Abstract
Past studies have shown inconsistent results on whether there is an association between multiple sclerosis (MS) and rheumatoid arthritis. To investigate the possible relationship between the 2 autoimmune diseases, we performed a nationwide cohort study utilizing the National Health Insurance Research Database and the Registry of Catastrophic Illness.
A total of 1456 newly diagnosed patients with MS and 10,362 control patients were matched for age, sex, and initial diagnosis date. Patients with MS had a higher incidence of rheumatoid arthritis (age-adjusted standardized incidence ratio: 1.72; 95% confidence interval = 1.01–2.91). There was a positive correlation in being diagnosed with rheumatoid arthritis in patients previously diagnosed with MS when stratified by sex and age. The strength of this association remained statistically significant after adjusting for sex, age, and smoking history (hazard ratio: 1.78, 95% confidence interval = 1.24–2.56, P = 0.002).
In conclusion, this study demonstrates that a diagnosis of MS increased the likelihood of a subsequent diagnosis of rheumatoid arthritis in patients, independent of sex, age, and smoking history.
Keywords: cohort studies, multiple sclerosis, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a common and widespread inflammatory arthritis that has burdened individuals and societies in terms of health[1] and financial[2] costs. Its exact cause remains unknown. Many genetic and environmental risk factors are implicated in its development, 1 of which, smoking, is well established.[3] Interestingly, numerous articles suggest a possible link between multiple sclerosis (MS) and RA.[4,5] The relationship between MS and RA is further supported by their shared immunologic autoantibodies (antibodies to myelin basic protein)[6] and common genetic backgrounds.[7] However, past epidemiological studies have been inconsistent, with some reporting that a greater number of patients with MS have RA compared with control patients,[4,5] while others showing contradictory results.[8,9] There are many possible reasons for the conflicting data, including sample size, studied populations, and even the definition of RA used. Additionally, the cross-sectional nature of these studies made it impossible to infer causality between MS and RA. It is still unknown from these studies whether MS is associated with increased incidence of RA or vice versa. Thus, we conducted this cohort study to further explore the association between MS and RA.
2. Methods
This was a retrospective cohort study that utilized the National Health Insurance Research Database (NHIRD) and the Registry of Catastrophic Illness. Before being released, data that could be used to identify patients were scrambled. Thus, the researchers were blinded to the identity of the patients from the databases. The Institutional Review Board of Kaohsiung Medical University Hospital exempted this study from the ethical approval (KMUHIRB-EXEMPT[I]-20150051).
2.1. Data source
In 1995, Taiwan established the single-payer National Health Insurance (NHI) program, obligatory for all citizens. The NHI program has a coverage rate of >99.6%.[10] Healthcare data on ambulatory care and inpatient services were recorded in the NHI medical claims database. For this study, Taiwan's National Health Research Institutes offered NHIRD of 1 million random individuals, which consisted of data on 1,000,000 insured persons randomly selected from all enrollees in the insurance program. This representative national subset has comparable distribution of sex, age, or insured amount to all enrollees in the original NHIRD.[11]
The NHIRD consisted of comprehensive NHI-related administrative and claims data. To ensure that the copayments do not discourage patients from seeking necessary medical attention, insured enrollees with certain catastrophic illnesses, including MS and RA, are eligible for a catastrophic illness certificate. Such certificates would exempt patients from inpatient and outpatient copayments. A catastrophic illness certificate for RA was issued after rheumatologists, commissioned by the Bureau of National Health Insurance (BNHI), validated the diagnosis of RA based on the 1987 American College of Rheumatology classification criteria. To get a catastrophic illness certificate for MS, the attending physician must provide relevant clinical information, laboratory results, and imaging reports as part of the application for review. The review committee of BNHI assessed applications according to both the Posner criteria and the McDonald criteria.
