Abstract
Previous studies reported a 2- to 17-fold higher risk of aortic complications (dilation or dissection) in patients with giant-cell arteritis (GCA). We aimed to determine whether or not GCA patients with large-vessel involvement demonstrated by positron emission tomography with 18F-fluorodeoxyglucose combined with computed tomography (FDG-PET/CT) have a higher risk of aortic complications. We conducted a retrospective multicenter study between 1995 and 2014. Patients were included if they fulfilled at least 3 American College of Rheumatology criteria for GCA, or 2 criteria associated with extratemporal biopsy-proven giant-cell vasculitis; they underwent at least 1 FDG-PET/CT scan at diagnosis or during follow-up; and the morphology of the aorta was assessed by medical imaging at diagnosis. Patients with an aortic complication at the time of diagnosis were excluded. Of the 130 patients included [85 women (65%), median age 70 (50–86)], GCA was biopsy proven in 77 (59%). FDG-PET/CT was performed at diagnosis in 63 (48%) patients and during the follow-up period in the 67 (52%) remaining patients. FDG-PET/CT was positive in 38/63 (60%) patients at diagnosis and in 31/67 (46%) patients when performed during follow-up (P = NS). One hundred four patients (80%) underwent at least 1 morphological assessment of the aorta during follow-up. Nine (9%) patients developed aortic complications (dilation in all and dissection in 1) at a median time of 33 (6–129) months after diagnosis. All of them displayed large-vessel inflammation on previous FDG-PET/CT. A positive FDG-PET/CT was significantly associated with a higher risk of aortic complications (P = 0.004).
In our study, a positive FDG-PET/CT was associated with an increased risk of aortic complications at 5 years.
Keywords: 18FDG-PET/CT, aortic complications, aortic dilation, giant-cell arteritis, prognostic tool
1. Introduction
Giant-cell arteritis (GCA) is the most common form of vasculitis in patients over 50 years old and is mainly identified through cephalic symptoms. However, evidence of extracephalic large-vessel involvement has emerged in recent decades, especially with the development of new imaging tools, making it possible to distinguish extracephalic forms from typical cephalic forms.[1–5] Positron emission tomography combined with computed tomography (PET/CT) with 18F-fluorodeoxyglucose (FDG) can be used to assess large-vessel inflammation and has demonstrated high sensitivity in detecting extracephalic forms, even in patients without clinical symptoms.[6–9]
Previous studies reported a 2- to 17-fold higher risk of aortic complications (dilation or dissection) in patients with GCA, occurring in 8% to 22% of patients during the first years of the disease.[10–14] In a population-based study of GCA patients followed over 50 years, aortic complications occurred in 18 of the 168 patients 5.1 to 10.9 years after diagnosis.[15] Blockmans et al[16] showed that patients with aortitis revealed by FDG-PET at diagnosis were more likely to develop a late increase in the volume of the thoracic aorta. It has also been suggested that aortic inflammation may be associated with a higher risk of cardiovascular death and a higher relapse rate.[17] Moreover, pathological evidence of active giant-cell aortitis has been reported in patients with GCA undergoing aortic surgical procedures.[18,19] Overall, there is probably a small subset of patients who are more predisposed to large-vessel inflammation and subsequent aortic complications, and early identification of these patients remains a clinical challenge.
We conducted a retrospective multicenter study on a large cohort of patients with GCA to evaluate whether or not patients with aortic involvement, demonstrated by FDG-PET/CT, have a higher risk of aortic complications.
2. Patients and methods
2.1. Study design and patients
This retrospective cohort study from 1995 to 2014 enrolled patients from 3 university hospitals (Caen University Hospital, Limoges University Hospital, and Lille University Hospital). The inclusion criteria were as follows: patients had to fulfill at least 3 of the 5 American College of Rheumatology (ACR) criteria for GCA,[20] or 2 criteria associated with a positive biopsy of arterial tissue other than the temporal artery, showing a vascular transmural infiltrate with the presence of giant cells; at least 1 FDG-PET/CT scan had been performed at diagnosis or during follow-up; and the morphology of the aorta was assessed by medical imaging at diagnosis. We excluded patients who already showed an aortic complication at the time of diagnosis, or patients who presented an aortic complication before the FDG-PET/CT scan was performed. Patients with isolated polymyalgia rheumatica (PMR), with no symptoms of GCA, were not included.
All the data were retrieved through a search of the computerized patient-record system at each institution, using the keywords “giant-cell arteritis” and “positron emission tomography.” The same investigator (HdB) extracted data from all the charts and collected detailed information using a standardized form.
This study was conducted in compliance with good clinical practices and the Declaration of Helsinki principles. In accordance with French law, formal approval from an ethics committee is not required for this type of study.
2.2. Study variables and definitions
We defined an aortic complication as the occurrence of aortic dilation or aortic dissection. Aortic dilation was defined as an increase in aorta diameter or a loss of parallelism of the aortic wall demonstrated by means of aortic CT or MR angiography, FDG-PET/CT scan, or an echocardiography of the aortic root. Adapted from previous report,[4] we considered the aortic root, aortic arch, and descending aorta to be dilated when the aortic diameter was ≥4.5, ≥4, and ≥3.5 cm, respectively. We analyzed the occurrence of aortic complications occurring within 5 years of the FDG-PET/CT scan and recorded the interval between the first FDG-PET/CT and diagnosis of an aortic complication.
