased risk of dementia (when converted from tremor dominant PD to the PIGD subtype).[5,6] However, the pathophysiologic differences between PD and PIGD remain unclear. Postmortem studies suggest that PIGD is associated with more varied pathologies such as vascular insults beyond the SN.[7] Patients who present initially with predominant postural instability with Parkinsonism such as bradykinesia and rigidity pose a diagnostic challenge as it is not clear if they behave like typical PD patients. Most of them respond to some extent to levodopa. Presence of any supporting imaging marker to differentiate PIGD from typical PD would be clinically useful for diagnosis and for clinical evaluation and prognostication.
Iron is essential for physiologic processes in the brain, such as facilitating cellular aerobic metabolism. Aside from the iron in hemoglobin, the 2 most important forms of nonheme iron are transferrin for transportation and ferritin for storage.[8] However, iron can accumulate as a consequence of abnormal regulation, leading to an overload of ferrous iron which reacts with hydrogen peroxide. The resulting hydroxyl radicals will cause oxidant stress and subsequently lead to the apoptosis of the neurons.[8–10] Furthermore, iron seems to induce conformational changes and aggregation of alpha-synuclein which is the primary component of Lewi bodies.[11] However, the cause and effect relationship between iron and neurodegeneration is still unclear.
Susceptibility-weighted imaging (SWI) is an MR sequence that measures magnetic susceptibility differences.[8–12] It is a 3-dimensional, high spatial resolution, fully flow-compensated gradient echo sequence that contains both magnitude and phase information. Due to their large magnetic moment and adequate stores in the brain, ferritin, hemosiderin, and deoxyhemoglobin influence the MR signal in SWI. These paramagnetic substances increase the magnetic field and therefore create a positive phase shift compared with the surrounding tissues. A direct correlation between brain iron concentration and SWI phase shifts has been shown in several studies,[8,13,14] enhancing SWI contrast. SWI is also highly sensitive for the depiction of cerebral microbleeds, which are caused by small vessel diseases.[15]
We hypothesize that there is differential damage of the brain structures due to iron deposition between PD and PIGD and magnetic resonance imaging (MRI) SWI sequences may be a useful and sensitive imaging tool for these patients. In this study, we investigated if the extrapyramidal brain structures are differentially affected on SWI in PD and PIGD patients and if the differences correlate with the clinical gait scores.
2. Materials and methods
This study was approved by the institutional ethics committee (year 2006, grant number 2006/007/c) and informed consent was obtained from every study subject.
2.1. Subjects
Patients diagnosed with PD and PIGD were recruited from the Movement Disorders Clinic of a tertiary hospital. The subjects were part of a cohort who had participated in a previous imaging study.[16] The diagnosis of PD was made in patients who presented initially with rest tremor, bradykinesia and rigidity based on the United Kingdom PD Brain Bank clinical criteria, by a neurologist specializing in PD. Parkinsonian patients who presented initially with predominant postural instability, frequent falls, freezing, and walking difficulty were defined as PIGD Parkinsonism following criteria used in literature.[5] Information on age, gender, age of onset of disease, family history, and severity of PD (Hoehn and Yahr scale [H&Y] stage 1; unilateral involvement, stage 2; bilateral involvement with no postural instability, stage 3; bilateral involvement with postural instability, stage 4; wheelchair bound, stage 5; bedbound) were collected, tabulated, and analyzed. Exclusion criteria included those with features of progressive supranuclear palsy and other neurodegenerative disorders, history of head injury, encephalitis, exposure to neuroleptic drugs, those with life threatening medical conditions or evidence of organ failure. All subjects underwent gait assessment using the Tinetti score (balance, gait, and total) on the same day as the MRI study of the brain. The Tinetti score is a performance-oriented mobility test, which evaluates physical, cognitive, and psychologic ability by direct observation and gives information about the risk for falls (25–28 = low fall risk, 19–24 = medium fall risk, and <19 = high fall risk).[17]
2.2. MRI
The MRI examinations were performed on a 3 Tesla MR system (TrioTim, Siemens, Erlangen, Germany) using a 12-channel matrix head coil as previously described.[16] The following MR sequences were obtained: T1 MPRAGE (TE/TR/TI = 3/2200/900, matrix = 240/256, isovoxel 0.9 mm in all 3 directions), DTI (TE/TR = 86/8200, b = 0, 800), T2 TSE TRA (TE/TR = 84/453, FOV = 240, matrix = 448, slice thickness = 2 mm), FLAIR (TE/TR/TI = 74/7150/2535, FOV = 240, slice thickness = 4 mm), SPACE 3D (TE/TR/TI = 198/6850/450, slice thickness = 4 mm), and fMRI, TOF 3D (TE/TR = 3,9/22, 4 slabs). The SWI sequence comprises of the following parameters: TE/TR = 20/33, flip angle = 15, bandwidth = 120, FOV = 240, matrix = 256, slice thickness = 4 mm, and number of slices = 32. Flow compensation was applied. The images were acquired in the transaxial plane, parallel to the anterior/posterior commissure (AC–PC) line. Images were reviewed on a commercially available Leonardo workstation (Siemens).
