Abstract
Single-incision laparoscopic surgery is cosmetically beneficial, but technically challenging. In this study, the learning curve (LC) for single-incision laparoscopic right hemicolectomy (SILRC), incorporating complete mesocolic excision to resect right-sided colon cancer, was investigated through multidimensional techniques. Between December 2009 and May 2015, 64 patients each underwent SILRC of right-sided colon cancer at Severance Hospital, performed in all instances by the same surgeon. Moving average and cumulative sum control chart (CUSUM) were used for LC analyses retrospectively. Surgical failure was defined as conversion to conventional laparoscopic surgery, postsurgical morbidity within 30 days, harvested lymph node count <12, or local tumor recurrence. Both moving average and CUSUM graphics of operative time registered nadirs at the 24th patient, with slight ascent thereafter, reaching a plateau at the 40th patient. The CUSUM for surgical success peaked at the 23rd patient. Operative time for 23 patients in phase 1 (1–23) and for 41 patients in phase 2 (24–64) of the LC did not differ significantly. By comparison, significant differences in patients of phase 2 included larger tumor size, higher harvested lymph node counts, longer proximal resection margins, and more advanced disease. As indicated by multidimensional statistical analyses, the LC for SILRC of right-sided colon cancer was 23 patients. In terms of operative time and surgical success, SILRC is feasible for surgeons experienced in LS, but may prove more challenging for novices, given the fundamental technical difficulties of this procedure.
Keywords: complete mesocolic excision, CUSUM, learning curve, right colectomy, right colon cancer, single port, single-incision laparoscopic surgery
1. Introduction
Laparoscopic surgery (LS) has improved short-term outcomes in patients with colorectal cancer, without undermining oncologic effects.[1–3] Patients likewise prefer the cosmetic benefits of LS, given no difference in anticipated results. However, the multiple entry sites commonly used create a potential for complications, such as hematomas and incisional hernias.[4,5].A fair number of colorectal surgeons consequently have gravitated towards single-incision laparoscopic surgery (SILS), as an even less invasive technique in this setting.
Although few reports are available for corroboration, SILS has compared favorably with more traditional methods, achieving similar postoperative and oncologic outcomes in treatment of colorectal cancer.[6–8] However, even colorectal surgeons experienced in conventional LS may find SILS challenging, due to colliding surgical instruments, difficulty in optimizing angles for surgery, and crowding of personnel. These features make both experienced surgeons and novices hesitant to use SILS procedures. Despite a re-emphasis on complete mesocolic excision (CME) with central vascular ligation (CVL) in surgery of colon cancer[9] and its proven laparoscopic feasibility,[10–14] it may be difficult for colorectal surgeons to perform CME via SILS.
Data on the learning curve (LC) for SILS resection of colon cancer by CME are needed to corroborate feasibility studies and to confirm that surgeons indeed are capable of adapting. Nonetheless, there are no published reports on the LC for single-incision laparoscopic right hemicolectomy (SILRC) incorporating principles of CME. Only a few researchers have examined the LC for SILRC.[15–18] This study was conducted to delineate the LC for CME of right-sided colon cancer as a SILS procedure, based on 2 statistical analytic methods: moving average and cumulative sum control chart (CUSUM).
2. Methods
2.1. Patients
Between December 2009 and May 2015, 64 elective SILRCs were performed for right-sided colon cancer resection, all conducted by a single surgeon (HH) at Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea. Patient data were collected prospectively and reviewed retrospectively. At study onset, the acting surgeon was already credited with >100 open surgical and conventional LS resections of colorectal cancer. In this cohort, all lesions were adenocarcinomas of the right colon, confirmed by histopathology and situated no further than the mid-transverse colon clinically (see prior definition).[12] All patients were informed in detail regarding both single-incision and conventional LS for right-sided colon cancer, but only those electing SILRC and granting signed informed consent were included. Initially, SILRC was reserved for patients with relatively early-stage cancers clinically, but the study was later broadened to include subjects with advanced disease. Patients undergoing other concurrent surgeries and those with distant metastases were excluded. The study was approved by the institutional review board in Severance Hospital.
