Supplemental Digital Content is available in the text
Keywords: aspergillosis, cutaneous, fungal infection, infectious diseases
Abstract
Primary cutaneous aspergillosis (PCA) is an uncommon infection of the skin. There is a paucity of organized literature regarding this entity in regard to patient characteristics, associated Aspergillus species, and treatment modalities on outcome (disease recurrence, disease dissemination, and mortality).
We reviewed all published reports of PCA from 1967 to 2015. Cases were deemed eligible if they included the following: patient baseline characteristics (age, sex, underlying condition), evidence of proven or probable PCA, primary treatment strategy, and outcome.
We identified 130 eligible cases reported from 1967 to 2015. The patients were predominantly male (63.8%) with a mean age of 30.4 ± 22.1 years. Rates of PCA recurrence, dissemination, and mortality were 10.8%, 18.5%, and 31.5%, respectively. In half of the cases, there was an association with a foreign body. Seven different Aspergillus species were reported to cause PCA. Systemic antifungal therapy without surgery was the most common form of therapy (60% of cases). Disease dissemination was more common in patients with underlying systemic conditions and occurred on average 41.4 days after PCA diagnosis (range of 3–120 days). In a multivariate linear regression model of mortality including only patients with immunosuppressive conditions, dissemination and human immunodeficiency virus/acquired immune deficiency syndrome were statistically significantly associated with increased mortality.
Nearly one-third of patients with PCA die with the disease. Dissemination and host status are critical in patient outcome.
1. Introduction
Primary cutaneous aspergillosis (PCA) is a rare but life-threatening invasive fungal infection of the skin caused by the mold Aspergillus. It is defined as aspergillosis in which an infected skin lesion is the initial source of disease.[1] This is in contrast to secondary cutaneous aspergillosis, the more common form, which involves the hematogenous spread of Aspergillus infection from a distal site (e.g., the lungs) to the skin. Particularly in immunocompromised patients, PCA has the potential to progress to systemic infection (disease dissemination) and is frequently lethal.[1,2] As the population of immunocompromised patients is expanding, it is expected that PCA will also rise in prevalence.[2] Due to the challenges associated with diagnosing fungal disease, especially in complex patients with multiple comorbidities, it is likely that PCA is underdiagnosed and underreported in the literature.[3,4]
Due to the rarity of the disease, the patient characteristics, rates of disease recurrence, dissemination, and mortality are understudied. To that end, we undertook a systematic review of the epidemiological, clinical, diagnostic, and therapeutic aspects of this serious infection with particular emphasis on the underlying condition, associated species of Aspergillus, primary treatment strategy, and rates of recurrence, dissemination, and mortality. Our underlying hypothesis is that factors such as host condition and disease dissemination may significantly affect patient outcome in PCA.
2. Methods
A MEDLINE search using the phrase “primary cutaneous aspergillosis” was conducted as recently as March 12, 2016. All English-language manuscripts from this search were then reviewed for cases of PCA. Only cases that provided the following information were included in this analysis: patient baseline characteristics (age, sex, underlying condition), evidence of proven or probable PCA[5] with no indications of primary infection at another site, primary treatment strategy, and patient outcome (mortality with disease). As a systemic review and analysis of the literature, no institutional review board approval was required for this study.
2.1. Database development
JMP Pro 11.0.0 (SAS Institute Inc, Cary, NC) was utilized to build a database of patients eligible as described earlier. The following information was recorded in the database as was available in the literature: year of publication; patient age at presentation, underlying condition (further divided into local underlying condition, systemic underlying condition, and no underlying condition), and patient sex; diagnosis modality; associated Aspergillus species; noted association with a foreign body (e.g., catheter, wound dressing); primary treatment method (none, antifungal, surgical, antifungal, or combination); occurrence of disease recurrence, dissemination, and mortality with disease; time from appearance of disease until mortality; dissemination diagnosis modality, time from PCA diagnosis to dissemination diagnosis, and site of dissemination. We excluded cases in which the age, sex, or underlying host condition was unclear; cases in which diagnosis could not be confirmed; case reports with diagnoses but no discussion of treatment and outcome; and patients lost to follow-up before outcome was noted.
2.2. Statistical analyses
JMP Pro 11.0.0 was utilized for all statistical analyses performed. For univariate analysis, categorical data were compared by a 2-tailed Fisher's exact test (α = 95%). To study which factors affect mortality in immunocompromised patients, a multivariate linear regression model was built with the inclusion of all variables that had P < 0.2 in univariate analysis.[6] Patients with no known immunodeficiencies in the database were excluded from this model.
3. Results
Out of 113 manuscripts resulting from the literature search, 78 contained eligible patients, resulting in a total of 130 unique eligible PCA cases.[7–84] The cases were reported from the years 1967 to 2015 (Supplementary Table 1). Most of the manuscripts consisted of single case reports or small case series. When manuscripts reported multiple patients, most often these patients shared the same risk factor (blood malignancy, stem cell transplantation [SCT], etc.) and occasionally the same species (particularly in cases of outbreak).[9]
3.1. Patient characteristics
The majority of the patient population was male (63.8%) (Table 1). The mean, median, and standard deviation were 30.4, 30, and 22.1 years of age, respectively. Ages ranged from neonatal to 81 years. The majority (61.5%) of patients were adults (between 18 and 65 years of age).
Table 1.
Characteristics of patients reported with PCA.