Aortitis was defined as a homogeneous circumferential thickening of ≥3 mm of new onset on CT angiography or an increase in FDG metabolism, with inflammatory biological parameters. Relapse was defined as the reoccurrence of clinical symptoms and/or increase in inflammatory parameters not attributable to a condition other than GCA. During follow-up, aortitis was considered to be a relapse only when imaging findings were associated with an increase in CRP and one of the following symptoms: constitutional and/or GCA symptoms and/or back pain.
The following data were recorded at diagnosis and during follow-up: demographics, cephalic manifestations (headaches, ophthalmologic disorders), extracephalic symptoms (PMR, limb claudication, vascular bruits, pulseless limb), and fever (>38.5°C). Laboratory test findings, temporal artery biopsy (TAB) status (or any other vascular histology), and FDG-PET/CT results were also collected.
At diagnosis, all patients underwent aortic imaging (aortic CT angiography, FDG-PET/CT scan, or abdominal echography combined with an echocardiogram) to identify large-vessel involvement or an aortic complication. For those patients who did not undergo an FDG-PET/CT scan at diagnosis (before or within 10 days of starting treatment), the examination was performed during follow-up. CRP rate and corticosteroid dose were recorded at the time of the FDG-PET/CT. We analyzed the occurrence of aortic complications in the whole cohort and in both subgroups of patients according to the time at which FDG-PET/CT was performed (i.e., at diagnosis or during follow-up).
2.3. FDG-PET protocol
FDG-PET/CT was performed using a Biograph 6 TrueV-HD (Siemens Medical Solutions, Erlangen, Germany) at Caen and Limoges University Hospitals, and a Discovery RX HD 16 (GE Healthcare, Buc, France) at Lille University Hospital. At each center, after a delay of 40 to 45 minutes following injection, an FDG-PET emission scan was obtained with 3 minutes per bed position in 3D mode. Images were acquired according to standard procedural guidelines (4–6 hours of fasting prior to injection of 4 MBq/kg of FDG and glycemia levels below 1.8 g/L). The 3D FDG-PET data were reconstructed using an iterative algorithm.
FDG-PET/CT results were extracted from Nuclear Medicine reports. According to our institutional guidelines, the analysis of vascular uptake was qualitative (i.e., positive or negative) and an FDG-PET/CT was deemed positive when it recorded circumferential FDG vascular uptake superior to the liver uptake as defined by Hautzel et al[21] in at least 1 of the following 8 vascular segments: thoracic, abdominal aorta, subclavian, axillary, carotid, iliac/femoral, and upper and lower limb arteries. Isolated uptake from the abdominal aorta and/or iliac/femoral and/or lower limb arteries was considered a negative result considering the high prevalence of atherosclerosis in such localizations. Focal (noncircumferential) FDG uptake was considered to be an atherosclerotic lesion and thus classified as a negative FDG-PET/CT. Patients with positive FDG-PET/CTs were considered to have large vascular extracephalic involvement.
2.4. Statistical analyses
Categorical variables are expressed as numbers (%) and quantitative variables as medians (range). Categorical variables were analyzed with the Chi-square or Fisher exact test, as appropriate, and quantitative variables with Wilcoxon rank-sum test.
Aortic complication-free survival rates, analyzed using life tables and the Kaplan–Meier method, were compared with log-rank tests. The date of FDG-PET/CT acquisition defined as T0 on survival curves and events, consisted in the occurrence of aortic complications. The date of the latest news or time of death was considered to be a censored point. Statistical analyses were computed using GraphPad Prism (5.0c, GraphPad Software, La Jolla, USA), except for survival, which was analyzed with JMP v9.0.1 (2010 SAS Institute, Inc. Cary, USA). A P-value ≤0.05 defined statistical significance.
