Abstract
To describe the features and clinical implications of computed tomography (CT), positron emission tomography (PET), and percutaneous needle aspiration biopsy (PCNB) in pulmonary nontuberculous mycobacterial (NTM) disease manifesting as a solitary nodule, mass, or mass-like consolidation mimicking malignancy.
Among a cohort of 388 patients with NTM pulmonary disease, 14 patients with clinically and radiologically suspected lung cancer were included in our study. Two chest radiologists evaluated CT features, including lesion type (nodule, mass, or mass-like consolidation), morphologic features (margin, degree of enhancement, calcification), and presence of accompanying findings suggestive of NTM pulmonary disease (bronchiectasis with clustered centrilobular nodules or upper-lobe cavitary lesions) by consensus. Diagnostic procedures for microbiologic diagnosis of NTM disease and clinical outcome were reviewed.
Incidence of NTM pulmonary disease presenting as solitary nodule/mass (n = 8) or mass-like consolidation (n = 6) was 3.6% (14 of 388). Most lesions were detected incidentally during routine health check-up or evaluation of other disease (11 of 14, 79%). Lesions typically showed poor contrast-enhancement (9 of 12) and internal calcification (6 of 14). No lesions had CT features suggestive of NTM pulmonary disease. All 4 lesions for which PET/CT imaging was performed showed strong fluorodeoxyglucose uptake simulating malignant lesions (mean, 4.9; range, 3.6–7.8). PCNB revealed mycobacterial histology in 6 of 11 specimens and positive culture results were obtained for 7 of 7 specimens.
NTM pulmonary disease may present as a solitary nodule, mass, or mass-like consolidation mimicking malignancy. CT features and PCNB are important to diagnose NTM disease mimicking lung cancer to avoid unnecessary surgery.
Keywords: lung, needle biopsy, nontuberculous mycobacteria, solitary pulmonary nodule, x-ray computed tomography
1. Introduction
Nontuberculous mycobacterial (NTM) pulmonary disease is an important cause of chronic pulmonary infection and the prevalence of NTM disease is increasing worldwide. According to the criteria of the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA), radiologic evidence of disease is as important as clinical and microbiologic criteria to make a diagnosis of NTM pulmonary disease.[1] NTM pulmonary diseases are classified into upper-lobe fibrocavitary disease and nodular bronchiectatic disease according to radiologic patterns. Characteristic radiologic features of fibrocavitary disease include heterogeneous nodular and cavitary opacities in the upper lobes, whereas those of nodular bronchiectatic disease are bronchiectasis and branching centrilobular nodules in the middle lobe and lingula.[2–4]
However, radiologic manifestations of NTM pulmonary disease are protean and may manifest as less common or well-recognized patterns. As with other granulomatous diseases such as tuberculosis, NTM pulmonary disease can manifest as a solitary nodule or mass mimicking primary lung cancer. Several earlier studies have described solitary granuloma caused by the Mycobacterium avium complex (MAC). One study compared computed tomography (CT) features between tuberculoma and MAC disease, and reported no significant difference in CT features.[5] Another study compared the clinical and CT features of solitary granuloma caused by MAC with those of granulomas caused by other NTM species, and reported that female sex, pleural indentation, and lobulation were associated with MAC disease.[6] However, to the best of our knowledge, the exact incidence and clinical and radiologic features of NTM pulmonary disease mimicking lung cancer have not been reported in detail.
Therefore, our goal in this study was to describe the features and clinical implications of CT, positron emission tomography (PET), and percutaneous needle aspiration biopsy (PCNB) findings in NTM pulmonary disease manifesting as a solitary nodule, mass, or mass-like consolidation mimicking malignancy.
2. Methods
2.1. Patient selection
This retrospective study was approved by the institutional review board of Seoul National University Bundang Hospital Institutional Review Board, and informed consent was waived. Between March 2007 and December 2011, a total of 437 patients in our institution (1300-bed university-affiliated tertiary referral hospital) were confirmed to have NTM pulmonary disease according to the criteria of the ATS and the IDSA.[1] Among them, 388 patients who underwent chest CT scan(s) were evaluated in this study. Two chest radiologists (S.J.H. and T.J.K.) screened the CT scans and identified patients with a solitary pulmonary nodule (SPN), solitary mass, or mass-like consolidation mimicking primary lung cancer.