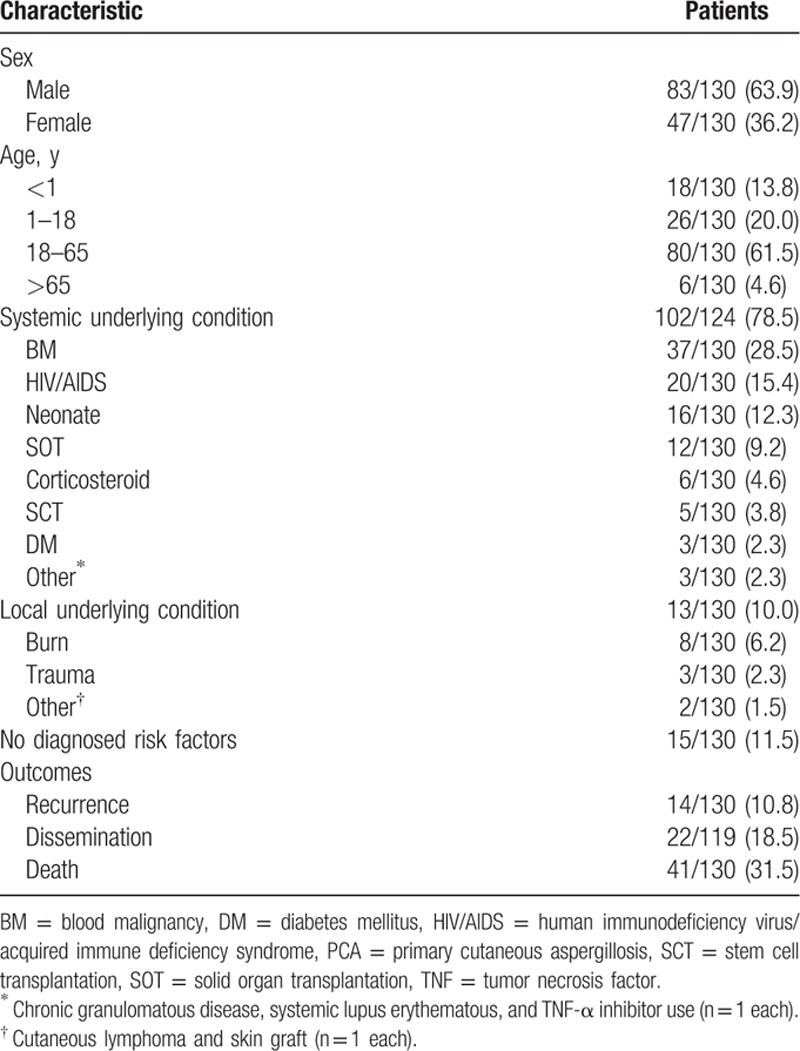
A variety of underlying conditions were encountered, the most common being blood malignancy (28.5%), followed by human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) (15.4%) and neonatal status (12.3%). A total of 11.5% of cases were reported with no diagnosed risk factors (presumably immunocompetent). Other risk factors included solid organ transplantation (9.2%), burns (6.2%), corticosteroid use (4.6%), SCT (3.8%), and diabetes mellitus and trauma (2.3% each). In the 3 patients with diabetes, poor control was suggested.[44,58] In 64 of 130 cases (49.2%), the infection was noted to be associated with a foreign body (most commonly catheters or wound dressings). In the 10 adults (ages 18–65) with no diagnosed immunodeficiencies or risk factors, occupational information was provided in 5 of 10 cases. Four of these patients were agricultural workers[18,42,73,76] and 1 was a researcher in a laboratory studying Aspergillus infection.[79] Infection was noted to be preceded by minor trauma in 3 of 10 of these cases.[73,76,79]
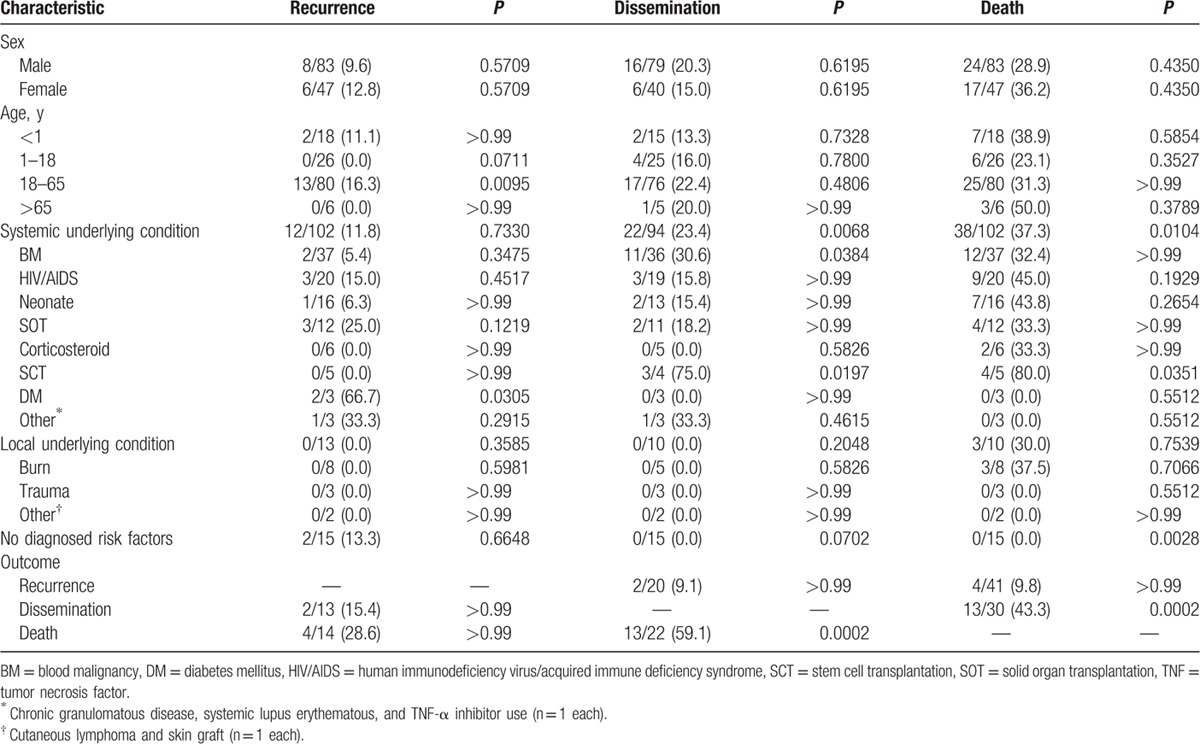
Outcomes appear to be associated with specific patient characteristics (Table 2). Compared to patients from all other age groups, adults were significantly more likely to have disease recurrence (16%, P = 0.0095). Patients with an underlying condition were more likely to suffer from disease dissemination (24.7%, P = 0.0035) and death (39.2%, P = 0.0056). Patients with diabetes mellitus were significantly associated with increased disease recurrence (66.7%, P = 0.0335), blood malignancy was significantly associated with disease dissemination (34.4%, P = 0.0395), and SCT was significantly associated with both disease dissemination (80.0%, P = 0.0066) and death (80.0%, P = 0.0407). In 24 of 41 (58.5%) cases of fatal PCA, information pertaining to time from PCA appearance to death was included. The median, mean, and standard deviation were 46.4, 79.1, and 99.27 days, with a range of 3 to 420 days.
