Abstract
To study the performance of serum and pleural lactate dehydrogenase (LDH) level in predicting survival in patients with adenocarcinoma lung presenting with malignant pleural effusions (MPE) at initial diagnosis.
Retrospective cohort study of the patient hospitalized for adenocarcinoma lung with MPE in year 2012.
Univariate analyses showed lower pleural fluid LDH 667 (313–967) versus 971 (214–3800), P = 0.04, female gender 9 (100%) versus 27 (41.5%), P = 0.009, never smoking status 9 (100%) versus 36 (55.3%), P = 0.009, and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy 8 (89%) versus 26 (40%), P = 0.009 to correlate with survival of more than 1.7 year versus less than 1.7 year. In multivariate analysis, low pleural fluid LDH and female gender maintained significance. The pleural LDH level of ≤1500 and >1500 U/L discriminated significantly (P = 0.009) between survival.
High pleural LDH (>1500 IU/L) predicts shorter survival (less than a year) in patients with adenocarcinoma lung presenting with MPE at the time of initial diagnosis. This marker may be clinically applied for selecting therapeutic modality directed at prevention of reaccumulation of MPE. Patients with low pleural LDH may be considered suitable for measures that provide more sustained effect on prevention of reaccumulation such as chemical pleurodesis or tunneled pleural catheter.
Keywords: adenocarcinoma, Asian, cancer (lung), pleura, pleurodesis, tunneled pleural catheter
1. Introduction
Up to 50% of patients with various metastatic malignancies develop para-malignant or malignant pleural effusion (MPE). MPE tend to recur rapidly after an initial thoracentesis in most patients.[1,2] Reported median survival in patients with MPE from various malignancies ranges from 4 to 7 months.[3–5] The management of these patients is 2-fold, one targeted at the cancer itself such as chemotherapy, radiotherapy, or tyrosine kinase inhibitor (TKI), and the other targeted at the drainage and prevention of reaccumulation of pleural effusion with tunneled pleural catheter, chemical pleurodesis, pleurectomy, or pleuroperitoneal shunt.[6–10] Among approaches for the prevention of reaccumulation of pleural effusion after initial thoracentesis, rate of reaccumulation of the pleural effusion, patient's prognosis, expandability of the lung, and patient preference are the main determinant factors for the choice of therapy.
Repeat therapeutic thoracentesis is recommended for patients with an expected survival of <1–3 months and in whom effusion reaccumulates slowly.[11] Tunneled pleural catheter is recommended for patients with trapped (unexpandable) lung and with an expected survival of <6 months.[11] Talc slurry instilled via small bore chest tube is recommended for patients with an expected survival of more than 6 months, and thoracoscopic talc insufflation is recommended for those with longer survival and when lysis of adhesions is needed.[11] However, difficulty in identifying a particular patient's survival accurately puts a limit on this method of management. Furthermore, improving survival trends for example in patients with adenocarcinoma lung treated with epidermal growth factor receptor (EGFR)-TKI necessitates greater adoption of methods that allow sustained effect on prevention of reaccumulation.
Where literature on predictors of survival in patients with MPE from mixed cancer group is available, such information in newly diagnosed lung cancer patients presenting with MPE is sparse. Most studies examining the prognostic factors affecting survival in patients with MPE have been done on mixed cancer groups. These studies have identified high pleural fluid lactate dehydrogenase (LDH) (>1500 IU/L),[12] high eastern cooperative oncology group (ECOG) score (3–4),[12–14] high neutrophil: lymphocyte ratio (>9),[12] cancer type (lung),[12] low pleural fluid pH (<7.28),[15] and high sVEGFR-1[16] pleural fluid level as the factors predicting poor survival. In studies assessing prognostic factors affecting survival specifically in lung cancer patients presenting with MPE, high ECOG score has been described to predict poor survival, and EGFR mutation and EGFR-TKI therapy to predict longer survival.[17,18]
LDH level has been shown to be raised in cancer and predict survival.[19–23] However, their role in adenocarcinoma lung, the most common histological subtype of lung cancer, has not been studied. We studied the performance of serum and pleural fluid LDH in predicting survival in patients with adenocarcinoma lung presenting with MPE at initial diagnosis.
