Abstract
The role of hepatic resection in hepatocellular carcinoma (HCC) with accompanying portal vein tumor thrombus (PVTT) remains controversial. This study aimed to evaluate the surgical outcomes of hepatic resection compared with those of transarterial chemoembolization (TACE) in HCC patients. A retrospective study was conducted using the medical records of 230 HCC patients with portal vein invasion who underwent hepatic resection (96 patients) or TACE (134 patients). The baseline characteristics, tumor characteristics, clinicopathological parameters, and overall survival rates were compared between the 2 groups. The baseline and tumor characteristics were comparable between the hepatic resection and TACE groups. The overall complication rate was 35.4% in the hepatic resection group, which was significantly lower than that in the TACE group (73.0%, P <0.001). However, the serious complication rate (grade ≥3) in the hepatic resection group was 13.5%, which was significantly higher than that in the TACE group (P = 0.003). The cumulative overall survival rates at 1, 3, and 5 years in the hepatic resection group were 86.5%, 60.4%, and 33.3%, respectively. These rates were much higher than those in the TACE group (1-year: 77.6%; 3-year: 47.8%; and 5-year: 20.9%; P = 0.021). The long-term survival was notably better in the patients with types I and II PVTT than in the patients with types III and IV PVTT (P <0.05). The univariate and multivariate analyses indicated that types III and IV PVTT and TACE may have contributed to the poor overall survival following surgery. In HCC patients with PVTT and compensated liver function, hepatic resection is a safe and effective surgical protocol, particularly for patients with type I or II PVTT.
Keywords: hepatocellular carcinoma, liver resection, thrombus, transarterial chemoembolization
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common and life-threatening neoplasms worldwide because of its high incidence and mortality,[1] particularly in Asia and Africa; however, the incidence of HCC is increasing in the Western world.[2] The clinical outcomes of HCC have improved as a result of curative therapies, such as hepatic resection, liver transplantation (LT), and radiofrequency ablation (RFA).[3] Because of the shortage of liver grafts, the inclusion criteria for LT are limited.[4] In addition, the high mortality and overall cost of LT have limited its widespread use as a treatment for HCC. The effectiveness of RFA for HCC has only been demonstrated in small liver cancers with a diameter ≤3 cm. Therefore, hepatic resection may be the treatment of choice for most HCC patients. However, because of tumor multifocality, portal vein invasion, and underlying advanced cirrhosis, only 30% of HCCs are amenable to hepatic resection at the time of diagnosis.[5]
The Barcelona Clinic for Liver Cancer (BCLC) staging system and treatment guidelines have been widely accepted, particularly by the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of the Liver (EASL).[4] According to these guidelines, HCCs with portal vein invasion should be graded as advanced, and the proposed treatment option for patients with advanced HCC is sorafenib. However, because of the severe and common adverse effects of sorafenib, many groups have recommended transarterial chemoembolization (TACE) for advanced HCC patients. According to the Hong Kong Liver Cancer staging system, HCC cases with intrahepatic venous invasion and compensated liver function should be graded as locally advanced tumors, and TACE is recommended as the first-line therapy for these patients.[6] Because of recent advances in surgical techniques and perioperative management, liver resection has become a reasonably safe treatment option with an acceptable mortality rate.[7,8] Aggressive surgical resection for HCC with vascular invasion (including the portal vein) has been proposed by several centers.[5,9,10] In this study, we aimed to compare the long-term survival outcomes of hepatic resection with those of TACE in HCC patients with portal vein tumor thrombus (PVTT). In our center, only 6.6% of patients with PVTT at the time of diagnosis of HCC underwent surgical resection. The PVTT was graded by using the Shi's classification[11]: tumor thrombi formation was found under microscopy, which was defined as type I0; segmental branches of portal vein or above vein's tumor thrombi, which was defined as Type I; right/left portal vein's tumor thrombi, which was defined as Type II; the main portal vein trunk's tumor thrombi, which was defined as Type III; the superior mesenteric vein's tumor thrombi, which was defined as Type IV.
