Supplemental Digital Content is available in the text
Keywords: alcoholic, chronic liver failure-sequential organ failure assessment, hepatic encephalopathy, liver cirrhosis, systemic inflammatory response syndrome
Abstract
Hepatic encephalopathy (HE) is a complication associated with worst prognosis in decompensated liver cirrhosis (LC) patients. Previous studies have identified prognostic factors for HE, and recent studies reported an association between systemic inflammatory response syndrome (SIRS) and liver disease. This study aimed to identify prognostic factors for 30-day mortality in alcoholic LC patients with HE who visited the emergency department (ED).
This was a retrospective study of alcoholic LC patients with HE from January 1, 2010, to April 30, 2015. The baseline characteristics, complications of portal hypertension, laboratory values, Child–Pugh class, Model for End-stage Liver Disease (MELD) score, chronic liver failure-sequential organ failure assessment (CLIF–SOFA) score, and SIRS criteria were assessed. The presence of 2 or more SIRS criteria was considered SIRS. The primary outcomes were 30-day mortality and prognostic factors for patients with HE visiting the ED.
In total, 105 patients who met the inclusion criteria were analyzed. Overall, the 30-day mortality rate was 6.7% (7 patients).
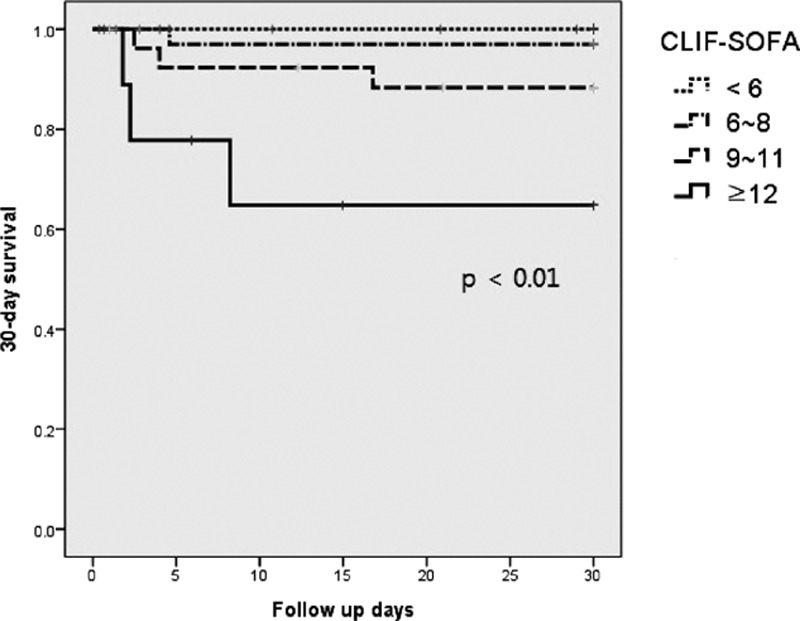
Significant variables were hepatorenal syndrome, international normalized ratio, white blood cell count, total bilirubin level, MELD score CLIF–SOFA score, and SIRS in univariate analysis. CLIF–SOFA score and SIRS were the significant factors in the multivariate analysis (hazard ratio 5.56, 15.98; 95% confidence interval 1.18–26.18, 1.58–161.37; P = 0.03, P = 0.02). The mortality rates differed according to the CLIF–SOFA score (P < 0.01).
The CLIF–SOFA score and SIRS in alcoholic LC patients with HE visiting the ED are independent predictors of 30-day mortality.
1. Introduction
Hepatic encephalopathy (HE) is a debilitating complication of decompensated liver cirrhosis (LC) that manifests as various neuropsychiatric symptoms.[1,2] Patients with HE use medical facilities more frequently.[2] HE has a worse prognosis than do the other complications of portal hypertension in decompensated LC.[3] Moreover, HE is a predictor of mortality independent of the Model for End-stage Liver Disease (MELD) score in patients with LC.[4–6] Few studies on prognostic factors for HE in LC patients have been performed.
