Supplemental Digital Content is available in the text
Keywords: comorbidity, pneumonia, pulmonary tuberculosis, retrospective cohort study
Abstract
Pulmonary tuberculosis (PTb) and pneumonia are diseases that may exist concomitantly. Population study investigating the subsequent pneumonia development in PTb patients is limited. This study compares the risk of pneumonia between cohorts with and without PTb.
We used the claims data of the Taiwan National Health Insurance to identify a cohort with PTb (N = 3417) newly diagnosed in 2000–2006 without pneumonia history, and a randomly selected comparison cohort (N = 6834) free of PTb and pneumonia, frequency matched by propensity score. Incidence rates and hazard ratios of pneumonia were calculated by sex, age, and comorbidity starting in the 7th month after the cohorts being established until the end of 2011.
We found the incidence of pneumonia to be 1.9-fold higher in the PTb cohort than in the PTb free cohort (51.6 vs 27.0 per 1000 person-years). The PTb cohort had a Cox method estimated adjusted hazard ratio of 2.14 (95% confidence interval = 1.96–2.32). We also found that the risk was greater for men than for women, but lower for young adults aged 20–39 years. Comorbidity interacted with PTb by aggravating the pneumonia risk, particularly for those with asthma. For PTb patients comorbid with asthma, the pneumonia incidence was 2.5-fold higher than for PTb patients free of comorbidities (75.9 vs 29.3 per 1000 person-years).
Our results display that PTb patients have an elevated risk of developing pneumonia. Adequate follow-up should be provided to the PTb patients, especially those with comorbidity.
1. Introduction
Pneumonia is an infectious disease that inflames the alveoli in the lung affecting the population worldwide, including developed and developing countries.[1–7] Bacteria, virus, and fungi are the most common pathogens of pneumonia, with bacteria and virus as the most susceptible for development.[2–11] Approximately 4 million deaths annually are attributed to pneumonia worldwide.[1,4] It is the second leading cause of hospitalization even in the United States, with a fatality of 3.4 per 100 among inpatients.[8,9] In Asian countries, community-acquired pneumonia alone is associated with near 1 million death in adults.[10] The incidence of all types of pneumonia has been estimated as high as 3.7 per 1000 annually in Taiwan.[11]
The risk of pneumonia is well known to be elevated in patients with a chronic health condition. Chronic respiratory diseases,[12,13] diabetes,[14] heart diseases,[15] chronic kidney disease,[16] and other conditions that weaken the immune system[17] have been reported as disorders of concern in associating with pneumonia development.[18] An earlier study from Finland[5] have reported that the more common comorbid conditions among pneumonia patients were heart diseases, lung diseases, immunosuppressive therapy, alcoholism, and occurrence of institutionalization.[15] Patients with lung diseases are also at an increased risk of developing pneumonia. Among chronic respiratory system diseases, asthma,[12] and chronic obstructive pulmonary disease (COPD)[13,18] have attracted more attentions in association with developing pneumonia.
Tuberculosis (Tb) is the most widespread chronic infectious disease and has been a major public health concern. Approximately 1.5 million world deaths (mainly in less developed areas) in 2014 had been infected with various mycobacteria resulting in Tb.[19] Approximately 90% of Tb cases may experience pulmonary tuberculosis (PTb), mostly caused by Mycobacterium tuberculosis.[20,21] After the infection is cured, PTb patients may remain at risk for other complications including impaired lung function and residual anatomic changes.[22,23] These impairments include bronchial, parenchymal, pleural, and chest wall lesions. Bronchovascular distortion, fibrotic bands, emphysematous changes, and bronchiectasis may still exist in patients after PTb treatment,[23] leading patients to become more susceptible to develop pneumonia. The incidence of PTb in Taiwan has declined to 54.5 cases per 100,000, with an annual mortality of 3.6 per 100, making it remains as one of leading causes of deaths among infectious diseases.[24] We attempted to evaluate the subsequent risk of pneumonia for people with PTb.
Owing to the lack of population studies investigating the occurrence of subsequent pneumonia for patients with PTb, we therefore, used Taiwan's insurance claims data to establish cohorts with and without PTb to compare the incidences of pneumonia between the 2 cohorts in a follow-up observation.