2. Patients and methods
2.1. Patients
We retrospectively collected data from all 1360 HCC in patients who were admitted to our center from January 2000 to December 2008. The preoperative diagnosis of HCC was based on the guidelines in “China's Common Malignancy Specifications: Primary Liver Cancer.[12]” Preoperative biopsy is not recommend as a routine approach to diagnose of HCC in China. The criteria for a noninvasive diagnosis of HCC in cirrhotic liver cases include contrast medium uptake during the arterial phase and washout during the portal-venous or late-venous phase. When these characteristics were not observed, dynamic contrast-enhanced ultrasonography or magnetic resonance imaging (MRI) was performed to confirm the HCC diagnosis. Additionally, an alpha-fetoprotein (AFP) level more than 400 ng/mL was considered a diagnostic characteristic of HCC when the imaging scan was not definitive. All of the HCC cases in the hepatic resection group were confirmed by pathology and hepatic arterial angiography.
The inclusion criteria were as follows: primary HCC, an age of 18 to 80 years, hepatic resection or TACE as the first treatment protocol, the presence of PVTT on a preoperative contrast-enhanced CT or MRI imaging scan, Child class A or B liver function, and an Eastern Cooperative Oncology Group (ECOG) score of 0 or 1. The exclusion criteria were as follows: hepatic vein thrombus; extrahepatic metastasis; invasion of the peripheral organ or tissue; Child class C liver function; liver cancers other than HCC; first-line treatments other than hepatic resection or TACE, such as LT or RFA; only with microvascular invasion (Type I0); cases lost to follow-up (time to death after operation cannot be got: 3 cases in the hepatic resection group, and 5 cases in the TACE group); postoperative therapies, such as RFA, resection or LT, in patients who underwent TACE; and postoperative therapies, such as RFA or LT, in patients who underwent hepatic resection. Based on the inclusion and exclusion criteria, a total of 230 patients were enrolled in this study and were divided into the following 2 groups according to the treatment protocols: the hepatic resection group (96 patients) and the TACE group (134 patients).
2.2. Surgical procedure
All of the patients underwent a routine physical examination, and the results were evaluated and discussed in our department. Before the surgical procedures, written informed consent was obtained from all of the patients or their families. This retrospective study was performed with approval by the Ethics Committee of our hospital. The patient records were anonymized and deidentified before the analysis. PVTT was classified according to Shi's classification based on the extent of the tumor thrombus.[11] Using this classification, the degree of disease severity in PVTT patients is graded in ascending order from type I to IV. The type of treatment differs according to the type of PVTT. Portal hypertension was defined as the presence of esophageal varices or a platelet count <100 × 109/L in association with splenomegaly.[10]
The hepatectomy procedure was defined according to the Brisbane 2000 terminology for liver anatomy and resection.[13] Surgery was performed via the abdominal approach in all of the patients through a right subcostal incision with a midline extension after mobilization of the liver, and intraoperative ultrasonography was routinely performed to estimate the location of the tumor and the extent of the tumor thrombus. The Pringle maneuver was applied to occlude the blood inflow in the liver, and the resection was performed using the clamp-crushing method. A thrombectomy was performed according to the location and extent of the PVTT. The thrombectomy procedure was performed based on the experience of Shi et al.[11] PVTT that was located within the resected area was resected en bloc within the tumor. PVTT that protruded into the main portal vein beyond the resection line was extracted from the open stump of the portal vein. For cases with PVTT that extended into the main portal trunk with its primary branches on both sides of the vein, the main portal trunk was exposed and clamped distal to the PVTT. In the hepatic resection group, R0 and R1 resections were defined by the absence (a tumor-free margin ≥1 mm for all detected lesions) or presence (a tumor-free margin ≤0 mm) of microscopic tumor invasion in the resection margins, respectively. An R2 resection was defined by the presence of gross residual tumor invasion in the resection margins.[5]
Routine TACE was performed by experienced physicians. Details of the procedure have been presented in previous reports.[14] We routinely perform TACE through the femoral artery under local anesthesia. Hepatic arteriography was performed to collect information on the tumor number, type, location, size, and arterial supply. The tip of the catheter was directed toward the tumor-feeding arteries for the superselective embolization of all tumors that were detected by digital subtraction angiography. An emulsified suspension was injected into the tumor vessels, followed by embolization with a gelatin sponge. The injection was continued until stasis was confirmed in the feeding artery. The postoperative complications were graded and compared by using the Clavien system.[15]
2.3. Follow-Up
All of the patients were followed up at our outpatient clinic in a standardized manner every 2 to 3 months in the first year after surgery and every 3 to 5 months after 1 year. Follow-up included an analysis of the tumor marker AFP, a liver function test and ultrasonography (first choice). Contrast-enhanced computed tomography (CT) or MRI was recommended when ultrasonography indicated a likely tumor recurrence or when AFP levels persistently increased. Suspicious intrahepatic lesions were further investigated using enhanced CT, MRI, or positron emission tomography (PET)-CT. When extrahepatic metastasis was suspected, corresponding imaging studies, such as chest CT, bone scintigraphy, and PET-CT, were performed. Once recurrence was confirmed, repeated resection or TACE and other therapies were recommended for patients according to their clinical status, liver function, and tumor characteristics. For recurrent cases, multidisciplinary treatments may be the first choice.