Systemic inflammatory response syndrome (SIRS) has long been used as a marker of the response to inflammation.[7] SIRS is also considered an important factor for prognosis in patients with liver disease,[8–10] and its association with HE in LC has been addressed.[11,12] One study showed that inflammation affects the severity of minimal HE in patients with LC.[12] Another study including patients who were admitted to the intensive care unit (ICU) regardless of etiology showed that SIRS differed between survivors and nonsurvivors in LC patients with severe HE.[13] However, the inflammatory status differs between viral and alcoholic LC.[14] Moreover, the status of patients could change from the time of presentation to the emergency department (ED) to admission to the ICU.
We evaluated the utility of clinical variables in the ED, including scoring systems and SIRS, as predictors of mortality in alcoholic LC patients with HE because they are frequently admitted to the ED.[15] The objective of this study was to identify predictive factors for 30-day mortality in alcoholic LC patients with HE who visited the ED.
2. Patients and methods
2.1. Study design and setting
This was a retrospective study of alcoholic LC patients with HE. This study was conducted in the ED of a tertiary hospital with an annual census of 35,000 patients. The patients were treated by board-certified emergency attending physicians. This study was approved by the Gyeongsang National University hospital institutional review board (number 2015-12-019-001).
2.2. Participants
Patients who visited the ED with LC from January 1, 2010, to April 30, 2015, were considered for inclusion in the study. The inclusion criteria were patients with alcoholic LC who presented with or showed clinical characteristics of HE in the ED. The diagnosis of HE was based on ammonia levels and the clinical characteristics of the patient.[2] Because ammonia levels could be increased in chronic liver disease, high levels only are not diagnosis for HE.[2] Treating physicians identified the symptoms of HEP and other causes changing mental. Abnormal behaviors reported by caregivers were also used to diagnosis. If a patient visited the hospital multiple times, only the first visit during the study period was considered in this study. Patients were excluded if they meet the following criteria: hepatocellular carcinoma, uncertain causes of LC, carrier of chronic viral hepatitis, and symptoms of HE occurring in the ward. Alcohol etiology was based on reported year-long alcohol abuse of >60 g/day for males and >40 g/day for females.[16] Hepatorenal syndrome was defined as the criteria of hepatorenal syndrome by the the International Ascites Club.[17] Spontaneous bacterial peritonitis were defined as elevated neutrophil count in ascitic fluid (>250 cells/mm3) and/or positive bacterial culture of ascitic fluid.[18]
2.3. Data collection
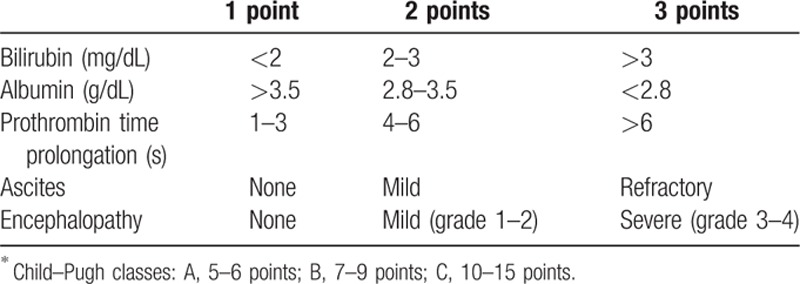
The electronic medical records of the enrolled patients were reviewed. The data were collected using standard patient record forms. Baseline characteristics—such as age, sex, diabetes mellitus, hypertension, alcohol abstinence, dialysis, and a transjugular intrahepatic portosystemic shunt—were assessed. Alcohol abstinence was defined as not consuming alcohol for >1 month. Portal hypertensive complications other than HE—such as varix bleeding, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome on presentation—were investigated. The HE grade was categorized according to the West Haven criteria.[2] Grades 1 and 2 were categorized as low grade and grades 3 and 4 as high grade. Ascites was classified as controlled or uncontrolled. The following laboratory values, which are routinely tested in the ED, were assessed: white blood cell count (WBC), platelet count, international normalized ratio (INR), levels of hemoglobin, protein, albumin, bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), sodium, potassium, ammonia, blood urea nitrogen (BUN), creatinine, and C-reactive protein, and pH. The results of cultures from sputum, urine, ascites, and blood collected within 2 days after ED presentation were evaluated. Three scoring systems are identified. The Child–Pugh score was calculated and the class determined (Table 1).[19] The MELD score was calculated from the results at the time of presentation to the ED according to the following formula[20]:
Table 1.