3. Results
3.1. Patient characteristics at diagnosis and during follow-up
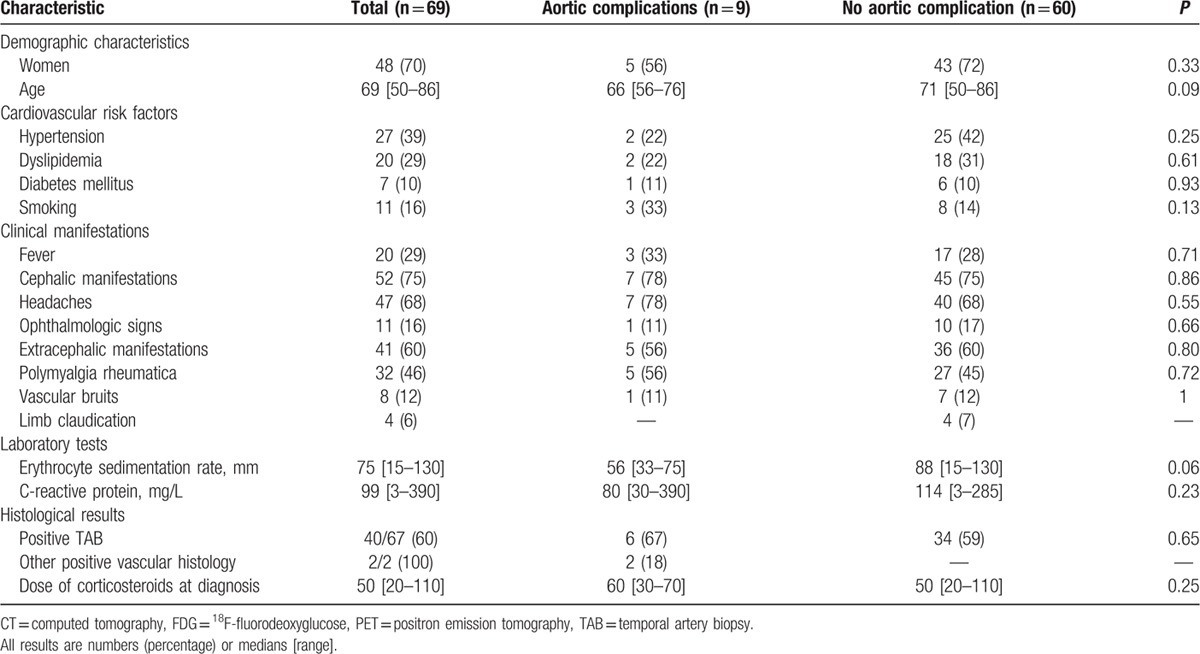
The clinical and imaging data were retrieved for 130 patients [85 women (65%), median age 70 (50–86)] diagnosed as having GCA between 1995 and 2014 and meeting the inclusion criteria. All patients satisfied at least 3 ACR criteria except for 2 who had 2 criteria and 1 extratemporal biopsy-proven giant-cell vasculitis on a mesenteric and femoral artery sample, respectively. GCA was biopsy proven in 77 (59%) patients (including 75 positive TAB). The patient characteristics at diagnosis are given in Table 1.
Table 1.
Patient characteristics at diagnosis and according to PET/CT results.
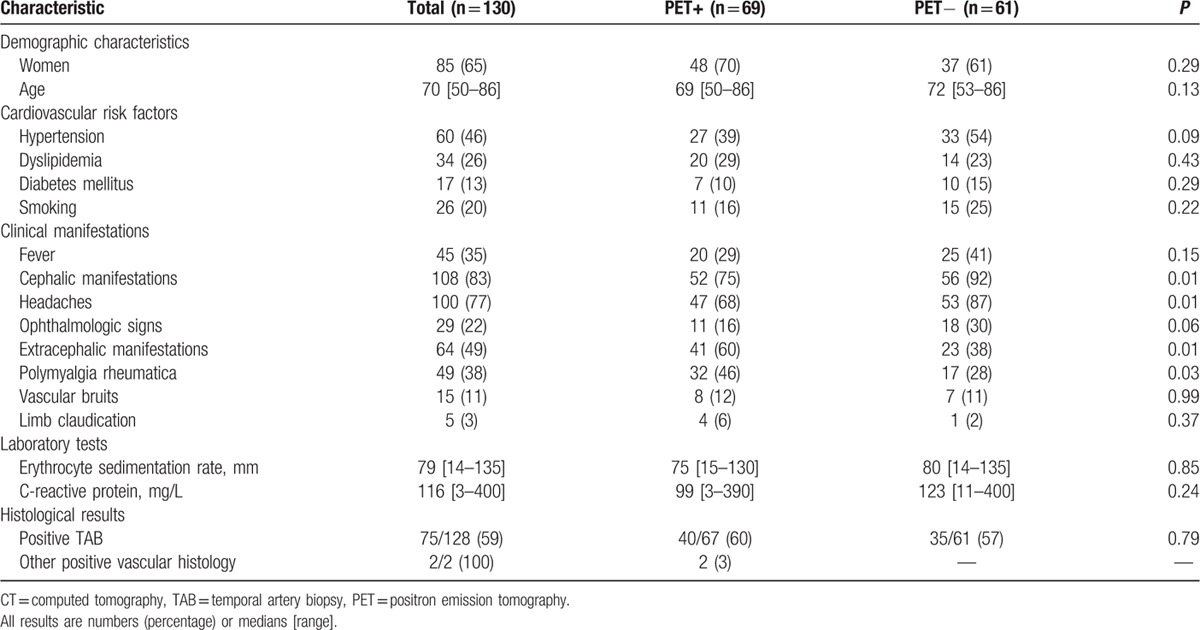
At presentation, 52 patients had isolated cephalic symptoms, 8 isolated extracephalic manifestations, 56 suffered from both, and 14 did not have any clinical symptoms. Of the 22 patients with no cephalic manifestations, GCA was biopsy proven in 18 (positive TAB in 17 cases and positive mesenteric artery sample in 1) and 17 patients had a positive FDG-PET/CT demonstrating large-vessel involvement. The 4 patients with negative TAB had a positive FDG-PET/CT and presented cephalic manifestations on disease relapse. They were retrospectively considered as fulfilling ACR criteria for GCA. Follow-up duration in these 4 patients was 62 [47–97] months and none of them was diagnosed with another condition during follow-up. PMR was present in 49 (38%) patients. All the patients had high inflammatory parameters on lab tests except for 4 patients who had a CRP < 15 mg/L but a positive TAB.