2.4. Statistical analysis
The statistical analyses were performed using Statistical Package for the Social Sciences (SPSS Inc, Chicago, IL) software (version 17.0). Descriptive statistics are provided as the means ± standard deviations, the categorical variables were analyzed using the χ2 test or Fisher exact test, the continuous variables were analyzed using Student t test or the Mann–Whitney U test. The overall survival curves were determined using the Kaplan–Meier and log rank test. Cox proportional hazard models were used for the multivariate analysis of the factors that were considered significant in the univariate analysis, the criteria of regression was mainly based on the previous studies that had reported the risk factors of the HCC patients’ survival. The inclusion of variables in the final models was based on biological and statistical considerations. A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Baseline demographic and tumor characteristics of the 2 patient groups
The clinical data for the 2 groups of patients are summarized in Table 1. No significant differences were observed in the baseline characteristics of the patients, such as age, gender, body mass index (BMI), and ethnicity. Most of the patients (95.2%) were of Han ethnicity. HBV infection (88.2%) was the most common cause of HCC in this study; 62.2% of the patients were administered preoperative antiviral therapy. These variables did not differ significantly between the 2 groups (P >0.05). Because of the high HBV infection rate, most of the patients (87.0%) were diagnosed with HCC and accompanying portal hypertension. All of the patients had compensated liver function; therefore, no Child class C cases were detected. The patients had a very good performance status, which was evaluated using the ECOG guidelines. Additionally, the liver function and ECOG scores were comparable between the 2 groups (P >0.05).
Table 1.
A comparison of the baseline data and the liver function results between the resection group and the TACE group.

Table 2 shows that the tumor characteristics in the hepatic resection group and the TACE group did not significantly vary according to the total tumor diameter and the tumor number. Additionally, the preoperative AFP levels and the classification of the AFP levels were comparable between the 2 groups. Typically, most of the tumors were located in the center of the liver rather than at the edge. The PVTT types according to the Shanghai criteria did not significantly differ between the 2 groups (P >0.05).
Table 2.
A comparison of the oncological features in the hepatic resection group and the TACE group.

3.2. TACE and hepatic resection outcomes
In the TACE group, 134 patients received a mean of 2.9 (range, 1–7) sessions of TACE. The most serious complications that occurred in these sessions were recorded. The overall complication rate was 73.0% in the TACE group; however, most of these complications were related to TACE toxicity, including pain in the upper quadrant, nausea/emesis, and fever. Four patients (3.0%) suffered from serious complications (grade = 3a, 1 case suffered femoral pseudoaneurysm, 1 case suffered refractory ascites, 2 cases suffered refractory pleural effusion) that required treatment under local anesthesia. No TACE-related deaths occurred in this study.
The overall complication rate in the hepatic resection group was 35.4%, which was significantly lower than that in the TACE group (P <0.001); however, 13 patients (13.5%) suffered the serious complication in the hepatic resection group: 3 cases suffered refractory pelural effusion and 3 cases suffered persistent bile leak which required treatment under local anesthesia (grade 3a); 2 cases suffered postoperative intraperitoneal hemorrhage and 2 cases suffered gastrointestinal bleeding and 1 case suffered biloma that required treatment under general anesthesia (grade 3b), and 1 case suffered liver failure (grade 4a), and the serious complication rate (grade ≥3), 1 in-hospital postoperative death (1.0%) occurred in the hepatic resection group and was caused by a serious postoperative infection. The serious complication rate in the hepatic resection group was significantly higher than that in the TACE group (P = 0.003). A total of 87 patients received an R0 resection. Additionally, 7 patients underwent an R1 resection, and 2 patients underwent an R2 resection.