Table 2.
Patient characteristics and relationships with outcomes (univariate analysis).
3.2. Aspergillus species
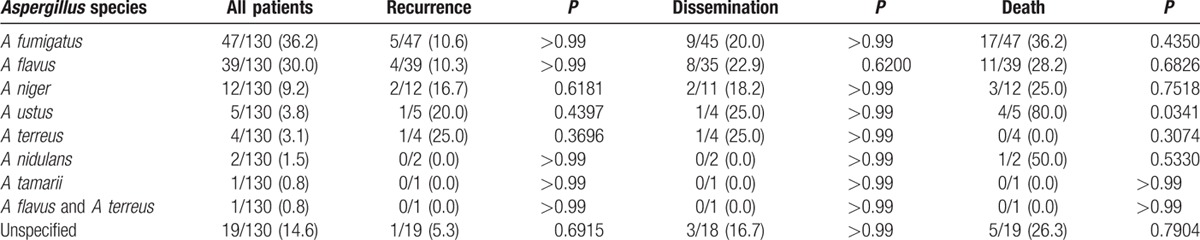
In 111 of 130 (85.4%) reported cases, the associated species of Aspergillus was identified with a total of 7 unique species (Table 3). In 1 case, a coinfection with both A flavus and A terreus was reported.[80] Out of the cases in which the species was identified, the most commonly reported species were A fumigatus (42.3%), A flavus (35.1%), and A niger (10.8%). In addition to the species reported in Table 3, A glaucus was identified in 1 case but was excluded from this analysis as the patient was lost to follow-up.[85] Compared to other species, A ustus was significantly associated with greater mortality (80%, P = 0.0341). Particularly in immunocompromised patients, it was not unusual to have concomitant infections such as concurrent dissemination (Mycobacterium tuberculosis,[8]Escherichia coli and Pneumocystis jirovecii,[36] cytomegalovirus,[40] and Mycobacterium avium complex),[56] localized PCA during dissemination of other pathogens (M avium complex,[40]Pseudomonas aeruginosa, and cytomegalovirus),[77] and localized coinfection (Staphylococcus capitis).[12]
Table 3.
Associated Aspergillus species and relationships with outcomes (univariate analysis).
3.3. Primary treatment strategy
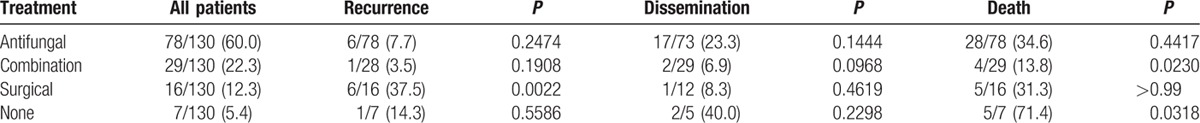
The most common treatment strategy was systemic Aspergillus-active antifungal therapy with no surgery (60.5%), followed by antifungals in combination with surgery (21.8%), surgery alone (12.9%), and no treatment (4.8%) (Table 4). Compared to the other treatment modalities, surgery alone was significantly associated with increased disease recurrence (37.5%, P = 0.0022) and a combination of surgery and systemic Aspergillus-active antifungal therapy was significantly associated with decreased mortality (13.8%, P = 0.0230). Patients receiving no treatment had greater mortality (71.4%, P = 0.0318).
Table 4.
Primary treatment strategy and relationships with outcomes (univariate analysis).
3.4. Dissemination
In instances in which dissemination was described, the modality for making a diagnosis of PCA dissemination was reported in 20 of 22 (90.9%) cases and included histological analysis through means such as biopsy or sputum culture (50.0%), chest x-ray (25.0%), computed tomography (10.0%), and a high index of clinical suspicion (10.0%).[16,39] The site of dissemination was described in 20 of 22 cases. Of these, the most common site of dissemination was the lungs (70.0%). In two cases, dissemination was reported at multiple organ sites: lungs, pericardium, stomach, liver, thyroid, gland, and brain in one patient;[41] and lung, spleen, and mesentery in the other.[62] In individual cases, there were reports of dissemination to the bone marrow[8] and kidney.[50] The time to dissemination (time from initial PCA diagnosis to diagnosis of dissemination) was reported in 10 of 22 cases (45.5%) and varied widely with a range of 3 to 120 days. The mean, median, and standard deviation were 41.4, 26.5, and 39.8 days, respectively.
Serum galactomannan antigen, a component of the Aspergillus cell wall, can be assayed for diagnosis of hematological involvement of disease.[86] Use of this assay was reported in only 8 of 130 (6.15%) cases. Of those cases, the assay was positive in 5 of 8 (62.5%).[14,16,59] In 2 of those cases, increasing levels of serum galactomannan antigen correlated with clinical signs of disease dissemination.[16,59] No dissemination was observed in the other 3 positive cases.[14]
3.5. Multivariate model of disease mortality in immunocompromised patients
Factors that affected occurrence of mortality (in a positive or negative fashion) in univariate analysis with P < 0.2 were included in a multivariate linear regression model[6] (Table 5). Patients with no diagnosed risk factors (otherwise known to be immunocompetent) were excluded from the model, resulting in a total n = 115. HIV/AIDS and disease dissemination were statistically significantly associated with greater mortality in this model of immunocompromised patients with PCA. The odds ratio (and 95% confidence interval) was 5.0736 (1.3781–29.4174) for HIV/AIDS and 5.3821 (1.7503–17.3991) for dissemination.