All the patients received only corticosteroids after diagnosis [median dose at introduction 50 (20–110) mg] and the median follow-up was 47 (6–273) months after diagnosis. In the whole cohort, 76 (58%) patients relapsed and 13 (10%) died (4 from vertebrobasilar stroke, 4 from acute myocardial infarction, 2 from infections, 1 from multivisceral organ failure due to acute lower limb ischemia, 1 from leukemia, and 1 during cardiac surgery for aortic complications). Twenty-two patients with disease relapse received methotrexate along with an increased dose of corticosteroids.
3.2. FDG-PET/CT findings
FDG-PET/CT was performed at diagnosis in order to search for large-vessel involvement in 63 (48%) patients, with 32 having extracephalic manifestations and 48 (76%) cephalic symptoms. The 67 remaining patients [52%; 32 (48%) with extracephalic manifestations and 60 (90%) with cephalic symptoms] were referred for FDG-PET/CT during the follow-up period due to suspected relapse in 54 and evaluation of the aorta and its branches in 13 (median time after diagnosis 29 [2–261] months). Among patients who underwent FDG-PET/CT during follow-up, 51 were receiving corticosteroids (median dose: 15 [1–60] mg), associated with methotrexate in 17 cases.
FDG-PET/CT was positive in 38/63 (60%) patients at diagnosis and in 31/67 (46%) patients when performed during follow-up (P = 0.11). The 17 patients who were taking corticosteroids combined with methotrexate had a positive FDG-PET/CT. Overall, a total of 69 (53%) patients had a positive FDG-PET/CT, the characteristics of which are shown in Table 2. The thoracic section of the aorta was the area mostly affected (78%), followed by the subclavian arteries (72%) and the abdominal aorta (55%). No patient had isolated involvement of lower or upper limbs.
Table 2.
PET/CT findings in patients with positive scan (n = 69).
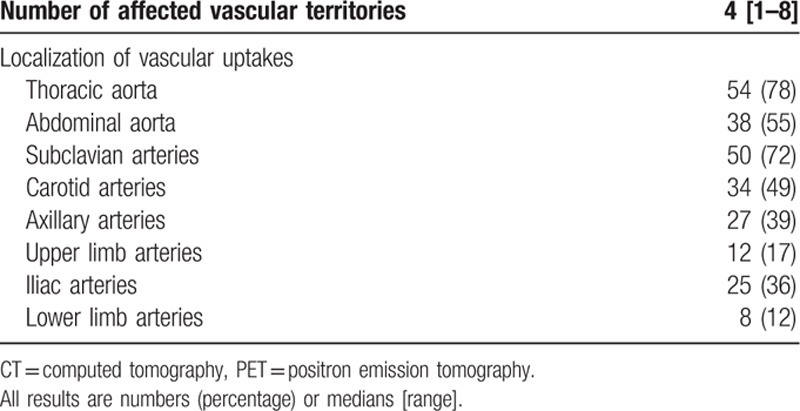
For all the FDG-PET/CTs performed, extravascular uptake was observed in 32 patients, including 15 with concomitant vascular uptakes. In 20 patients, extravascular uptake was identified as corresponding to cancer in 5 (lung, colon, prostate, mandible, and uterus), subacromial bursitis in 8, colic polyps in 3, mediastinal lymphadenopathy in 2, uterus in 1 (large fibroma), and thyroid gland in 1 (benign nodule). For the remaining 12 patients, no investigation was undertaken to explore extravascular uptake.
3.3. Factors associated with a positive FDG-PET/CT and aortic complications
Compared with patients whose results were negative, patients with a positive FDG-PET/CT had more extracephalic manifestations including PMR and fewer cephalic symptoms (60% vs 38%, P = 0.01 and 75% vs 92%, P = 0.01 respectively; Table 1). Extracephalic manifestations were associated with positive PET findings. Of the 108 patients with cephalic symptoms, we observed a higher rate of positive FDG-PET/CT in patients with associated extracephalic manifestations: 35/56 (63%) compared to 17/52 (33%) in patients with isolated cephalic symptoms (P = 0.002).
At the time of the FDG-PET/CT, patients with a positive scan were receiving a lower dose of corticosteroids than patients with negative scans [0.5 (0–70) mg vs 15 (0–80) mg, P = 0.01] but did not have higher inflammatory parameters [median CRP 35 (1–200) mg/L vs 20 (1–250) mg/L, P = 0.1]. FDG-PET/CT results did not differ according to TAB status.
One hundred four patients (80%) had undergone a morphological assessment of the aorta after the FDG-PET/CT, at a median time of 27 (4–77) months in patients with a positive baseline FDG-PET/CT (n = 53), and at 25 (6–107) months (P = NS) in patients with a negative baseline FDG-PET/CT (n = 51). The imaging technique for this follow-up assessment consisted of a new FDG-PET/CT in 30 patients, CT angiography in 66, echocardiography in 7, and MR angiography in 1.