2.3. Image analysis
The MR images were read by a neuroradiologist for the presence of infarcts and hemorrhages. Quantitative image analysis on the SWI images was performed by 2 independent raters, blinded to subject data. Six regions of interest (ROIs) were drawn bilaterally according to the anatomical structures, in the midbrain (SN and RN), basal ganglia (PUT, GP, and head of caudate [CA]) and thalamus (TH) on the slice where they were visualized best. In addition, 4 small circular ROIs (5 cm2) were drawn in the 4 quadrants of the PUT (anterior-medial, anterior-lateral, posterior-medial, and posterior-medial) to account for the inhomogenity of its mineralization. The T2-weighted and FLAIR +_ FA DTI images were used in conjunction for better localization and delineation of the structures. The average signal intensity values and slice position for each ROI were recorded on each side. The presence of microbleeds inside the ROIs or elsewhere in the brain was noted as well as infarcts of the basal ganglia.
2.4. Statistical analysis
The data were analyzed using the Statistical Package for Social Science software (Version 17.0. Chicago: SPSS Inc.) for Windows. The interclass correlation coefficient (ICC) for inter-rater reliability was obtained for the SWI ROIs for every structure to ascertain reproducibility of the results between the 2 raters. A 2-sample chi-squared test for categorical variables and t test for continuous variables were used to compare the demographic and SWI ROI data. A multivariate logistical regression analysis was performed with SWI ROI as the dependent variable and with age, gender, and duration of disease as independent variables. P values of <0.05 were considered statistically significant. Clinical correlation of SWI ROI values with the 2 subsets of the Tinetti score (balance and gait) and the Tinetti total score was done using Pearson correlation.
3. Results
3.1. Patients
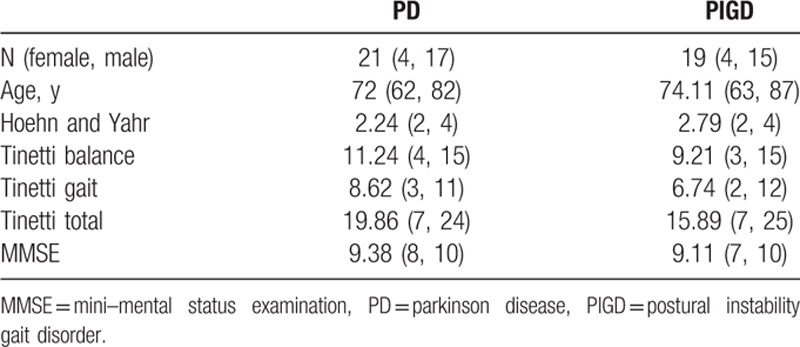
Forty patients (21 PD and 19 PIGD) participated in the study. The mean age of the PD and PIGD patients was 72 and 74 years, respectively. There were 4 female patients in each group, compared with 17 PD and 15 PIGD male patients. In the Tinetti score, PD patients scored on average 11.24, 8.62, and 19.86 points for balance, gait, and total, respectively, while patients with PIGD obtained lower scores in all 3 categories (9.21, 6.74, and 15.89, respectively) (P = 0.01). On the H&Y patients scored between 2 and 4, the average values being 2.24 for the PD patients and 2.79 for the PIGD patients (Table 1).
Table 1.
Demographics of subjects.