2. Methods and materials
2.1. Data source
The Taiwan Health Insurance Bureau has authorized the Taiwan National Health Research Institutes to manage the insurance claim data for public use. We obtained from the Institutes a subset data containing the registration and medical records of 1 million insured persons randomly selected from the whole population of approximately 23 million persons for the period of 1996–2011. Personal baseline demographic status including sex, birth date, occupation, income, and residential area are available in the registration file. The Taiwan National Health Research Institutes used surrogate numbers to substitute the identification numbers of insured people for privacy protection to fulfill the principles of the Declaration of Helsinki. The medical records including health care services provided at health care institutions for inpatients and outpatients were available in the database. Diseases diagnosed were coded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Patients were identified for the disease with at least 2 diagnoses in the outpatient records or 1 diagnosis in the inpatient records in 1 year. Because all personal identifications have been replaced with surrogate numbers, no consent was required from the study population for the present study.
2.2. Study cohorts
From the insurance claims data, patients newly diagnosed with PTb (ICD-9-CM 011) from 2000 to 2006 (N = 3417) without the history of pneumonia (ICD-9-CM 480–488) or other types tuberculosis (ICD-9-CM 010, 012–018) were the PTb cohort selected. After excluding patients with the history of pneumonia and/or tuberculosis, or with a missing demographic information, the rest of claims data were used to randomly select a 2-fold of none PTb subjects as the comparison cohort, matched propensity score of PTb cases. Baseline comorbidities were also identified for both cohorts, including COPD (ICD 491, 492 and 496), asthma (ICD 493), heart diseases (ICD 410–429), diabetes (ICD 250), chronic liver disease and cirrhosis (ICD 571); depression or anxiety (ICD 300 and 311), chronic kidney disease (CKD, ICD 585), and autoimmune diseases (ICD 710, 714), and malignant neoplasm of trachea, bronchus and lung (ICD 162).
2.3. Statistical analysis
We first compared the distributions of sex, age, and specific baseline comorbidities between the 2 cohorts. These were then used to estimate the incident pneumonia for the cohorts. We then began calculating the incident pneumonia cases and divided by the follow-up time from the 7th month after the patient's date of selection and placement in the appropriate cohort group. Calculations ceased once pneumonia was diagnosed, or if the patient was censored due to death, if cancellation of insurance policy occurred, or at the end of 2011.
The PTb cohort to none PTb cohort crude hazard ratio (HR) and adjusted hazard ratio (aHR) of pneumonia were measured (with 95% confidence interval [CI]), using univariate and multivariable Cox proportional hazards regression analyses, respectively. Using the Kaplan–Meier method, we estimated the cumulative incidence proportions of pneumonia for the 2 cohorts, and used the log-rank test to examine the distribution difference. Comorbidity may interact with PTb to affect the pneumonia development. We also performed a series of analysis to measure the interactions between PTb and comorbidities. The related aHRs of pneumonia were then calculated using those without any of these conditions as reference. In Discussion section, we further created 2 supplement tables. One was to compare deaths and insurance cancellations between PTb and comparison cohorts. The other one was to perform case–control analysis comparing sex and age differences, and frequency of PTb diagnosis, between PTb patients with and without pneumonia. We used SAS statistical software (version 9.3 Windows; SAS Institute Inc, Cary, NC) for data analyses with a significance level of 0.05.
3. Results
Both the PTb cohort and comparison cohort had similar distributions of age and propensity score, more so with men and with the elderly (Table 1). The prevalence rates of most comorbidities were also similar in both cohorts, except that asthma was more prevalent in PTb cohort than in none PTb comparison cohort. The cumulative incidences of pneumonia was 16% greater in the PTb cohort than in the comparison cohort by the end of follow-up (P < 0.0001) (Fig. 1).
Table 1.
Distributions of demographic characteristics and comorbidities in PTb cohort and propensity score-matched comparison cohort.
Figure 1.