2.2. Perioperative and pathologic outcomes
Analysis of baseline characteristics and perioperative outcomes included the following variables: sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, alcohol intake, smoking status, prior abdominal surgery, neoadjuvant and adjuvant chemotherapy, tumor location, incision length, conversion to conventional LS, operative time (OT), estimated blood loss, numeric pain intensity scale (NPIS), time to bowel movement, time to resumption of soft diet, in-hospital length of stay (LOS), and postoperative morbidity and mortality. Conversion to LS was defined as insertion of additional port(s). Tumor size, harvested lymph node count, status of resection margins (proximal and distal), clinical stage (as stipulated by American Joint Committee on Cancer guidelines, seventh edition), and local recurrences were recorded to assess pathologic outcomes.
2.3. Surgical technique
Having properly prepared the bowel (i.e., polyethylene glycol cathartic the day before surgery), each patient was placed in a supine position, and a single longitudinal incision (3–5 cm) was made at umbilicus. Pneumoperitoneum was then induced, insufflating with carbon dioxide gas. A multichannel Octo port (DalimsurgNET Co Ltd, Seoul, Korea) was routinely used for SILRC, incorporating the medial-to-lateral modified CME protocol adopted by our institution. The latter stipulates minor differences, promoting oncologic outcomes commensurate with standard CME. Basic SILS technique and our modified CME procedure are detailed in previous studies.[8,12]
2.4. Statistical analysis
Student t test or Mann–Whitney U test was applied for analysis of continuous variables, whereas categorical variables were subjected to chi-square or Fisher exact test, setting statistical significance at P < 0.05. The LC for OT was evaluated by moving average and CUSUM. The moving average of 20 consecutive patients was calculated as follows:
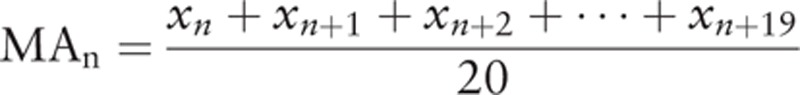 |
The CUSUM was applied to show the sequential difference of OT between each case and the mean value. xi represents the OT of each case and μ represents the mean overall OT in the following equation:
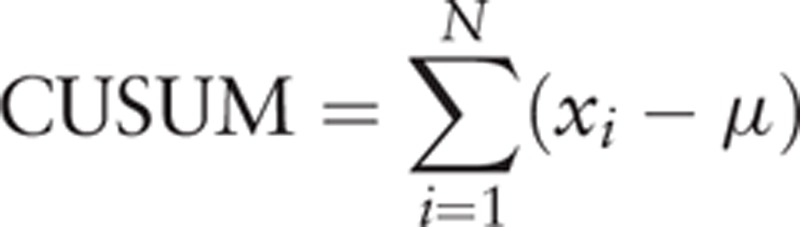 |
Surgical success was analyzed by CUSUM and risk-adjusted CUSUM (RA-CUSUM) to more appropriately reflect LC. Surgical failure was equated with any of the following: procedural conversion, postoperative complications, harvested lymph node count <12, or local recurrence. The acceptable failure rate was set to 10%. The surgical success CUSUM ascended graphically as successful surgeries accrued, whereas surgical failures resulted in a decline. Our intent was to reproduce methods applied in our similar, previous analysis, addressing LC for single-incision laparoscopic anterior resection (SILAR) of sigmoid colon cancer.[19] A comprehensive account of statistical methods was offered in that study. All statistical computations relied on standard software: SPSS v20.0 (SPSS Inc, Chicago, IL) and R package v3.1.2. (http://www.R-project.org).