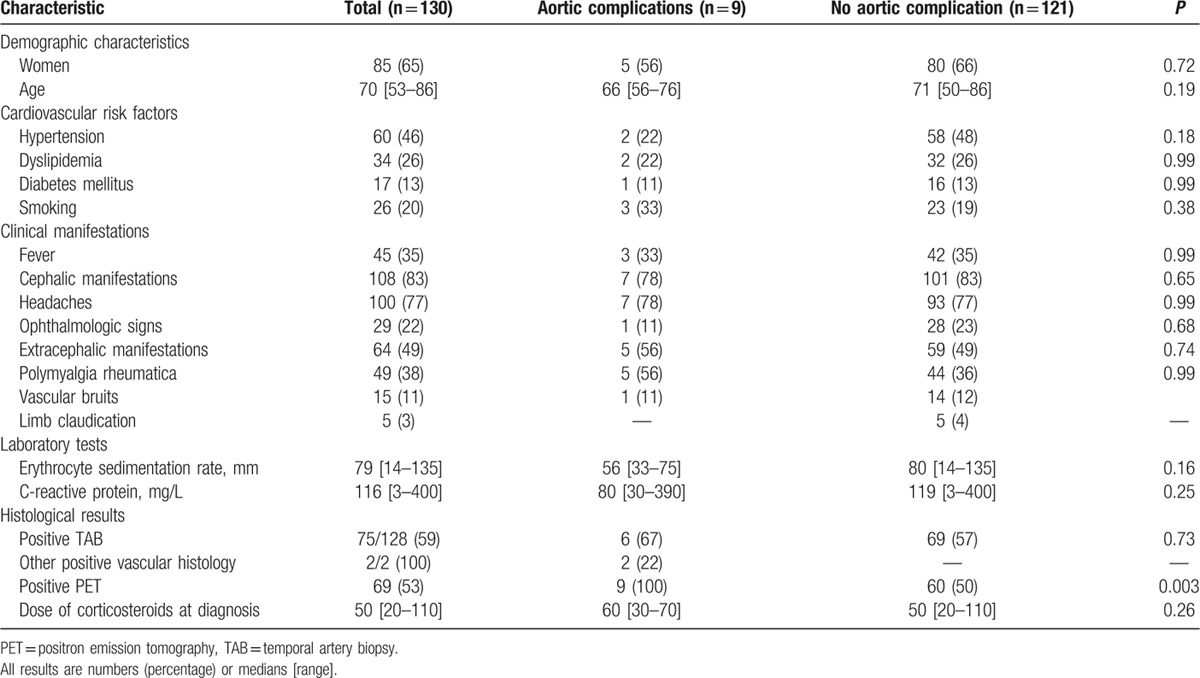
Nine patients (9%) were diagnosed with aortic complications (aortic dilation in all and aortic dissection in 1) at a median time of 33 (6–129) months after diagnosis. The characteristics of these patients are shown in Table 3. The aortic dilation involved the thoracic aorta in 7 patients, the abdominal aorta in 1 and both in 1 (median diameter of thoracic aorta: 44 (40–54) mm; the diameter of the abdominal aorta in the 2 patients with abdominal aorta dilation: 38 and 40 mm). The patient with isolated abdominal localization was a corticosteroid-dependent woman who presented isolated infrarenal aortic dilation, with no evidence of atherosclerosis. The 9 patients had a previous positive FDG-PET/CT performed 25 (6–54) months before the detection of aortic complications. A median of 3 (1–6) vascular segments was positive. In 8 patients, aortic dilation occurred in the same region that had been inflammatory on the previous FDG-PET/CT. There was no difference between patients with or without aortic complications regarding cardiovascular risk factors, initial clinical presentation, TAB status, inflammatory parameters, therapeutic management, and relapse rate (Table 4).
Table 3.
Detailed characteristics of the 9 patients with giant-cell arteritis who experienced aortic complications 25 [6–54] months after positive PET.
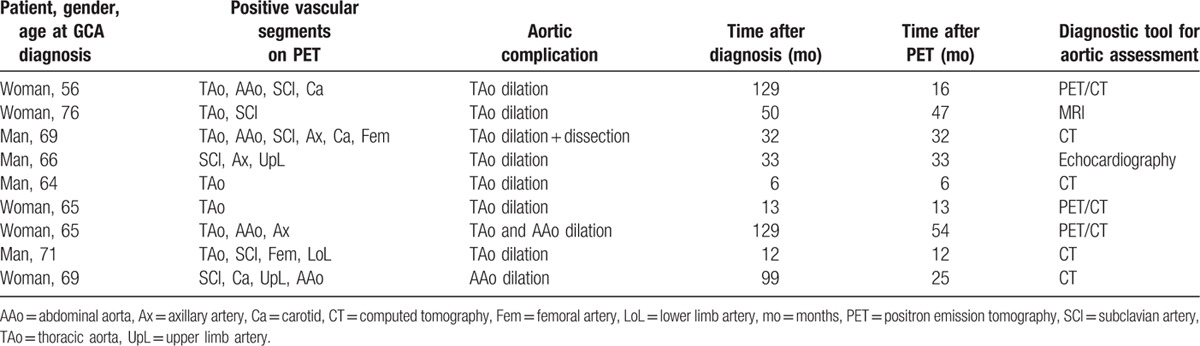
Table 4.
Patient characteristics according to the presence or absence of aortic complications.
As shown in the Kaplan–Meier curves, a positive FDG-PET/CT at baseline was associated with a significantly higher risk of aortic complications, in the whole cohort (Fig. 1A), in patients who underwent the procedure at diagnosis (Fig. 1B) or during follow-up (Fig. 1C) (log rank: P = 0.004, P = 0.04, and P = 0.05, respectively).
Figure 1.
