Abstract
Diagnosis of giant-cell arteritis (GCA) is challenging in the absence of cardinal cranial symptoms/signs. We aimed to describe the clinical presentation, diagnostic process, and disease course of GCA patients without cranial symptoms, and to compare them to those of patients with typical cranial presentation. In this retrospective multicenter study, we enrolled patients with GCA who satisfied at least 3 of the 5 American College of Rheumatology criteria for GCA, or 2 criteria associated with contributory vascular biopsy other than temporal artery biopsy or with demonstration of large-vessel involvement; underwent iconographic evaluation of large arterial vessels (aortic CT scan or a positron emission tomography with 18F-fluorodeoxyglucose combined with computed tomography (FDG-PET/CT) scan or cardiac echography combined with a large-vessel Doppler) at diagnosis. We divided the cohort into 2 groups, distinguishing between patients without cranial symptoms/signs (i.e., headaches, clinical temporal artery anomaly, jaw claudication, ophthalmologic symptoms) and those with cranial symptoms/signs. In the entire cohort of 143 patients, all of whom underwent vascular biopsy and vascular imaging, we detected 31 (22%) patients with no cranial symptoms/signs. In the latter, diagnosis was biopsy proven in an arterial sample in 23 cases (74% of patients, on a temporal site in 20 cases and on an extratemporal site in 3). One-third of these 31 patients displayed extracranial symptoms/signs whereas the remaining two-thirds presented only with constitutional symptoms and/or inflammatory laboratory test results. Compared to the 112 patients with cardinal cranial clinical symptoms/signs, patients without cranial manifestations displayed lower levels of inflammatory laboratory parameters (C-reactive level: 68 [9–250] mg/L vs 120 [3–120] mg/L; P < 0.01), highest rate of aorta and aortic branch involvement identified (19/31 (61%) vs 42/112 (38%); P = 0.02) and also a lower rate of disease relapse (12/31 (39%) vs 67/112 (60%); P = 0.04). Our results suggest that patients without cranial symptoms/signs are prone to lower inflammatory laboratory parameters, fewer relapses, and more large-vessel involvement than those displaying cardinal cranial manifestations. Further studies are therefore required in order to determine whether these 2 subgroups of patients have a different prognosis, and therefore warrant different therapeutic and monitoring regimens.
Keywords: cephalic symptoms, extracephalic involvement, FDG-PET/CT, giant-cell arteritis, temporal artery biopsy
1. Introduction
Giant-cell arteritis (GCA) is the most frequent type of systemic vasculitis in people aged over 50 in western countries. External carotid branch involvement accounts for the high frequency of cranial symptoms.[1] The 1990 Criteria of the American College of Rheumatology (ACR) are still used for disease classification and 3 of the 5 criteria describe cranial findings, that is, new-onset localized headache, temporal artery tenderness or decreased temporal artery pulse, and positive temporal artery biopsy (TAB).[2] The presence of cranial symptoms/signs increases the probability of GCA diagnosis and thereby that of TAB implementation, which remains the most potent criterion for confirming diagnosis. However, 1 subset of patients with GCA did not display any cranial symptoms/signs—diagnosis was therefore delayed in these patients.[3] Definite diagnosis is thus confirmed either on positive arterial histology (on a temporal or an extratemporal vascular biopsy site displaying giant-cell vasculitis), or more recently on the evidence on imaging of extracranial large-vessel involvement. Incidentally, the development of imaging tools such as aortic angiography CT scans or 18F-fluorodeoxyglucose (FDG) positron emission tomography (FDG-PET/CT) facilitates the assessment of large-vessel inflammation with a high degree of sensitivity in detecting the extracranial forms, even in patients without clinical symptoms.[4–7] Like other investigators, we showed in a previous study that large-vessel involvement demonstrated on FDG-PET/CT was frequent and affected half of our patients.[8] A few authors consider clinical GCA disease patterns other than constitutional symptoms, with most GCA patients displaying cardinal cranial manifestations. A few other GCA patients may exhibit only extracranial vascular manifestations (e.g., aorta and aortic branch involvement) or remain asymptomatic in terms of vascular presentation.[9,10] Moreover, these different patient subgroups may have a different clinical course and outcome.[3,9,11,12]
To validate, implement, and perhaps challenge those findings, we conducted a retrospective multicenter study on a large cohort of GCA patients, all of whom underwent extracranial vascular imaging at diagnosis. We therefore aimed to describe the clinical presentation, diagnostic process, and outcome in GCA patients without cranial symptoms/signs, and, finally, to compare them to those of patients exhibiting cranial presentation.