3. Results
3.1. Patients
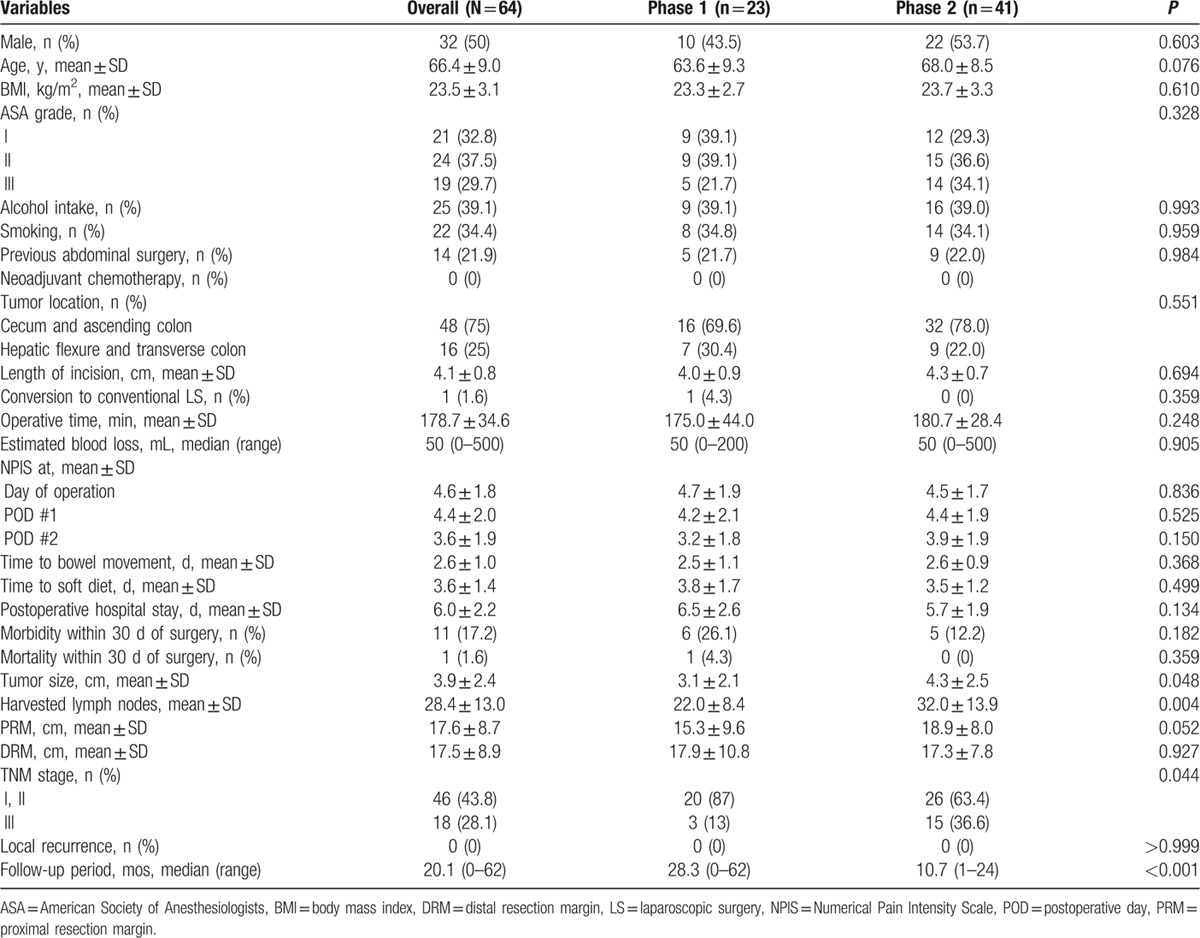
A total of 64 patients with right-sided colon cancers each underwent SILRC (Table 1). Mean analytic parameters of note were as follows: age 66.4 years; BMI 23.5 kg/m2; OT 178.7 minutes; incision length 4.1 cm; harvested lymph node count 28.4; and postoperative in-hospital LOS 6.0 days. Median blood loss was 50 mL, with 1 procedure (1.6%) converted to LS. There were no local recurrences during the median follow-up period of 20.1 months.
Table 1.
Patient characteristics and perioperative outcomes compared by learning phases.
3.2. Learning curve
Both moving average and CUSUM charting of OT reached nadirs at the 24th patient (Figs. 1 and 2). Similarly, the CUSUM graph of surgical success peaked at the 23rd patient (Fig. 3). Unfortunately, adjustment for prior risk (RA-CUSUM) could not be done. To identify predictors of surgical failure (defined by conversion, complications, harvested lymph node count <12, and local recurrence), logistic regression analysis was conducted, based on the following variables: sex, age, BMI, alcohol intake, smoking status, ASA status, prior abdominal surgery, OT, estimated blood loss, tumor size, tumor (T) stage, and pathologic stage. Had any of these emerged as significant variables (P < 0.05), which they did not, a model for probability of surgical failure could have been generated.