Table 5.
Multivariate model of factors on immunocompromised patient mortality.
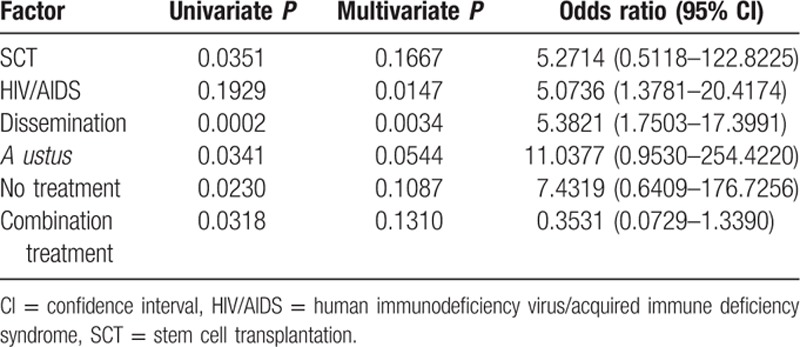
4. Discussion
This is the largest and more comprehensive analysis of published PCA cases to date, composed of 130 patients. The largest previous database of PCA patients collected from the literature consisted of 14 patients and focused on underlying patient diseases, associated Aspergillus species, and histological features but not on outcome.[14] In the current study, the age and sex distribution is consistent with other large collections of case reports in the literature of aspergillosis in general.[87,88] However, the proportion of PCA patients with no apparent risk factors (15 of 130, 11.5%) in our study was higher than what has been reported in other forms of aspergillosis (e.g., pulmonary, sinus, cerebral, or bone/joint aspergillosis). It is possible that colonization of the skin is more common than other organ systems given its high exposure to environmental conidia; this is supported by our finding that cases of PCA in immunocompetent patients were associated with occupation (agricultural work) and, in some cases, local trauma preceding infection. Prior minor injury may have been underreported due to recall biases.
Aspergillosis remains a disease with high lethality. In other large collections of patient cases from the literature, mortality with disease for cerebral aspergillosis, sinus aspergillosis, pulmonary aspergillosis, and bone/joint aspergillosis has been reported as 88.1%,[87] 65.6%,[89] 60.2%,[87] and 25%,[88] respectively. In this study, mortality in patients with PCA was found to be 31.5% (or 35.7% with the exclusion of immunocompetent patients). Of note, none of the 15 cases of patients with presumed immunocompetence reported dissemination or death. In univariate analysis, systemic underlying conditions (often with accompanying immunodeficiencies) were significantly associated with increased likelihood of mortality (P = 0.0104). These findings further support the recommendation that reversal of immunosuppression is key to improving outcomes in Aspergillus-related disease.[5]
Only 42.3% of identified isolates were due to A fumigatus in our review of PCA (35.1% of identified isolates were A flavus). This is in contrast to other forms of invasive aspergillosis. For example, in 1 large collection of 261 cases of invasive aspergillosis (including pulmonary, skin, sinus, central nervous system, and disseminated cases), 66% of those identified were A fumigatus (14% were A flavus).[90] It has been hypothesized that A fumigatus is the predominant pathogen associated with pulmonary aspergillosis (the most common form of aspergillosis[87]) due to the ability of its relatively smaller conidia to evade pulmonary clearance.[91] As PCA occurs due to direct fungal inoculation after disruption of the epithelial barrier,[2] conidia size differences between species of Aspergillus may have less relevance in developing primary skin infection. Understanding the increased distribution of species associated with PCA might be important for treatment, as resistance patterns differ between species; for example, A terreus is generally considered resistant to amphotericin B and A ustus has demonstrated increased azole resistance.[5,92] In this study, A ustus was significantly associated with greater mortality in univariate analysis (P = 0.0341). However, as A ustus was involved in only 5 of 130 cases, further investigation is required.
The most current treatment guidelines discuss both surgical intervention and systemic antifungal therapy for cutaneous aspergillosis.[5] In this study, univariate analysis indicated that a combination of systemic Aspergillus-active antifungal therapy and surgery was associated with significantly less mortality (P = 0.0230) and no treatment at all was associated with significantly greater mortality (P = 0.0318). PCA often results in a necrotic lesion, and Aspergillus has been demonstrated to both destroy blood vessels and mitigate host angiogenesis and wound healing.[93] Combined, these factors may inhibit systemic delivery of antifungals to the wound bed. Therefore, reducing as much fungal burden as possible by surgical resection and debridement of the cutaneous lesion may synergistically improve patient outcome. Surgery alone was associated with significantly increased rates of recurrence (P = 0.0022). Others have observed that surgical treatment alone in the absence of immunocompetency is often ineffective in the treatment of cutaneous aspergillosis.[94] Analogous to tumor resection, it is possible that surgery without systemic antifungal use may allow the survival of trace amounts of pathogen located at the wound margins or already spread distal to the wound through the vasculature or lymphatics. Surgical resection of large lesions was often associated with significant scarring and disfiguration.[17,21,23,29,38,68,72,75] Ultimately, the optimal timing and extent of surgery remains unclear as patient selection is a confounding variable for better outcomes with surgery.
Angioinvasion and dissemination of Aspergillus to other organ systems are predictive of poor response to therapy and is associated with increased mortality.[87,90] In this study, 59.1% of PCA patients with dissemination died and in univariate analysis, dissemination was significantly associated with mortality (P = 0.0002). Immune status and dissemination are clearly linked; there were cases in which immunocompetent patients in resource-poor settings had PCA lesions without treatment for over >10 years without disease dissemination.[45,53] The most common site of dissemination was the lungs (70.0% of cases), but a wide range of organs have been reported. The time to dissemination varied greatly, with a median of approximately 1 month, standard deviation of 39.8 days, and range from 3 to 120 days. From a clinical standpoint, these data in sum (high lethality associated with dissemination and high variance in the timeline of dissemination development) support aggressive vigilance for dissemination throughout the treatment of PCA, especially as it may not be clear when a patient's primary lesion began developing. Serum galactomannan antigen was assayed in only 8 patients; in the 5 patients with a positive assay, only 2 had signs of disseminated disease. Based on these limited data, it is unclear if serum galactomannan antigen is useful for monitoring dissemination in PCA.