2. Patients and methods
2.1. Study design and patients
This 1995 to 2015 retrospective cohort study enrolled patients from 3 university hospitals in North West France (Caen University Hospital, Limoges University Hospital, and Lille University Hospital). This cohort was created for a previous study which set out to analyze the occurrence of aortic complications in GCA patients.[8] The following criteria were used for this study: diagnosis of GCA was confirmed in the presence of at least 3 of the 5 ACR criteria for GCA.[13] We also included patients with two criteria associated with vascular biopsy other than temporal artery biopsy and displaying giant cell vasculitis. The few patients without cranial symptoms/signs and a negative TAB (i.e., with only 2 ACR criteria) could be enrolled if vascular imaging showed large-vessel involvement and provided that no other condition appeared during follow-up; aortic morphology evaluation by medical imaging (aortic computed tomography or an FDG-PET/CT scan or cardiac echography combined with a Doppler scan of large vessel including the carotid, axillary, and subclavian arteries) on diagnosis.
All data were retrieved through a search of the computerized patient-record system of each institution. The same investigator (HdB) extracted data from all records and collected detailed information using a standardized form.
This study was conducted in compliance with good clinical practices and the Declaration of Helsinki principles. In accordance with French law, formal approval from an ethics committee is not required for this type of study. The manuscript was prepared in accordance with STROBE guidelines.
2.2. Study variables and definitions
Clinical presentation at disease onset for each patient was retrieved, and led to the definition of 2 separate groups of patients—those with cranial manifestations (i.e., headaches, scalp tenderness, jaw claudication, ophthalmologic symptoms) on the one hand and, on the other hand, those without. We also retrieved extracranial symptoms (i.e., PMR, limb claudication, vascular bruits, and pulseless limb). Altogether, using clinical presentation and large-vessel imaging at diagnosis, we identified 4 subgroups of patients: patients with isolated cranial symptoms/signs (i.e., no extracranial manifestations or large-vessel involvement on imaging); patients with isolated large-vessel involvement (i.e., clinical extracranial symptoms and/or large-vessel imaging on imaging); patients with both involvements (i.e., cranial symptoms/signs and large-vessel involvement); patients without cranial symptoms/signs or large-vessel involvement.
Demographic data, past medical history, laboratory parameters (including erythrocyte sedimentation rate, C-reactive protein), TAB (or other vascular biopsy) results, imaging reports, treatment and outcomes were also retrieved for each patient. Imaging results were extracted from radiology reports. According to our institutional guidelines, the analysis of vascular uptake on FDG-PET/CT was qualitative (i.e., positive or negative) and an FDG-PET/CT was positive for diagnosis of vasculitis when showing a circumferential and smooth-line FDG vascular uptake superior to that of the liver, as defined by Hautzel et al,[14] in at least 1 of the 8 following vascular segments: thoracic aorta, abdominal aorta, subclavian, axillary, carotid, iliac/femoral, and upper and lower limb arteries. However, isolated uptakes from the abdominal aorta and/or iliac/femoral and/or lower limb arteries were not considered to be positive results in terms of large-vessel vasculitis, considering the high prevalence of atherosclerosis in such localizations. On CT angiography, aortitis and inflammation of the aortic branches were defined as homogeneous circumferential thickening ≥3 mm. No other condition known to trigger large-vessel inflammation was observed in our patients, including other forms of systemic vasculitis, Behçet disease, Hyper-IgG4 syndrome, Erdheim–Chester disease, sarcoidosis, syphilis, or mycobacteriosis.
Corticosteroid dependency was defined at prednisone dose levels >20 mg/day for 6 months or >10 mg/day for 1 year in order to prevent recurrence. Relapse consisted of recurrence of symptoms and/or inflammatory parameters on laboratory findings, attributable to GCA, which required a sustained increase in treatment. During follow-up, the observation of aortitis on imaging was deemed to be a relapse only if the GCA symptoms and CRP levels increased.