To guarantee diagnosis accuracy, the BNHI commissioned respective specialists to regularly review the medical record by randomly sampling patient charts, and imposing fines for false claims if erroneous coding was found. These 2 databases have been the source for multiple epidemiological studies, including MS and RA.[12,13]
2.2. Cohort selection
We identified all newly diagnosed patients with MS (International Classification of Diseases, Ninth Revision: 340) with a certificate of catastrophic illness[12,13] between January 1, 1998, and December 31, 2011. The entry date for the MS cohort was identified as the date on which patients were first diagnosed with MS as documented in the Registry of Catastrophic Illness. Patients younger than 20 years old or having a diagnosis of RA before or within 1 year of a MS diagnosis were excluded in this study. At most 8 control patients were randomly selected for each patient with MS from the NHIRD, a pool of roughly 1 million individuals. For the control patients, the entry date corresponded to the date on which control patients utilized NHI services. Control patients matched the MS cohort for age, sex, and entry date. Individuals aged <20 years or having RA before or within 1 year of the entry date were excluded. The goal of this matching process was to guarantee similar follow-up time at baseline. To assess long-term exposure status,[14] we excluded patients who followed up <1 year from diagnosis.
2.3. Outcome definition
In this study, we identified RA as any participant with a catastrophic illness certificate of RA diagnosis (International Classification of Diseases, Ninth Revision: 7140) using the Registry of Catastrophic Illness.[12,13] Follow-up was terminated on the occurrence of RA recorded in the Registry of Catastrophic Illness. For those who did not have RA recorded in the Registry of Catastrophic Illness, the last day of follow-up was defined as the date of last inpatient or outpatient service before December 31, 2011.
2.4. Assessment of covariates
Seen as a risk factor for both RA[3] and MS,[4] smoking was a confounding variable. The patient's smoking history was obtained from records in the NHI database.
2.5. Statistical analysis
Patients with the diagnosis of RA preceding that of MS or being diagnosed with RA within a year after being diagnosed of MS were excluded. Therefore, the first year after entry date was not counted as follow-up time and follow-up time was considered starting from 1 year after being diagnosed with MS. The follow-up period was calculated from 1 year after the cohort entry date to the end of follow-up.
We first calculated the incidence of RA (cases per 1000 person-years) in patients with MS and control patients stratified by sex and age. To examine whether patients with MS had a higher incidence of developing RA, we computed the standardized incidence ratio (SIR) and corresponding 95% confidence intervals (95% CIs) for RA in patients with MS. After confirming the assumption of proportional hazards by Schoenfeld residuals trend tests, multivariate Cox hazard regression was used to examine the hazard ratio (HR) of MS after adjusting for sex, age, and smoking. We used Kaplan–Meier analysis to estimate the cumulative incidence of RA and compared the difference between 2 groups by log-rank tests. A 2-tailed P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19.3.
3. Results
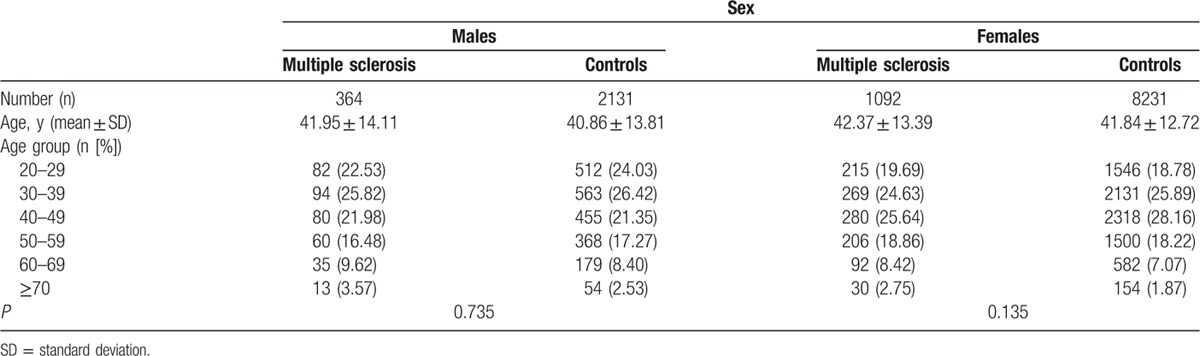
A total of 1456 patients with MS were selected from the Registry of Catastrophic Illness and 10,362 control patients from NHIRD. The mean age of male patients was 41.95 ± 14.11 years in the MS group and 40.86 ± 13.81 years in control patients. The mean age of female patients was 42.37 ± 13.39 years in the MS group and 41.84 ± 12.72 years in control patients (Table 1), similar to those reported.[15] The age distribution between the MS group and control patients was similar in both male (P = 0.735) and female (P = 0.135) cohorts.
Table 1.