In our multivariate linear regression model of immunocompromised patients, HIV/AIDS (P = 0.0147) and disease dissemination (P = 0.0034) were significantly associated with increased mortality. In a previously published multivariate model of factors affecting mortality in immunocompromised patients with invasive aspergillosis in general, dissemination and underlying host disease were also significantly correlated with mortality (in addition to other factors such as creatinine clearance, steroid usage, and monocyte count).[95] Of note, in multivariate analysis, no therapeutic modality demonstrated improved patient outcome compared to the others. Due to the complexity of Aspergillus infection pathophysiology (e.g., the aforementioned host angiogenic suppression),[93] development of novel therapies that improve wound healing and reverse these disease phenotypes may be warranted.
4.1. Strengths and limitations
One of the main strengths of the study is the number of cases included that allowed us to develop a robust multivariate model that showed that dissemination of disease and underlying host diseases (HIV/AIDS) are significant in contributing to patient mortality. In addition, we were able for the first time in PCA to characterize key disease outcomes in addition to mortality, such as recurrence and dissemination. However, this study is not without limitations, chiefly because of publication, ascertainment, and classification biases. Due to the complexity of the patient population that is afflicted by PCA, it was hard to dissect PCA-attributable mortality. As occurs whenever patient data are collected from multiple studies across multiple decades, inconsistencies occur in the literature as medicine evolves. New diagnostic technologies (e.g., computed tomography, polymerase chain reaction, and galactomannan assays) as well as therapeutic modalities (e.g., the introduction of potent new triazoles such as voriconazole) were introduced between the first and last cases reported (1967–2015). Likewise, resistance patterns in Aspergillus species have also evolved over this time period and patient outcome associated with resistant species is poor.[96] In 1 case series, several patients were diagnosed via histopathology but cultures were negative.[24] This may introduce classification bias as other hyalohyphomycetes, such as Fusarium, have been mistaken as Aspergillus by histopathology.[97] Cutaneous mucormycosis, another skin-based fungal disease accompanied by local necrosis, shares a similar clinical presentation to PCA but can be differentiated via immunohistological and/or molecular analysis.[98,99]
PCA diagnosis was made by evidence of a cutaneous lesion infected with Aspergillus in the absence of infection elsewhere. It is possible that some cases of PCA were in fact secondary cutaneous aspergillosis that had become disseminated from undetected lesions in the lungs or elsewhere. This possibility was largely excluded by lack of radiologic findings; however, it is impossible to definitely rule out spread from a nidus in the lungs too small to detect. Likewise, the time between PCA diagnosis and dissemination diagnosis may not be a reliable indicator of the timescale of dissemination, as it is not always clear when the primary lesion first developed or when dissemination actually occurred. Lastly, reporting on patient immune status as reflected by markers such as white blood cell count at different stages of disease would have complemented many of the findings in this study. However, given the variation of detail surrounding immunocompetence presented in the literature, these markers were not included in this analysis.
5. Conclusions
Although relatively uncommon, PCA is a condition associated with extensive morbidity and mortality and significant rates of disease recurrence and dissemination in a heterogeneous group of patients with varying degrees of immune suppression. Dissemination and HIV/AIDS status were significantly associated with increased mortality. Early diagnosis and prevention of dissemination of PCA is key to improved outcomes. Although further investigation is required, these findings suggest that aggressive therapy, stimulating immunocompetency, holding a high suspicion for dissemination even early in the disease process, and the development of new therapeutic modalities are of importance in the treatment of PCA.
Acknowledgments
AMT would like to thank the Baylor College of Medicine Medical Scientist Training Program (NIH T32 GM007330) and the Barrow Scholars Program. The authors would also like to thank Ms Ying Jiang, MS, for her expertise in biostatistics.
Supplementary Material
Footnotes
Abbreviations: AIDS = acquired immune deficiency syndrome, HIV = human immunodeficiency virus, PCA = primary cutaneous aspergillosis, SCT = stem cell transplantation.
Supplemental Digital Content is available for this article.
Part of this work was presented as a poster at the Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC) 2015 meeting.
This work was supported by the John S. Dunn Foundation.
The authors have no conflict of interest to disclose.
