Abstract
Little is known on the association between appendectomy and pyogenic liver abscess. The objective of this study was to investigate the association between appendectomy and the risk of pyogenic liver abscess in Taiwan.
This population-based retrospective cohort study was conducted using the hospitalization dataset of the Taiwan National Health Insurance Program. There were 212,530 subjects age 20 to 84 years with newly diagnosed appendectomy as the appendectomy group since 1998 to 2010, and 850,099 randomly selected subjects without appendectomy as the nonappendectomy group. Both appendectomy and nonappendectomy groups were matched with sex, age, comorbidities, and index year of diagnosing appendectomy. The incidence of pyogenic liver abscess at the end of 2011 was estimated in both groups. The multivariable Cox proportional hazards regression model was applied to investigate the hazard ratio (HR) and 95% confidence interval (CI) for risk of pyogenic liver abscess associated with appendectomy and other comorbidities including alcoholism, biliary stone, chronic kidney disease, chronic liver diseases, and diabetes mellitus.
The overall incidence of pyogenic liver abscess was 1.73-fold greater in the appendectomy group than that in the nonappendectomy group (3.85 vs 2.22 per 10,000 person-years, 95% CI 1.71, 1.76). The multivariable regression analysis disclosed that the adjusted HR of pyogenic liver abscess was 1.77 for the appendectomy group (95% CI 1.59, 1.97), when compared with the nonappendectomy group.
Appendectomy is associated with increased hazard of pyogenic liver abscess. Further studies remain necessary to confirm our findings.
Keywords: alcoholism, appendectomy, biliary stone, diabetes mellitus, pyogenic liver abscess
1. Introduction
The role of the human appendix is still not clearly identified. Recent literature shows that the human appendix might be regarded as a part of the immune system because many immunoglobulin-producing cells can be detected in normal appendix mucosa.[1–5] Therefore, due to the change of immune function after removing the human appendix, people with appendectomy are found to be associated with increased risk of pulmonary tuberculosis, colorectal cancer, rheumatoid arthritis, and ischemic heart disease,[6–9] but pyogenic liver abscess has not yet been included for study.
In the other hand, previous studies have shown that elevated total bilirubin levels were found in patients with acute appendicitis.[10,11] Several case reports have shown that acute appendicitis could proceed to the development of pyogenic liver abscess.[12–14] These above findings suggest that the inflammation signal potentially caused by infective focus could be transmitted from the inflamed appendix to the liver.
Pyogenic liver abscess is a major public health problem because of its severe morbidity and mortality. It is an infective disease of the liver caused by various pathogens. Particularly, pyogenic liver abscess often occurs in patients with immunocompromised status, such as diabetes mellitus, malignancy, and splenectomy.[15–17] However, the role of appendectomy has not yet been included for study.
Based on the aforementioned literature review, we think that there could a link between appendectomy and pyogenic liver abscess based on 2 plausible hypotheses. The first, there could be a bacteremia from the inflamed appendix to the liver. Then, consecutive pyogenic liver abscess might develop later. The second, the change of immune function after removing the human appendix could increase the possibility of pathogens attacking the liver. Then, pyogenic liver abscess might develop later. At present, the information is insufficient on the association between appendectomy and pyogenic liver abscess. If the association really exists, more evidence can be added to the knowledge on appendectomy and pyogenic liver abscess. Therefore, we conducted a population-based retrospective cohort study using the hospitalization dataset of the Taiwan National Health Insurance Program to investigate the following questions: Whether there is a link between appendectomy and pyogenic liver abscess? and What are the possible mechanisms underlying the association between appendectomy and pyogenic liver abscess?
2. Methods
2.1. Design and data source
This population-based retrospective cohort study was conducted using the hospitalization dataset of the Taiwan National Health Insurance Program. Short speaking, this program began in March 1, 1995, which covered about 99% of 23 million people living in Taiwan.[18] The details of the program have been well written in previous studies.[19–31] This study was approved by the Ethics Review Board of China Medical University and Hospital in Taiwan (CMUH-104-REC2-115).