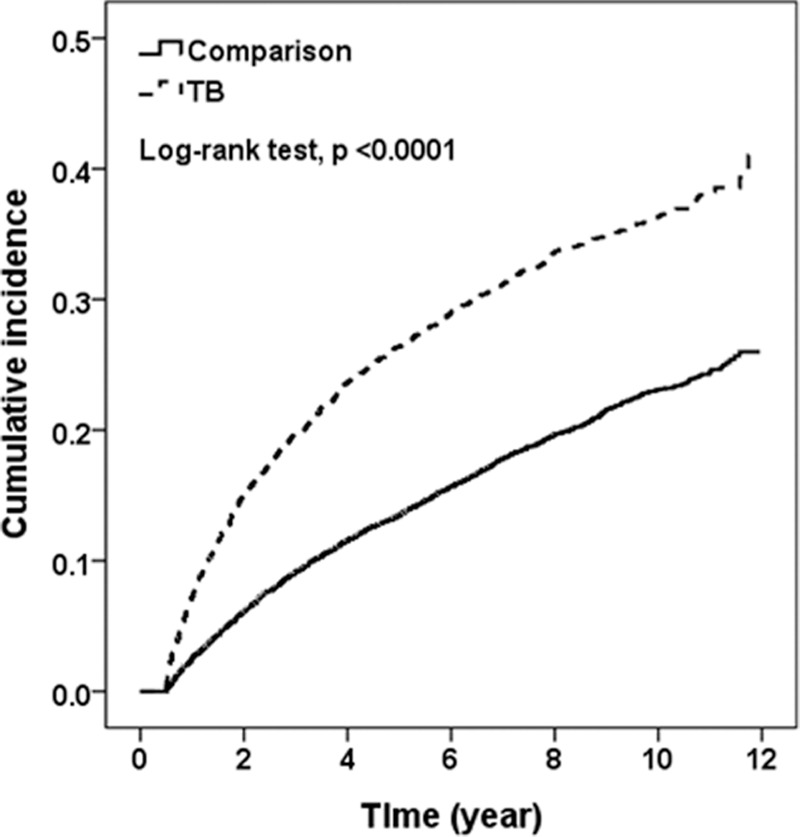
Kaplan–Meier method estimated cumulative incidence of pneumonia for tuberculosis and comparison cohorts. Dotted line: TB cohort; Solid line: Comparison cohort.
Overall, the incidence of pneumonia was 1.9-fold higher in the PTb cohort than in the comparison cohort (51.6 vs 27.0 per 1000 person-years), with an aHR of 2.14 (95% CI, 1.96–2.32) (Table 2). Incidences in both cohorts increased with age, being higher in males than in females. Comorbidities also increased the incidence rates of pneumonia in both cohorts, having a greater impact in the rate increase in the PTb cohort than in comparison cohort, particularly for PTb patients with asthma. The age-specific PTb cohort to comparison cohort relative hazard was lower in young adults aged 20–34 years and the elderly, but the highest in those aged 35–49 years. The sex-specific PTb cohort to comparison cohort relative hazard of pneumonia was greater in males than in females. The aHR was also found to be greater in the cohorts without comorbidity than the cohorts with a comorbidity.
Table 2.
Incidences of pneumonia and multivariable Cox proportional hazards regression analysis estimated tuberculosis cohort to comparison cohort hazard ratios by sex, age, and comorbidity.
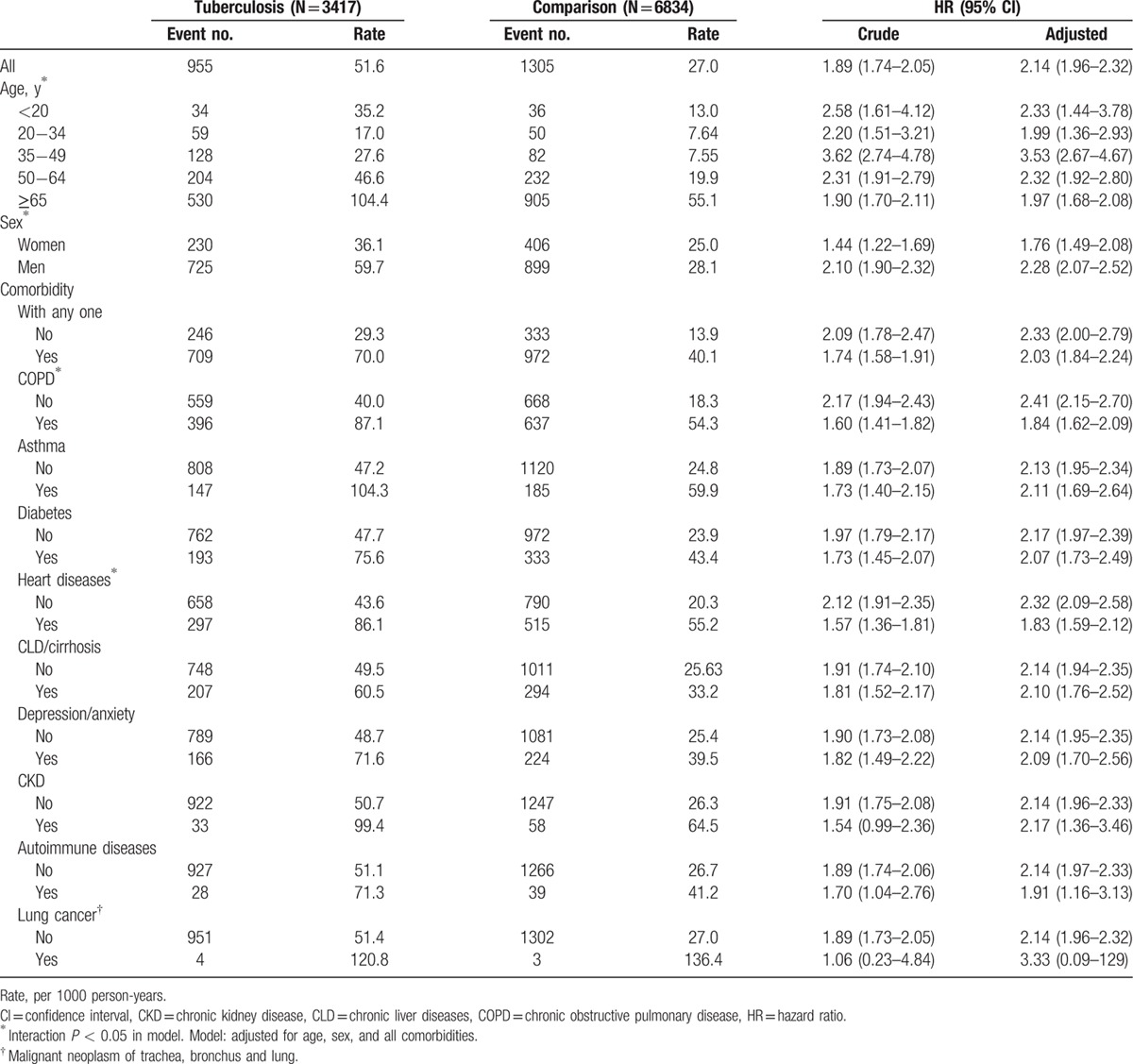
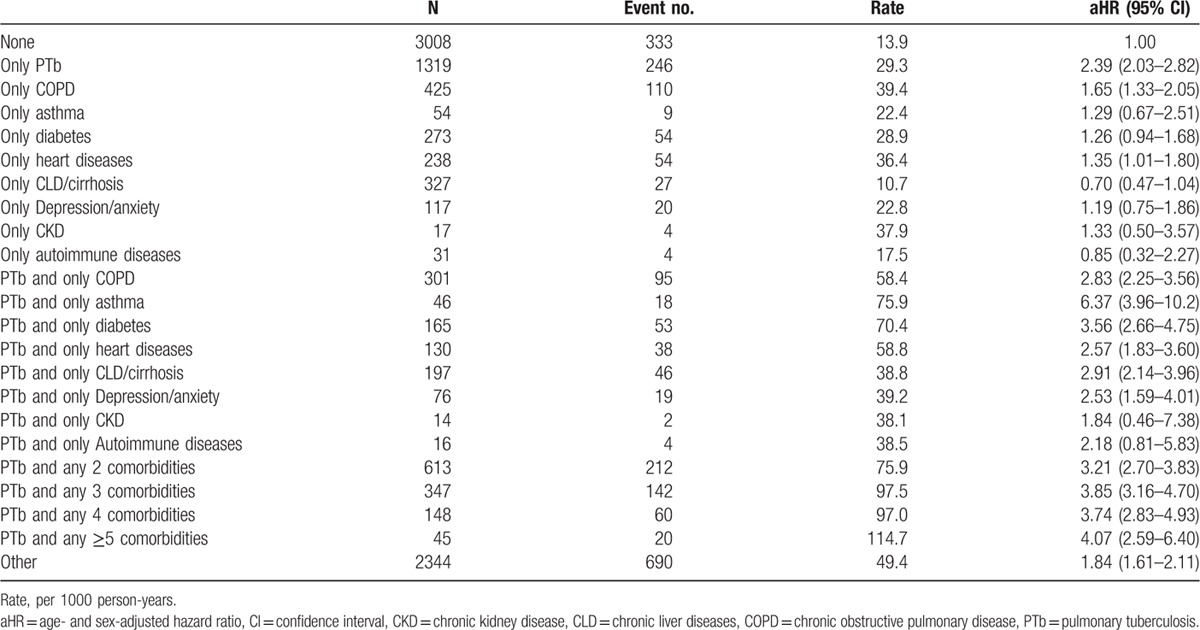
Table 3 shows the pneumonia risk associated with PTb, individual comorbidity, and with PTb joined by 1 comorbidity or multiple comorbidities. COPD, heart diseases, or CKD alone exhibited a stronger relationship leading to pneumonia than PTb. The incidence of pneumonia in PTb patients increased as the number of comorbidity increased. Incidence was found to be 2.6-fold higher for PTb patients with the comorbidity of asthma than PTb patients free of comorbidities (75.9 vs 29.3 per 1000 person-years), with an aHR of 6.37 (95% CI = 3.96–10.2) comparing with subjects free of PTb and comorbidities.