The age distribution of patients with multiple sclerosis and the controls in baseline.
3.1. Increased incidence of rheumatoid arthritis in multiple sclerosis
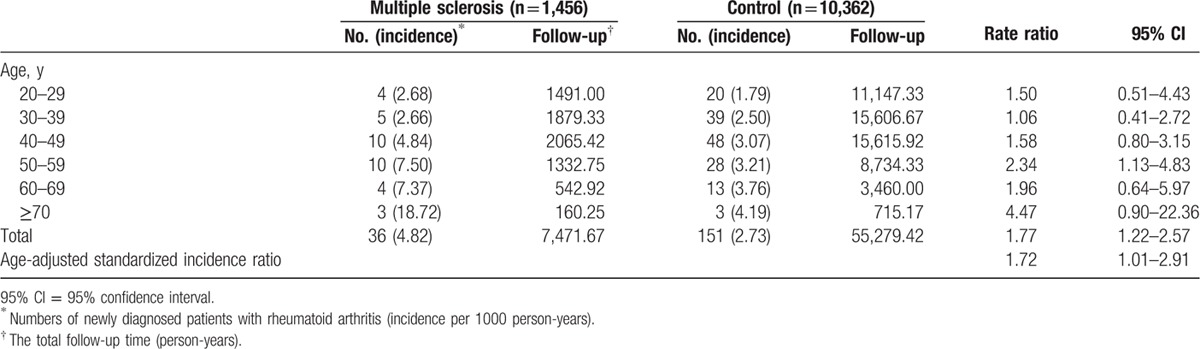
There was an increased incidence of RA in patients with MS when compared to control patients (age-adjusted SIR: 1.72, 95% CI = 1.01–2.91) (Table 2). When stratified by age, a consistent trend was noticeable. However, due to the limited cases of RA, the 95% CI oftentimes included the null value.
Table 2.
Age-adjusted standardized incidence ratio of rheumatoid arthritis in patients with multiple sclerosis stratified by age.
3.2. Standardized incidence ratio of rheumatoid arthritis in the male cohort
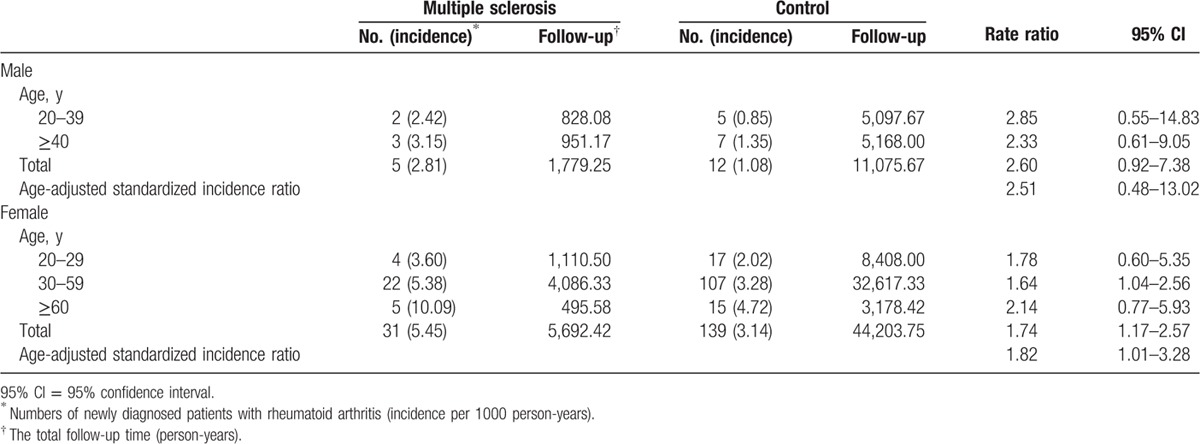
To clarify the relationship between SIR and sex, we further analyzed SIR of RA in male and female cohorts separately. In the male cohort, a higher incidence of RA was observed in patients with MS compared to that in the control group, but was not statistically significant (age-adjusted SIR: 2.51, 95% CI = 0.48–13.02) (Table 3). When stratified by age, a consistent trend was also present. However, due to the limited cases of RA, the 95% CI oftentimes included the null value.
Table 3.
Age-adjusted standardized incidence ratio of rheumatoid arthritis in male and female patients with multiple sclerosis stratified by age.