References
- 1.Van Burik J-AH, Colven R, Spach DH. Cutaneous aspergillosis. J Clin Microbiol 1998; 36:3115–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TJ. Primary cutaneous aspergillosis—an emerging infection among immunocompromised patients. Clin Infect Dis 1998; 27:453–457. [DOI] [PubMed] [Google Scholar]
- 3.Rolston KV, Bodey GP, Safdar A. Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clin Infect Dis 2007; 45:228–233. [DOI] [PubMed] [Google Scholar]
- 4.Georgiadou SP, Kontoyiannis DP. Concurrent lung infections in patients with hematological malignancies and invasive pulmonary aspergillosis: how firm is the Aspergillus diagnosis? J Infect 2012; 65:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:327–360. [DOI] [PubMed] [Google Scholar]
- 6.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41:634–653. [DOI] [PubMed] [Google Scholar]
- 7.Ajith C, Dogra S, Radotra BD, et al. Primary cutaneous aspergillosis in an immunocompetent individual. J Eur Acad Dermatol Venereol 2006; 20:738–739. [DOI] [PubMed] [Google Scholar]
- 8.Aksoy DY, Turker A, Altundag MK, et al. Concomitant Mycobacterium tuberculosis and Aspergillus niger infection in a patient with acute myeloid leukemia. Chemotherapy 2003; 49:264–266. [DOI] [PubMed] [Google Scholar]
- 9.Allo MD, Miller J, Townsend T, et al. Primary cutaneous aspergillosis associated with Hickman intravenous catheters. N Engl J Med 1987; 317:1105–1108. [DOI] [PubMed] [Google Scholar]
- 10.Amod FC, Coovadia YM, Pillay T, et al. Primary cutaneous aspergillosis in ventilated neonates. Pediatr Infect Dis J 2000; 19:482–483. [DOI] [PubMed] [Google Scholar]
- 11.Anderson A, Foster RS, Brand R, et al. Acute onset of pustules at the site of tape placement in an immunocompromised infant with acute myeloid leukemia. Diagnosis: primary cutaneous aspergillosis. Pediatr Dermatol 2014; 31:609–610. [DOI] [PubMed] [Google Scholar]
- 12.Andresen J, Nygaard EA, Stordal K. Primary cutaneous aspergillosis (PCA)—a case report. Acta Paediatr 2005; 94:761–762. [DOI] [PubMed] [Google Scholar]
- 13.Arikan S, Uzun O, Cetinkaya Y, et al. Primary cutaneous aspergillosis in human immunodeficiency virus-infected patients: two cases and review. Clin Infect Dis 1998; 27:641–643. [DOI] [PubMed] [Google Scholar]
- 14.Bernardeschi C, Foulet F, Ingen-Housz-Oro S, et al. Cutaneous invasive aspergillosis: retrospective multicenter study of the French invasive-aspergillosis registry and literature review. Medicine (Baltimore) 2015; 94:e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borbujo J, Jara M, Barros C, et al. Primary cutaneous aspergillosis in a patient with acquired immunodeficiency syndrome. J Eur Acad Dermatol Venereol 1999; 12:268–270. [DOI] [PubMed] [Google Scholar]
- 16.Bretagne S, Bart-Delabesse E, Wechsler J, et al. Fatal primary cutaneous aspergillosis in a bone marrow transplant recipient: nosocomial acquisition in a laminar-air flow room. J Hosp Infect 1997; 36:235–239. [DOI] [PubMed] [Google Scholar]
- 17.Buescher TM, Moritz DM, Killyon GW. Resection of chest wall and central veins for invasive cutaneous Aspergillus infection in an immunocompromised patient. Chest 1994; 105:1283–1285. [DOI] [PubMed] [Google Scholar]
- 18.Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol 1967; 96:545–547. [PubMed] [Google Scholar]
- 19.Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol 1978; 114:78–80. [PubMed] [Google Scholar]
- 20.Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect 1998; 37:24–27. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Liao W, Wen H, et al. Primary cutaneous aspergillosis in a patient with systemic lupus erythematosus. Mycoses 2012; 55:e42–e44. [DOI] [PubMed] [Google Scholar]
- 22.Cho WH, Kim JE, Jeon DS, et al. Tracheobronchial aspergillosis following primary cutaneous aspergillosis in a lung-transplant recipient. Intern Med 2011; 50:131–134. [DOI] [PubMed] [Google Scholar]
- 23.Craiglow B, Hinds G, Antaya R, et al. Primary cutaneous aspergillosis in an immunocompetent patient: successful treatment with oral voriconazole. Pediatr Dermatol 2009; 26:493–495. [DOI] [PubMed] [Google Scholar]
- 24.D’Antonio D, Pagano L, Girmenia C, et al. Cutaneous aspergillosis in patients with haematological malignancies. Eur J Clin Microbiol Infect Dis 2000; 19:362–365. [DOI] [PubMed] [Google Scholar]
- 25.Epstein MD, Segalman KA, Mulholland JH, et al. Successful treatment of primary cutaneous Aspergillus flavus infection of the hand with oral itraconazole. J Hand Surg Am 1996; 21:1106–1108. [DOI] [PubMed] [Google Scholar]
- 26.Erisir-Oygucu S, Akcan AB, Oygur N. Primary cutaneous aspergillosis in an extremely low birth weight preterm. Turk J Pediatr 2009; 51:621–623. [PubMed] [Google Scholar]
- 27.Estes SA, Hendricks AA, Merz WG, et al. Primary cutaneous aspergillosis. J Am Acad Dermatol 1980; 3:397–400. [DOI] [PubMed] [Google Scholar]
- 28.Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit. J Hosp Infect 2011; 79:344–348. [DOI] [PubMed] [Google Scholar]
- 29.Frankenbusch K, Eifinger F, Kribs A, et al. Severe primary cutaneous aspergillosis refractory to amphotericin B and the successful treatment with systemic voriconazole in two premature infants with extremely low birth weight. J Perinatol 2006; 26:511–514. [DOI] [PubMed] [Google Scholar]
- 30.Galimberti R, Kowalczuk A, Hidalgo Parra I, et al. Cutaneous aspergillosis: a report of six cases. Br J Dermatol 1998; 139:522–526. [DOI] [PubMed] [Google Scholar]
- 31.Gedela K, Nelson M, Francis N, et al. Cutaneous aspergillosis associated with HIV infection. Int J STD AIDS 2012; 23:679–680. [DOI] [PubMed] [Google Scholar]