Child–Pugh classification.∗
MELD score = 3.8∗loge(bilirubin [mg/dL]) + 11.2∗loge (INR) + 9.6∗loge (creatinine [mg/dL]) + 6.4∗ (etiology: 0 if cholestatic or alcoholic; 1 otherwise).
Chronic liver failure-sequential organ failure assessment (CLIF–SOFA) score was calculated as Table 2.[21]
Table 2.
Chronic liver failure-sequential organ failure assessment (CLIF–SOFA) score.
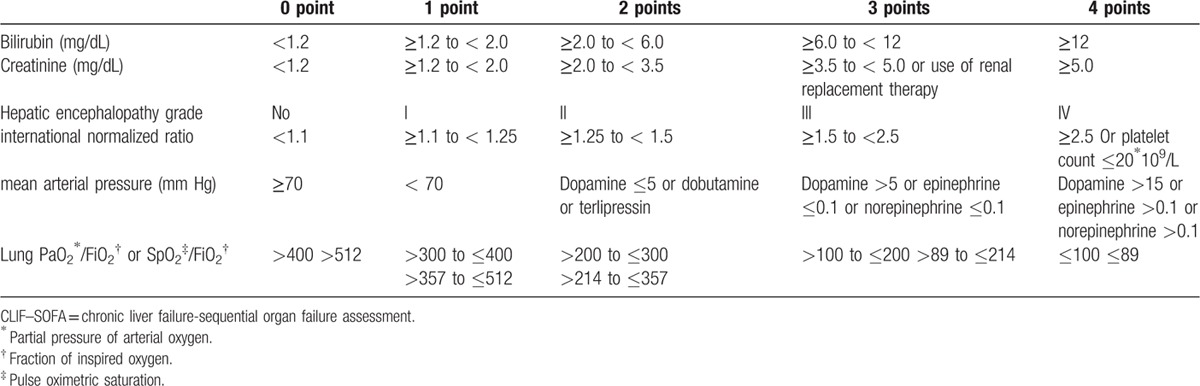
The SIRS was identified based on vital signs at the time of presentation to the ED. The SIRS variables were as follows: heart rate >90 beats per minute; temperature >38°C or <36°C, respiration rate >20 breaths per minute or PaCO2 < 32 mm Hg, and WBC >12,000/mm3 or < 4000/mm3, or >10% immature neutrophils.[7] An SIRS score of ≥2 was categorized as SIRS.[7] Death within 30 days after visiting the ED was assessed.
2.4. Outcomes
The primary outcomes were 30-day mortality and prognostic factors for patients with HE visiting the ED.
2.5. Data analysis
The SPSS 21.0 statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis. Continuous variables are presented as means (±SD) and categorical variables as numbers (percentages). Univariate and multivariate Cox proportional hazard analyses were used to identify prognostic factors for 30-day mortality. The variables were divided into 2 groups according to previous studies or the receiver operating characteristic (ROC) curve. Variables with a P-value < 0.05 in the univariate analysis, together with other variables considered relevant, were included in the multivariate analysis. A P-value < 0.05 was considered to indicate statistical significance. Kaplan–Meier survival curves were generated to evaluate survival according to the CLIF–SOFA score.
3. Results
A total of 1779 patients with LC visited the ED during the 5-year study period. Among them, 1395 patients visited due to problems related to LC other than HE. A further 269 patients were excluded due to a repeat visit to the ED, hepatocellular carcinoma, or nonalcoholic LC. Ten patients who showed symptoms of HE on the ward were also excluded. In total, 105 patients who met the inclusion criteria were enrolled in the study (Fig. 1).
Figure 1.