Table 3.
Incidences and hazard ratios of pneumonia associating with PTb and comorbidities.
4. Discussion
In the association between infectious and noninfectious chronic disorders, it is more common for patients with a variety of respiratory disorders to develop pneumonia.[2,3,12,13] Our study used the retrospective cohort study instead of cross-sectional study to evaluate the subsequent risk of pneumonia for patients with PTb. Previous studies have shown pneumonia can also exist concomitantly with PTb patients and vice versa.[17,25–28] A recent study using medical records from 6 medical centers revealed a strong evidence of the concomitant occurrence of PTb in patients with pneumonia.[26] The risk of PTb is stronger for hospitalized health care-associated pneumonia than for community-acquired pneumonia, as well as for patients with a more severe pneumonia. A Korean study using hospital medical records showed that among 90 PTb patients on mechanical ventilation for acute respiratory failure, 66 cases had tuberculous pneumonia and 24 cases had miliary TB.[29] The study reflects that PTb patients with acute respiratory failure are more likely to become complicated with the development of pneumonia. These clinical data studies demonstrate a strong relationship between PTb and pneumonia, with Tb patients having a higher risk of developing pneumonia. Another study using medical records of 2016 tuberculosis patients at medical centers, Lin et al[30] found pneumonia the most common predictor (39.5%) among 43 cases of tuberculosis-related mortality.