2.3. Statistical analyses
Categorical variables are expressed as number (%) and quantitative variables as median (range). Categorical variables were analyzed according to the Chi-square or Fisher exact test, as appropriate and quantitative variables with Wilcoxon rank-sum test.
Quantitative variables among the four subgroups were analyzed using the Kruskal–Wallis test. For categorical analysis, the Chi-square for trend was used.
Statistical analyses were computed with JMP 9.0.1. A P-value ≤0.05 defined statistical significance.
3. Results
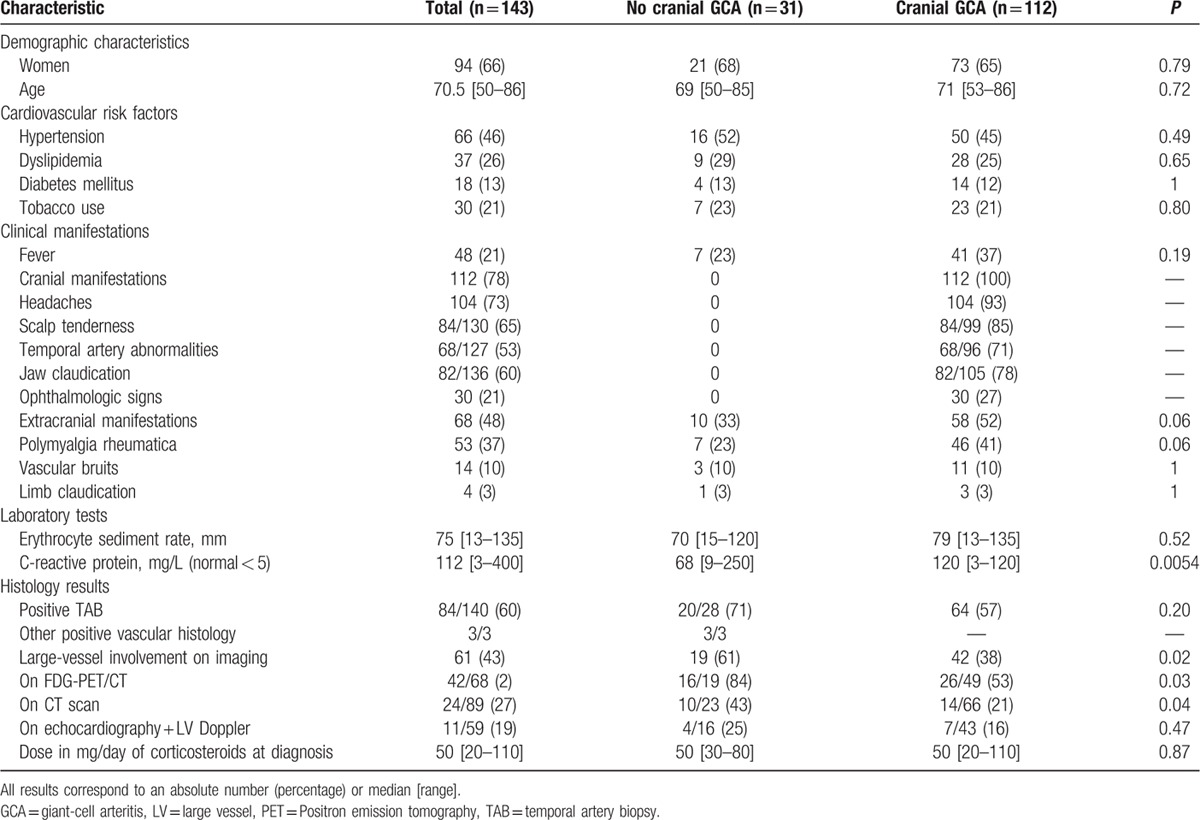
One hundred forty-three patients satisfied the inclusion criteria. Patients’ characteristics on diagnosis and during follow-up are shown in Table 1.
Table 1.
Characteristics of patients with and without cephalic clinical symptoms/signs of giant-cell arteritis at diagnosis.