Figure 1.
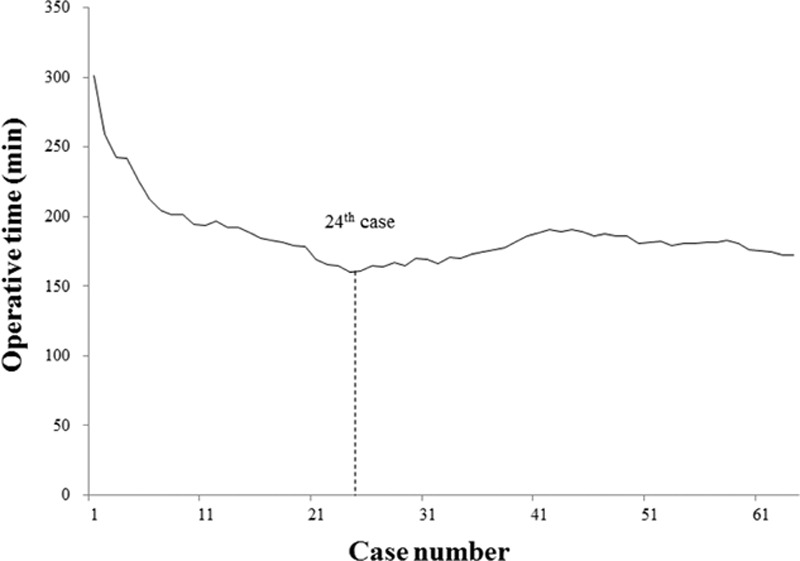
Moving average method for operative time: nadir at 24th patient.
Figure 2.
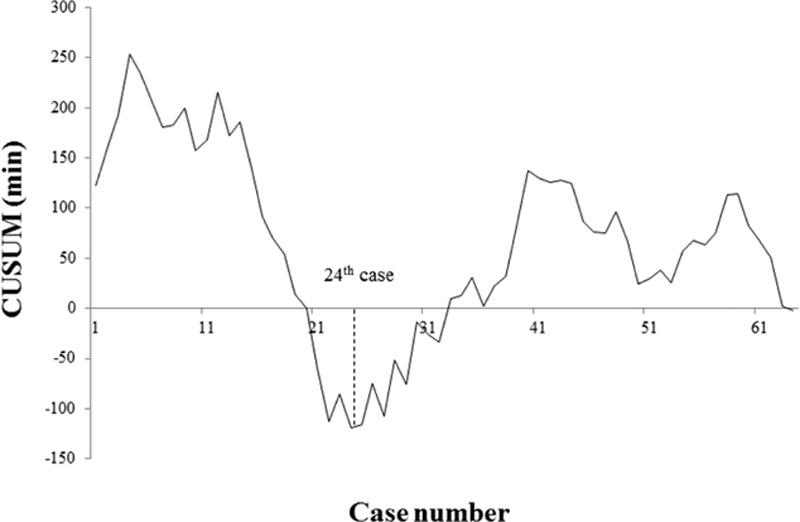
CUSUM for operative time: nadir at 24th patient. CUSUM = cumulative sum control chart.
Figure 3.
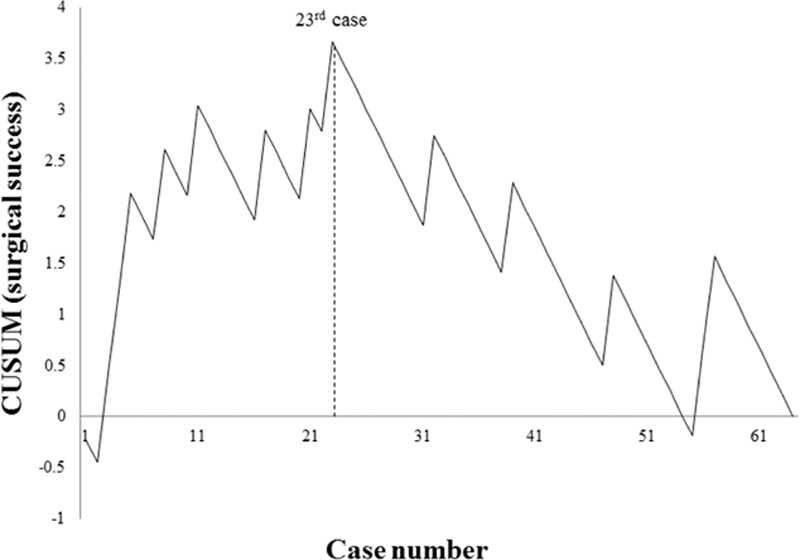
CUSUM for surgical success: peak at 23rd patient. CUSUM = cumulative sum control chart.