3.3. Long-term survival
In the 5-year follow-up period, 170 patients died: 64 patients in the hepatic resection group and 106 patients in the TACE group. The primary cause of death during follow-up was tumor recurrence (160 patients, 94.1%), liver failure (8 patients, 4.7%), infection (1 patient, 0.6%), and a car accident (1 patient, 0.6%). The cumulative overall survival rates at 1, 3, and 5 years in the hepatic resection group were 86.5%, 60.4%, and 33.3%, respectively. These rates were much higher than those in the TACE group (1-year: 77.6%; 3-year: 47.8%; and 5-year: 20.9%; P = 0.021; Fig. 1).
Figure 1.
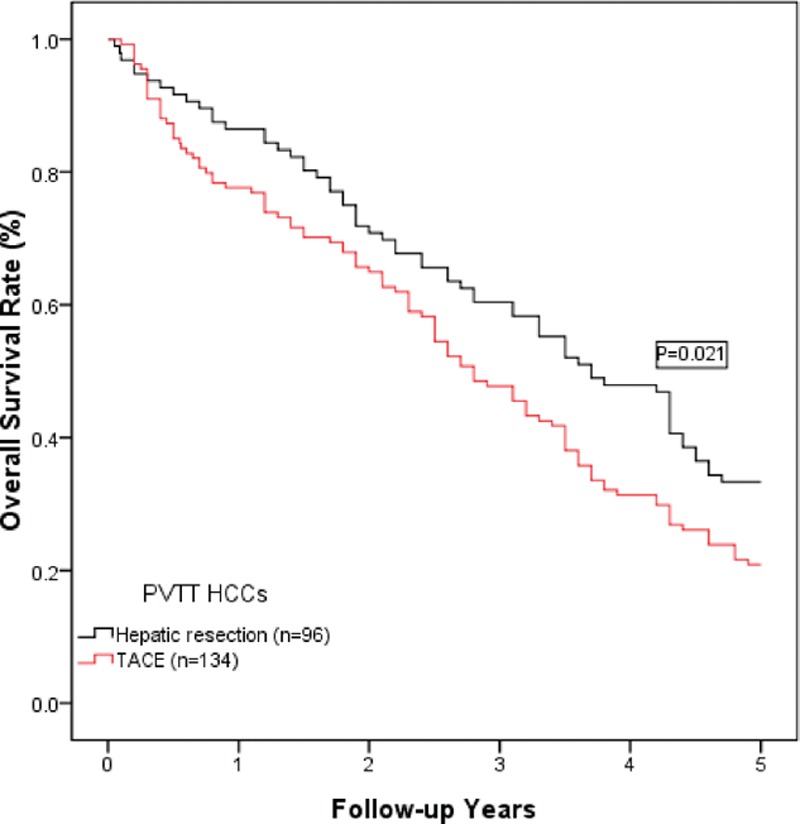
A comparison of the overall survival curves for HCC patients with PVTT who were treated with hepatic resection or TACE. The 1-, 3-, and 5-year overall survival rates in the hepatic resection group were 86.5%, 60.4%, and 33.3%, respectively. These rates were significantly higher than those in the TACE group (1-year: 77.6%; 3-year: 47.8%; and 5-year: 20.9%; P = 0.021).
As shown in Fig. 2, a subgroup analysis of the patients according to PVTT type indicated that the overall survival of patients with type II PVTT was comparable to that of patients with type I PVTT (P >0.05); however, the long-term survival of patients with type II PVTT was much better than that of patients with type III PVTT (P <0.05). Additionally, the overall survival of patients with types III and IVPVTT was comparable (P >0.05).
Figure 2.