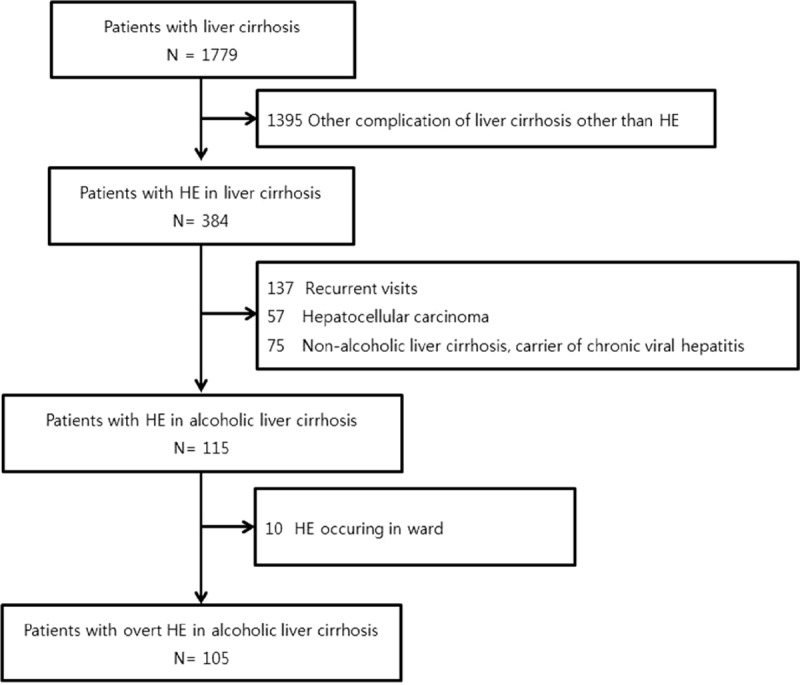
The study patients.
Males accounted for 91.4% of the total patients, and the mean age was 57.8 (± 9.9) years. More than half of the patients still consumed alcohol (53.3%). Forty-seven patients had high-grade HE. Hepatorenal syndrome was found in 6 patients, spontaneous bacterial peritonitis in 4, and variceal bleeding in 11. The baseline characteristics of the patients are shown in Table 3. Cultures were performed in 58 patients, of whom 16 showed positive results. Mean MELD and CTP scores were 13.1 (± 8.5) and 10.8 (± 2.3), respectively. The mean CLIF–SOFA score was 7.7 (±2.5). All of the patients were treated with lactulose; enemas were performed in the majority of patients.
Table 3.
Baseline characteristics of the patients.
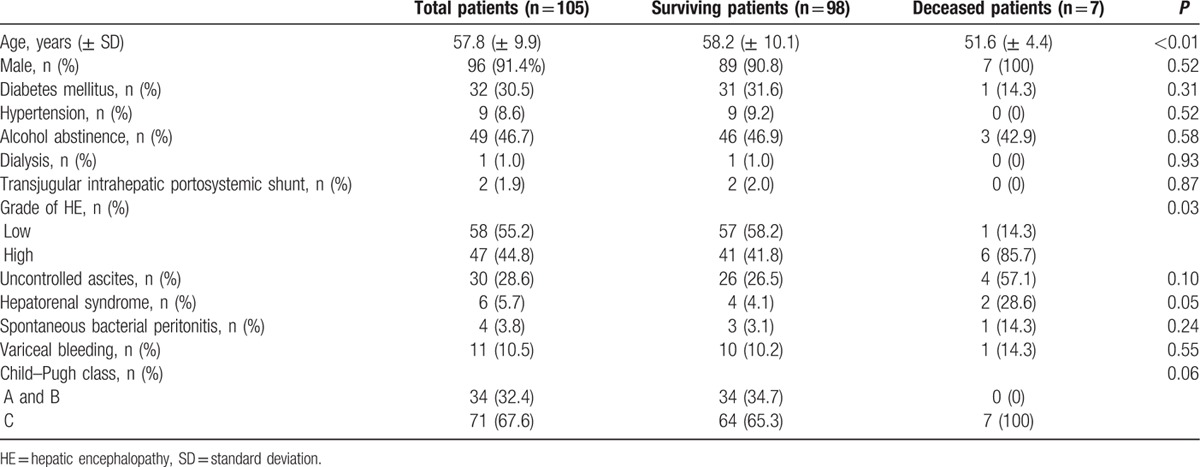
Overall, the 30-day mortality rate was 6.7% (7 patients). Clinical and laboratory variables—age, gender, diabetes mellitus, hypertension, alcohol abstinence, dialysis, transjugular intrahepatic portosystemic shunt, HE grade, ascites, hepatorenal syndrome, spontaneous bacterial peritonitis, variceal bleeding, laboratory results (WBC count, platelet, INR, levels of