For graphic depictions of LCs by OT and by surgical success, we divided patients into 2 groups as follows: phase 1, patients 1 to 23; and phase 2, patients 24 to 64 (Table 1). There were no significant intergroup differences for OT, estimated blood loss, or local recurrence. However, in patients of phase 2 (vs those of phase 1), tumor size was larger (4.3 vs 3.1 cm; P = 0.048) and harvested lymph node counts were higher (32 vs 22; P = 0.004). Patients of phase 2 also harbored more advanced disease, determined by pathologic staging (36.6% vs 13%; P = 0.044). Likewise, patients of phase 2 were older, with shorter LOS, less morbidity, and longer proximal resection margins, albeit statistical significance was marginal at best.
4. Discussion
Since the introduction of the SILS technique, the feasibility and safety of its use for colorectal cancer has been documented in several publications, a few reporting the LC for SILRC. To our knowledge, this is the first comprehensive statistical analysis of the LC for single-incision laparoscopic resection of right colon cancer by means of CME with CVL. Our results show increases in pathologic stage, tumor size, and harvested lymph node counts that indicate patients with more difficult and advanced disease were increasingly selected for SILRC. However, we found that the CUSUM for OT declined in phase 1 and increased in phase 2 of the LC to peak at the 40th patient, with no further ascent thereafter (Fig. 2). Similar patterns were demonstrated in graphs of moving averages (Fig. 1). Because the final 15 subjects (patients 24–40) displayed increasing OT, but differed from the mean by only 10.4 minutes (189.1 vs 178.7 minutes), overall OT appeared to stabilize as the surgeon's experience increased. Surgical failures occurred in 8 patients (34.8%) of phase 1, with only 5 surgical failures (12.2%) in phase 2. These findings suggest that a minimum of 23 patients are needed for surgical success, although a number of factors may certainly impact OT.
Once feasibility and safety of a newly developed procedure are established, data on LC are important and practical, providing clinical clues to aid novices and experienced surgeons in adopting the new technique. Earlier studies reporting LCs in colorectal surgery simply assessed performance by dichotomizing patients for arithmetic comparisons. Given the now greater sophistication of statistical analytics, more suitable techniques, including CUSUM and RA-CUSUM, have been applied in this setting.
In assessing the LC for laparoscopic sigmoid colon resection, Dincler et al[20] reported a decline in OT after 90 to 110 patients, with intraoperative complications and conversion rates declining after 70 to 80 patients. Although they did use moving average and CUSUM for analysis, indications for surgery were heterogeneous. Tekkis et al[21] similarly used RA-CUSUM to report the LC for LS of colorectal diseases, again in a number of conditions, including benign polyps, cancer, inflammatory bowel disease, and diverticulitis. However, Bege et al[22] applied RA-CUSUM and moving average to assess the LC for LS of rectal cancer, showing that 50 patients were needed to achieve surgical success. As in our study, surgical success was defined by the absence of conversion to open surgery, postoperative morbidity, R1 resection, and local recurrence.
Since our prior reporting of the LC for SILAR of sigmoid colon cancer,[19] there have been few other publications addressing the LC for SILS in colorectal surgery. Based on 20 patients, Hopping and Bardakcioglu[16] reported a 10-patient LC for SILS right hemicolectomy. However, they merely dichotomized subjects and compared groups, with no real statistical rationale. Kirk et al[17] otherwise have established a LC of 40 patients by dividing 70 patients into 7 groups of 10 patients each. However, multidimensional statistical techniques similarly were lacking, and indications for surgery in both of these studies were uncertain. Using CUSUM method amidst heterogeneous surgical indications, Haas et al[15] reported a LC in this setting between 30 and 36 patients. Park et al[18] also determined that 31 patients were needed for learning phase completion, with another 10 required for complication-free steady-state performance, assessed by moving average and CUSUM methods, respectively. Although their study was unique, comparing LCs for single-incision and for conventional LS resection of right-sided colon cancer, their cohort (n = 35) was relatively small.