3.3. Standardized incidence ratio of rheumatoid arthritis in the female cohort
In the female cohort, patients with MS had a higher incidence of RA compared to the control group (age-adjusted SIR: 1.82, 95% CI = 1.01–3.28) (Table 3). When stratified by age, a similar trend was observed and conserved throughout. However, due to the limited cases of RA, some of the 95% CIs included the null value.
3.4. Cumulative incidences of rheumatoid arthritis for multiple sclerosis and the control cohorts
Cumulative incidences of RA in MS and the control cohorts are shown in Fig. 1. Patients in the MS cohort were associated with a significantly higher cumulative incidence of RA than that found in the control cohort (P = 0.002). In males, the MS cohort was associated with a higher cumulative incidence of RA than that in the control cohort, but was not statistically significant (P = 0.053; Fig. 2). In females, the MS cohort was associated with a statistically significant higher cumulative incidence of RA than that in the control cohort (P = 0.006; Fig. 3).
Figure 1.
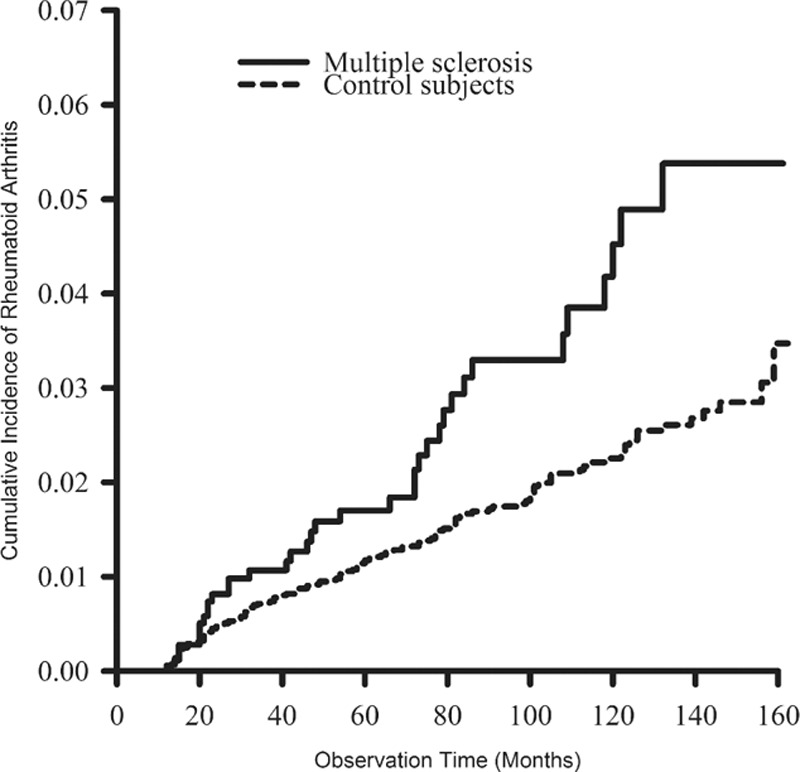
The cumulative incidence rates of rheumatoid arthritis were higher in patients with multiple sclerosis than in control patients (P = 0.002, estimated by the log-rank test).
Figure 2.