hemoglobin, protein, albumin, total bilirubin, AST, ALT, sodium, potassium, ammonia, BUN, creatinine, and C-reactive protein, pH and culture results), Child-Pugh class, MELD score, CLIF–SOFA score and SIRS —were compared by univariate Cox regression analysis. Of these factors, hepatorenal syndrome, WBC, INR, total bilirubin level, MELD score, CLIF–SOFA score, and SIRS were found to be significant (Table 4, Supplemental Digital Content). Among them, we assumed inter-correlations between significant variables; therefore, we selected CLIF–SOFA score and SIRS for multivariate Cox regression to identify independent prognostic factors for 30-day mortality. These factors were adjusted for age, gender, alcohol abstinence, and culture results. CLIF–SOFA score and SIRS were significant factors (hazard ratio 5.56, 15.98; 95% confidence interval 1.18–26.18, 1.58–161.37; P = 0.03, P = 0.02). Survival according to the CLIF–SOFA score was evaluated by Kaplan–Meier analysis (Fig. 2); higher CLIF–SOFA scores were associated with higher 30-day mortality rates (P < 0.01).
Table 4.
Univariate Cox proportional hazard models for 30-day mortality.
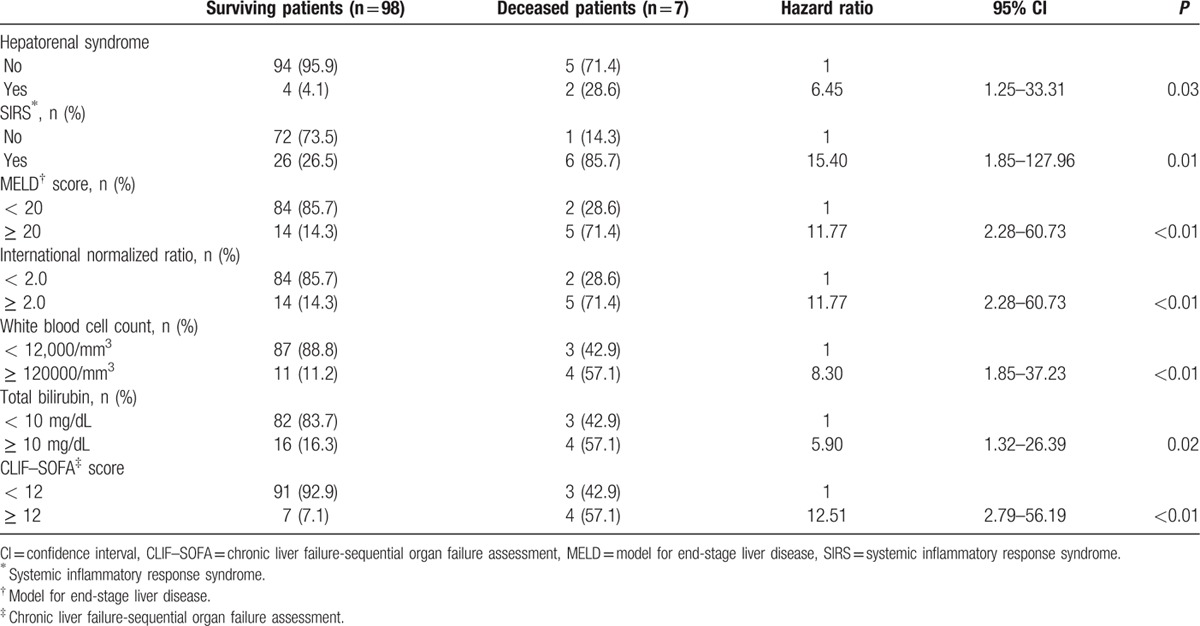
Figure 2.