- 32.Gene J, Azon-Masoliver A, Guarro J, et al. Cutaneous infection caused by Aspergillus ustus, an emerging opportunistic fungus in immunosuppressed patients. J Clin Microbiol 2001; 39:1134–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous Aspergillus. Am J Dermatopathol 2001; 23:224–226. [DOI] [PubMed] [Google Scholar]
- 34.Googe PB, DeCoste SD, Herold WH, et al. Primary cutaneous aspergillosis mimicking dermatophytosis. Arch Pathol Lab Med 1989; 113:1284–1286. [PubMed] [Google Scholar]
- 35.Granstein RD, First LR, Sober AJ. Primary cutaneous aspergillosis in a premature neonate. Br J Dermatol 1980; 103:681–684. [DOI] [PubMed] [Google Scholar]
- 36.Grossman ME, Fithian EC, Behrens C, et al. Primary cutaneous aspergillosis in six leukemic children. J Am Acad Dermatol 1985; 12:313–318. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Khaira A, Lata S, et al. Primary cutaneous aspergillosis in renal transplant recipient. Saudi J Kidney Dis Transpl 2009; 20:848–849. [PubMed] [Google Scholar]
- 38.Hashmi KU, Ahmed P, Satti TM, et al. Cutaneous aspergillosis as a first manifestation of systemic infection in allogeneic haematopoietic stem cell transplantation. J Pak Med Assoc 2007; 57:324–326. [PubMed] [Google Scholar]
- 39.Herron MD, Vanderhooft SL, Byington C, et al. Aspergillosis in a 24-week newborn: a case report. J Perinatol 2003; 23:256–259. [DOI] [PubMed] [Google Scholar]
- 40.Hunt SJ, Nagi C, Gross KG, et al. Primary cutaneous aspergillosis near central venous catheters in patients with the acquired immunodeficiency syndrome. Arch Dermatol 1992; 128:1229–1232. [PubMed] [Google Scholar]
- 41.Khardori N, Hayat S, Rolston K, et al. Cutaneous Rhizopus and Aspergillus infections in five patients with cancer. Arch Dermatol 1989; 125:952–956. [PubMed] [Google Scholar]
- 42.Kotwal A, Biswas D, Kakati B, et al. Aspergillus nidulans causing primary cutaneous aspergillosis in an immunocompetent patient. Cutis 2015; 95:E1–E3. [PubMed] [Google Scholar]
- 43.Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine. Diagn Microbiol Infect Dis 2008; 62:443–446. [DOI] [PubMed] [Google Scholar]
- 44.Lai CS, Lin SD, Chou CK, et al. Aspergillosis complicating the grafted skin and free muscle flap in a diabetic. Plast Reconstr Surg 1993; 92:532–536. [DOI] [PubMed] [Google Scholar]
- 45.Lakhanpal S, Pandhi RK, Khaitan BK, et al. Primary cutaneous aspergillosis in an immunocompetent host. Acta Derm Venereol 2000; 80:74–75. [DOI] [PubMed] [Google Scholar]
- 46.Larkin JA, Greene JN, Sandin RL, et al. Primary cutaneous aspergillosis: case report and review of the literature. Infect Control Hosp Epidemiol 1996; 17:365–366. [DOI] [PubMed] [Google Scholar]
- 47.Lohana P, Hogg FJ. Vacuum-assisted closure and primary cutaneous aspergillosis in a burn—a management dilemma!. Ann Burns Fire Disasters 2010; 23:48–50. [PMC free article] [PubMed] [Google Scholar]
- 48.Loria KM, Salinger MH, Frohlich TG, et al. Primary cutaneous aspergillosis in a heart transplant recipient treated with surgical excision and oral itraconazole. J Heart Lung Transplant 1992; 11:156–159. [PubMed] [Google Scholar]
- 49.Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis 1999; 29:1594–1596. [DOI] [PubMed] [Google Scholar]
- 50.Mallat SG, Aoun M, Moussalli A, et al. Cutaneous aspergillosis in a renal transplant recipient. J Med Liban 2004; 52:111–114. [PubMed] [Google Scholar]
- 51.Martin EB, Gastanaduy PA, Camacho-Gonzalez AF, et al. Primary cutaneous aspergillosis in two pediatric trauma patients. Pediatr Infect Dis J 2012; 31:427–428. [DOI] [PubMed] [Google Scholar]
- 52.McCarty JM, Flam MS, Pullen G, et al. Outbreak of primary cutaneous aspergillosis related to intravenous arm boards. J Pediatr 1986; 108:721–724. [DOI] [PubMed] [Google Scholar]
- 53.Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol 2009; 27:367–370. [DOI] [PubMed] [Google Scholar]
- 54.Mowad CM, Nguyen TV, Jaworsky C, et al. Primary cutaneous aspergillosis in an immunocompetent child. J Am Acad Dermatol 1995; 33:136–137. [DOI] [PubMed] [Google Scholar]
- 55.Munn S, Keane F, Child F, et al. Primary cutaneous aspergillosis. Br J Dermatol 1999; 141:378–380. [DOI] [PubMed] [Google Scholar]
- 56.Murakawa GJ, Harvell JD, Lubitz P, et al. Cutaneous aspergillosis and acquired immunodeficiency syndrome. Arch Dermatol 2000; 136:365–369. [DOI] [PubMed] [Google Scholar]
- 57.Mysorekar VV, Eshwarappa M, Lingaraj U. Opportunistic infections in a renal transplant recipient. Infect Dis Rep 2012; 4:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagarkar KM, Dey AB, Ray R, et al. Prolonged pyrexia in a diabetic due to systemic aspergillosis. J Assoc Physicians India 1997; 45:887–888. [PubMed] [Google Scholar]
- 59.Nakai K, Kanda Y, Mineishi S, et al. Primary cutaneous aspergillosis caused by Aspergillus ustus following reduced-intensity stem cell transplantation. Ann Hematol 2002; 81:593–596. [DOI] [PubMed] [Google Scholar]
- 60.Nakashima K, Yamada N, Yoshida Y, et al. Primary cutaneous aspergillosis. Acta Derm Venereol 2010; 90:519–520. [DOI] [PubMed] [Google Scholar]
- 61.Osorio F, Magina S, Azevedo F. Primary cutaneous aspergillosis complicating tumor necrosis factor-alpha blockade therapy in a patient with psoriasis. Actas Dermosifiliogr 2012; 103:939–941. [DOI] [PubMed] [Google Scholar]
- 62.Palmero ML, Pope E, Brophy J. Sporotrichoid aspergillosis in an immunocompromised child: a case report and review of the literature. Pediatr Dermatol 2009; 26:592–596. [DOI] [PubMed] [Google Scholar]
- 63.Papouli M, Roilides E, Bibashi E, et al. Primary cutaneous aspergillosis in neonates: case report and review. Clin Infect Dis 1996; 22:1102–1104. [DOI] [PubMed] [Google Scholar]
- 64.Park SB, Kang MJ, Whang EA, et al. A case of primary cutaneous aspergillosis in a renal transplant recipient. Transplant Proc 2004; 36:2156–2157. [DOI] [PubMed] [Google Scholar]