2.2. Participants, comorbidities, and major outcome
Using the hospitalization dataset of the Taiwan National Health Insurance Program, the appendectomy group was defined as subjects age 20 to 84 years with newly diagnosed appendectomy since 1998 to 2010 (the International Classification of Diseases [ICD] 9th Revision, ICD-9 procedure codes 47.0 and 47.1). To increase a statistical power, for each appendectomy subject, 4 subjects without appendectomy were randomly selected as the nonappendectomy group. Both appendectomy and nonappendectomy groups were matched by sex, age (every 5-year span), comorbidities, and index year of diagnosing appendectomy. The index date was defined as the date of diagnosing appendectomy. Subjects with history of pyogenic liver abscess, amebic liver abscess, or liver transplantation before the index date were excluded from the study.
The comorbidities potentially related to pyogenic liver abscess were included as follows: alcoholism, biliary stone, chronic kidney disease, diabetes mellitus, as well as chronic liver diseases including cirrhosis, alcoholic liver damage, hepatitis B, hepatitis C, and other chronic hepatitis. All comorbidities were diagnosed with ICD-9 codes.
The major outcome was a new diagnosis of pyogenic liver abscess (ICD-9 code 572.0) according to hospital discharge diagnosis during the follow-up period. All study subjects were followed until they were diagnosed with pyogenic liver abscess or to the end of 2011.
2.3. Statistical analysis
The distributions of sex, age, and comorbidities were compared between the appendectomy and nonappendectomy groups using the Chi-squared test for categorized variables and t test for continuous variables. The incidence of pyogenic liver abscess was estimated as the event number of pyogenic liver abscess identified during the follow-up period, divided by the total follow-up person-years for each group. The proportional hazard model assumption was also estimated using a test of scaled Schoenfeld residuals. In the model evaluating the risk of pyogenic liver abscess throughout overall follow-up period, results of the test revealed a significant relationship between Schoenfeld residuals for appendectomy and follow-up period, suggesting the proportionality assumption was violated (P value < 0.001). In the subsequent analyses, we stratified the follow-up period to deal with the violation of proportional hazard assumption. Initially, all variables were included in univariable model. Next step, only those found to be significant in the univariable model were further included in the multivariable model. The multivariable Cox proportional hazards regression model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for risk of pyogenic liver abscess associated with appendectomy and other comorbidities including alcoholism, biliary stone, chronic kidney disease, chronic liver diseases, and diabetes mellitus. All analyses were performed by SAS software version 9.2 (SAS Institute Inc., Cary, NC). Two-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the study population
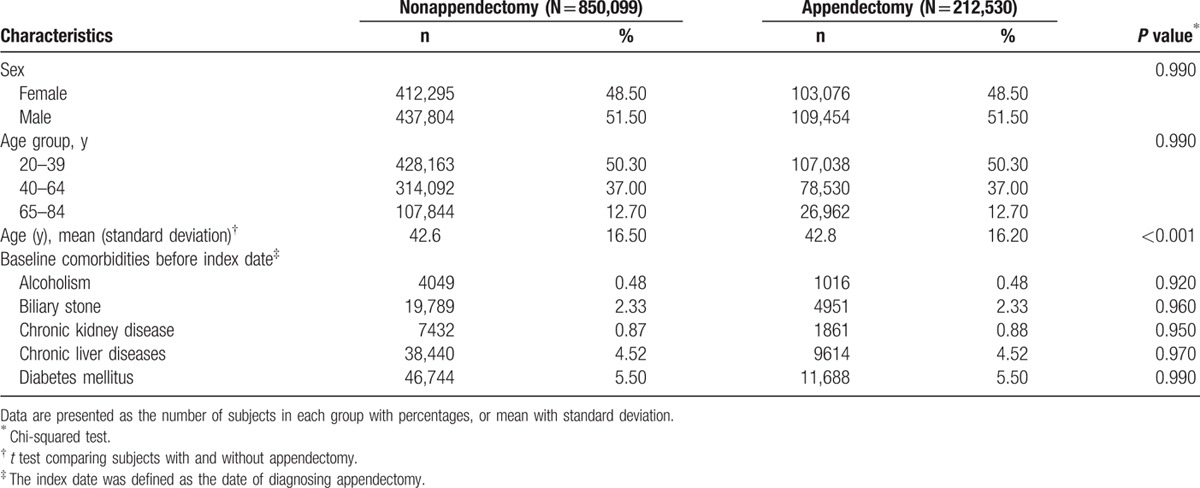
Table 1 shows the distributions of sex, age, and comorbidities between the appendectomy and nonappendectomy groups. There were 212,530 subjects in the appendectomy group and 850,099 subjects in the nonappendectomy group, with similar distribution of sex. The mean ages (standard deviation) of the study subjects were 42.8 ± 16.2 years for the appendectomy group and 42.6 ± 16.5 years for the nonappendectomy group (t test, P < 0.001). The proportions of alcoholism, biliary stone, chronic kidney disease, chronic liver diseases, and diabetes mellitus were equally distributed in both groups (Chi-squared test, P > 0.05 for all).