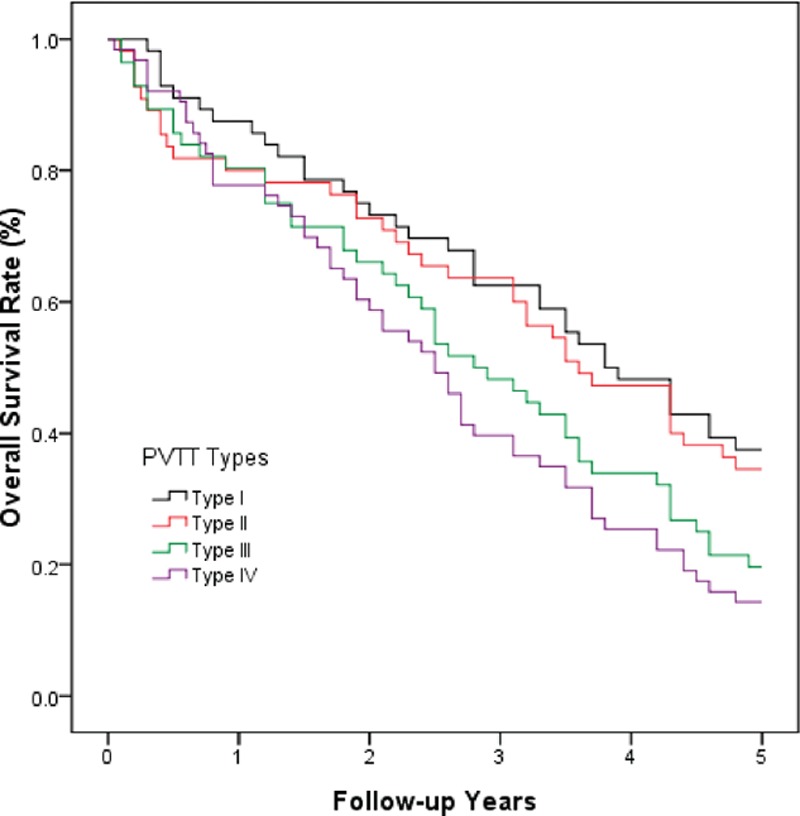
A comparison of the overall survival curves for HCC patients with different types of PVTT. Types I and II PVTT were associated with comparable long-term survival (P > 0.05). Additionally, types III and IV PVTT were associated with comparable long-term survival. However, the long-term survival in patients with type II PVTT was much better than that in patients with type III PVTT (P < 0.05).
3.4. Univariate and multivariate analyses of overall survival
Additional survival analyses were performed, and the results are shown in Tables 3 and 4. These analyses included the following factors that were associated with survival: age; gender; race; BMI; cause of liver disease; preoperative anti-viral therapy; HBV-DNA; Child–Pugh score; portal hypertension; ECOG performance status; neutrophil-lymphocyte ratio (NLR); albumin (ALB), creatinine, platelet (PLT), and AFP levels; total tumor diameter; tumor number; tumor location; PVTT type; and therapy protocol (hepatic resection or TACE). The univariate analyses identified the following 21 prognostic factors that predicted poor overall survival: age; gender; BMI; race; cause of liver disease; preoperative antiviral therapy; HBV-DNA; Child score; ECOG score; portal hypertension; tumor number; tumor diameter; tumor location; PVTT type; NLR; AFP, creatinine, ALB, hemoglobin (Hb), and PLT levels; and treatment protocol. Multivariate Cox regression analyses were performed using these significant factors. The results of the analyses revealed that types III and IV PVTT and TACE were significant risk factors for the overall survival of HCC patients after surgery.
Table 3.
The risk factors for the overall survival of patients according to the univariate analyses.

Table 4.
The results of the multivariate analyses of factors that contributed to the overall survival rates.