3.1. Characteristics of the subgroup of patients without any cranial symptoms
We identified a subgroup of 31 (22%) patients (21 women (68%), median age 69 [50–85]) who did not show any cranial symptoms at presentation. Ten (33%) of them presented extracranial clinical manifestations, whereas the other 21 patients had no vascular, rheumatologic or other organic symptom, but constitutional symptoms and/or isolated inflammatory parameters. Diagnosis was confirmed on the basis of histological features in 23 (74%) patients, on TAB in 20 and on a vascular site other than temporal in three patients (mesenteric vascular sample in 1 patient due to mesenteric ischemia requiring emergency surgery, aortic biopsy due to an inaugural aortic dissection in another patient, and femoral artery biopsy due to inaugural lower limb ischemia in the third patient). The eight other patients, whose median follow-up was 31 [22–47] months, had a negative TAB but exhibited large-vessel inflammation on FDG-PET/CT at diagnosis and complete investigations ruled out any other diagnosis.
All 31 patients had high inflammatory parameters with elevated C-reactive protein (CRP) levels, except 2 with a positive TAB who had CRP levels <15 mg/L (normal range < 5 mg/L).
3.2. Imaging details of patients without any cranial symptom/sign
At diagnosis, 8 patients underwent an FDG-PET/CT, 12 others an aortic CT scan and the final 11 underwent both an aortic CT scan and an FDG-PET/CT. Large-vessel Doppler scans and echocardiography were performed in 16 patients, all of whom underwent another aortic imaging procedure.
Altogether, a total of 19 (61%) patients presented with large-vessel inflammation on imaging of the aorta and main aortic branches. In the 11 patients who had both FDG-PET/CT and aortic CT scans, the results were concordant in 6 patients, whereas FDG-PET/CT highlighted large-vessel involvement that was not evident on aortic CT scan in 5 patients. A median of 4 [1–7] vascular territories were affected according to FDG-PET/CT.
All 4 patients with abnormal Doppler scans had subclavian artery involvement. Two had carotid involvement and 1 humeral artery involvement. All displayed diffuse inflammatory involvement on FDG-PET/CT.
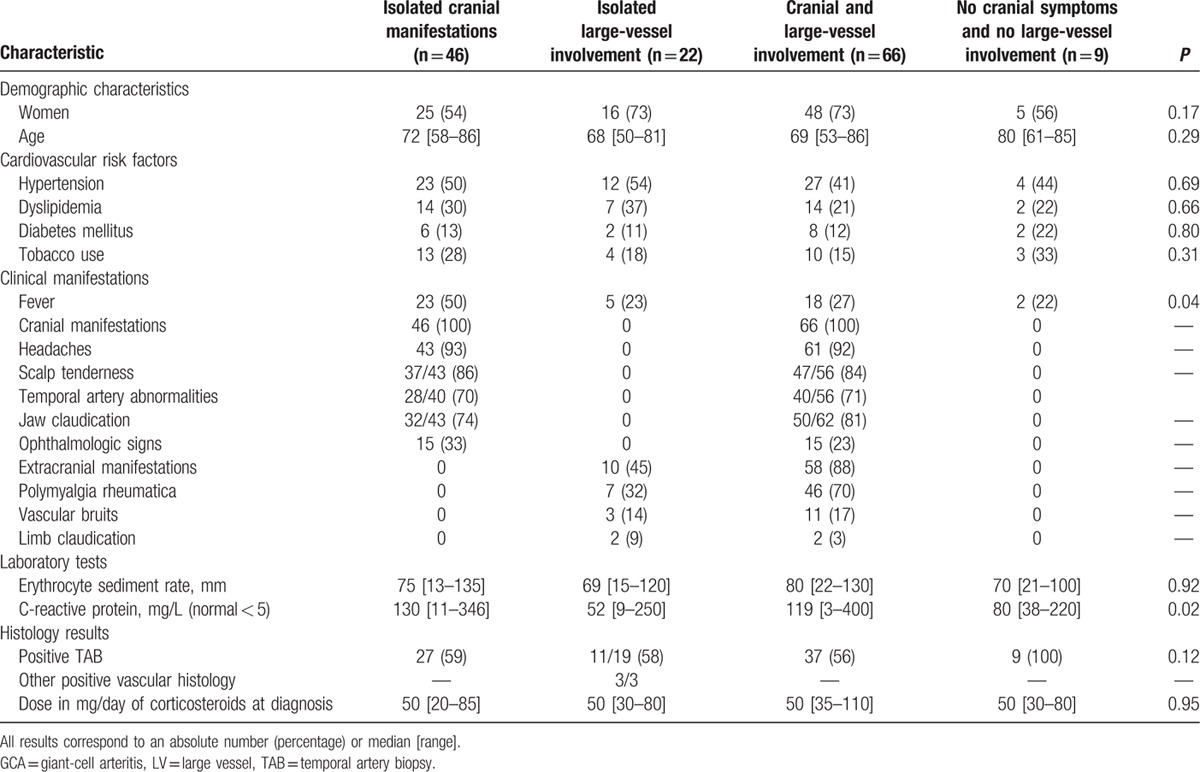
Altogether, among the 31 patients without cranial symptoms/signs, 22 had isolated large-vessel involvement (10 with extracranial manifestations and 19 with large-vessel inflammation on imaging), whereas the 9 other patients did not exhibit any cranial symptoms/signs or extracranial involvement (Table 2).