Table 1.
Characteristics between appendectomy and nonappendectomy groups.
3.2. Incidence of pyogenic liver abscess of the study population stratified by sex, age, and follow-up period
Table 2 shows that the overall incidence of pyogenic liver abscess was 1.73-fold greater in the appendectomy group than that in the nonappendectomy group (3.85 vs 2.22 per 10,000 person-years, 95% CI 1.71, 1.76). The incidence rates of pyogenic liver abscess, as stratified by sex, age, and follow-up period, were all higher in the appendectomy group than those in the nonappendectomy group. The appendectomy group age 65 to 84 years had the highest incidence rate of pyogenic liver abscess (11.1 per 10,000 person-years). The analysis stratified by follow-up period disclosed that the risk of pyogenic liver abscess persisted over time, even after 5 years of diagnosing appendectomy (incidence rate ratio 1.20, 95% CI 1.17, 1.23). However, the risk appeared to be higher during the first 1 year of follow-up, particularly during the first 3 months (incidence rate ratio 9.14, 95% CI 8.98, 9.30).
Table 2.
Incidence of pyogenic liver abscess between appendectomy and nonappendectomy groups stratified by sex, age, and follow-up period.
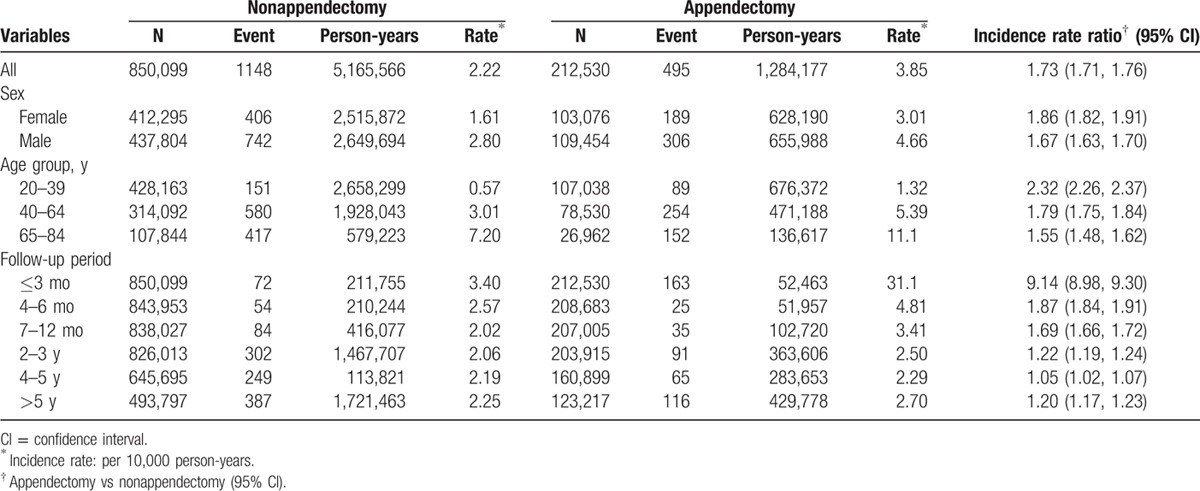
Fig. 1 shows the Kaplan–Meier cumulative incidences of pyogenic liver abscess in the appendectomy and nonappendectomy groups (0.34% vs 0.22% at the end of follow-up; P < 0.001).
Figure 1.