An SPN or solitary mass was defined as a solitary, rounded or oval lesion measuring up to 3 cm (nodule) or larger (mass) in diameter.[7] Mass-like consolidation was defined as a localized consolidative lesion with a typically rounded or oval shape that showed no change or increase in size on a follow-up CT scan taken at an interval of at least 1 month. Patients with the following radiologic findings were excluded: typical radiologic manifestations of NTM pulmonary disease such as upper-lobe cavitary or nodular bronchiectatic form,[2–4] nonspecific inflammatory lesions, sequelae of pulmonary tuberculosis, and normal lung parenchyma.
In total, 14 patients with radiologically and clinically suspected primary lung cancer were included in our study, and their medical records were analyzed retrospectively. One author (S.J.H.) reviewed the electronic medical records and analyzed the presenting symptoms, underlying diseases, previous history of tuberculosis, laboratory findings, diagnostic work-ups for microbiologic confirmation for NTM disease, treatment methods such as antibiotic therapy, surgical excision, and follow-up or treatment outcomes.
2.2. Microbiologic studies
Respiratory specimens including sputum, bronchial washing fluid, tissue, or aspirates from PCNB or specimens from surgical excision were used for microbiologic diagnosis of NTM pulmonary disease. PCNB was done with a 20-or 22-gauge Westcott needle (Becton Dickinson, Franklin Lakes, NJ). Mycobacterial smear and culture were performed using standard methods.[1] All mycobacterial isolates were analyzed to distinguish between M tuberculosis and NTM using a commercially available polymerase chain reaction (PCR)-based assay system (MTB-ID, M&D Inc, Gangwon, Korea). NTM species were identified using PCR and restriction fragment length polymorphism methods based on the rpoB gene.[8]
2.3. Imaging techniques and interpretation
CT examinations were performed with 1 of 3 scanners (iCT, Brilliance 64, or Mx-8000 IDT; Philips Medical Systems, Best, the Netherlands) using automatic exposure control with the following parameters: tube voltage, 120 kVp; tube current, 100 to 150 mAs, detector collimation, 0.625 to 1.5 mm; beam pitch, 0.75 to 1.75; and rotation time, 0.5 to 0.75 seconds. Contrast-enhanced CT scans were performed in 12 patients, with a 45-second delay after attenuation of the ascending aorta had reached 200 HU after intravenous injection of 80 to 100 mL iopromide (Ultravist 370; Schering, Berlin, Germany). Noncontrast CT scans were performed in 2 patients using the same protocol except for use of contrast media. Images were reconstructed using a lung reconstruction algorithm with 1 mm (thin section) and 3 mm (thick section) thicknesses. All images were displayed with lung (level setting, −600 HU; width setting, 1500 HU) and mediastinal (level setting, 30 HU; width setting, 400 HU) window settings.
Two thoracic radiologists (S.J.H. and T.J.K.) analyzed CT images independently, and consensus was reached by discussion when discordance was present. NTM pulmonary disease mimicking malignancy was grouped into 2 patterns according to CT features: SPN/mass and mass-like consolidation. With regard to CT features, lesion size, location, margin (well-defined, poorly defined), border (smooth, lobulated, spiculated), degree of enhancement, presence of internal calcification, air-bronchogram, cavitation, pleural retraction, and satellite lesions around the main lesion were evaluated. The degree of enhancement was classified as follows: poor, enhancement less than that of intrathoracic muscle (<50 HU); moderate, enhancement similar to that of intrathoracic muscle (50–70 HU); and strong, enhancement greater than that of intrathoracic muscle (>70 HU). Presence of clustered centrilobular nodules with or without bronchiectasis, which are typical imaging findings of nodular bronchiectatic NTM disease,[3] was also evaluated. Follow-up CT findings were correlated with treatment methods.