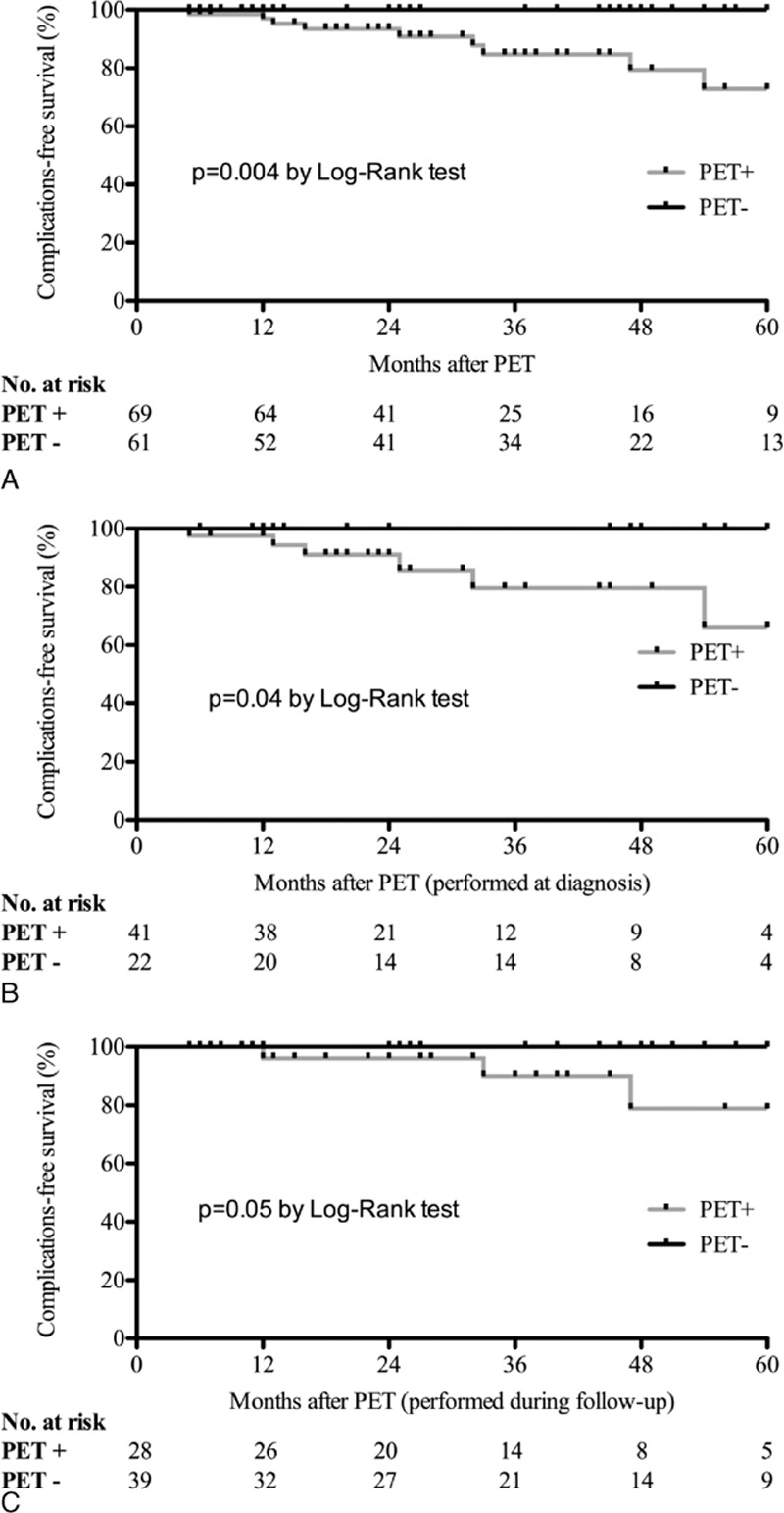
Kaplan–Meier curves of aortic complication-free survival in patients with giant-cell arteritis. The x axis shows the follow-up time after positron emission tomography (PET) in 12-month intervals, with the number of participants (positive or negative PET) who are still at risk. The aortic complication-free survival is shown in the entire cohort (A), and in patients who underwent FDG-PET/CT at diagnosis (B) or during follow-up (C). Comparison between patients with positive PET vs those with negative PET found a statistically significant increase in aortic complications (dilation and/or dissection) in patients with a positive PET result, regardless of when PET is performed.
In univariate analysis, only a positive FDG-PET/CT scan was associated with aortic complications. We did not observe any other significant predictive factors of aortic complications in the entire cohort (Table 4) or in patients with positive FDG-PET/CT (Table 5).
Table 5.
Characteristics of 69 patients with positive FDG-PET/CT depending on the presence or absence of aortic complications.
Aortic complications led to surgery in 2 patients. Both of them showed evidence of vascular inflammation on surgical vascular samples. The first patient underwent an emergency Bentall operation with aortic root replacement for an acute aortic dissection, and had histological evidence of giant-cell vasculitis with transmural inflammation. He died a few hours after surgery. The second patient had tube graft surgery for a thoracic aortic dilation measured at 54 mm. Histology showed perivascular infiltration of lymphocytes but no giant cells. Of the 13 patients who died, a vascular event was involved in 11, including 6 patients who had a positive FDG-PET (P = NS).