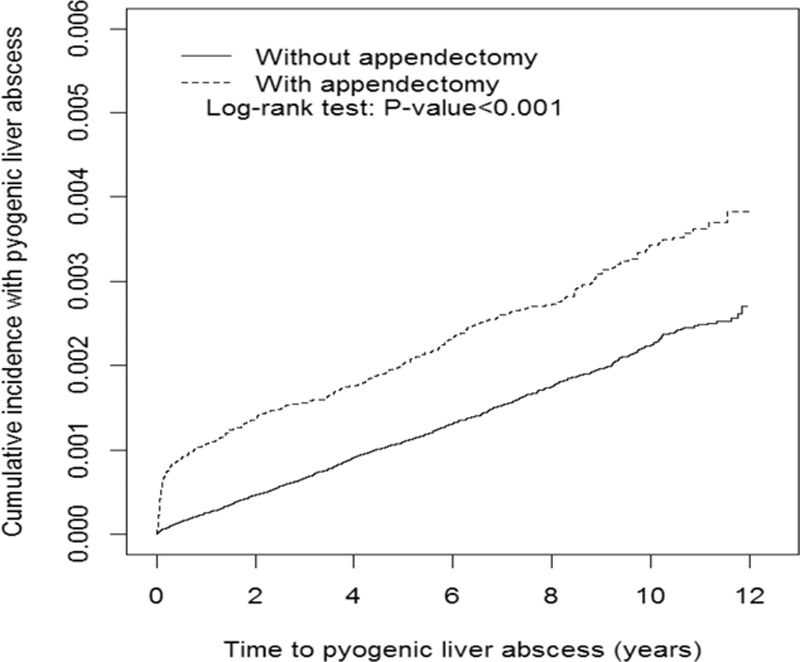
The Kaplan–Meier cumulative incidences of pyogenic liver abscess in the appendectomy and nonappendectomy groups (0.34% vs 0.22% at the end of follow-up; P < 0.001).
3.3. Pyogenic liver abscess associated with appendectomy and other comorbidities
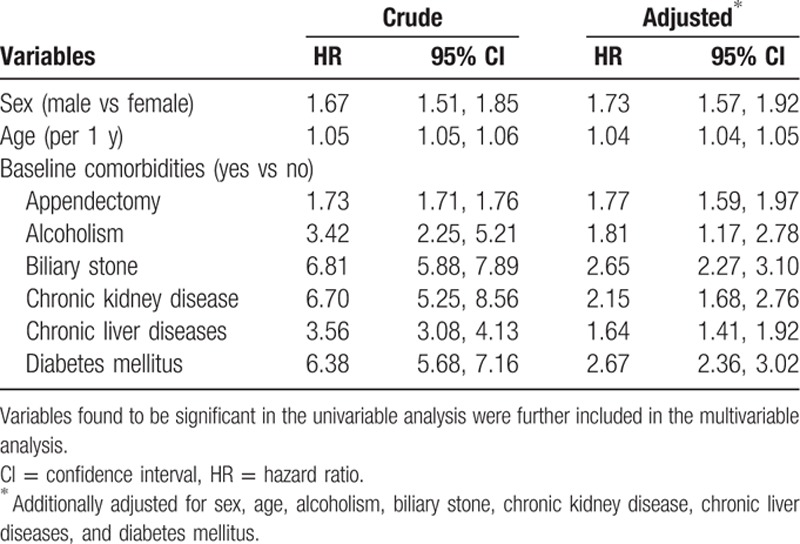
Only those found to be significant in the univariable analysis were further included in the multivariable analysis. After adjusted for sex, age, alcoholism, biliary stone, chronic kidney disease, chronic liver diseases, and diabetes mellitus, the multivariable Cox proportional hazards regression model disclosed that the adjusted HR of pyogenic liver abscess was 1.77 for the appendectomy group (95% CI 1.59, 1.97), when compared with the nonappendectomy group. In addition, male (adjusted HR 1.73, 95% CI 1.57, 1.92), age (per 1 year increase, adjusted HR 1.04, 95% CI 1.04, 1.05), alcoholism (adjusted HR 1.81, 95% CI 1.17, 2.78), biliary stone (adjusted HR 2.65, 95% CI 2.27, 3.10), chronic kidney disease (adjusted HR 2.15, 95% CI 1.68, 2.76), chronic liver diseases (adjusted HR 1.64, 95% CI 1.41, 1.92), and diabetes mellitus (adjusted HR 2.67, 95% CI 2.36, 3.02) were other factors significantly associated with pyogenic liver abscess (Table 3).