18F-fluorodeoxyglucose (FDG) PET/CT was performed in 5 patients using a dedicated PET/CT scanner (DVCT, GE Healthcare, Milwaukee, WI). All patients were fasted for at least 6 hours before the PET scan, and peripheral blood glucose level was <200 mg/dL. 18F-FDG (0.14 mCi/kg) was injected intravenously, and whole-body scanning was performed 50 minutes after administration. The maximum standardized uptake value (SUVmax) of the pulmonary lesion was considered representative of FDG uptake.
3. Results
3.1. Clinical characteristics
In our cohort of patients with NTM pulmonary disease from a single tertiary referral center, the incidence of NTM pulmonary disease mimicking lung cancer clinically and radiologically was 3.6% (14 of 388). Among these 14 patients, 10 (71%) were women (mean age, 60 years; range, 37–78 years) and 4 were men (mean age, 61 years; 47–85 years). Pulmonary lesions were mostly incidentally detected during a routine health check-up or evaluation of other disease in 11 patients (79%), whereas 3 lesions were detected during evaluation of symptoms such as cough (n = 2, 14%) or fever (n = 1, 7%). No patient exhibited hemoptysis. All women were never smokers, but all men were either ex-smokers or current smokers (mean pack years, 33; range, 15–60). Five patients had hypertension, 3 had diabetes mellitus, and 1 had a history of treatment for pulmonary tuberculosis. Laboratory findings were unremarkable in all patients: white blood cell count (mean, 6235/μL; range, 4320–9580/μL), absolute neutrophil count (mean, 3767/μL: range, 2127–7760/μL), erythrocyte sedimentation rate (mean, 10.7 mm/hour; range, 2–38 mm/hour), and C-reactive protein (mean, 0.33 mg/L; range, 0.01–2.28 mg/L).
3.2. Imaging features
NTM pulmonary disease mimicking primary lung cancer presented as an SPN/mass (n = 8) or mass-like consolidation (n = 6). Mean SPN/mass size was 2.3 cm (range, 1.3–3.4 cm), whereas mean mass-like consolidation size was 4.5 cm (range, 2.6–7.1 cm). Lesions were distributed in various locations. The lesion was in the right middle lobe in 1 patient and the lingular segment in another patient; both these sites are common sites of nodular bronchiectatic NTM pulmonary disease.
Eleven lesions had a well-defined margin and 10 lesions had a lobulated border. Internal calcification was seen in 6 lesions (43%) (Figs. 1 and 2). Cavity and pleural retraction were found in 4 patients each (Fig. 3). Satellite lesions (i.e., small branching nodules) around the main lesion were not found. Degree of contrast enhancement was poor in 9 (range, 17–46 HU) (Figs. 1–4) and moderate in 3 (range, 55–63 HU) lesions. Mild bronchiectasis in the right middle lobe and/or lingular segment was seen in 3 patients.
Figure 1.
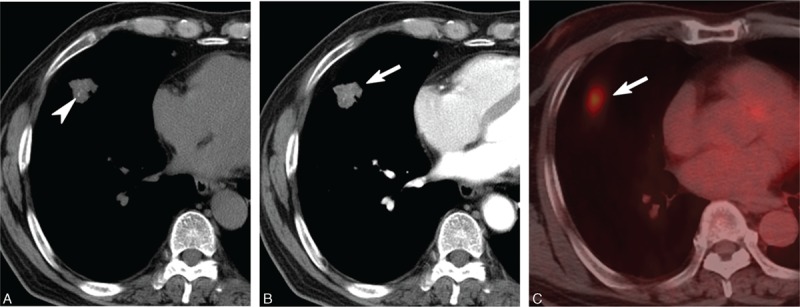
A 60-year-old asymptomatic man with an SPN. A, Noncontrast CT showed an SPN with internal calcification (arrowhead) in the right middle lobe. B, Contrast-enhanced CT showed a nodule (arrow) with poor contrast enhancement with a mean attenuation value of 46 HU. C, Fused axial image from 18F-FDG PET/CT showed significant FDG uptake (SUVmax, 3.7). D, CT-guided PCNB was done using a 22-gauge Westcott needle (arrowheads). PCNB specimen showed chronic granulomatous inflammation. Culture of PCNB aspirates was positive for Mycobacterium avium. Follow-up CT obtained after completion of anti-MAC complex antibiotic treatment showed minimal residual fibrotic change (not shown). CT = computed tomography, FDG = fluorodeoxyglucose, MAC = Mycobacterium avium, PCNB = percutaneous needle aspiration biopsy, PET = positron emission tomography, SPN = solitary pulmonary nodule, SUVmax = maximum standardized uptake value.