In the present follow-up study, the population data confirms that patients with PTb are associated with a near 2-fold increase for subsequent incident pneumonia. With respect in comparing individual comorbidity, our findings showed the incidence of pneumonia for patients with PTb alone was less than that for patients with COPD, heart diseases, or CKD alone. But, the aHR of pneumonia associated with patients with PTb was 2.39 (95% CI = 2.03–2.82), which was higher than that for patients with another type of disease. Our data also showed that the presence of comorbidities increase the incident pneumonia in both cohorts, with a greater increase in the PTb cohort. The greatest increase is associated with asthma, with an excess incidence of 104.3 per 1000 person-years in the PTb cohort (Table 2). These data imply that there is an additional greater risk of pneumonia due to asthma for PTb patients. McKeever et al[12] assessed the risk of respiratory health for asthma patients and found those on inhaled corticosteroids had a 2-fold increased risk of pneumonia.
The excess incidence rates of pneumonia associated with COPD and heart diseases are lower than the rate associated with asthma. However, COPD and heart diseases are more prevalent than asthma in this study, and could have greater population attributable risks than asthma have for the development of pneumonia. In a recent retrospective study, Lee et al [31] found that PTb patients had a near 2-fold elevated risk to develop COPD. In a meta-analysis study, Torres et al[18] found the HRs of contracting pneumonia associated with chronic respiratory diseases based on cohort studies was 2.9, or 1.0–1.9 in association with diabetes, and 1.5–3.9 in association with chronic heart diseases. These hazards of pneumonia in relation to comorbidities are consistent with our findings.