To avoid the limitations of similar earlier efforts, right-sided colon cancer was the sole surgical indication in this study, and the LC was derived from 2 accepted statistical methods: moving average and CUSUM. As opposed to our previous study of LC for SILAR, we did not find continuous decline in OT, reflecting gradual OT reduction.[19] Instead, we encountered a fluctuating OT that eventually stabilized, despite more advanced disease and greater degrees of technical difficulty over time. Although considered a major LC determinant, OT inevitably reaches a lower limit. Furthermore, there are many other factors impacting surgical success, namely procedural conversion, specimen quality, and morbidity/mortality. Inherent pathologic factors, including tumor size, harvested lymph node count, status of resection margins, and local recurrence, must also be taken into account. Bege et al[22] and Tekkis et al[21] used 4 factors to define surgical success, whereas Park et al[23] identified 5 criteria for surgical failure. In another study by Park et al,[18] CUSUM was used to chart major complications, including anastomotic leakage, intra-abdominal abscess, or fluid collection. For our purposes, procedural conversion, postoperative complications, harvested lymph node count <12, and local recurrence were measures of surgical failure. However, no significant variables emerged from logistic regression analysis, so RA-CUSUM was not an option. The CUSUM chart for surgical success in our study showed a gradual decline in surgical failure that stabilized after the 23rd patient, which was similar to the CUSUM for OT (Fig. 3).
Ultimately, we found that successful SILRC was achieved after 23 patients. These results were aligned with but not directly comparable to similar studies of SILRC, given the general dearth of statistical analysis, the heterogeneity of surgical indications, or overall differences in surgical technique (no prior usage of CME with CVL). Although our LC was shorter for SILRC than for SILAR (61–65 patients), the surgeon had already adapted somewhat to SILS technique, initiating SILAR procedures 4 months before attempting SILRC. SILS is a challenging and demanding technique, marked by uncomfortable situations in a highly restricted operative field. Even for surgeons experienced in LS, OT may consequently be prolonged.
This study has acknowledged limitations, the first being its reliance on retrospective single-center data generated by 1 surgeon. Hence, selection bias is inevitable and its generalizability is in question. Moreover, because our surgeon had amassed over 100 LS procedures and 14 SILARs in advance of attempting SILRC, a longer LC for SILRC may be expected of novice surgeons. Another limitation is that parameters defining surgical failure may not be uniformly applicable. Additionally, the median follow-up period for phase 2 patients was <2 years. Because most local recurrences of colorectal cancer occur within a 2-year time frame, it is possible that some patients in phase 2 may have experienced local recurrences. Nevertheless, this study provides useful information to surgeons who are contemplating the addition of SILS to their professional repertoire.
5. Conclusions
Our investigation enabled delineation of a 2-phase LC for SILS CME of right-sided colon cancer, with a requirement of approximately 23 patients. According to multidimensional statistical analyses, short-term and pathologic outcomes stabilize after this point, implying that surgical success is thereafter ensured. Surgeons experienced in LS may readily acquire skills needed to resect cancers via SILRC. However, novices will likely require more time for becoming proficient, given the innate challenges of this procedure.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, CME = complete mesocolic excision, CUSUM = cumulative sum control chart, CVL = central vascular ligation, DRM = distal resection margin, LC = learning curve, LOS = length of stay, LS = laparoscopic surgery, NPIS = Numeric Pain Intensity Scale, OT = operative time, POD = postoperative day, PRM = proximal resection margin, SILRC = single-incision laparoscopic right hemicolectomy, SILS = single-incision laparoscopic surgery.
Funding: This work was supported by the Students’ Association of the Graduate School of Yonsei University funded by the Graduate School of Yonsei University.
The authors report no conflicts of interest.