2. Methods
The current study was conducted on the patients hospitalized for the management of MPE as the first manifestation of the adenocarcinoma lung in year 2012. We retrospectively collected data on biomarkers such as serum LDH, and the pleural fluid analysis results, done within 24 hours of admission of these patients. Additionally, we collected data on smoking status, performance status, epidermal growth factor mutation result, type of therapy, and survival on these patients. EGFR status was established by isolating DNA from the prepared sections of the formalin fixed, paraffin-embedded tissue with the DNeasy Blood & Tissue Kit (Qiagen GmbH, Germany), according to the manufacturer's protocol. Serum and pleural fluid LDH levels were measured using the enzymatic rate method described as P to L (pyruvate to lactate) with NADH/NAD+ monitoring method. Approval from institutional review board (DSRB) was obtained.
2.1. Data analysis
We used software (SPSS, version 17; SPSS, Chicago, IL) for all statistical analyses. The results were compared using a Wilcoxon 2-sample test or Fisher exact test. P values were 2 sided and considered indicative of a significant difference if less than 0.05. Various clinical variables such as gender, smoking status, ECOG status, therapy received, biochemical variables such as serum LDH, serum C-reactive protein, and pleural fluid variables such as pleural LDH, and pleural adenosine deaminase were analyzed using the Univariate analysis and multivariate analysis.
3. Results
One hundred patients with MPE were screened. Out of these, 74 patients had MPE from adenocarcinoma lung. Clinical characteristics are presented in Table 1.
Table 1.
Clinical characteristics of patients (n = 74).
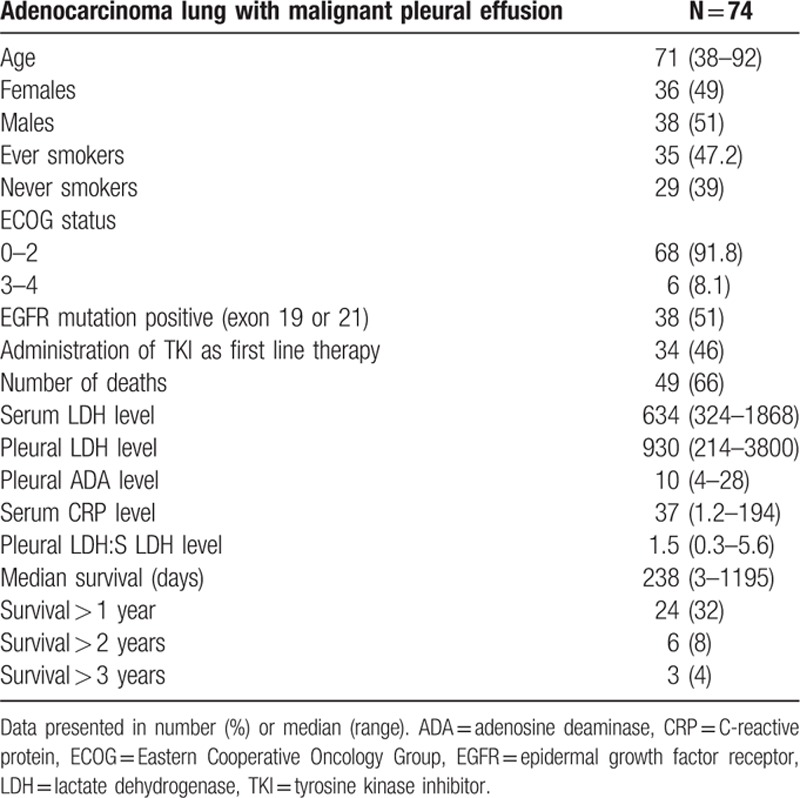
Univariate analyses showed lower pleural fluid LDH 667 (313–967) versus 971 (214–3800), P = 0.04, female gender 9 (100%) versus 27 (41.5%), P = 0.009, never smoking status 9 (100%) versus 36 (55.3%), P = 0.009, and EGFR-TKI therapy 8 (89%) versus 26 (40%), P = 0.009 to correlate with survival of more than 1.7 year versus less than 1.7 year (Table 2).