We conducted additional data analyses and found the mortality was near 2.6-fold greater in the PTb patients than in comparisons (25.2 vs 9.61 per 100) with a slightly lower insurance cancellation (16.0 vs 16.5 per 100) (Supplement table 1). We further performed a case–control analysis within the PTb patients to compare characteristics between pneumonia cases and those without pneumonia developed (Supplement table 2). There was a u-shape relationship between PTb and pneumonia development among age groups, pneumonia cases were younger or older and men were at higher risk. The pneumonia risk increased with the frequency of tuberculosis cares.
This study encountered limitations on available data in our research. Among the limitations, laboratory tests were unavailable to detect the etiological pathogens for pneumonia cases. However, the claims for pneumonia in the insurance system require the diagnosis confirmed by chest radiographic manifestations or with the growth of microorganisms from cultures of respiratory specimens or clinical improvement after antibiotic therapy. Physicians are also obligated to report the Tb diagnosis to the Taiwan Centers for Disease Control. Tuberculosis treatment is required to follow the World Health Organization guidelines.[32] Second, the lifestyle information was also unavailable from the claims data. Smoking habits, for example, were unavailable and have been found as a risk factor associated with the development of pneumonia.[2] However, the smoking rate might be much lower in PTb patients than in comparisons. In Taiwan, less than 5% of women smoke. Third, the risk of developing pneumonia may increase with the severity of PTb. However, patients with severe PTb may have shorter follow-up time because they are censored from premature death. The competing risk associated with death in patients with severe PTb could underestimate the pneumonia risk. Fourth, we calculated the incident pneumonia cases from the 7th month after the date the patient selected to avoid the immortal time bias. Some incident pneumonia cases before the 7th month could be excluded. Delayed diagnosis of Tb and early pneumonia occurrence caused by Tb might be possible.
To the best of our knowledge, there is a lack of literature on the temporal relationship between PTb and pneumonia risk based on population data. In conclusion, this study has confirmed our hypothesis that PTb patients have a near 2-fold elevated risk of developing pneumonia. The elderly and younger patients are at higher risk. PTb patients need to be closely monitored to prevent the development of pneumonia and to prevent deaths from this complication, especially for those that have a comorbidity.
Supplementary Material
Footnotes
Abbreviations: aHR = adjusted hazard ratio, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, Non-PTb = none pulmonary tuberculosis, PTb = pulmonary tuberculosis.
FY Wu and FC Sung have equal contribution.
Authorship: conception and design: TMC, CHM, TCS, MHY, FCS; administrative support: FYW, FCS; collection and assembly of data: all authors; data analysis and interpretation: TMC, CHM, FCS; manuscript writing and revision: all authors; final approval of manuscript: all authors.
Funding/support: The present study was supported by the National Sciences Council, Executive Yuan (grant numbers NSC 100-2621-M-039-001), China Medical University Hospital (grant number 1MS1), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019). China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan).