Figure 2.
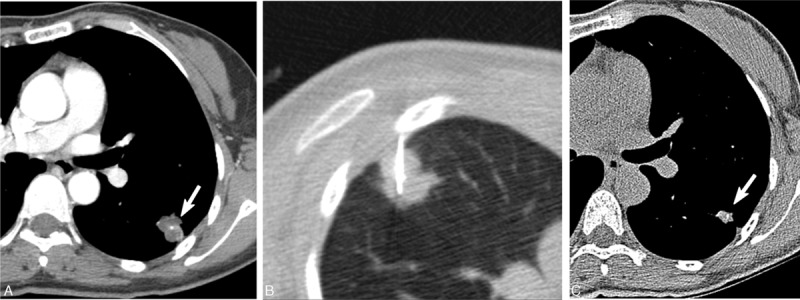
A 73-year-old asymptomatic woman with an SPN. A, CT showed a lobulated nodule (arrow) with poor contrast enhancement (HU, 41) in the left lobe. Note the internal dot-like calcification. B, CT-guided PCNB using a 20-gauge Westcott needle showed chronic granulomatous inflammation. Culture of PCNB aspirates showed the presence of Mycobacterium avium. PCNB aspirates were negative for tuberculosis-PCR and positive for NTM-PCR. C. Follow-up CT obtained 2 years later without anti-MAC antibiotic treatment showed a decrease in the size of the NTM nodule (arrow). CT = computed tomography, MAC = Mycobacterium avium, NTM = nontuberculous mycobacterial, PCNB = percutaneous needle aspiration biopsy, PCR = polymerase chain reaction.
Figure 3.
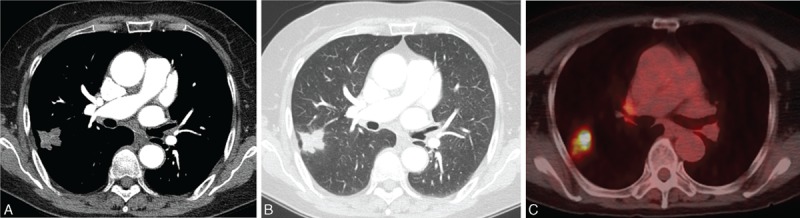
A 73-year-old asymptomatic woman with an SPN. A, Contrast-enhanced CT shows a lobulated nodule with poor contrast enhancement with a mean attenuation value of 33 HU. B, Lung window CT image showed a nodule with a lobulated contour in the right upper lobe. No abnormal findings such as bronchiectasis or clustered small nodules in the remaining lungs were noted. C, Fused axial image from 18F-FDG PET/CT showed strong FDG uptake (SUVmax, 7.8). This patient underwent surgical resection (right upper lobectomy) without PCNB. Surgical specimen showed chronic granulomatous inflammation and was positive for NTM-PCR. D, Fused axial image. CT = computed tomography, FDG = fluorodeoxyglucose, MAC = Mycobacterium avium, NTM = nontuberculous mycobacterial, PCNB = percutaneous needle aspiration biopsy, PCR = polymerase chain reaction, SUVmax = maximum standardized uptake value.
Figure 4.