Table 2.
Univariate analyses of survival.
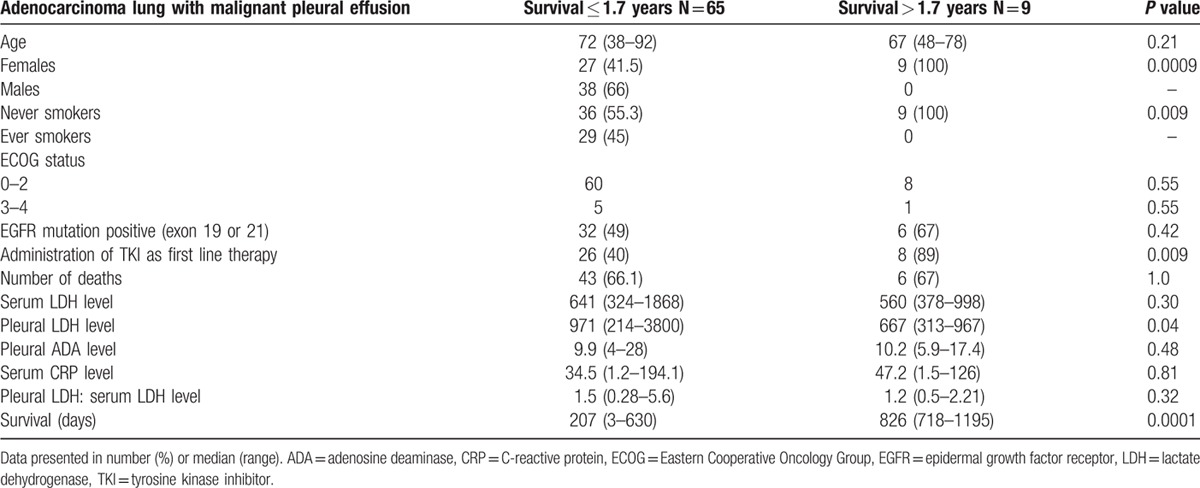
In multivariate analysis, low pleural fluid LDH maintained significance. The pleural LDH of ≤1500 and >1500 U/L showed significant discrimination (P = 0.009) between survival (Table 3). Based on the results of multivariate analysis and previously published evidence of association of pleural LDH of >1500 U/L with the poor survival,[12] 1500 U/L was identified as the cut-off for survival difference.
Table 3.
Survival difference between various levels of serum lactate dehydrogenase (LDH) and pleural LDH.

4. Discussion
Our findings show that high pleural LDH (>1500 IU/L) predicts shorter survival (less than a year) in patients with adenocarcinoma lung presenting with MPE at the time of initial diagnosis. This marker may be clinically applied for selecting therapeutic modality directed at prevention of reaccumulation of pleural effusion. Patients with low pleural LDH may be considered suitable for measures that provide more sustained effect on prevention of reaccumulation such as chemical pleurodesis or tunneled pleural catheter. Repeated therapeutic thoracentesis or best supportive care (BSC) may be reserved for those with high pleural LDH.
4.1. Survival
Median survival was 238 days (7.9 months) in our cohort with 1 year survival of 32%. The survival was higher (13.3 months) in those receiving EGFR-TKIs. This is in contrast to studies published prior to advent of TKIs. Median survival in patients with MPE from nonsmall cell lung cancer has been 6.5 to 8 months prior to the advent of EGFR-TKIs.[24] In the studies comparing survival in MPE from various cancer types, the lung cancer has been associated with poorest survival. Pilling et al[4] reported median survival of 138 days in the lung cancer patients versus 258 and 297 days in breast and malignant mesothelioma, respectively. Clive et al[12] reported median survival of 74 days in the lung cancer versus 192 and 339 days in breast and mesothelioma, respectively. The better survival in our cohort is attributable to high prevalence (51%) of EGFR mutation in adenocarcinoma and high proportion of such patients (46%) receiving EGFR-TKI therapy. Nonsmokers and females treated with EGFR-TKI had a better survival in our cohort due to greater prevalence of EGFR mutation in this subgroup consistent with previous reports describing high prevalence of EGFR mutation in females and never smokers in Asian population.[18,25–27] Multivariate analysis however limited the better survival to low pleural fluid LDH and female gender.