4. Discussion
PVTT is one of the most important prognostic factors for the survival of HCC patients.[7,16–18] The optimal treatment for HCC with PVTT remains controversial, particularly regarding hepatic resection. HCC with PVTT is classified as advanced-stage according to the AASLD/BCLC staging system, and surgical resection is not recommended for patients with advanced HCC. TACE or sorafenib is recommended as the first-line treatment for these patients; however, several groups have argued that surgical resection in HCC patients with PVTT has produced significantly better results than in unresectable patients who are treated with sorafenib.[19] However, the use of TACE for the treatment of advanced HCC is limited by potential adverse events, high costs, and reduced efficiency. TACE is commonly used to treat patients with advanced HCC according to the BCLC staging system. Previous studies have demonstrated that TACE had better efficacy than conservative treatment in HCC patients with PVTT; however, the outcomes of TACE were poor.[20,21] Surgical resection with complete extirpation of the tumor may be the only viable treatment option for patients with HCC, particularly due to the shortage of liver grafts for transplantation. Previous reports have suggested that hepatic resection is a safe and effective treatment for HCC with PVTT when patients are carefully selected. In patients with all types of PVTT, the median survival has ranged from 8.9 to 33 months and the operative mortality has ranged from 0% to 5.9%.[5,7,11,22–24] The potential benefits of surgical resection in patients with HCC and PVTT include decreased portal venous pressure, improved liver function, prolonged survival, and improved quality of life.[11]
This study demonstrated better long-term outcomes in the HCC patients with PVTT who were treated with hepatic resection compared with the HCC patients who were treated with TACE as the initial treatment. In patients with types I and II PVTT, better outcomes were particularly observed. Our results are consistent with Kondo's study, in which liver resection showed better outcomes in HCC patients with the first- or higher-order branch of portal vein when comparing with those patients with the main portal venous trunk PVTT.[25] In the report by Chen et al,[24] the HCC patients with the first portal branch PVTT showed significantly better long-term overall survival rates when comparing with those patients with PVTT that extended into the main trunk. Lower degree of disease severity may contribute to the better outcomes in the types I and II PVTT patients with HCC R0 resection or thrombectomy. When the tumor thrombi involve the main portal vein trunk or the superior mesenteric vein, it was defined as Type III or IV PVTT, and these kinds of PVTT may lead to intraoperative difficulties in resecting the thrombi. Meanwhile, portal vein wall invasion may lead to thrombi residue and high risk of postoperative recurrence. It was reported tumor thrombus extends to the main portal vein may lead to extremely poor prognosis due to the following reasons: portal hypertension due to tumor thrombi portal vein obstruction may lead to worse liver function or liver failure, esophageal variceal bleeding, intractable ascites. Meanwhile, more extensive intrahepatic metastases due to the tumor cells spread along the portal vein may also contribute to the poor prognosis.[11,26]
To identify the risk factors that impacted the long-term survival of the patients, univariate and multivariate Cox proportional hazard regression models were constructed. In the univariate analysis, an AFP level ≥400 ng/mL, an NLR ≥4, and a tumor diameter >10 cm were associated with significant P values. However, no significant differences were observed in the multivariate analysis. Tumor number and tumor size may not be considered contraindications for hepatic resection: it is generally accepted that patients should receive hepatic resection for HCC irrespective of the number of tumors when a reasonably sized functional liver remnant is available. Hepatic resection for a single large HCC is associated with favorable long-term survival.[5,27] In the present study, the univariate and multivariate analyses indicated that the tumor number and the tumor diameter were not contributing factors to the overall survival of the HCC patients with PVTT. Additionally, other biomarkers that may contribute to tumor recurrence or may be risk factors for the overall survival of these patients, such as AFP levels and the NLR, were included in the univariate and multivariate analyses. These factors were statistically significant in the univariate analysis; however, the P value for these factors did not reach 0.05 in the multivariate analysis. The main reason for this result may be the impact of the PVTT type and the treatment protocol, which may be more important for long-term survival than other biomarkers.[28]
This study had several limitations. First, this study was retrospective and was conducted at a single center. Second, the pathological examinations were not included in the analysis because of a lack of data in the TACE group. Tumor differentiation, microvascular invasion, or other biomarkers may contribute to tumor recurrence after surgery. Third, this single-center analysis was performed in China, which has a high prevalence of HBV infection; therefore, the results of this analysis may not be applicable to patients with HCV infection or alcoholic cirrhosis. Fourth, ultrasonography but not enhanced CT or MRI is the first choice to monitor the HCC recurrence is also a limiting factor in our study. Larger, randomized multicenter studies are needed to confirm these results.