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.WHO. Pneumonia Fact Sheet No. 331: Updated November 2014. http://www.who.int/mediacentre/factsheets/fs331/en/ (accessed October 5, 2015). [Google Scholar]
- 2.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssens JP, Krause KH. Pneumonia in the very old. Lancet Infect Dis 2004; 4:112–124. [DOI] [PubMed] [Google Scholar]
- 4.Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet 2011; 377:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray JF. Viral infection. Murray and Nadel's Textbook of Respiratory Medicine. Chapter 31. 5th ed2010; Philadelphia, PA: Saunders/Elsevier, ISBN 1416047107. [Google Scholar]
- 6.Anevlavis S, Bouros D. Community acquired bacterial pneumonia. Expert Opin Pharmacother 2010; 11:361–374. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo LTM. Viral pneumonia: epidemiological, clinical, pathophysiological, and therapeutic aspects. J Bras Pneumol 2009; 35:899–906. [DOI] [PubMed] [Google Scholar]
- 8.Pfuntner A, Wier LM, Stocks C. Most frequent conditions in U.S. hospitals, 2011. HCUP Statistical Brief #162. Rockville, MD: Agency for Healthcare Research and Quality; September 2013. [PubMed] [Google Scholar]
- 9.Buie VC, Owings MF, DeFrances CJ, et al. National hospital discharge survey: 2006 summary. Vital Health Stat 2010; 13:1–79. [PubMed] [Google Scholar]
- 10.Peto L, Nadjm B, Horby P, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg 2014; 108:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou FH, Tsai KY, Chou YM. The incidence and all-cause mortality of pneumonia in patients with schizophrenia: a nine-year follow-up study. J Psychiatr Res 2013; 47:460–466. [DOI] [PubMed] [Google Scholar]
- 12.McKeever T, Harrison TW, Hubbard R, et al. Inhaled corticosteroids and the risk of pneumonia in people with asthma: a case-control study. Chest 2013; 144:1788–1794. [DOI] [PubMed] [Google Scholar]
- 13.Rouzé A, Cottereau A, Nseir S. Chronic obstructive pulmonary disease and the risk for ventilator-associated pneumonia. Curr Opin Crit Care 2014; 20:525–531. [DOI] [PubMed] [Google Scholar]
- 14.Jette B, Kornum JB, Thomsen RW, et al. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care 2007; 30:2251–2257. [DOI] [PubMed] [Google Scholar]
- 15.Koivula I, Sten M, Makela PH. Risk factors for pneumonia in the elderly. Am J Med 1994; 96:313–320. [DOI] [PubMed] [Google Scholar]
- 16.Chou CY, Wang SM, Liang CC, et al. Risk of pneumonia among patients with chronic kidney disease in outpatient and inpatient settings: a nationwide population-based study. Medicine 2014; 93:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med 2001; 164:2120–2126. [DOI] [PubMed] [Google Scholar]
- 18.Torres A, Blasi F, Dartois N, et al. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015; 70:984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Global Tuberculosis Control 2014. Geneva: World Health Organization; 2014. http:www.who.int/tb/publications/global_report/en/ Accessed July 9, 2015. [Google Scholar]
- 20.Lawn SD, Zumla AI. Tuberculosis. Lancet 2011; 378:57–72. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald DW, Sterling TR, Haas DW. Bennett JE, Dolan R, Blaser MJ. Mycobacterium tuberculosis. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Chapter 251 Elsevier Churchill Livingstone, 8th edPhiladelphia, PA:2015. [Google Scholar]
- 22.Pasipanova JG, Miller TL, Vecino M, et al. Pulmonary impairment after tuberculosis. Chest 2007; 131:1817–1824. [DOI] [PubMed] [Google Scholar]
- 23.Kim HY, Song KS, Goo JM, et al. Thoracic sequelae and complications of tuberculosis. Radiographics 2001; 21:839–858. [DOI] [PubMed] [Google Scholar]
- 24.Liao CM, Hsieh NH, Huang TL, et al. Assessing trends and predictors of tuberculosis in Taiwan. BMC Pub Health 2012; 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavallazzi R, Wiemken T, Christensen D, et al. Predicting Mycobacterium tuberculosis in patients with community-acquired pneumonia. Eur Respir J 2014; 43:178–184. [DOI] [PubMed] [Google Scholar]
- 26.Feng JY, Fang WF, Wu CL, et al. Concomitant pulmonary tuberculosis in hospitalized healthcare associated pneumonia in a tuberculosis endemic area: a multi-center retrospective study. PLoS One 2012; 7:e36832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liam CK, Pang YK, Poosparajah S. Pulmonary tuberculosis presenting as community-acquired pneumonia. Respirology 2006; 11:786–792. [DOI] [PubMed] [Google Scholar]
- 28.Long R, Chong H, Hoeppner V, et al. Empirical treatment of community-acquired pneumonia and the development of fluoroquinolone-resistant tuberculosis. Clin Infect Dis 2009; 48:1354–1360. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Pack KM, Jeong E, et al. Pulmonary tuberculosis with acute respiratory failure. Eur Respir J 2008; 32:1625–1630. [DOI] [PubMed] [Google Scholar]
- 30.Lin CH, Lin CJ, Kuo YW, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CH, Lee MC, Lin HH, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One 2012; 7:e37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luh KT. Taiwan Guidelines for TB Diagnosis and Treatment. 5th edTaipei, Taiwan: Centers for Disease Control; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