Table 2.
Characteristics at diagnosis of giant-cell arteritis patients with isolated cranial manifestations, isolated large-vessel involvement, both involvements, and those without cranial or extracranial involvement.
3.3. Outcomes of patients without any cranial symptoms
All 31 patients showed good initial outcomes under corticosteroids. During a follow-up of 30 [6–94] months, 12 (39%) patients experienced disease relapse (median delay: 18 [13–74] months), 5 had initial extracranial symptoms, and 7 exhibited initial constitutional symptoms and/or isolated inflammatory parameters (P = n.s.). Three of these 12 patients with disease relapse developed cranial symptoms/signs only in this setting. Nine showed corticosteroid dependency to control the disease and therefore required a sparing agent (methotrexate in 7 cases, disulone in 1 case, and azathioprine in another case). Nine patients underwent FDG-PET/CT during relapse and 5 tested positive (2 showed persistent large-vessel inflammation and 3 displayed new large-vessel involvement). The 4 others were negative but these patients had not initially demonstrated large-vessel involvement.
Two patients died—the first from a stroke within the 5th week of corticosteroid treatment and the second from multivisceral deficiency following lower limb ischemia during disease relapse confirmed upon surgical femoral artery biopsy. The histological examination confirmed active vasculitis. Two patients with a positive FDG-PET/CT at diagnosis developed aortic dilation that was detected 25 and 32 months after FDG-PET/CT, respectively.
3.4. Comparison of patients with cranial symptoms/signs to those without
When compared to patients with cardinal cranial symptoms/signs, patients devoid of cranial manifestations at disease onset exhibited significantly lower levels of inflammatory laboratory parameters (C-reactive level: 68 [9–250] mg/L vs 120 [3–120] mg/L; P < 0.01), highest rate of identified aorta and aortic branch involvement (19/31 (61%) vs 42/112 (38%); P = 0.02) and also a lower rate of disease relapse (12/31 (39%) vs 67/112 (60%); P = 0.04) (Tables 1 and 2).
When analyzing the 4 subgroups of patients (Table 2), lower inflammatory parameters were found in patients with isolated large-vessel involvement (P = 0.02). Conversely, patients with isolated cranial symptoms/signs had more fever at diagnosis than all other subgroups (P = 0.04).
Relapse rates in the 4 groups were 56%, 32%, 62%, and 56% in isolated cranial GCA, isolated large-vessel GCA, both cranial and large-vessel involvements, and GCA without cranial or large-vessel involvement, respectively (P = 0.10).
Otherwise, no difference was detected between these subgroups of patients in terms of demographics, cardiovascular risk factors, TAB status, and therapeutic management.
A total of 41 (37%) patients with cranial symptoms/signs became corticosteroid-dependent and required a sparing agent: methotrexate in 24 cases, disulone in 6, cyclophosphamide in 6, azathioprine in 2, and TNF alpha blockers in 3 cases (no statistical difference between both groups).