3.2. SWI analysis
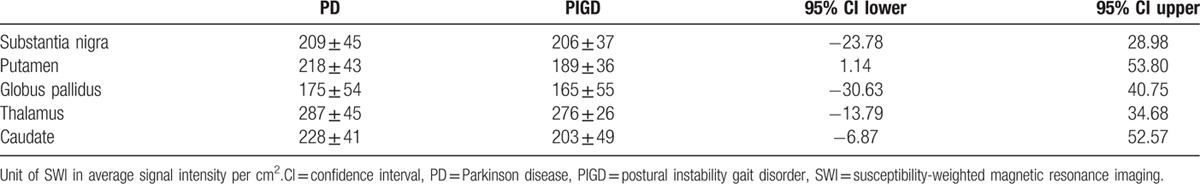
ICC showed good inter-rater reliability of 0.80 and above as detailed: SN 0.80, RN 0.92, PUT 0.95, GP 0.96, TH 0.99, CA 0.88, anterior-medial PUT 0.91, anterior-lateral PUT 0.8, posterior-medial PUT 0.83, and posterior-lateral PUT 0.8. For final statistical computation, an average signal intensity of the right and left ROI for each structure from Rater 1 was used. SWI intensity values in all regions were on average lower in PIGD than in PD patients with the lowest results found in GP. All the ROIs studied are shown in Fig. 1. The values (mean ± standard deviation) are summarized in Table 2 (unit of SWI in average signal intensity per cm2).
Figure 1.
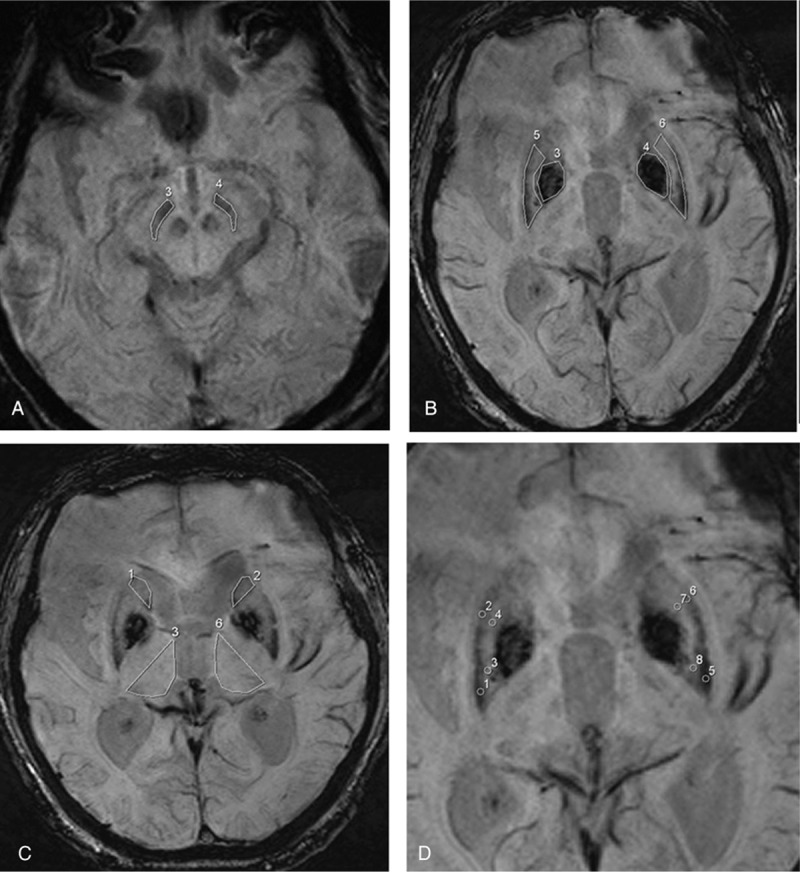
Axial susceptibility-weighted magnetic resonance imaging illustrating the region of interest placements in the (A) substantia nigra in the midbrain, (B) putamen (numbered 5,6) and globus pallidus (numbered 3,4), (C) head of caudate nucleus (numbered 1,2) and thalamus (numbered 3,6), and the 4 subregions of the putamen (D).
Table 2.
Mean SWI intensity in PD vs PIGD.
SN was best visualized 4 to 8 mm caudal to the AC–PC line, while in most cases PUT and GP are well seen on the same slice as the AC–PC line and TH and CA 4 mm above it.
It was noted that in some subjects the pulvinar was mineralized. Surprisingly, this was mostly the case in controls (12 out of 20), while there were fewer in the PD and PIGD group (6 out of 21 and 7 out of 19, respectively).
Multivariate analysis taking into account age, gender, and duration of disease (3 PD and 1 PIGD did not have exact disease duration and not included) showed statistically significant differences for the PUT (P = 0.04) and GP (P = 0.04) between PD and PIGD patients. The results for SN, RN, TH, and CA were not significant (Table 3).
Table 3.
Logistic regression in of SWI values in PD vs PIGD.