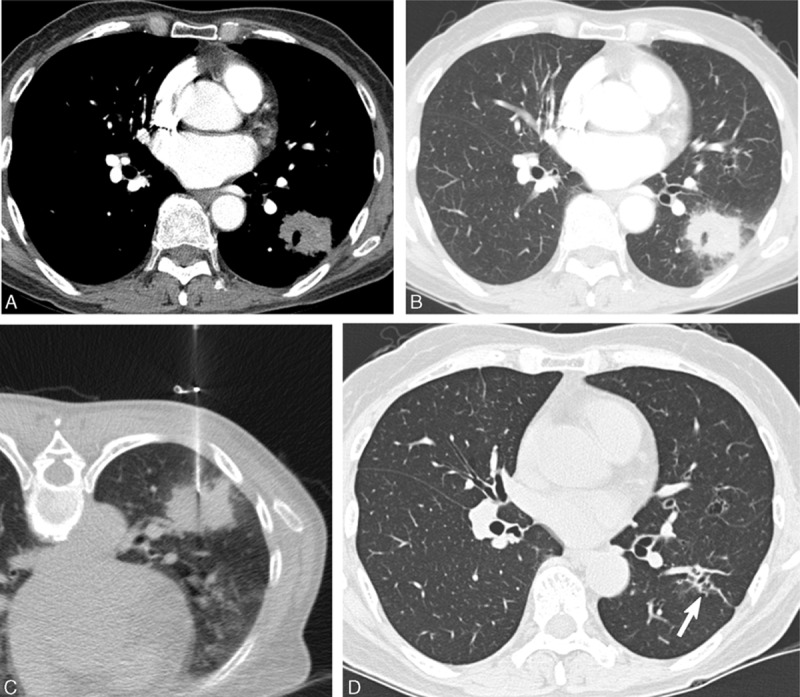
A 61-year-old asymptomatic woman with a mass-like consolidation. A, Contrast-enhanced CT showed a mass-like consolidation with poor contrast enhancement with a mean attenuation value of 39 HU. B, Lung window CT image showed an irregular border and ill-defined ground-glass opacity adjacent to the mass-like consolidation. C, CT-guided PCNB using a 22-gauge Westcott needle showed chronic granulomatous inflammation. Culture of PCNB aspirates revealed the presence of Mycobacterium intracellulare organisms. D, Follow-up CT obtained 2 years after anti-MAC antibiotic treatment showed complete resolution with minimal fibrotic change (arrow). CT = computed tomography, MAC = Mycobacterium avium, PCNB = percutaneous needle aspiration biopsy.
18F-FDG PET/CT was performed in 4 patients with a solitary nodule or mass and in 1 with mass-like consolidation. The mean SUVmax was 4.9 (range, 3.6–7.8) (Figs. 1 and 3).
In 5 patients who underwent surgical resection, no recurrence was found on follow-up imaging studies (range, 6–72 months). Five of the remaining patients received standard antibiotic therapy for NTM disease, whereas the other 4 were not based on ATS-IDSA guidelines,[1] and all showed radiologic improvement regardless of treatment (Figs. 2 and 4). The interval between the initial and follow-up CT examinations ranged from 65 to 775 days.
3.3. Microbiologic diagnosis and diagnostic modalities
Microbiologic diagnosis and diagnostic modalities used to evaluate the 14 patients with NTM pulmonary disease are summarized in Table 1. Microbiologic diagnoses were established by PCNB (n = 8) (Figs. 1, 2 and 4), surgery (n = 5), or culture of bronchial washing fluid (n = 1). Causative NTM species were M avium (n = 7), M intracellulare (n = 3), M abscessus (n = 1), and M kansasii (n = 1).
Table 1.
Diagnostic results in 14 patients with NTM pulmonary disease mimicking malignancy.