Table 3.
Crude and adjusted HR and 95% CI of pyogenic liver abscess associated with appendectomy and other comorbidities.
3.4. Risk of pyogenic liver abscess stratified by appendectomy and comorbidity
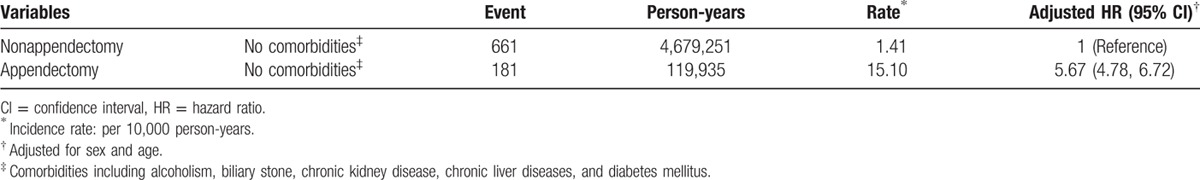
To reduce the potential confounding effect of comorbidity studied, as a reference of subjects without appendectomy and without any comorbidity, the adjusted HR of pyogenic liver abscess was 5.67 for subjects with appendectomy alone and without any comorbidity studied (95% CI 4.78, 6.72) (Table 4).
Table 4.
Risk of pyogenic liver abscess stratified by appendectomy and comorbidities.
4. Discussion
To the best of our knowledge, this is the first population-based cohort study investigating the association between appendectomy and pyogenic liver abscess. In the present study, we noted that overall incidence of pyogenic liver abscess was 1.73-fold greater in the appendectomy group than that in the nonappendectomy. We also noted that, after adjusted for confounding factors, people with appendectomy were associated with 1.77-fold increased hazard of pyogenic liver abscess (Table 3). However, the incidence rate of pyogenic liver abscess among people with appendectomy seems to be lower than that among people with inflammatory bowel disease by Lin et al's study in Taiwan (3.85 vs 6.72 per 10,000 person-years).[32] We also noted that the risk of pyogenic liver abscess persists over time, even after 5 years of performing appendectomy. However, the risk seems to be particularly higher during the first 3 months of follow-up (incidence rate ratio 9.14, Table 2). In the preantibiotic era, pyogenic liver abscess had ever been a feared complication of acute appendicitis. Based on previous studies showing elevated total bilirubin levels in patients with acute appendicitis, and previous case reports showing acute appendicitis potentially preceding to the development of pyogenic liver abscess,[10–14] in spite of no definite evidence available in this observational study, we think that the inflammation signal potentially related to infective focus could be transmitted from the inflamed appendix to the liver. In addition, procedural complications of appendectomy could be involved in the liver. Therefore, pyogenic liver abscess might develop later. That could partially explain why the risk of pyogenic liver abscess is particularly higher during the first 3 months of follow-up. However, these findings indicate a further research direction whether conservative treatment of acute appendicitis without surgery could reduce the development of consecutive pyogenic liver abscess.
In order to clarify whether there could be another plausible link between appendectomy and pyogenic liver abscess, not related to infective focus from the inflamed appendix or procedural complications, we made a further analysis. That is, subjects with diagnosis of pyogenic liver abscess within 1 year after performing appendectomy were excluded. We noted that there were 272 events of pyogenic liver abscess and 1,280,952 person-years in the appendectomy group, and 938 events of pyogenic liver abscess and 5,153,503 person-years in the nonappendectomy group. The incidence of pyogenic liver abscess was 1.16-fold greater in the appendectomy group than that in the nonappendectomy group (2.12 vs 1.82 per 10,000 person-years, 95% CI 1.15, 1.19, table not shown). Although the risk of pyogenic liver abscess seems to be slightly decreased (overall incidence rate ratio 1.77 vs 1.16), these findings further highlight that the risk of pyogenic liver abscess still exists among those with appendectomy 1 year later. The risk seems to be not related to infective focus or procedural complications. The risk substantially persists over time, even after 5 years of diagnosing appendectomy (incidence rate ratio 1.20, 95% CI 1.17, 1.23, Table 2).