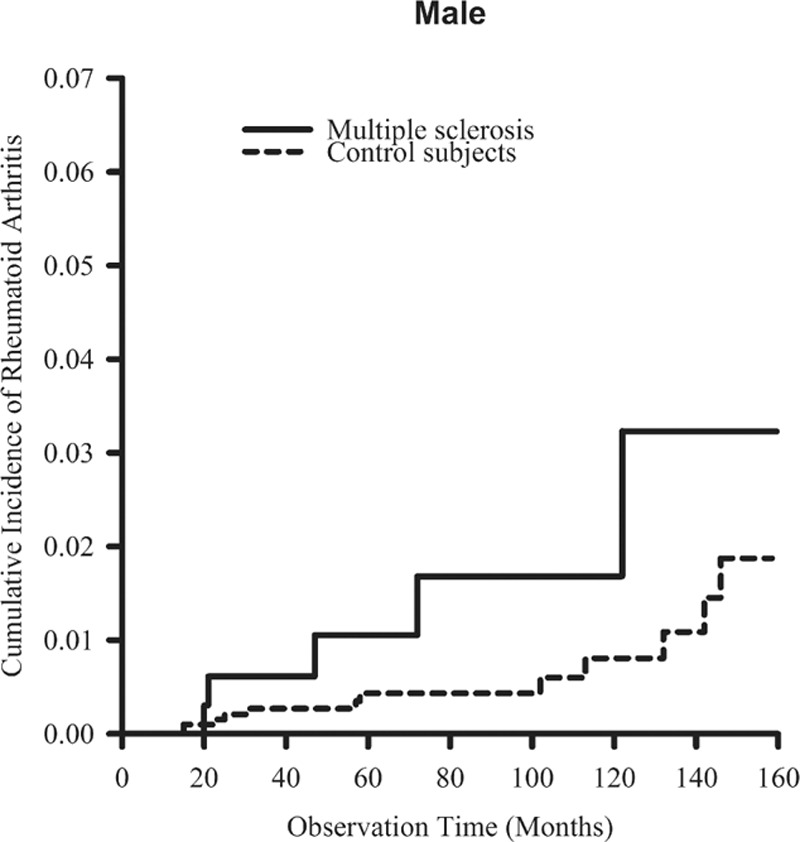
The cumulative incidence rates of rheumatoid arthritis in males were higher in patients with multiple sclerosis than in control patients, but the difference was not statistically significant (P = 0.053, estimated by the log-rank test).
Figure 3.
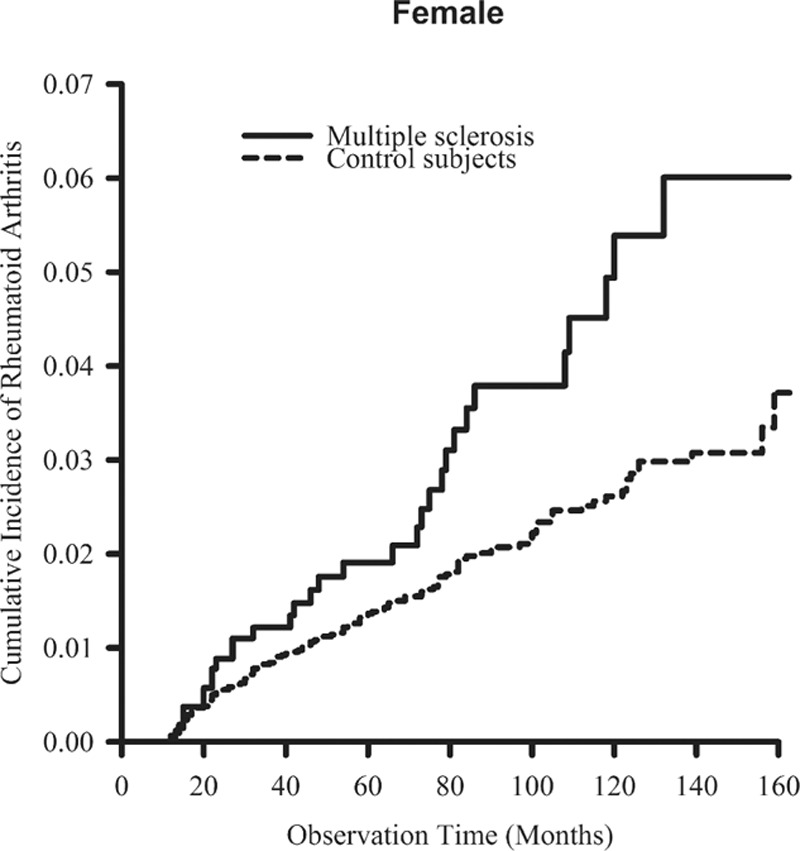
The cumulative incidence rates of rheumatoid arthritis in females were higher in patients with multiple sclerosis than in control patients (P = 0.006, estimated by the log-rank test).
3.5. The hazard ratio of rheumatoid arthritis in multiple sclerosis
Using multivariate Cox regression analysis, the HR for RA in patients with MS when compared to the control group was 1.77 (95% CI = 1.23–2.54, P = 0.002) after adjusting for sex and age. When we further adjusted for smoking, the MS effect estimate was essentially the same (HR: 1.78, 95% CI = 1.24–2.56, P = 0.002).