Survival according to the CLIF–SOFA score. CLIF–SOFA = chronic liver failure-sequential organ failure assessment.
4. Discussion
In this study, the 30-day mortality rate of alcoholic LC patients with HE was 6.7%, and the CLIF–SOFA score and SIRS were significant factors. Patients with higher CLIF–SOFA had a higher 30-day mortality rate.
Previous studies have reported that systolic blood pressure, MELD score, WBC, electrolytes, HE grade, renal function, and ammonia level are significant prognostic factors for HE.[5,22–27] These studies included patients regardless of the etiology of LC, such as viral infection or alcohol consumption; however, the clinical features of LC differ according to the etiology of the condition.[16,28,29] The clinical outcomes and prognosis of patients with alcoholic LC have been reported.[27,30–32] Limited studies on alcoholic LC patients with HE have been performed, and the results indicated that treatment should take place in the ICU.[33] This is, to our knowledge, the first study on prognostic factors for alcoholic LC patients with HE.
The CLIF–SOFA score was developed for acute decompensation of cirrhosis.[21] Acute decompensation is defined as acute development of major complication such as HE, ascites, gastrointestinal bleeding, and bacterial infection.[21] The SOFA score and CLIF–SOFA score were differed in coagulation and cerebral components.[21] Because hypersplensim by portal hypertension in LC cause sequestration of platelets, platelet is replaced by INR in coagulation component.[34] And cerebral component is changed from the Glasgow Coma Score to HE grade because of difficulties of assessment for lower grade of HE.[34] Other studies for all causes of LC validated its effectiveness for prognosis.[34,35] One study for the patients with alcoholic LC also validated scoring systems and showed that CLIF–SOFA could predict the 4-week mortality more accurate than other scoring systems.[36] Our study also showed CLIF–SOFA was a significant factor for 30-day mortality. Physician can easily calculated the CLIF–SOFA score and predict the prognosis.
SIRS is an uncontrolled inflammatory response.[7] An SIRS and the presence of infection are criteria used to define sepsis. Because the liver plays a major role in the response to infection, patients with liver disease are prone to infection.[37] Therefore, the relationship between SIRS and liver disease has been investigated for >10 years.[8] Rolando et al. reported that SIRS was associated with a poorer prognosis in acute liver failure.[8] Recent studies have also suggested that SIRS has a prognostic value in acute liver failure.[38] Chronic liver diseases are also associated with SIRS.[13,39] Michelena et al reported SIRS to be related to 90-day mortality in patients with alcoholic hepatitis.[39] These studies showed that SIRS is significantly associated with a poorer prognosis, irrespective of the presence of infection.[8,13,38,39] Our results also indicated that infection was not associated with 30-day mortality, possibly related to sterile inflammation in liver disease.[40] Sterile inflammation is an inflammatory response to stimuli in the absence of pathogens.[40] Studies have shown that sterile inflammation plays a role in alcoholic liver disease, nonalcoholic liver disease, and drug-induced liver injury.[40,41] Endogenous damage-associated molecular patterns released from damaged cells trigger an inflammatory response, resulting in immune-mediated fibrosis of the liver.[40] This sterile inflammation might be related to SIRS. In some cases, however, it is difficult to identify the site of infection, and the culturing of some pathogens requires a prolonged period. The availability of laboratory results also can take several hours. In contrast, the SIRS can be calculated within a few minutes and is thus a suitable initial prognostic factor for mortality in the ED.
Wong et al showed that the addition of HE to the MELD score increased its prognostic value, and the adjusted score was used to prioritize patients awaiting liver transplantation.[5] Our results will assist identification of more urgent cases and patients who require more aggressive treatment, such as liver transplantation. As mortality differed according to the CLIF–SOFA score, it might be possible more selective choices for treatment.