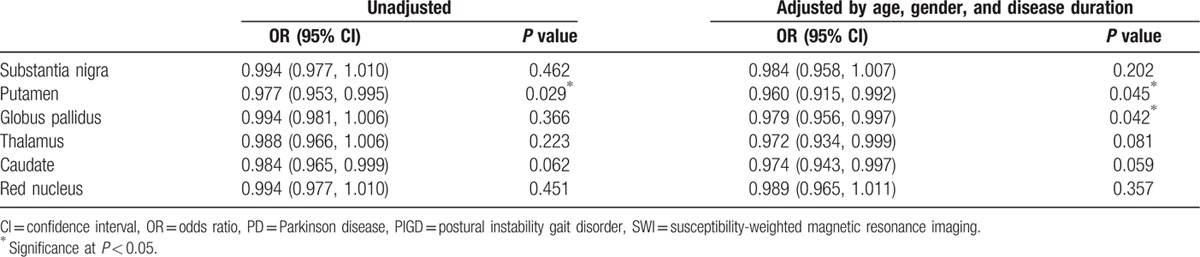
For the 4 subregions of the PUT, no significance was found though there was a trend toward significance in the lateral- and medial-posterior regions (both P = 0.08). The lateral- and medial-anterior regions showed a P value of 0.19 and 0.13, respectively.
3.3. Clinical correlation
Pearson correlation analysis showed that there was a statistically significant positive correlation between putaminal hypointensity and the Tinetti balance (r = 0.422, P = 0.007) and total score (r = 0.389, P = 0.013) in all the test subjects (PD and PIGD) as a group (Fig. 2). There was also a positive correlation found in the PIGD patients alone but this was not statistically significant (r = 0.354, P = 0.137).
Figure 2.
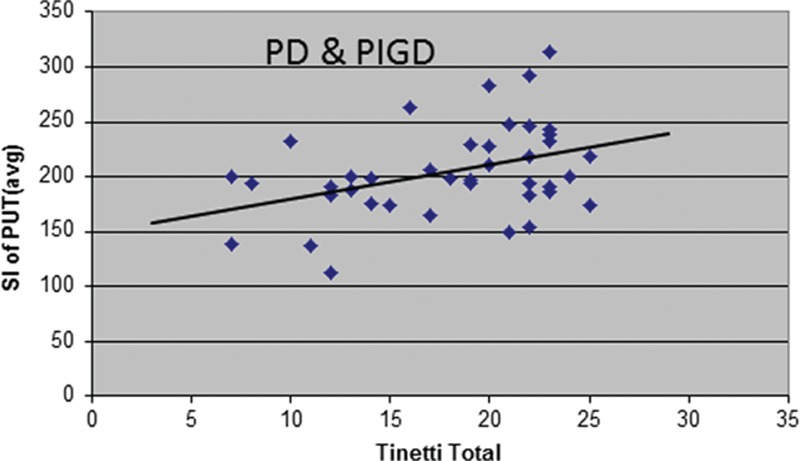
Scatter plots showing the clinical correlation between susceptibility-weighted magnetic resonance imaging intensity in the putamen and the Tinetti total score for all patients.
4. Discussion
In this study, we found that PIGD showed significantly lower intensities in the SWI MRI sequence in the PUT and GP than PD. Similarly, the SWI values in all the other structures also demonstrated lower intensity values even though the differences did not reach statistical significance (Table 3).
Different MRI techniques such as T2, T2∗, and T2’ have been used to visualize PD and brain mineralization. T2-weighted sequences use the transverse relaxation time of protons and the transverse relaxation rate (R2). R2 can be increased by iron and other paramagnetic molecules but is strongly influenced by the water concentration of a tissue, decreasing the MR signal. This is a major drawback in diseases such as PD where affected structures are likely to accumulate water as a result of neuronal loss.[8] SWI is a gradient echo sequence which uses R2’ (magnitude) and phase information. R2’ was able to show the increased iron content in the SN in PD which is validated by autopsy studies.[18,19] Additionally, R2’ and phase are not influenced by water content.
Low signal intensities in SW imaging are correlated with iron accumulation,[8] which is found in PD. Dysfunctional iron regulation and aggregation leads to oxidative stress and cell death. Whether this is primary or secondary to the disease remains controversial and whether there are other pathological mechanisms involved in PIGD as compared to PD still needs further evaluation. Other metals like zinc can have similar effects on the neurons but cannot be visualized with SWI.[20] Clinical differences between PD and PIGD could partly be due to differences in the composition of brain mineralization and may be a reason why a radiological correlate could not be found in other structures aside from the PUT. Brain mineralization is not only related to diseases such as PD but also comes physiologically with aging.[21] Therefore, we adjusted the multivariate analysis for age as well as gender and vascular risk factors. The latter can influence symptoms and severity in patients with Parkinsonism.[22] After the adjustment, we still found a statistically significant difference in the PUT for PD and PIGD.