4. Discussion
To our knowledge, this cohort study is the first to demonstrate a positive correlation between MS and the incidence of RA using nationwide longitudinal administrative data. In the male cohort, there was a trend toward an increased incidence of RA in patients with MS, although due to the limited sample, the associated CIs included the null value. In the female cohort, the effect of MS on the susceptibility of RA persisted after adjusting for age. Furthermore, similar trends were observed in individual age groups. Using multivariate Cox regression, there was a significant association between a history of MS and subsequent RA development, even after adjusting for sex, age, and smoking. These findings are in accordance with findings from prior epidemiological studies.[4,5]
Findings in this study may have some important clinical implications. Past studies have shown that pain associated with RA limited physical activity, disrupted normal activity,[16] and increased disability[17] in patients with MS. Moreover, depression[18] and anxiety,[19] more prevalent in MS, led to increased disease activity,[20] subclinical atherosclerosis,[21] and mortality[22] in patients with RA. Thus, co-occurrence of MS and RA may represent a disease entity distinct from either disease alone and may require more aggressive treatment.
There are several possible explanations for the apparent association of MS with RA. Serum interleukin-17 (IL-17) is increased in both patients with MS[23] and patients with RA.[24] IL-17, which plays a central role in the pathogenesis of MS,[23,25–28] is also involved in the pathogenesis of RA, namely, facilitating angiogenesis,[29] stimulating osteoclastogenesis and receptor activator of nuclear factor-κB ligand expression in fibroblast-like synoviocytes,[30] and increasing matrix metalloproteinase-3 and IL-6 in RA cartilage progenitor cells.[31] In addition, IL-17 involvement is used to correlate radiographic progression,[32] C-reactive protein, anticyclic citrullinated peptide antibody, histopathology changes,[33] and therapy effectiveness[34] in patients with RA. Furthermore, T-helper type 17 (Th17) cells expand in MS[35,36] and RA.[37] Th17, which promotes MS,[38–43] is also implicated in RA, by inducing proinflammatory cytokines, such as IL-6, and facilitating matrix metalloproteinase production by synovial fibroblasts.[44] There is a positive correlation between the percentage of Th17 cells and C-reactive protein levels as well as increasing the disease activity score[37] and decreasing treatment response.[34] Thus, common immunologic pathways, involving IL-17 and Th17, may be one of the mechanisms through which MS increases susceptibility to RA. Moreover, previous studies have shown tyrosine kinase 2 rs34536443 and MHC class II transactivator rs3087456 correlating with the susceptibility to MS[7,45] and RA.[7,46] Thus, common genetic factors, including tyrosine kinase 2 rs34536443 and MHC class II transactivator rs3087456, may be another contributing factor.
This study has some limitations. First, although the BNHI routinely and randomly checks patient charts to ensure the quality of claims from all medical institutions, the possibility of miscoding or misclassification cannot be totally ruled out. However, such bias would apply to both patients with MS and control patients, and thus would be expected to underestimate, rather than overestimate, the magnitude of the association between MS and RA. Second, there are concerns as to whether patients with MS might initiate earlier consultations with healthcare professionals because of their frequent contacts with the healthcare clinic, leading to increased findings of RA. However, past studies have shown a decreased incidence of gout[47] and polymyalgia rheumatica,[8] both inflammatory arthritic diseases, in patients with MS. These findings suggest that early consultations could not explain the findings in this study. Next, there are concerns that the association between MS and incidence of RA may have been introduced by a hitherto unmeasured Epstein–Barr virus infection status. However, recent meta-analysis reports refute the association between Epstein–Barr virus infection and RA.[48] Lastly, some important information about laboratory or clinical data was not readily available in the administrative database, namely, the subtypes of each disease. Thus, the relationship between the different subtypes of MS (relapsing-remitting, primary progressive, or secondary progressive) and their relationship between the subtypes of RA (seropositive and seronegative) remain unclear. Further studies to clarify these points are indeed necessary.
In conclusion, the nationwide retrospective cohort study showed an association between a standing diagnosis of MS and a subsequent diagnosis of RA. It is apparent that early treatment of the disease in patients with RA could improve outcomes[49] and reduce mortality.[50] Therefore, it behooves patients with MS and their physicians to the possibility of the co-occurrence of RA and treats accordingly. Further studies are needed to elucidate the natural history of RA coexisting with MS and to identify the risk factors that led to that relationship.