Fourteen patients died including the 2 previously described without cranial symptoms/signs and 12 others with initial cranial manifestations (4 from vertebrobasilar strokes, 4 from acute myocardial infarction, 2 from infections, 1 from leukemia, and 1 during heart surgery for aortic complications). We did not observe any differences in disease course (relapse, corticosteroid-dependency or mortality) in patients with different cranial symptoms/signs.
4. Discussion
This article describes, to the best of our knowledge, the largest series on GCA patients without any cranial symptoms/signs. In addition to this different atypical clinical presentation, patients without cranial symptoms/signs also appear to be a distinct pattern subgroup of GCA because they exhibit lower inflammatory laboratory parameters, more frequent large arterial involvement and less disease relapse compared to patients with typical cranial presentation.
Furthermore, this study is consistent with the following 3 paradigms, namely that patients without cardinal cranial presentation but with constitutional symptoms should be screened for GCA in the absence of any other obvious diagnosis; TAB remains the gold standard to retain a definite diagnosis of GCA even in patients without any cranial signs since more than two-thirds of our patients without cranial symptoms/signs displayed positive histology; the extremely beneficial properties of large arterial imaging, especially FDG-PET/CT in the subgroup of patients without cranial symptoms/signs who are more prone to large artery involvement.
The challenge and difficulty in diagnosing GCA in the absence of cardinal cranial manifestations was emphasized in studies conducted by Gonzalez-Gay et al[3] and Brack et al.[9] They found a longer interval between symptom onset and the time of diagnosis in patients without cranial symptoms/signs compared to patients with typical presentation.[3,9] TAB as well as whole-body imaging is currently used to investigate long-term, persistent, constitutional symptoms, and/or inflammatory laboratory tests without obvious diagnosis. Both of these can lead to the diagnosis of GCA by showing signs indicative of vasculitis. Some studies have pointed out predictive criteria for a positive TAB with several contradictory conclusions. For example, in a few studies, headaches, visual impairment, jaw claudication, abnormalities of temporal artery palpation, and high inflammatory parameters would be more frequent in patients with a positive TAB compared to patients with negative TAB.[15–17] Conversely, for others, headaches or PMR would be more common in patients with negative TAB.[17] In exclusive extracranial forms of GCA, Brack et al[9] suggest that TAB cannot be considered as the gold standard for confirming GCA diagnosis given the high rate of negative TAB. Our study contradicts this statement.
Since the development of new easy-access imaging tools such as FDG-PET/CT, aortic CT scan or aortic magnetic resonance angiography, testing for large-vessel involvement has become standard clinical practice in the diagnosis of GCA. The frequency of inflammatory aorta and aortic branch involvement varies from 22% to 85% of GCA cases according to the literature.[4,8,18–22] Our study detected a 43% rate of large-vessel involvement. This discrepancy could be attributed to the variability of imaging techniques used, all of which have different levels of sensitivity in detecting large-vessel involvement.
We previously showed that patients who exhibited large-vessel inflammation were more likely to develop early aortic complications.[8] These patients should probably be carefully screened in terms of aortic morphology after displaying large-vessel inflammation.
According to many authors, there are different disease patterns in GCA.[9,23,24] Indeed, patients with large-vessel involvement are believed to follow a different course compared to patients with typical cranial presentation. Kermani et al[25] and Espitia et al[11] showed that patients with large-vessel involvement have more relapses, decreased survival, and more cardiovascular events. The study by Brack et al[9] showed that the latter might have lower inflammatory laboratory parameters and a lower rate of positive TAB. Nuenninghoff et al[26] showed that there may also be more cases of hyperlipidemia and coronary artery disease and, as in our study, fewer cranial manifestations and lower inflammatory laboratory parameters. Muratore et al[12] showed a smaller proportion of cranial symptoms/signs and a higher rate of disease relapse in patients with large-vessel involvement, which is consistent with our findings. Overall, these studies remind us how heterogeneous the clinical forms of GCA can be. As in the study by Gonzalez-Gay et al,[24] our data suggest that GCA without cranial symptoms/signs, represents another independent disease pattern with a distinctly less inflammatory presentation and a lower rate of relapse.