Because the proportions of alcoholism, biliary stone, chronic kidney disease, chronic liver diseases, and diabetes mellitus were equally distributed in the appendectomy and nonappendectomy groups, the confounding effect caused by comorbidities studied could be minimized. In an additional analysis stratified by presence or absence of appendectomy and comorbidity, even in absence of any comorbidity studied, people with appendectomy were still associated with increased hazard of pyogenic liver abscess (adjusted HR 5.67, Table 4). These findings indicate the increased hazard associated with appendectomy cannot be entirely attributable to the effect of comorbidities studied. Appendectomy could play an important role on risk of pyogenic liver abscess, independent of comorbidities studied.
Moreover, the pathogenesis of appendectomy associated with pyogenic liver abscess could not be entirely discerned in this observational study. Yet no similar research can be compared with each other. We reviewed the relevant literature and summarized the plausible explanation of this association. Recent literature shows that the human appendix might be regarded as a part of the immune system because many immunoglobulin-producing cells can be detected in normal appendix mucosa, particularly with immunoglobulin-G and immunoglobulin-A.[1–5] Therefore, due to the change of immune function of the bowel after removing the human appendix, the bowel pathogens could more easily ascend to attack the liver via the bile duct system, and as a consequence, pyogenic liver abscess might develop later. Similarly, due to the change of immune function, people with appendectomy are also likely to be associated with increased risk of pulmonary tuberculosis, colorectal cancer, rheumatoid arthritis, and ischemic heart disease.[6–9] However, more studies are needed to clarify these associations.
Some limitations in this present study should be discussed. First, the diagnosis of appendectomy and pyogenic liver abscess is not directly adapted from clinical documentation. It is based on the ICD-9 codes recorded in the hospitalization dataset. Although we cannot provide sensitivity/specificity of single code for appendectomy or pyogenic liver abscess, according to the medical quality in Taiwan, the accuracy of diagnostic code based on hospitalization diagnosis could be confidently believed. Second, comorbidities included were all diagnosed with ICD-9 codes. The diagnosis accuracy based on the ICD-9 codes has been extensively assessed in previous studies.[33–40] Third, due to the inherent limitation of this dataset used, there was no record on types of acute appendicitis. We cannot distinguish between perforated and nonperforated appendicitis. Whether the perforation per se is a risk factor for pyogenic liver abscess cannot be determined in this observational study. Fourth, due to the same limitation, there was no record on the bilirubin levels in this dataset. We cannot differentiate whether patients with appendectomy have elevated total bilirubin levels or not. Fifth, the incidence rates of pyogenic liver abscess of 3.85, respectively, 2.22 per 10,000 person-years are low (Table 2). Consecutively the statistically significant HR of pyogenic liver abscess associated with appendectomy turns out to be tiny if expressed in the difference of absolute values. Nevertheless, this is the first time to find the association between appendectomy and pyogenic live abscess based on a systematic analysis.
Despite the limitations, some strengths of this study should be addressed. This is a novel observation using a large, population-based dataset with nice statistical analyses. We firstly suggest that appendectomy is associated with pyogenic liver abscess. The hypothesis, methodology, and discussion are well written. The results are impressive. It delivers up-to-date biomedical information on the knowledge of appendectomy and pyogenic liver abscess.
In conclusion, appendectomy is associated with increased hazard of pyogenic liver abscess, independent of alcoholism, biliary stone, chronic kidney disease, chronic liver diseases, and diabetes mellitus. The more plausible pathophysiological mechanisms underlying the association between appendectomy and pyogenic liver abscess might be related to infective focus transmitting from the inflamed appendix to the liver, or immunosuppressive status caused by appendectomy. More research is needed to examine issues related to the pathogenesis of appendectomy associated with pyogenic liver abscess.
Footnotes
Abbreviation: ICD-9 code = International Classification of Diseases, 9th Revision, Clinical Modification.
K-FL and S-WL equally contributed to this study.