Acknowledgments
We are indebted to all patients of the Taiwan's National Health Insurance Research Database and Registry of Catastrophic Illness database.
Footnotes
Abbreviations: BNHI = Bureau of National Health Insurance, 95% CI = 95% confidence interval, HR = hazard ratio, IL = interleukin, MS = multiple sclerosis, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, RA = rheumatoid arthritis, SIR = standardized incidence ratio, Th17 = T-helper type 17.
The authors have no conflicts of interest to disclose.
References
- 1.Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73:1316–1322. [DOI] [PubMed] [Google Scholar]
- 2.Gunnarsson C, Chen J, Rizzo JA, et al. The employee absenteeism costs of rheumatoid arthritis: evidence from US national survey data. J Occup Environ Med 2015; 57:635–642. [DOI] [PubMed] [Google Scholar]
- 3.Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case–control study, using incident cases. Ann Rheum Dis 2003; 62:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrie RA, Horwitz RI, Cutter G, et al. Smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases. Neuroepidemiology 2011; 36:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JH, Chen YH, Lin HC. Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur J Neurol 2010; 17:1215–1219. [DOI] [PubMed] [Google Scholar]
- 6.Terao C, Ohmura K, Katayama M, et al. Myelin basic protein as a novel genetic risk factor in rheumatoid arthritis—a genome-wide study combined with immunological analyses. PLoS One 2011; 6:e20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanberg M, Lidman O, Padyukov L, et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nat Genet 2005; 37:486–494. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen NM, Frisch M, Rostgaard K, et al. Autoimmune diseases in patients with multiple sclerosis and their first-degree relatives: a nationwide cohort study in Denmark. Mult Scler 2008; 14:823–829. [DOI] [PubMed] [Google Scholar]
- 9.Tremlett HL, Evans J, Wiles CM, et al. Asthma and multiple sclerosis: an inverse association in a case–control general practice population. QJM 2002; 95:753–756. [DOI] [PubMed] [Google Scholar]
- 10.Bureau of National Health Insurance. Universal Health Coverage in Taiwan [Internet]. http://www.nhi.gov.tw/Resource/webdata/21717_1_20120808UniversalHealthCoverage.pdf Accessed 15/12/2015. [Google Scholar]
- 11.Yang NP, Chen HC, Phan DV, et al. Epidemiological survey of orthopedic joint dislocations based on nationwide insurance data in Taiwan, 2000–2005. BMC Musculoskelet Disord 2011; 12:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo CF, Grainge MJ, Valdes AM, et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern Med 2015; 175:1518–1526. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CF, Grainge MJ, Valdes AM, et al. Familial risk of Sjögren's syndrome and co-aggregation of autoimmune diseases in affected families: a nationwide population study. Arthritis Rheumatol 2015; 67:1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CS, Gau SS, Lai MS. Long-term antidepressant use and the risk of type 2 diabetes mellitus: a population-based, nested case–control study in Taiwan. J Clin Psychiatry 2014; 75:31–38. [DOI] [PubMed] [Google Scholar]
- 15.Neva JL, Lakhani B, Brown KE, et al. Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behav Brain Res 2016; 297:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiest KM, Fisk JD, Patten SB, et al. Comorbidity is associated with pain-related activity limitations in multiple sclerosis. Mult Scler Relat Disord 2015; 4:470–476. [DOI] [PubMed] [Google Scholar]
- 17.Marrie RA, Horwitz R, Cutter G, et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009; 72:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry 2005; 76:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minden SL, Feinstein A, Kalb RC, et al. Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014; 82:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overman CL, Bossema ER, van Middendorp H, et al. The prospective association between psychological distress and disease activity in rheumatoid arthritis: a multilevel regression analysis. Ann Rheum Dis 2012; 71:192–197. [DOI] [PubMed] [Google Scholar]
- 21.Liu YL, Szklo M, Davidson KW, et al. Differential association of psychosocial comorbidities with subclinical atherosclerosis in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015; 67:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang DC, Choi H, Kroenke K, et al. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019. [PubMed] [Google Scholar]
- 23.Babaloo Z, Yeganeh RK, Farhoodi M, et al. Increased IL-17A but decreased IL-27 serum levels in patients with multiple sclerosis. Iran J Immunol 2013; 10:47–54. [PubMed] [Google Scholar]