4. Discussion
To the best of our knowledge, the present study describes the largest known cohort of patients with GCA referred for noninvasive vascular assessment of inflammation using FDG-PET/CT. This showed involvement of the aorta and its branches in one-half of the cohort. Aortic complications occurred in 9 of the patients within the first few years of large-vessel inflammation being identified on the FDG-PET/CT, regardless of whether the procedure was performed at diagnosis or during follow-up. In the present study, aortic complications occurred at a median time of 33 months after diagnosis, and 25 months after the FDG-PET/CT that showed large-vessel inflammation.
Extracephalic large-vessel involvement is more common than previously reported in GCA and is more frequently tested for, even in patients without extracephalic manifestations.[4,5] Early detection of the involvement of the aorta and its main branches is essential for patient management. It has been suggested that patients with large-vessel inflammation may have a poor clinical outcome, with increased vascular events and late dilation of the thoracic aorta, which may be associated with a higher risk of death, especially in relation to aortic dissection.[13,16,17]
In the study by Gonzalez-Gay et al,[12] 8.1% of patients (17 of 210) developed an aortic complication at the 3-year follow-up stage after diagnosis. In 44 patients followed for 5.4 years, Garcia-Martinez et al[11] reported a 22.2% complication rate. Kermani et al[13] reported a rate of 16% over a median time of 8.8 years and Nuenninghoff et al[15] a rate of 18% in a population of GCA followed for over 50 years.
However, none of these previous studies reported a relationship between previous evidence of large-vessel inflammation in patients who later developed aortic complications. Pathological studies in patients with GCA who underwent aortic surgery showed inflammation in aortic samples, suggesting that aortic complications might be a consequence of inflammatory damage of the aortic wall.[18,19] Garcia-Martinez et al[11] showed that patients who received a lower dose of steroids were more likely to develop aortic complications, suggesting that aortitis may possibly lead to aortic damage even in patients whose GCA was clinically quiescent. Our work suggests that careful attention to aortic morphology is required, especially in patients who showed large-vessel involvement on the FDG-PET/CT.
Many factors have been identified as being associated with aortic complications. Gonzalez-Gay et al[12] suggested that a younger age at diagnosis, hypertension, persistence of high inflammatory parameters and frequent relapses may be associated with a higher risk of aortic complications. Nuenninghoff et al[15] showed that patients with hyperlipidemia, aortic regurgitation murmur at GCA diagnosis, and coronary artery disease might be more likely to develop aortic complications. Other predictors have been suggested such as male gender or increasing time interval since GCA diagnosis.[16] We did not observe any such associations in our study, even with the cumulative dose of corticosteroids. Moreover, many of these predictors are known to be associated with aortic dilation or dissection in the general population (male gender, coronary risk factors, coronary or peripheral artery disease, and hypertension).[22,23]
In our study, apart from a previous positive FDG-PET/CT showing large-vessel inflammation, we did not observe any other predictive factors associated with aortic complications.
We did not observe a higher risk of death in our patients with aortic complications than other teams have.[13,15] However, our patients were followed up for 4 years, which may not be long enough to observe fatal complications. In our patients who died, a cardiovascular event was involved in more than three-quarters of cases, but only half of them had previous large-vessel involvement on the FDG-PET/CT.
Large vessel involvement has been reported in 13% to 83% of patients with GCA, with inconstant extracephalic manifestations.[3,4,6,11,24] These results suggest that large-vessel involvement should be tested for even in the absence of indicative clinical symptoms. Moreover, it has been reported that patients with extracephalic presentations may have less positive TAB.[1,5,12] Interestingly, we did not observe any such differences relating to TAB status and clinical presentation. Among the 22 patients who did not have any cephalic symptoms at presentation, we found 14 positive TABs, emphasizing the high usage of TAB in patients in whom GCA is suspected.
Besides FDG-PET/CT, aortic CT angiography and magnetic resonance imaging have shown high sensitivity in detecting large-vessel inflammation, but are poorly documented in patient prognosis assessments.[4,25] Further studies are required to assess the ability of noninvasive angiographic techniques to predict aortic complications in patients with GCA.
Our work has some major strengths. First, we describe a large population of patients with a GCA diagnosis. Indeed, all of them had at least 3 positive ACR criteria and all underwent a TAB (except 2 who had a biopsy-proven extratemporal GCA). Moreover, they all had an aortic morphology assessment at diagnosis, and over 80% of them were assessed again with whole-body imaging during follow-up.
Our study also has limitations, especially owing to its retrospective design that may limit the exhaustivity of data collection. The different timing of FDG-PET/CT and the various indications for referring patients to PET/CT are potential limitations. However, regardless of the time of and reasons for PET/CT, the risk of aortic complications was higher in patients with a positive scan.
The variability in time interval to follow-up imaging and in the imaging techniques used is a limitation of the retrospective design of the study. This may have underestimated the rate of long-term aortic complications since imaging was performed at a median interval of 27 months after FDG-PET/CT. Some patients may develop late complications, as suggested by studies reporting frequent complications observed more than 5 years’ postdiagnosis.[11–14] This study highlights the fact that a subset of GCA patients with large-vessel inflammation may develop early aortic complications after a positive PET/CT.
The small sample of patients with aortic complications did not allow multivariate analysis that might have identified other predictive factors of aortic complications at the time of PET/CT.
Cardiovascular risk factors may have played a role in aortic comorbidities but we did not observe increased cardiovascular risk factors in patients who developed aortic complications.