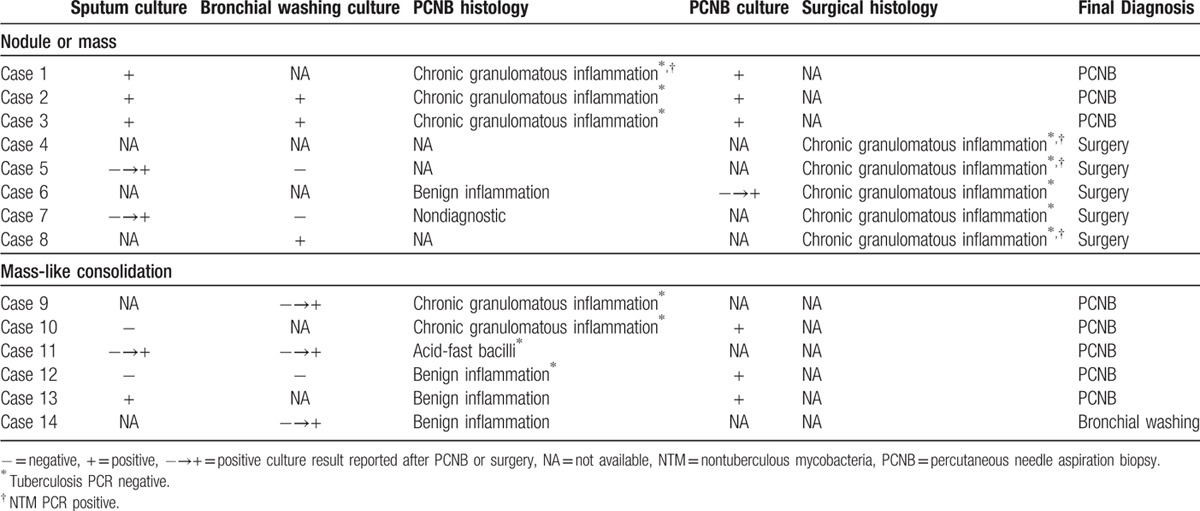
Sputum culture and bronchial washing fluid culture were positive in 6 and 9 patients, respectively. Sputum culture and bronchial washing culture results were available after PCNB or surgical excision in 2 and 3 patients, respectively. One patient with nondiagnostic PCNB was confirmed to have NTM disease by positive culture of bronchial washing fluid.
PCNB was performed in 11 patients. Histologic diagnoses of PCNB showed chronic granulomatous inflammation in 5, chronic inflammation nondiagnostic for NTM disease in 5, and acid-fast bacilli clusters of NTM species in 1. Culture of PCNB specimens yielded positive results in 7 patients, including 3 with nondiagnostic NTM disease, and NTM PCR was positive in 1 patient. In total, NTM disease was microbiologically diagnosed in 8 of 11 patients who underwent PCNB.
Surgical excision was performed in 5 patients; wedge resection in 4, and lobectomy in 1. Three patients underwent surgical excision without PCNB, and the results of sputum or bronchial washing fluid culture were negative in these patients. Two patients with nonspecific PCNB results underwent surgical excision before the positive culture reports from PCNB were available. All surgical specimens showed chronic granulomatous inflammation with or without caseous necrosis. Tuberculosis (TB)-PCR results were all negative, whereas NTM-PCR results were positive in 3 patients.
4. Discussion
Our study showed that NTM pulmonary disease can manifest as an SPN, solitary mass, or mass-like consolidation. These lesions presented with radiologic features overlapping those of primary lung cancer. In our cohort of NTM pulmonary disease from a single tertiary referral center, the incidence of NTM pulmonary disease mimicking lung cancer clinically and radiologically was 3.6% (14 of 388). The lesions typically showed poor contrast enhancement and frequent internal calcification on CT. PCNB was quite useful for microbiologic diagnosis of NTM disease manifesting as an SPN, mass, or mass-like consolidation.
Evaluation of SPNs is a common diagnostic dilemma that has become more prevalent with the increasing use of CT in current clinical practice.[9] The most common cause of benign SPNs is M tuberculosis, but recent advances in microbiologic diagnosis have led to increased confirmation of SPNs due to NTM disease. Gribetz et al[10] reported that 14 of 20 solitary nodular lesions were due to NTM disease, including M intracelluare, M fortuitum, and M gordonae in the United States. Yonemori et al[6] found that 24% of resected solitary pulmonary granulomas were due to the M avium-intracellulare (MAI) complex in Japan. However, to the best of our knowledge, the actual incidence of NTM pulmonary disease presenting as an SPN, mass, or mass-like consolidation and mimicking primary lung cancer has not been evaluated. The incidence of NTM pulmonary disease mimicking primary lung cancer in our study was 3.6% (14 of 388), which represents a small but meaningful subset of NTM pulmonary disease. Considering the increasing prevalence of NTM disease worldwide, the incidence in our study may not be negligible, and NTM disease should be included as one of the differential diagnoses of an SPN, mass, or mass-like consolidation in clinical practice.