These findings are clinically relevant in the context of strategies for the prevention of reaccumulation of MPE. They highlight the need for offering methods that provide long-lasting effect on prevention of reaccumulation of MPE such as chemical pleurodesis or tunneled pleural catheter as indicated, in contrast to methods that provide only episodic or one time relief such as thoracentesis and chest drain, and do not obviate the need for seeking medical aid repeatedly for reaccumulation.
4.2. Serum and pleural fluid LDH
LDH is an omnipresent cellular enzyme, the level of that rises as a result of tissue injury in a nonspecific manner.[28,43] Hence, it is elevated in several clinical conditions.[28,43] However, a disproportionately high and isolated serum LDH is specific to certain diagnostic groups such as sepsis and cancer patients. It is a marker of poor prognosis in these conditions.[19,20,29–37,43]
High level of pleural LDH in pleural space and its relationship with poor survival has been described in mixed cancer groups although underlying mechanism is not completely understood. Upregulation of the LDH enzyme to allow preferential use of glycolysis over oxidative phosphorylation for energy by tumor cells has been described. High rate of glycolysis is advantageous to growing cells because it is capable of producing adenosine triphosphate (ATP) considerably faster than oxidative phosphorylation.[23,43] The mechanism of poor survival is the association of high LDH level with high degree of necrosis in pleural cavity. Our findings confirm the relationship between pleural LDH and prognosis in the Asian population affected by adenocarcinoma lung with MPE and indicate validity of pleural LDH as a predictor of survival in this population.[38,41]
4.3. ECOG PS versus pleural LDH
ECOG status did not show any relationship with the short or long survival in our cohort. Performance status has been used for predicting life expectancy. However, it can be affected by age of onset of cancer (e.g., younger patient may be fitter than elderly with same stage) and cancer type (e.g., lung cancer being more debilitating than breast cancer). In addition, the ECOG performance status varies with management and hence may not be suitable for predicting prognosis.[12] Two large randomized controlled trials (RCTs) evaluating treatment strategies in MPE have indicated inaccuracy of clinicians’ ability in predicting survival in MPE. In the TIME2 trial, despite excluding patients with predicted survival of <3 months, 34% died within 3 months of trial entry. In another study, 17% of patients died within 30 days of trial entry who were predicted to survive >2 months.[39,40] This highlights that clinical judgement on which ECOG scale is based, alone is imprecise at estimating patient survival. Pleural LDH is more objective in that sense and may be more precise in identifying patients with poor survival.
Our study has following limitations. First, it is a retrospective single center study. Second, along with the low pleural LDH, we also found association of longer survival with EGFR-TKI therapy. Although in multivariate analysis, only low pleural LDH maintained significance, it is arguable that EGFR-TKI therapy would have contributed to longer survival. In the context of our study, this suggests that patients on TKI therapy should also be offered methods that carry the potential to have long-lasting effect on reaccumulation of MPE such as chemical pleurodesis. However, this is debatable. Investigators from Taiwan have reported significantly better pleural effusion recurrence-free survival in an EGFR positive group treated with TKIs versus EGFR negative group regardless of pleurodesis (7.1 vs 1.1 months), and concluded that pleurodesis can be withheld in nonsmall cell lung cancer patients taking TKIs because TKIs alone can control effusion and prevent it from reaccumulating.[42]
The strengths of our study are that it addresses the specific population of MPE, that is, adenocarcinoma lung which is the most prevalent histological subtype of lung cancer, and in which the profile of survival is changing. Application of our results to this group of patients may lead to more robust outcomes.