PIGD is a subtype of PD which is associated with a more rapid disease progression and the development of dementia. It is also less sensitive to L-Dopa treatment. We showed that a low SWI value in PUT could be used as a supportive imaging marker in addition to clinical assessment.
A study by Aramaki et al[23] suggests that difficult movements activate the PUT and this in turn facilitates the basal ganglia-thalamocortical circuit associated with gait initiation and improved initial motor performance. Ouchi et al[24] found that dopamine transporter availability is reduced in the nigrostriatal projection by gait exercises while in PD patients it was reduced in the caudate and orbitofrontal cortex. The results suggest that the PUT is most severely affected in gait.
There are a few other studies using SWI to distinguish different types of Parkinsonian disorders. Gupta et al[25] concluded that SWI was able to differentiate between PD, multiple system atrophy—Parkinson variant (MSA-P), and progressive supranuclear palsy (PSP). Hwang et al[26] and Meijer et al[27] showed that SWI can visualize putaminal atrophy and putaminal hypointensity has high specificity in differentiating MSA-P from PD. Wang et al[28] found that using SWI MSA-P shows higher putaminal as well as pulvinar iron than PD. Since both PIGD and MSA-P seem to show high putaminal iron accumulation, the mineralization of the pulvinar might be a means to differentiate. In our study, the pulvinar showed no more mineralization in PIGD patients than in PD patients or controls. Nonetheless, the studies used different imaging parameters and therefore are not fully comparable. Further studies on this subject will provide further insights.
We also found a positive correlation between SWI hypointensity and Tinetti balance and total score (which assess the severity of imbalance and risk of falls), suggesting that it may be useful for prospective evaluation of clinical progression. Furthermore, SWI is a simple sequence which is easy to collect and evaluate by both radiologists as well as neurologists. SWI measurements can also be utilized for other research to differentiate PD from PIGD.
Our study has some limitations. A longitudinal study would be needed to explore the development of the brain mineralization over time. A bigger sample size could show if the trend shown in the SN, caudate nucleus, and TH can be verified. We cannot totally exclude the possibility that PSP and other neurodegenerative features may develop in those with PIGD over time. Quantitative SWI values may not be easily applied in a busy radiological setting since it may be time consuming. Finally, the potential influence of microbleeds within the ROI or elsewhere in the brain cannot be excluded.
In conclusion, we demonstrated for the first time that SWI values in PIGD patients were lower in PUT and GP compared with PD, providing new clues to the pathophysiologic differences of these conditions. We also showed that there was a positive correlation between the SWI putaminal hypointensity and the Tinetti score in PD and PIGD, suggesting that this imaging marker may be useful in prospective evaluation for clinical progression for these Parkinsonian disorders. Further SWI studies to differentiate PIGD from vascular Parkinsonism or other atypical Parkinsonian disorders will be interesting.
Acknowledgments
We thank the support from National Medical Research Council (STaR award to E-KT and translational clinical research program in PD).
Footnotes
Abbreviations: CA = caudate nucleus, GP = globus pallidus, H&Y = Hoehn and Yahr scale, MRI = magnetic resonance imaging, PD = Parkinson disease, PIGD = postural instability gait disorder, PUT = putamen, RN = red nucleus, ROI = region of interest, SN = substantia nigra, SWI = susceptibility-weighted MRI, TH = thalamus.
E-KT, ES, and L-LC designed the study. ES drafted the main manuscript text. K-MN, C-SY and HR assisted in the collection of data. SF-C and H-HL conducted the statistical analysis. E-KT and L-LC revised the manuscript. L-LC supervised the study.
E-KT has received honoraria from Elsevier and Wiley publishers. The corresponding author declares that the authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research; that the author has full access to all of the data; and that the author has the right to publish any and all data, separate and apart from the guidance of any sponsor.