K-FL and S-HC planned and conducted this study. They substantially contributed to the conception of the article. They critically revised the article. S-WL substantially contributed to the conception of the article, initiated the draft of the article, and critically revised the article. C-LL conducted the data analysis and critically revised the article.
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019). This funding agency did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Spencer J, Finn T, Isaacson PG. Gut associated lymphoid tissue: a morphological and immunocytochemical study of the human appendix. Gut 1985; 26:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerke K, Brandtzaeg P, Rognum TO. Distribution of immunoglobulin producing cells is different in normal human appendix and colon mucosa. Gut 1986; 27:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazar KA, Lee PY, Yun AJ. An “eye” in the gut: the appendix as a sentinel sensory organ of the immune intelligence network. Med Hypotheses 2004; 63:752–758. [DOI] [PubMed] [Google Scholar]
- 4.Bollinger RR, Barbas AS, Bush EL, et al. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol 2007; 249:826–831. [DOI] [PubMed] [Google Scholar]
- 5.Laurin M, Everett ML, Parker W. The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken) 2011; 294:567–579. [DOI] [PubMed] [Google Scholar]
- 6.Lai SW, Lin CL, Liao KF, et al. Increased risk of pulmonary tuberculosis among patients with appendectomy in Taiwan. Eur J Clin Microbiol Infect Dis 2014; 33:1573–1577. [DOI] [PubMed] [Google Scholar]
- 7.Wu SC, Chen WT, Muo CH, et al. Association between appendectomy and subsequent colorectal cancer development: an Asian population study. PLoS ONE 2015; 10:e0118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzeng YM, Kao LT, Kao S, et al. An appendectomy increases the risk of rheumatoid arthritis: a five-year follow-up study. PLoS ONE 2015; 10:e0126816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CH, Tsai MC, Lin HC, et al. Appendectomy increased the risk of ischemic heart disease. J Surg Res 2015; 199:435–440. [DOI] [PubMed] [Google Scholar]
- 10.Khan S. Evaluation of hyperbilirubinemia in acute inflammation of appendix: a prospective study of 45 cases. Kathmandu Univ Med J (KUMJ) 2006; 4:281–289. [PubMed] [Google Scholar]
- 11.Estrada JJ, Petrosyan M, Barnhart J, et al. Hyperbilirubinemia in appendicitis: a new predictor of perforation. J Gastrointest Surg 2007; 11:714–718. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen MR, Baekgaard N, Kirkegaard P. Pyogenic liver abscess. A case report with a short review of current concepts of diagnosis and management. Acta Chir Scand 1983; 149:437–439. [PubMed] [Google Scholar]
- 13.Mahieu X, Boverie J, Lemaire JM, et al. Pyogenic liver abscess. Diagnostic and therapeutic approach: a case report. Acta Chir Belg 1993; 93:220–223. [PubMed] [Google Scholar]
- 14.Nasir AA, Adeniran JO, Abdur-Rahman LO, et al. Pyogenic liver abscess in children: is ruptured appendix still relevant as cause? Case report. Niger Postgrad Med J 2009; 16:176–178. [PubMed] [Google Scholar]
- 15.Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004; 2:1032–1038. [DOI] [PubMed] [Google Scholar]
- 16.Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 2008; 14:1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai SW, Lai HC, Lin CL, et al. Splenectomy correlates with increased risk of pyogenic liver abscess: a nationwide cohort study in Taiwan. J Epidemiol 2015; 25:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NHIR. Database. Taiwan: NHIR. http://nhird.nhri.org.tw/en/index.html (cited in April 1, 2016, English version). [Google Scholar]
- 19.Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore) 2010; 89:295–299. [DOI] [PubMed] [Google Scholar]
- 20.Hung SC, Liao KF, Lai SW, et al. Risk factors associated with symptomatic cholelithiasis in Taiwan: a population-based study. BMC Gastroenterol 2011; 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai SW, Muo CH, Liao KF, et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011; 106:1697–1704. [DOI] [PubMed] [Google Scholar]