A few studies have focused on GCA patients without cranial manifestations. Gonzalez-Gay et al[3] showed that patients with headaches, when compared to those without, had a shorter length of time to diagnosis, more frequent clinical abnormalities of the temporal arteries and a more frequent PMR picture.
Some studies suggest that there is probably a different immunological and inflammatory pathway depending on GCA patterns.[27] Brack et al[9] showed that patients with large-vessel involvement had a higher level of interleukin (IL)-2 but a lower level of IL-1β, IL-6, and TGF-β1 than patients with typical cranial presentation. Because IL-1 and IL-6 are the leading proinflammatory cytokines involved in systemic inflammation resulting in CRP increase, these pathophysiological differences may support the profile of lower inflammatory parameters in patients without cranial symptoms/signs.
ACR criteria for GCA diagnosis were satisfied in only 65% of our patients without cranial symptoms/signs, reminding us how challenging diagnosis actually is in patients without typical cranial manifestations. Our results are clinically relevant since they show that patients with no cranial symptoms/signs should even have a TAB, which still proved beneficial in two-thirds of cases. The working diagnosis of these patients should also include large-vessel imaging in order to analyze the inflammatory status of the aorta and aortic branches. Further investigations would be useful to revise ACR criteria taking cranial and extracranial symptoms/signs into consideration.
Our work does, however, carry some limitations, particularly because of its retrospective design. We did not retrieve the delay between symptom onset and time of diagnosis to highlight how challenging it is to confirm GCA diagnosis in the subgroup of patients without cranial signs, as evidenced in the studies of Gonzalez-Gay et al[3] and Brack et al.[9] Moreover, we did not retrieve enough data on clinical symptoms or biological parameters during follow-up, thus precluding any analysis on changes in symptoms during relapse. Despite being the largest patient series without cranial manifestations, our relatively moderate sample size might have precluded the identification of other differences with patients showing typical cranial GCA symptoms. Similarly, the imaging of extracranial vascular involvement was not homogeneous in our cohort. Half of the cohort underwent FDG-PET/CT, which is whole body imaging with the best reproducible data acquiring interpretation, whereas some of them only had a combination of echocardiography and a peripheral large-vessel Doppler scan, which is probably less sensitive for detecting subclavian artery or abdominal aorta involvement, for instance, and more prone to displaying interindividual variability under certain circumstances. While 62% of our patients underwent an aortic CT scan, we detected a relatively lower rate of large-vessel involvement compared to FDG-PET/CT. Moreover, we did not review these images centrally.
It may be argued that this study detected more extracranial vascular involvement in patients without cranial symptoms/signs because these patients were more likely to be referred to FDG-PET/CT for atypical presentation. However, all our patients were screened for large-vessel involvement and over 40% underwent an FDG-PET/CT scan with typical cranial presentation.
We only included patients who underwent large-vessel imaging at diagnosis. Although large-vessel evaluation is standard clinical practice in our centers, some patients did not undergo this procedure and were not enrolled in this study. Thus, our cohort was not formed with consecutive patients. We probably overestimated the proportion of patients without cranial symptoms/signs, who were more likely to be referred for whole-body imaging.
5. Conclusion
In our study, patients without cranial presentation showed lower inflammatory laboratory parameters, more frequent large-vessel involvement and less disease recurrence than patients with the cardinal cranial presentation. Despite the apparently more benign presentation and clinical course, our previous work revealed that patients with large-vessel inflammation are more prone to developing early aortic complications, such as dilation or dissection. Thus, patients without cranial symptoms/signs but with large-vessel involvement should probably be carefully screened in terms of aorta morphology during follow-up. Further prospective studies are required to determine whether these 2 patient subgroups have a different prognosis, and require different therapeutic and monitoring regimens.
Footnotes
Abbreviations: ACR = American College of Rheumatology, CRP = C-reactive protein, CT = computed tomography, FDG-PET/CT = positron emission tomography with 18F-fluorodeoxyglucose combined with computed tomography, GCA = giant-cell arteritis, PMR = polymyalgia rheumatica, TAB = temporal artery biopsy.
The authors have no conflicts of interest to disclose.