In conclusion, this retrospective study indicated that hepatic resection may lead to better long-term survival in HCC patients with PVTT, particularly in patients with type I or II PVTT, when compared with TACE as initial therapy.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Diseases, AFP = alpha-fetoprotein, BCLC = Barcelona Clinic Liver Cancer, EASLD = European Association for the Study of Liver Disease, Hb = hemoglobin, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, LT = liver transplantation, MELD = model for end-stage liver disease, NLR = neutrophil–lymphocyte ratio, PHT = portal hypertension, PLT = platelet, PVTT = portal vein tumor thrombus, RFA = radiofrequency ablation, TACE = transarterial chemoembolization, UCSF = University of California, San Francisco.
NZ and BD proposed the study. NZ performed research and wrote the first draft. NZ and BD collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. BD is the guarantor.
The authors have no conflicts of interest to disclose.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004; 127 (5 suppl 1):S5–S16. [DOI] [PubMed] [Google Scholar]
- 3.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 2011; 253:453–469. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014; 63:844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng ZW, Guo RP, Zhang YJ, et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2012; 118:4725–4736. [DOI] [PubMed] [Google Scholar]
- 6.Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014; 146:1691–1700. [DOI] [PubMed] [Google Scholar]
- 7.Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014; 61:583–588. [DOI] [PubMed] [Google Scholar]
- 8.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013; 257:929–937. [DOI] [PubMed] [Google Scholar]
- 9.Inoue Y, Hasegawa K, Ishizawa T, et al. Is there any difference in survival according to the portal tumor thrombectomy method in patients with hepatocellular carcinoma? Surgery 2009; 145:9–19. [DOI] [PubMed] [Google Scholar]
- 10.Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014; 260:329–340. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2010; 17:2073–2080. [DOI] [PubMed] [Google Scholar]
- 12.Lei J, Wang W, Yan L. Surgical resection versus open-approach radiofrequency ablation for small hepatocellular carcinomas within Milan criteria after successful transcatheter arterial chemoembolization. J Gastrointest Surg 2013; 17:1752–1759. [DOI] [PubMed] [Google Scholar]
- 13.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333–339. HPB 2002; 4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jianyong L, Jinjing Z, Wentao W, et al. Preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: a single center analysis. Ann Hepatol 2014; 13:394–402. [PubMed] [Google Scholar]
- 15.Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011; 254:907–913. [DOI] [PubMed] [Google Scholar]
- 16.Zhao JB, Feng C, Zhu QH, et al. Transjugular intrahepatic portosystemic shunt with covered stents for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2014; 20:1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song do S, Bae SH, Song MJ, et al. Hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2013; 19:4679–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogl TJ, Nour-Eldin NE, Emad-Eldin S, et al. Portal vein thrombosis and arterioportal shunts: effects on tumor response after chemoembolization of hepatocellular carcinoma. World J Gastroenterol 2011; 17:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 20.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006; 131:461–469. [DOI] [PubMed] [Google Scholar]
- 21.Kim KM, Kim JH, Park IS, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 2009; 24:806–814. [DOI] [PubMed] [Google Scholar]
- 22.Ikai I, Hatano E, Hasegawa S, et al. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg 2006; 202:431–438. [DOI] [PubMed] [Google Scholar]
- 23.Le Treut YP, Hardwigsen J, Ananian P, et al. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case-control series. J Gastrointest Surg 2006; 10:855–862. [DOI] [PubMed] [Google Scholar]
- 24.Chen XP, Qiu FZ, Wu ZD, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol 2006; 13:940–946. [DOI] [PubMed] [Google Scholar]
- 25.Kondo K, Chijiiwa K, Kai M, et al. Surgical strategy for hepatocellular carcinoma patients with portal vein tumor thrombus based on prognostic factors. J Gastrointest Surg 2009; 13:1078–1083. [DOI] [PubMed] [Google Scholar]
- 26.Lai EC, Lau WY. The continuing challenge of hepatic cancer in Asia. Surgeon 2005; 3:210–215. [DOI] [PubMed] [Google Scholar]
- 27.Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009; 249:118–123. [DOI] [PubMed] [Google Scholar]
- 28.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 2006; 12:7561–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]