- 22.Chen HY, Lai SW, Muo CH, et al. Ethambutol-induced optic neuropathy: a nationwide population-based study from Taiwan. Br J Ophthalmol 2012; 96:1368–1371. [DOI] [PubMed] [Google Scholar]
- 23.Cheng KC, Chen YL, Lai SW, et al. Patients with chronic kidney disease are at an elevated risk of dementia: a population-based cohort study in Taiwan. BMC Nephrol 2012; 13:1471–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng KC, Chen YL, Lai SW, et al. Risk of esophagus cancer in diabetes mellitus: a population-based case-control study in Taiwan. BMC Gastroenterol 2012; 12:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YL, Cheng KC, Lai SW, et al. Diabetes and risk of subsequent gastric cancer: a population-based cohort study in Taiwan. Gastric Cancer 2013; 16:389–396. [DOI] [PubMed] [Google Scholar]
- 26.Lai HC, Chang SN, Lin CC, et al. Does diabetes mellitus with or without gallstones increase the risk of gallbladder cancer? Results from a population-based cohort study. J Gastroenterol 2013; 48:856–865. [DOI] [PubMed] [Google Scholar]
- 27.Hung SC, Lai SW, Tsai PY, et al. Synergistic interaction of benign prostatic hyperplasia and prostatitis on prostate cancer risk. Br J Cancer 2013; 108:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai HC, Tsai IJ, Chen PC, et al. Gallstones, a cholecystectomy, chronic pancreatitis, and the risk of subsequent pancreatic cancer in diabetic patients: a population-based cohort study. J Gastroenterol 2013; 48:721–727. [DOI] [PubMed] [Google Scholar]
- 29.Lai HC, Lin CC, Cheng KS, et al. Increased incidence of gastrointestinal cancers among patients with pyogenic liver abscess: a population-based cohort study. Gastroenterology 2014; 146:e1129–e1137. [DOI] [PubMed] [Google Scholar]
- 30.Kuo SC, Lai SW, Hung HC, et al. Association between comorbidities and dementia in diabetes mellitus patients: population-based retrospective cohort study. J Diabetes Complications 2015; 29:1071–1076. [DOI] [PubMed] [Google Scholar]
- 31.Yang SP, Muo CH, Wang IK, et al. Risk of type 2 diabetes mellitus in female breast cancer patients treated with morphine: a retrospective population-based time-dependent cohort study. Diabetes Res Clin Pract 2015; 110:285–290. [DOI] [PubMed] [Google Scholar]
- 32.Lin JN, Lin CL, Lin MC, et al. Pyogenic liver abscess in patients with inflammatory bowel disease: a nationwide cohort study. Liver Int 2016; 36:136–144. [DOI] [PubMed] [Google Scholar]
- 33.Lai SW, Liao KF, Lai HC, et al. Proton pump inhibitors and risk of hepatocellular carcinoma: a case-control study in Taiwan. Acta Gastroenterol Belg 2013; 76:348–350. [PubMed] [Google Scholar]
- 34.Lai SW, Lin CL, Liao KF, et al. No association between depression and risk of hepatocellular carcinoma in older people in Taiwan. ISRN Psychiatry 2013; 2013:901987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai SW, Liu JC, Tseng CH, et al. Chronic osteomyelitis and hepatocellular carcinoma: an observation in Taiwan. Kuwait Med J 2013; 45:159–160. [Google Scholar]
- 36.Liao K-F, Lin C-L, Lai S-W. Schizophrenia correlates with increased risk of hepatocellular carcinoma in men: a cohort study in Taiwan. Int Med J 2015; 22:273–276. [Google Scholar]
- 37.Liao K-F, Lin C-L, Lai S-W, et al. Parkinson's disease and risk of pancreatic cancer: a population-based case-control study in Taiwan. Neurol Asia 2015; 20:251–255. [Google Scholar]
- 38.Liao KF, Lin CL, Lai SW, et al. Zolpidem use associated with increased risk of pyogenic liver abscess: a case-control study in Taiwan. Medicine (Baltimore) 2015; 94:e1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai SW, Lin CL, Liao KF. Atrial fibrillation associated with acute pancreatitis: a retrospective cohort study in Taiwan. J Hepatobiliary Pancreat Sci 2016; 23:242–247. [DOI] [PubMed] [Google Scholar]
- 40.Lai SW, Chen PC, Liao KF, et al. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol 2012; 107:46–52. [DOI] [PubMed] [Google Scholar]