In conclusion, pleural LDH level in patients with adenocarcinoma lung presenting with MPE at the time of diagnosis may be used to select the therapeutic modality directed at prevention of reaccumulation. This parameter in patients with rapid reaccumulation rate of pleura effusion may be used to offer methods providing sustained effect on the prevention of reaccumulation of pleural effusion such as chemical pleurodesis or intrapleural catheter as indicated, whereas chest drain alone, repeated thoracentesis, or best supportive care may be considered more suitable alternative for patients with pleural LDH >1500 IU, and slow reaccumulation rate. As survival is improving in lung cancer, future studies should seek to identify methods, or pleural sclerosing agents that can offer prolonged effect on prevention of reaccumulation.
Acknowledgements
The authors thank Ms Ivy Yu Ling Ling for her valuable contribution in editing the figures and administrative work, and Dr Robert Hawkins for his valuable help in providing information on the biochemical tests.
Footnotes
Abbreviations: ECOG = eastern cooperative oncology group, EGFR = epidermal growth factor receptor, LDH = lactate dehydrogenase, MPE = malignant pleural effusion, TKI = tyrosine kinase inhibitor.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008; 83:235. [DOI] [PubMed] [Google Scholar]
- 2.Shaw PH, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2013; 11:CD002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 2000; 117:79. [DOI] [PubMed] [Google Scholar]
- 4.Pilling JE, Dusmet ME, Ladas G, et al. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5:1544. [DOI] [PubMed] [Google Scholar]
- 5.Schulze M, Boehle AS, Kurdow R, et al. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg 2001; 71:1809. [DOI] [PubMed] [Google Scholar]
- 6.Dresler CM, Olak J, Herndon JE, 2nd, et al. Cooperative groups cancer and leukemia group B, eastern cooperative oncology group, north central cooperative oncology group, radiation therapy oncology group. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005; 127:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004; CD002916. [DOI] [PubMed] [Google Scholar]
- 8.Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419. [DOI] [PubMed] [Google Scholar]
- 9.Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014; 384:1118. [DOI] [PubMed] [Google Scholar]
- 10.Gupta D, Ross K, Piacentino V, 3rd, et al. Use of LeVeen pleuroperitoneal shunt for refractory high-volume chylothorax. Ann Thorac Surg 2004; 78:e9. [DOI] [PubMed] [Google Scholar]
- 11.Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65 suppl 2:ii32–ii40. [DOI] [PubMed] [Google Scholar]
- 12.Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014; 69:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamboni MM, da Silva CT, Jr, Baretta R, et al. Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med 2015; 15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anevlavis S, et al. Prognostic factors in patients presenting with pleural effusion revealing malignancy. Respiration 2014; 87:311–316. [DOI] [PubMed] [Google Scholar]
- 15.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 2000; 117:79. [DOI] [PubMed] [Google Scholar]
- 16.Hooper CE, Elvers KT, Welsh GI, et al. VEGF and sVEGFR-1 in malignant pleural effusions: association with survival and pleurodesis outcomes. Lung Cancer 2012; 77:443–449. [DOI] [PubMed] [Google Scholar]
- 17.Kasapoglu US, Arınç S, Gungor S, et al. Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J 2015; 9:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Wu SG, Yu CJ, Tsai MF, et al. Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J 2013; 41:1409–1418. [DOI] [PubMed] [Google Scholar]
- 19.Swan F, Velasquez WS, Tucker S, et al. A new serologic staging system for large cell lymphomas based on initial (2-microglobulin and lactate dehydrogenase levels. J Clin Oncol 1989; 7:1518–1527. [DOI] [PubMed] [Google Scholar]
- 20.Terpos E, Katodritou E, Roussou M, et al. High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents. Eur J Haematol 2010; 85:114–119. [DOI] [PubMed] [Google Scholar]