This study had several limitations. First, because this was a retrospective study, data regarding some variables, such as culture results and precipitating factors, were missing for a proportion of the patients. Because the presence of infection was reported previously not to be a significant factor, the absence of culture results likely did not influence our findings. Predicting mortality based on the present status of the patients is crucial, regardless of the precipitating factors. Second, we did not compare patients with LC due to other causes. Third, alcohol abstinence might be a related factor; however, this information may have been erroneous as the patients provided the data. Fourth, this study was conducted at a single center. Therefore, further prospective and multicenter studies should be conducted.
5. Conclusion
In conclusion, the CLIF–SOFA score and SIRS in alcoholic LC patients with HE visiting the ED are independent predictors of 30-day mortality.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate aminotransferase, BUN = blood urea nitrogen, CLIF–SOFA = chronic liver failure-sequential organ failure assessment, HE = hepatic encephalopathy, INR = international normalized ratio, LC = liver cirrhosis, MELD = Model for End-stage Liver Disease, SIRS = systemic inflammatory response syndrome.
Authorship: JHJ conceptualized and designed the study, analyzed the data, drafted the initial manuscript, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. SP conceptualized and designed the study, coordinated and supervised data collection, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. DHK conceptualized and designed the study, interpreted data, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. SCK conceptualized and designed the study interpreted data and critically reviewed the manuscript, interpreted data, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. CK interpreted data, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. SHL interpreted data, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. TYK interpreted data, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. SBL interpreted data, critically reviewed the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Sharma P, Sharma BC, Sarin SK. Predictors of nonresponse to lactulose in patients with cirrhosis and hepatic encephalopathy. Eur J Gastroenterol Hepatol 2010; 22:526–531. [DOI] [PubMed] [Google Scholar]
- 2.American Association for the Study of Liver Diseases. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014; 61:642–659. [DOI] [PubMed] [Google Scholar]
- 3.Zhang JY, Kuai JH, Jia JD, et al. The effect of portal hypertension on prognosis in patients with decompensated liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi 2009; 17:263–265. [PubMed] [Google Scholar]
- 4.Said A, Williams J, Holden J, et al. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol 2004; 40:897–903. [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Gish RG, Ahmed A. Hepatic encephalopathy is associated with significantly increased mortality among patients awaiting liver transplantation. Liver Transpl 2014; 20:1454–1461. [DOI] [PubMed] [Google Scholar]
- 6.Stewart CA, Malinchoc M, Kim WR, et al. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl 2007; 13:1366–1371. [DOI] [PubMed] [Google Scholar]
- 7.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874. [PubMed] [Google Scholar]
- 8.Rolando N, Wade J, Davalos M, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000; 32:734–739. [DOI] [PubMed] [Google Scholar]
- 9.Maiwall R, Chandel SS, Wani Z, et al. SIRS at admission is a predictor of AKI development and mortality in hospitalized patients with severe alcoholic hepatitis. Dig Dis Sci 2016; 61:920–929. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Khalek EE, El-Fakhry A, Helaly M, et al. Systemic inflammatory response syndrome in patients with liver cirrhosis. Arab J Gastroenterol 2011; 12:173–177. [DOI] [PubMed] [Google Scholar]
- 11.Shawcross DL, Davies NA, Williams R, et al. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 2004; 40:247–254. [DOI] [PubMed] [Google Scholar]
- 12.Shawcross DL, Wright G, Olde Damink SW, et al. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis 2007; 22:125–138. [DOI] [PubMed] [Google Scholar]
- 13.Shawcross DL, Sharifi Y, Canavan JB, et al. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 2011; 54:640–649. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Esparza M, Tristan-Manzano M, Ruiz-Alcaraz AJ, et al. Inflammatory status in human hepatic cirrhosis. World J Gastroenterol 2015; 21:11522–11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25 suppl 1:3–9. [DOI] [PubMed] [Google Scholar]