References
- 1.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008; 372:234–245. [DOI] [PubMed] [Google Scholar]
- 2.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990; 33:1122–1128. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Gay MA, Garcia-Porrua C, Amor-Dorado JC, et al. Giant cell arteritis without clinically evident vascular involvement in a defined population. Arthritis Rheum 2004; 51:274–277. [DOI] [PubMed] [Google Scholar]
- 4.Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006; 55:131–137. [DOI] [PubMed] [Google Scholar]
- 5.Prieto-Gonzalez S, Depetris M, Garcia-Martinez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73:1388–1392. [DOI] [PubMed] [Google Scholar]
- 6.Besson FL, Parienti JJ, Bienvenu B, et al. Diagnostic performance of (1)(8)F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011; 38:1764–1772. [DOI] [PubMed] [Google Scholar]
- 7.Puppo C, Massollo M, Paparo F, et al. Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. Biomed Res Int 2014; 2014:574248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boysson H, Liozon E, Lambert M, et al. [18F] FDG PET in giant-cell arteritis: a prognostic tool for aortic complications. Ann Rheum Dis 2014; 73 (suppl. 2):60. [Google Scholar]
- 9.Brack A, Martinez-Taboada V, Stanson A, et al. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42:311–317. [DOI] [PubMed] [Google Scholar]
- 10.Forster S, Tato F, Weiss M, et al. Patterns of extracranial involvement in newly diagnosed giant cell arteritis assessed by physical examination, colour coded duplex sonography and FDG-PET. Vasa 2011; 40:219–227. [DOI] [PubMed] [Google Scholar]
- 11.Espitia O, Neel A, Leux C, et al. Giant cell arteritis with or without aortitis at diagnosis. A retrospective study of 22 patients with longterm followup. J Rheumatol 2012; 39:2157–2162. [DOI] [PubMed] [Google Scholar]
- 12.Muratore F, Kermani TA, Crowson CS, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology 2015; 54:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994; 37:187–192. [DOI] [PubMed] [Google Scholar]
- 14.Hautzel H, Sander O, Heinzel A, et al. Assessment of large-vessel involvement in giant cell arteritis with 18F-FDG PET: introducing an ROC-analysis-based cutoff ratio. J Nucl Med 2008; 49:1107–1113. [DOI] [PubMed] [Google Scholar]
- 15.Mari B, Monteagudo M, Bustamante E, et al. Analysis of temporal artery biopsies in an 18-year period at a community hospital. Eur J Intern Med 2009; 20:533–536. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum 2001; 30:249–256. [DOI] [PubMed] [Google Scholar]
- 17.Duhaut P, Pinede L, Bornet H, et al. Biopsy proven and biopsy negative temporal arteritis: differences in clinical spectrum at the onset of the disease. Ann Rheum Dis 1999; 58:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agard C, Barrier JH, Dupas B, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum 2008; 59:670–676. [DOI] [PubMed] [Google Scholar]
- 19.Aschwanden M, Kesten F, Stern M, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2×11 arterial regions. Ann Rheum Dis 2010; 69:1356–1359. [DOI] [PubMed] [Google Scholar]
- 20.Blockmans D, De Ceuninck L, Vanderschueren S, et al. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: a prospective study in 35 patients. Rheumatology 2007; 46:672–677. [DOI] [PubMed] [Google Scholar]
- 21.Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 2012; 71:1170–1176. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt WA, Seifert A, Gromnica-Ihle E, et al. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology 2008; 47:96–101. [DOI] [PubMed] [Google Scholar]
- 23.Cid MC, Prieto-Gonzalez S, Arguis P, et al. The spectrum of vascular involvement in giant-cell arteritis: clinical consequences of detrimental vascular remodelling at different sites. APMIS Suppl 2009; 127:10–20. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Gay MA, Barros S, Lopez-Diaz MJ, et al. Giant cell arteritis: disease patterns of clinical presentation in a series of 240 patients. Medicine 2005; 84:269–276. [DOI] [PubMed] [Google Scholar]
- 25.Kermani TA, Warrington KJ, Crowson CS, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis 2013; 72:1989–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003; 48:3522–3531. [DOI] [PubMed] [Google Scholar]
- 27.Weyand CM, Tetzlaff N, Bjornsson J, et al. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum 1997; 40:19–26. [DOI] [PubMed] [Google Scholar]