In our study, NTM pulmonary disease presenting as a solitary lesion frequently showed poor contrast enhancement (9 of 12, 75%) and internal calcification (6 of 14, 43%), which could be helpful for differentiation from lung cancer. Although specific subtypes of lung cancer such as invasive mucinous adenocarcinoma and hypovascular lung cancer may have overlapping features, poor contrast enhancement on CT should raise the suspicion of NTM disease in the differential diagnosis of solitary pulmonary lesions.
Calcification can be seen in both benign and malignant lesions, but is more frequently observed in chronic inflammatory diseases such as tuberculosis or histoplasmosis. Relatively high incidence of calcification in our patients may aid in the correct radiologic diagnosis of NTM disease. It is also noteworthy that NTM pulmonary disease may manifest as a solitary lesion without alleged typical imaging features of NTM disease such as cellular bronchiolitis with or without bronchiectasis (i.e., nodular bronchiectatic form) or cavitary lesions with upper lung predominance (i.e., upper-lobe cavitary form). None of the solitary lesions in our study had concomitant typical radiologic findings suggestive of NTM pulmonary disease, in agreement with the results reported in a previous study.[6]
Other CT features of solitary lesions in our study overlapped with those of primary lung cancer. Lobulated border (71%) and pleural retraction (28%) are common findings of malignant pulmonary lesions. FDG PET/CT does not provide additional information to help differentiation from lung cancer because NTM disease can cause false-positive results like other chronic inflammatory diseases such as tuberculosis, fungal infection, and sarcoidosis.[11] All 4 lesions for which PET/CT images were available showed strong FDG uptake simulating malignant lesions (mean, 4.9; range, 3.6–7.8), consistent with the results of previous studies.[12,13]
Microbiologic procedures are key for the diagnosis of NTM disease. However, the results of sputum or bronchial washing culture in patients with an SPN, mass, or mass-like consolidation should be interpreted with caution. NTM species are often saprophytes and may colonize airways rather than infect them, and therefore, cultures of sputum or bronchial washing fluid can be falsely positive. Thus, a positive culture result for sputum or bronchial washing fluid does not exclude the possibility of concomitant lung cancer. Simultaneous lung cancer and NTM pulmonary disease may occur, considering the recent increase in incidence of NTM disease. In addition, the diagnostic yield of culture studies in patients with solitary lesion may be lower than that of typical CT features of NTM pulmonary disease. In our study, 3 of 9 (33%) sputum and bronchial washing cultures, respectively, were falsely negative. Previous studies reported negative culture results in 25% to 67% of patients with an SPN caused by the MAI complex.[5,14]
Because NTM pulmonary nodules or masses are likely to have a peripheral rather than central location,[6] PCNB can be an effective procedure to obtain a sufficient amount of specimen for culture and histologic diagnosis of NTM disease. ATS/IDSA criteria include mycobacterial histologic features (granulomatous inflammation or acid-fast bacilli) and positive culture for NTM of biopsy specimens.[1] In our study, mycobacterial histology was observed in 6 of 11 PCNB specimens (55%), and positive culture results were obtained in all specimens (7 of 7, 100%). Our results highlight the importance of culture study using aspirates or tissues obtained from PCNB for microbiologic confirmation of NTM disease.
Five of 14 patients in our study had surgical biopsies. Three patients underwent surgical excision without PCNB, whereas the other 2 patients underwent surgery before a positive culture report from PCNB was available. The decision to perform surgical excision without histologic confirmation depends on the likelihood of malignancy. Surgery may have significant complications and morbidities including infection and postoperative pain. Real-time PCR evaluation of NTM disease might be a useful alternative to conventional culture studies in NTM disease.[15] In our study, TB or NTM PCR studies were performed in the majority of PCNB and surgical specimens, and showed promising results for the diagnosis of NTM disease. Characteristic CT features in our study such as low attenuation, poor contrast-enhancement, and internal calcification may suggest the possibility of NTM pulmonary disease, whereas appropriate use of PCNB may allow histological diagnosis of NTM disease and avoidance of unnecessary surgery.