References
- 1.Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 2010; 97:1638–1645. [DOI] [PubMed] [Google Scholar]
- 2.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010; 11:637–645. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw PF, Allardyce RA, Frampton CM, et al. Long-term outcomes of the Australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg 2012; 256:915–919. [DOI] [PubMed] [Google Scholar]
- 4.Kadar N, Reich H, Liu CY, et al. Incisional hernias after major laparoscopic gynecologic procedures. Am J Obstet Gynecol 1993; 168:1493–1495. [DOI] [PubMed] [Google Scholar]
- 5.Marcovici I. Significant abdominal wall hematoma from an umbilical port insertion. JSLS 2001; 5:293–295. [PMC free article] [PubMed] [Google Scholar]
- 6.Poon JT, Cheung CW, Fan JK, et al. Single-incision versus conventional laparoscopic colectomy for colonic neoplasm: a randomized, controlled trial. Surg Endosc 2012; 26:2729–2734. [DOI] [PubMed] [Google Scholar]
- 7.Yun JA, Yun SH, Park YA, et al. Oncologic outcomes of single-incision laparoscopic surgery compared with conventional laparoscopy for colon cancer. Ann Surg 2016; 263:973–978. [DOI] [PubMed] [Google Scholar]
- 8.Kim CW, Cho MS, Baek SJ, et al. Oncologic outcomes of single-incision versus conventional laparoscopic anterior resection for sigmoid colon cancer: a propensity-score matching analysis. Ann Surg Oncol 2015; 22:924–930. [DOI] [PubMed] [Google Scholar]
- 9.Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation: technical notes and outcome. Colorectal Dis 2009; 11:354–364.[discussion 364-355]. [DOI] [PubMed] [Google Scholar]
- 10.Mori S, Kita Y, Baba K, et al. Laparoscopic complete mesocolic excision via reduced port surgery for treatment of colon cancer. Dig Surg 2015; 32:45–51. [DOI] [PubMed] [Google Scholar]
- 11.Galizia G, Lieto E, De Vita F, et al. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int J Colorectal Dis 2014; 29:89–97. [DOI] [PubMed] [Google Scholar]
- 12.Cho MS, Baek SJ, Hur H, et al. Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg 2015; 261:708–715. [DOI] [PubMed] [Google Scholar]
- 13.Liang JT, Lai HS, Huang J, et al. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc 2015; 29:2394–2401. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Kim IK, Kang SI, et al. Laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 2014; 28:2747–2751. [DOI] [PubMed] [Google Scholar]
- 15.Haas EM, Nieto J, Ragupathi M, et al. Critical appraisal of learning curve for single incision laparoscopic right colectomy. Surg Endosc 2013; 27:4499–4503. [DOI] [PubMed] [Google Scholar]
- 16.Hopping JR, Bardakcioglu O. Single-port laparoscopic right hemicolectomy: the learning curve. JSLS 2013; 17:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirk KA, Boone BA, Evans L, et al. Analysis of outcomes for single-incision laparoscopic surgery (SILS) right colectomy reveals a minimal learning curve. Surg Endosc 2015; 29:1356–1362. [DOI] [PubMed] [Google Scholar]
- 18.Park Y, Yong YG, Yun SH, et al. Learning curves for single incision and conventional laparoscopic right hemicolectomy: a multidimensional analysis. Ann Surg Treat Res 2015; 88:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CW, Kim WR, Kim HY, et al. Learning curve for single-incision laparoscopic anterior resection for sigmoid colon cancer. J Am Coll Surg 2015; 221:397–403. [DOI] [PubMed] [Google Scholar]
- 20.Dincler S, Koller MT, Steurer J, et al. Multidimensional analysis of learning curves in laparoscopic sigmoid resection: eight-year results. Dis Colon Rectum 2003; 46:1371–1378.[discussion 1378-1379]. [DOI] [PubMed] [Google Scholar]
- 21.Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005; 242:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bege T, Lelong B, Esterni B, et al. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg 2010; 251:249–253. [DOI] [PubMed] [Google Scholar]
- 23.Park EJ, Kim CW, Cho MS, et al. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 2014; 28:2821–2831. [DOI] [PubMed] [Google Scholar]