National Medical Research Council and Singhealth Foundation. The funders have no role in the preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 1999; 122:1437–1448. [DOI] [PubMed] [Google Scholar]
- 2.Berg D, Hochstrasser H. Iron metabolism in Parkinsonian syndromes. Mov Disord 2006; 21:1299–1310. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes SL, Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson's disease. Neurobiol Dis 2008; 32:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham JM, Paley MN, Grünewald RA, et al. Brain iron deposition in Parkinson's disease imaged using the PRIME magnetic resonance sequence. Brain 2000; 123:2423–2431. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990; 40:1529–1534. [DOI] [PubMed] [Google Scholar]
- 6.Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006; 21:1123–1130. [DOI] [PubMed] [Google Scholar]
- 7.Rajput AH, Pahwa R, Pahwa P, et al. Prognostic significance of the onset mode in Parkinsonism. Neurology 1993; 43:829–830. [DOI] [PubMed] [Google Scholar]
- 8.Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 2005; 23:1–25. [DOI] [PubMed] [Google Scholar]
- 9.Loeffler DA, Connor JR, Juneau PL, et al. Transferrin and iron in normal, Alzheimer's disease, and Parkinson's disease brain regions. J Neurochem 1995; 65:710–724. [DOI] [PubMed] [Google Scholar]
- 10.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol 2003; 53 suppl 3:S26–S36.discussion S36–S38. [DOI] [PubMed] [Google Scholar]
- 11.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem 2001; 23:44284–44296.276. [DOI] [PubMed] [Google Scholar]
- 12.Chavhan GB, Babyn PS, Thomas B, et al. Principles, techniques, and applications of T2∗-based MR imaging and its special applications. Radiographics 2009; 29:1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 2005; 22:439–450. [DOI] [PubMed] [Google Scholar]
- 14.Ogg RJ, Langston JW, Haacke EM, et al. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging 1999; 17:1141–1148. [DOI] [PubMed] [Google Scholar]
- 15.Charidimou A, Jäger HR, Werring DJ. Cerebral microbleed detection and mapping: Principles, methodological aspects and rationale in vascular dementia. Exp Gerontol 2012; 47:843–852. [DOI] [PubMed] [Google Scholar]
- 16.Chan LL, Ng KM, Rumpel H, et al. Transcallosal diffusion tensor abnormalities in predominant gait disorder Parkinsonism. Parkinsonism Relat Disord 2014; 20:53–59. [DOI] [PubMed] [Google Scholar]
- 17.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986; 34:119–126. [DOI] [PubMed] [Google Scholar]
- 18.Ordidge RJ, Gorell JM, Deniau JC, et al. Assessment of relative brain iron concentrations using T2-weighted and T2∗-weighted MRI at 3 Tesla. Magn Reson Med 1994; 32:335–341. [DOI] [PubMed] [Google Scholar]
- 19.Dexter DT, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991; 114:1953–1975. [DOI] [PubMed] [Google Scholar]
- 20.Jomova K, Vondrakova D, Lawson M, et al. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 2010; 345:91–104. [DOI] [PubMed] [Google Scholar]
- 21.Harder SL, Hopp KM, Ward H, et al. Mineralization of the deep gray matter with age: a retrospective review with susceptibility-weighted MR imaging. Am J Neuroradiol 2008; 29:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonini A, Vitale C, Barone P, et al. The relationship between cerebral vascular disease and Parkinsonism: the VADO study. Parkinsonism Relat Disord 2012; 18:775–780. [DOI] [PubMed] [Google Scholar]
- 23.Aramaki Y, Haruno M, Osu R, et al. Movement initiation-locked activity of the anterior putamen predicts future movement instability in periodic bimanual movement. J Neurosci 2011; 31:9819–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouchi Y, Kanno T, Okada H, et al. Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson's disease. Brain 2001; 124:784–792. [DOI] [PubMed] [Google Scholar]
- 25.Gupta D, Saini J, Kesavadas C, et al. Utility of susceptibility-weighted MRI in differentiating Parkinson's disease and atypical Parkinsonism. Neuroradiology 2010; 52:1087–1094. [DOI] [PubMed] [Google Scholar]
- 26.Hwang I, Sohn CH, Kang KM, et al. Differentiation of Parkinsonism-predominant multiple system atrophy from idiopathic Parkinson disease using 3T susceptibility-weighted MR imaging, focusing on putaminal change and lesion asymmetry. AJNR Am J Neuroradiol 2015; 36:2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijer FJ, van Rumund A, Fasen BA, et al. Susceptibility-weighted imaging improves the diagnostic accuracy of 3T brain MRI in the work-up of Parkinsonism. Am J Neuroradiol 2015; 36:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Butros SR, Shuai X, et al. Different iron-deposition patterns of multiple system atrophy with predominant Parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. Am J Neuroradiol 2012; 33:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]