- 24.Gullick NJ, Abozaid HS, Jayaraj DM, et al. Enhanced and persistent levels of interleukin (IL)-17+ CD4+ T cells and serum IL-17 in patients with early inflammatory arthritis. Clin Exp Immunol 2013; 174:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999; 5:101–104. [DOI] [PubMed] [Google Scholar]
- 26.Hedegaard CJ, Krakauer M, Bendtzen K, et al. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 2008; 125:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med 2009; 206:313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med 2008; 205:1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, Chen Z, Chamberlain ND, et al. Angiogenesis in rheumatoid arthritis is fostered directly by toll-like receptor 5 ligation and indirectly through interleukin-17 induction. Arthritis Rheum 2013; 65:2024–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KW, Kim HR, Kim BM, et al. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am J Pathol 2015; 185:3011–3024. [DOI] [PubMed] [Google Scholar]
- 31.Schminke B, Trautmann S, Mai B, et al. Interleukin 17 inhibits progenitor cells in rheumatoid arthritis cartilage. Eur J Immunol 2016; 46:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndongo-Thiam N, Miossec P. A cell-based bioassay for circulating bioactive IL-17: application to destruction in rheumatoid arthritis. Ann Rheum Dis 2015; 74:1629–1631. [DOI] [PubMed] [Google Scholar]
- 33.Roşu A, Mărgăritescu C, Stepan A, et al. IL-17 patterns in synovium, serum and synovial fluid from treatment-naïve, early rheumatoid arthritis patients. Rom J Morphol Embryol 2012; 53:73–80. [PubMed] [Google Scholar]
- 34.Chen DY, Chen YM, Chen HH, et al. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther 2011; 13:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang SC, Fan XH, Pan QM, et al. Decreased expression of IL-27 and its correlation with Th1 and Th17 cells in progressive multiple sclerosis. J Neurol Sci 2015; 348:174–180. [DOI] [PubMed] [Google Scholar]
- 36.Tao Y, Zhang X, Chopra M, et al. The role of endogenous IFN-β in the regulation of Th17 responses in patients with relapsing-remitting multiple sclerosis. J Immunol 2014; 192:5610–5617. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Li YG, Li YH, et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One 2012; 7:e31000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008; 172:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durelli L, Conti L, Clerico M, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol 2009; 65:499–509. [DOI] [PubMed] [Google Scholar]
- 40.Wojkowska DW, Szpakowski P, Ksiazek-Winiarek D, et al. Interactions between neutrophils, Th17 cells, and chemokines during the initiation of experimental model of multiple sclerosis. Mediators Inflamm 2014; 2014:590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 2007; 13:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jäger A, Dardalhon V, Sobel RA, et al. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 2009; 183:7169–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez NE, Sato F, Kawai E, et al. Th17-biased RORγt transgenic mice become susceptible to a viral model for multiple sclerosis. Brain Behav Immun 2015; 43:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Hamburg JP, Corneth OB, Paulissen SM, et al. IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann Rheum Dis 2013; 72:1700–1707. [DOI] [PubMed] [Google Scholar]
- 45.Couturier N, Bucciarelli F, Nurtdinov RN, et al. Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility. Brain 2011; 134:693–703. [DOI] [PubMed] [Google Scholar]
- 46.Diogo D, Bastarache L, Liao KP, et al. TYK2 protein-coding variants protect against rheumatoid arthritis and autoimmunity, with no evidence of major pleiotropic effects on non-autoimmune complex traits. PLoS One 2015; 10:e0122271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo CF, Grainge MJ, Mallen C, et al. Comorbidities in patients with gout prior to and following diagnosis: case–control study. Ann Rheum Dis 2016; 75:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ball RJ, Avenell A, Aucott L, et al. Systematic review and meta-analysis of the sero-epidemiological association between Epstein–Barr virus and rheumatoid arthritis. Arthritis Res Ther 2015; 17:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moura CS, Abrahamowicz M, Beauchamp ME, et al. Early medication use in new-onset rheumatoid arthritis may delay joint replacement: results of a large population-based study. Arthritis Res Ther 2015; 17:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Symmons DP, Jones MA, Scott DL, et al. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol 1998; 25:1072–1077. [PubMed] [Google Scholar]