Patients with atypical presentations (e.g., with no cephalic symptoms) are more likely to be referred for FDG-PET/CT, a situation that may overestimate PET positivity in this subset of patients. However, 40% of the patients in our study had typical and isolated cephalic symptoms and underwent an FDG-PET/CT, given the ease the ease with which PET examinations are performed at our institutions.
5. Conclusions
In this study, GCA patients with a positive FDG-PET/CT were more likely to develop aortic complications within 5 years of presenting with large-vessel inflammation. These results suggest that FDG-PET/CT imaging should be performed after GCA diagnosis. As the risk of aortic complications continues to increase over time, further prospective studies are required to investigate the potential benefit of periodic FDG-PET/CT assessment.
Footnotes
Abbreviations: ACR = American College of Rheumatology, CRP = C-reactive protein, CT = computed tomography, FDG-PET/CT = positron emission tomography with 18F-fluorodeoxyglucose combined with computed tomography, GCA = giant-cell arteritis, MR = magnetic resonance, PMR = polymyalgia rheumatica, TAB = temporal artery biopsy.
The authors have no conflicts of interest to disclose.
BB and AM contributed equally to this study.
References
- 1.Agard C, Barrier JH, Dupas B, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum 2008; 59:670–676. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz T, Matteson EL. Large-vessel involvement in giant cell arteritis. Curr Opin Rhematol 2006; 18:10–17. [DOI] [PubMed] [Google Scholar]
- 3.Klein RG, Hunder GG, Stanson AW, et al. Large artery involvement in giant cell (temporal) arteritis. Ann Intern Med 1975; 83:806–812. [DOI] [PubMed] [Google Scholar]
- 4.Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 2012; 71:1170–1176. [DOI] [PubMed] [Google Scholar]
- 5.Brack A, Martinez-Taboada V, Stanson A, et al. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42:311–317. [DOI] [PubMed] [Google Scholar]
- 6.Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006; 55:131–137. [DOI] [PubMed] [Google Scholar]
- 7.Prieto-Gonzalez S, Depetris M, Garcia-Martinez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73:1388–1392. [DOI] [PubMed] [Google Scholar]
- 8.Besson FL, Parienti JJ, Bienvenu B, et al. Diagnostic performance of (1)(8)F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011; 38:1764–1772. [DOI] [PubMed] [Google Scholar]
- 9.Puppo C, Massollo M, Paparo F, et al. Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. Biomed Res Int 2014; 2014:574248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995; 122:502–507. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martinez A, Hernandez-Rodriguez J, Arguis P, et al. Development of aortic aneurysm/dilatation during the followup of patients with giant cell arteritis: a cross-sectional screening of fifty-four prospectively followed patients. Arthritis Rheum 2008; 59:422–430. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, et al. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine 2004; 83:335–341. [DOI] [PubMed] [Google Scholar]
- 13.Kermani TA, Warrington KJ, Crowson CS, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis 2013; 72:1989–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robson JC, Kiran A, Maskell J, et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis 2015; 74:129–135. [DOI] [PubMed] [Google Scholar]
- 15.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003; 48:3522–3531. [DOI] [PubMed] [Google Scholar]
- 16.Blockmans D, Coudyzer W, Vanderschueren S, et al. Relationship between fluorodeoxyglucose uptake in the large vessels and late aortic diameter in giant cell arteritis. Rheumatology 2008; 47:1179–1184. [DOI] [PubMed] [Google Scholar]
- 17.Espitia O, Neel A, Leux C, et al. Giant cell arteritis with or without aortitis at diagnosis. A retrospective study of 22 patients with longterm followup. J Rheumatol 2012; 39:2157–2162. [DOI] [PubMed] [Google Scholar]
- 18.Miller DV, Isotalo PA, Weyand CM, et al. Surgical pathology of noninfectious ascending aortitis: a study of 45 cases with emphasis on an isolated variant. Am J Surg Pathol 2006; 30:1150–1158. [DOI] [PubMed] [Google Scholar]
- 19.Evans JM, Bowles CA, Bjornsson J, et al. Thoracic aortic aneurysm and rupture in giant cell arteritis. A descriptive study of 41 cases. Arthritis Rheum 1994; 37:1539–1547. [DOI] [PubMed] [Google Scholar]
- 20.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994; 37:187–192. [DOI] [PubMed] [Google Scholar]
- 21.Hautzel H, Sander O, Heinzel A, et al. Assessment of large-vessel involvement in giant cell arteritis with 18F-FDG PET: introducing an ROC-analysis-based cutoff ratio. J Nucl Med 2008; 49:1107–1113. [DOI] [PubMed] [Google Scholar]
- 22.Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000; 117:1271–1278. [DOI] [PubMed] [Google Scholar]
- 23.Senaratne JM, Raggi P. Screening for aortic aneurysms in patients with coronary artery disease: should it be done? Expert Rev Cardiovasc Ther 2015; 13:735–737. [DOI] [PubMed] [Google Scholar]
- 24.Daumas A, Rossi P, Bernard-Guervilly F, et al. [Clinical, laboratory, radiological features, and outcome in 26 patients with aortic involvement amongst a case series of 63 patients with giant cell arteritis]. Rev Med Intern 2014; 35:4–15. [DOI] [PubMed] [Google Scholar]
- 25.Meller J, Strutz F, Siefker U, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging 2003; 30:730–736. [DOI] [PubMed] [Google Scholar]