- 65.Perzigian RW, Faix RG. Primary cutaneous aspergillosis in a preterm infant. Am J Perinatol 1993; 10:269–271. [DOI] [PubMed] [Google Scholar]
- 66.Prasad PV, Babu A, Kaviarasan PK, et al. Primary cutaneous aspergillosis. Indian J Dermatol Venereol Leprol 2005; 71:133–134. [DOI] [PubMed] [Google Scholar]
- 67.Rabbani MZ, Amir M, Khan MY, et al. Primary aspergillosis of the cheek. A diagnostic dilemma. J Pak Med Assoc 2007; 57:613–615. [PubMed] [Google Scholar]
- 68.Raszka WV, Jr, Shoupe BL, Edwards EG. Isolated primary cutaneous aspergillosis of the labia. Med Pediatr Oncol 1993; 21:375–378. [DOI] [PubMed] [Google Scholar]
- 69.Ricci RM, Evans JS, Meffert JJ, et al. Primary cutaneous Aspergillus ustus infection: second reported case. J Am Acad Dermatol 1998; 38:797–798. [DOI] [PubMed] [Google Scholar]
- 70.Richards KA, Mancini AJ. A painful erythematous forearm nodule in a girl with Hodgkin disease. Diagnosis: primary cutaneous aspergillosis. Arch Dermatol 2000; 136:1165–1170. [DOI] [PubMed] [Google Scholar]
- 71.Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis 2011; 87:197–200. [PubMed] [Google Scholar]
- 72.Rogdo B, Kahlert C, Diener PA, et al. Primary cutaneous aspergillosis in a preterm neonate. BMJ Case Rep 2014; 2014: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romano C, Miracco C. Primary cutaneous aspergillosis in an immunocompetent patient. Mycoses 2003; 46:56–59. [DOI] [PubMed] [Google Scholar]
- 74.Romero LS, Hunt SJ. Hickman catheter-associated primary cutaneous aspergillosis in a patient with the acquired immunodeficiency syndrome. Int J Dermatol 1995; 34:551–553. [DOI] [PubMed] [Google Scholar]
- 75.Santos RP, Sanchez PJ, Mejias A, et al. Successful medical treatment of cutaneous aspergillosis in a premature infant using liposomal amphotericin B, voriconazole and micafungin. Pediatr Infect Dis J 2007; 26:364–366. [DOI] [PubMed] [Google Scholar]
- 76.Sharma S, Yenigalla BM, Naidu SK, et al. Primary cutaneous aspergillosis due to Aspergillus tamarii in an immunocompetent host. BMJ Case Rep 2013; 2013:010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanford D, Boyle M, Gillespie R. Human immunodeficiency virus-related primary cutaneous aspergillosis. Australas J Dermatol 2000; 41:112–116. [DOI] [PubMed] [Google Scholar]
- 78.Stiller MJ, Teperman L, Rosenthal SA, et al. Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. J Am Acad Dermatol 1994; 31:344–347. [DOI] [PubMed] [Google Scholar]
- 79.Tahir C, Garbati M, Nggada HA, et al. Primary cutaneous aspergillosis in an immunocompetent patient. J Surg Tech Case Rep 2011; 3:94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tak V, Mathur P, Xess I, et al. A case of dual infection in a paediatric trauma victim of primary cutaneous aspergillosis caused by Aspergillus flavus and Aspergillus terreus. Indian J Med Microbiol 2013; 31:193–196. [DOI] [PubMed] [Google Scholar]
- 81.Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis 2008; 81:127–130. [PubMed] [Google Scholar]
- 82.Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol 2002; 19:439–444. [DOI] [PubMed] [Google Scholar]
- 83.Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma. Mycoses 2009; 52:462–464. [DOI] [PubMed] [Google Scholar]
- 84.Zhang QQ, Li L, Zhu M, et al. Primary cutaneous aspergillosis due to Aspergillus flavus: a case report. Chin Med J (Engl) 2005; 118:255–257. [PubMed] [Google Scholar]
- 85.Venugopal TV, Venugopal PV. Primary cutaneous aspergillosis from Tamilnadu diagnosed by fine needle aspiration cytology. Med Mycol Case Rep 2012; 1:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 2006; 42:1417–1427. [DOI] [PubMed] [Google Scholar]
- 87.Lin S-J, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 2001; 32:358–366. [DOI] [PubMed] [Google Scholar]
- 88.Gabrielli E, Fothergill A, Brescini L, et al. Osteomyelitis caused by Aspergillus species: a review of 310 reported cases. Clin Microbiol Infect 2014; 20:559–565. [DOI] [PubMed] [Google Scholar]
- 89.Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis 1996; 23:608–615. [DOI] [PubMed] [Google Scholar]
- 90.Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis disease spectrum, treatment practices, and outcomes. Medicine 2000; 79:250–260. [DOI] [PubMed] [Google Scholar]
- 91.Mullins J, Seaton A. Fungal spores in lung and sputum. Clin Exp Allergy 1978; 8:525–533. [DOI] [PubMed] [Google Scholar]
- 92.Balajee SA, Houbraken J, Verweij PE, et al. Aspergillus species identification in the clinical setting. Stud Mycol 2007; 59:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ben-Ami R, Lewis RE, Leventakos K, et al. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009; 114:5393–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reischies F, Hoenigl M. The role of surgical debridement in different clinical manifestations of invasive aspergillosis. Mycoses 2014; 57 suppl 2:1–14. [DOI] [PubMed] [Google Scholar]
- 95.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 2008; 47:1176–1184. [DOI] [PubMed] [Google Scholar]
- 96.van der Linden JW, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 2011; 17:1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sangoi AR, Rogers WM, Longacre TA, et al. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institution. Am J Clin Pathol 2009; 131:364–375. [DOI] [PubMed] [Google Scholar]
- 98.Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 2012; 367:2214–2225. [DOI] [PubMed] [Google Scholar]
- 99.Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Cutaneous mucormycosis: mycological, clinical, and therapeutic aspects. Curr Fungal Infect Rep 2015; 9:229–237. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