- 21.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4:891–899. [DOI] [PubMed] [Google Scholar]
- 22.Warburg O, Wind F, Neglers E. The Metabolism of Tumours. 1930; London: Arnold Constable, 254–70. [Google Scholar]
- 23.Pfeiffer P, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001; 292:504–507. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura S, Ando Y, Minami H, et al. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res 1997; 3:47–50. [PubMed] [Google Scholar]
- 25.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361:947–957. [DOI] [PubMed] [Google Scholar]
- 26.Kim ES, Hirsch V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008; 372:1809–1818. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 2006; 24:5034–5042. [DOI] [PubMed] [Google Scholar]
- 28.Lott JA, Nemensanszky E. Lott JA, Wolf PL. Lactate dehydrogenase. Clinical Enzymology, a Case Oriented Approach. New York: Year Book Medical; 1987. 213–244. [Google Scholar]
- 29.Suárez-Santamaría M, Santolaria F, Pérez-Ramírez A, et al. Prognostic value of inflammatory markers (notably cytokines and procalcitonin), nutritional assessment, and organ function in patients with sepsis. Eur Cytokine Netw 2010; 21:19–26. [DOI] [PubMed] [Google Scholar]
- 30.Tredan O, Ray-Coquard I, Chvetzoff G, et al. Validation of prognostic scores for survival in cancer patients beyond first-line therapy. BMC Cancer 2011; 11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steyerberg EW, Keizer HJ, Fossa SD, et al. Resection of residual retroperitoneal masses in testicular cancer: evaluation and improvement of selection criteria. The ReHiT study group. Reanalysis of histology in testicular cancer. Br J Cancer 1996; 74:1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youa B, Tranchand B, Girarda P, et al. Etoposide pharmacokinetics and survival in patients with small cell lung cancer: a multicentre study. Lung Cancer 2008; 62:261–272. [DOI] [PubMed] [Google Scholar]
- 33.Spiess PE, Pettaway CA, Vakar-Lopez F, et al. Treatment outcomes of small cell carcinoma of the prostate. Cancer 2007; 110:1729–1737. [DOI] [PubMed] [Google Scholar]
- 34.Johnson PWM, Joel SP, Love S, et al. Tumour markers for prediction of survival and monitoring of remission in small cell lung cancer. Br J Cancer 1993; 67:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fussenich LM, Desar IM, Peters ME, et al. A new, simple and objective prognostic score for phase I cancer patients. Eur J Cancer 2011; 47:1152–1160. [DOI] [PubMed] [Google Scholar]
- 36.Swan F, Velasquez WS, Tucker S, et al. A new serologic staging system for large cell lymphomas based on initial β2-microglobulin and lactate dehydrogenase levels. J Clin Oncol 1989; 7:1518–1527. [DOI] [PubMed] [Google Scholar]
- 37.Terpos E, Katodritou E, Roussou M, et al. High serum lactate dehydrogenase adds prognostic value to the international myeloma staging system even in the era of novel agents. Eur J Haematol 2010; 85:114–119. [DOI] [PubMed] [Google Scholar]
- 38.Bielsa S, Salud A, Martinez M, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med 2008; 19:334–339. [DOI] [PubMed] [Google Scholar]
- 39.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389. [DOI] [PubMed] [Google Scholar]
- 40.Dresler CM, Olak J, Herndon JE, II, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005; 127:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Moragon E, Aparicio J, Sanchis J, et al. Malignant pleural effusion: prognostic factors for survival and response to chemical pleurodesis in a series of 120 cases. Respiration 1998; 65:108–113. [DOI] [PubMed] [Google Scholar]
- 42.Chi-Hsien Chen, Chien-Hung Gow, Chong-Jen Yu, et al. Clinical response of gefitinib on malignant pleural effusions in patients with non-small cell lung cancer. J Cancer Mol 2008; 4:23–28. [Google Scholar]
- 43.Verma A, Abisheganaden J, Light RW. Identifying malignant pleural effusion by a cancer ratio (serum LDH: pleural fluid ADA ratio). Lung 2016; 194:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