Our study had several limitations. First, this retrospective study was conducted at a single referral center in East Asia. In addition, NTM species included in this study were mostly M avium or M intracelluare, which might be different from the species prevalent in other regions. Second, although we tried to include as many as possible subjects from a relatively large cohort over a 5-year period, the number of patients with NTM disease mimicking lung cancer was small and not sufficient for robust statistical analyses.
In summary, NTM pulmonary disease can lead to radiologic findings similar to lung cancer. The prevalence of NTM pulmonary disease is increasing significantly, hence the need to increase awareness of this condition. Characteristic CT features together with appropriate use of percutaneous needle biopsy can help identify NTM pulmonary disease mimicking lung cancer and may reduce unnecessary surgery.
Footnotes
Abbreviations: CT = computed tomography, FDG = fluorodeoxyglucose, NTM = nontuberculous mycobacteria, PCNB = percutaneous needle aspiration biopsy, PCR = polymerase chain reaction, PET = positron emission tomography, SUVmax = maximum standardized uptake value.
The authors report no conflicts of interest.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 2.Moore E. Atypical mycobacterial infection in the lung: CT appearance. Radiology 1993; 187:777–782. [DOI] [PubMed] [Google Scholar]
- 3.Miller WT., Jr Spectrum of pulmonary nontuberculous mycobacterial infection. Radiology 1994; 191:343–350. [DOI] [PubMed] [Google Scholar]
- 4.Kim TS, Koh W-J, Han J, et al. Hypothesis on the evolution of cavitary lesions in nontuberculous mycobacterial pulmonary infection: thin-section CT and histopathologic correlation. Am J Roentgenol 2005; 184:1247–1252. [DOI] [PubMed] [Google Scholar]
- 5.Hahm CR, Park HY, Jeon K, et al. Solitary pulmonary nodules caused by Mycobacterium tuberculosis and Mycobacterium avium complex. Lung 2010; 188:25–31. [DOI] [PubMed] [Google Scholar]
- 6.Yonemori K, Tateishi U, Tsuta K, et al. Solitary pulmonary granuloma caused by Mycobacterium avium-intracellulare complex. Int J Tuberc Lung Dis 2007; 11:215–221. [PubMed] [Google Scholar]
- 7.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Park H-J, Cho S-N, et al. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol 2000; 38:2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erasmus JJ, Connolly JE, McAdams HP, et al. Solitary pulmonary nodules: part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000; 20:43–58. [DOI] [PubMed] [Google Scholar]
- 10.Gribetz AR, Damsker B, Bottone EJ, et al. Solitary pulmonary nodules due to nontuberculous mycobacterial infection. Am J Med 1981; 70:39–43. [DOI] [PubMed] [Google Scholar]
- 11.Goo JM, Im J-G, Do K-H, et al. Pulmonary tuberculoma evaluated by means of FDG PET findings in 10 cases. Radiology 2000; 216:117–121. [DOI] [PubMed] [Google Scholar]
- 12.Demura Y, Tsuchida T, Uesaka D, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur J Nucl Med Mol Imaging 2009; 36:632–639. [DOI] [PubMed] [Google Scholar]
- 13.Kawate E, Yamazaki M, Kohno T, et al. Two cases with solitary pulmonary nodule due to non-tuberculous mycobacterial infection showing intense 18F-fluorodeoxyglucose uptake on positron emission tomography scan. Geriatr Gerontol Int 2010; 10:251–254. [DOI] [PubMed] [Google Scholar]
- 14.Lim J, Lyu J, Choi C, et al. Non-tuberculous mycobacterial diseases presenting as solitary pulmonary nodules. Int J Tuberc Lung Dis 2010; 14:1635–1640. [PubMed] [Google Scholar]
- 15.Yun EY, Cho SH, Go SI, et al. Usefulness of real-time PCR to detect mycobacterium tuberculosis and nontuberculous mycobacteria. Tuberc Respir Dis 2010; 69:250–255. [Google Scholar]