- 16.Wiegand J, Kuhne M, Pradat P, et al. Different patterns of decompensation in patients with alcoholic vs. non-alcoholic liver cirrhosis. Aliment Pharmacol Ther 2012; 35:1443–1450. [DOI] [PubMed] [Google Scholar]
- 17.Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53:397–417. [DOI] [PubMed] [Google Scholar]
- 19.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 20.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33:464–470. [DOI] [PubMed] [Google Scholar]
- 21.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144:1426–1437.1437.e1421-1429. [DOI] [PubMed] [Google Scholar]
- 22.Hung TH, Tseng CW, Tseng KC, et al. Effect of renal function impairment on the mortality of cirrhotic patients with hepatic encephalopathy: a population-based 3-year follow-up study. Medicine 2014; 93:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung TH, Lay CJ, Chang CM, et al. The effect of infections on the mortality of cirrhotic patients with hepatic encephalopathy. Epidemiol Infect 2013; 141:2671–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaduputi V, Chandrala C, Abbas N, et al. Prognostic significance of hypokalemia in hepatic encephalopathy. Hepatogastroenterology 2014; 61:1170–1174. [PubMed] [Google Scholar]
- 25.Qureshi MO, Khokhar N, Shafqat F. Ammonia levels and the severity of hepatic encephalopathy. J Coll Physicians Surg Pak 2014; 24:160–163. [PubMed] [Google Scholar]
- 26.Udayakumar N, Subramaniam K, Umashankar L, et al. Predictors of mortality in hepatic encephalopathy in acute and chronic liver disease: a preliminary observation. J Clin Gastroenterol 2007; 41:922–926. [DOI] [PubMed] [Google Scholar]
- 27.Benhaddouch Z, Abidi K, Naoufel M, et al. Mortality and prognostic factors of the cirrhotic patients with hepatic encephalopathy admitted to medical intensive care unit. Ann Fr Anesth Reanim 2007; 26:490–495. [DOI] [PubMed] [Google Scholar]
- 28.Kotoh K, Fukushima M, Horikawa Y, et al. Serum albumin is present at higher levels in alcoholic liver cirrhosis as compared to HCV-related cirrhosis. Exp Ther Med 2012; 3:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iino S. Differentiation alcoholic liver cirrhosis from viral liver cirrhosis. Nihon Rinsho 1994; 52:174–180. [PubMed] [Google Scholar]
- 30.Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010; 51:1675–1682. [DOI] [PubMed] [Google Scholar]
- 31.Kojima S, Ito H, Takashimizu S, et al. The outcome of patients with alcoholic liver cirrhosis. Nihon Arukoru Yakubutsu Igakkai Zasshi 2014; 49:136–141. [PubMed] [Google Scholar]
- 32.Janicko M, Veseliny E, Senajova G, et al. Predictors of hepatorenal syndrome in alcoholic liver cirrhosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015. [DOI] [PubMed] [Google Scholar]
- 33.Piechota M. Hepatic encephalopathy in the course of alcoholic liver disease–treatment options in the intensive care unit. Anaesthesiol Intensive Ther 2014; 46:34–36. [DOI] [PubMed] [Google Scholar]
- 34.Pan HC, Jenq CC, Tsai MH, et al. Scoring systems for 6-month mortality in critically ill cirrhotic patients: a prospective analysis of chronic liver failure–sequential organ failure assessment score (CLIF–SOFA). Aliment Pharmacol Ther 2014; 40:1056–1065. [DOI] [PubMed] [Google Scholar]
- 35.Silva PE, Fayad L, Lazzarotto C, et al. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF–SOFA for prediction of mortality in cirrhosis. Liver Int 2015; 35:1516–1523. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Lee JH, Oh S, et al. CLIF–SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int 2015; 35:46–57. [DOI] [PubMed] [Google Scholar]
- 37.Lin KH, Wang FL, Wu MS, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis 2014; 80:72–78. [DOI] [PubMed] [Google Scholar]
- 38.Leithead JA, Ferguson JW, Bates CM, et al. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non-paracetamol-induced acute liver failure. Gut 2009; 58:443–449. [DOI] [PubMed] [Google Scholar]
- 39.Michelena J, Altamirano J, Abraldes JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015; 62:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology 2012; 143:1158–1172. [DOI] [PubMed] [Google Scholar]
- 41.Hoque R, Farooq A, Mehal WZ. Sterile inflammation in the liver and pancreas. J Gastroenterol Hepatol 2013; 28 suppl 1:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


