Supplemental Digital Content is available in the text
Keywords: erectile dysfunction, nationwide population-based cohort study, osteoporosis
Abstract
In this study, we aimed to investigate the risk of osteoporosis in patients with erectile dysfunction (ED) by analyzing data from the Taiwan National Health Insurance Research Database (NHIRD). From the Taiwan NHIRD, we analyzed data on 4460 patients aged ≥40 years diagnosed with ED between 1996 and 2010. In total, 17,480 age-matched patients without ED in a 1:4 ratio were randomly selected as the non-ED group. The relationship between ED and the risk of osteoporosis was estimated using Cox proportional hazard regression models. During the follow-up period, 264 patients with ED (5.92%) and 651 patients without ED (3.65%) developed osteoporosis. The overall incidence of osteoporosis was 3.04-fold higher in the ED group than in the non-ED group (9.74 vs 2.47 per 1000 person-years) after controlling for covariates. Compared with patients without ED, patients with psychogenic and organic ED were 3.19- and 3.03-fold more likely to develop osteoporosis. Our results indicate that patients with a history of ED, particularly younger men, had a high risk of osteoporosis. Patients with ED should be examined for bone mineral density, and men with osteoporosis should be evaluated for ED.
1. Introduction
Erectile dysfunction (ED) is a neurovascular process dependent on the vascular health of the erectile tissue and the health of the central and peripheral nervous systems.[1] Thus, changes or alterations in the fibroelastic properties of the neural, vascular, and erectile tissue can cause. ED is defined as the inability to attain and maintain adequate erection for satisfactory sexual intercourse.[2] ED is the most common sexual problem in men, often causing serious distress and prompting them to seek medical attention. ED primarily affects men over 40 years of age.[3] Various medical, psychological, environmental, and lifestyle factors, such as cardiovascular disease (CVD), diabetes mellitus (DM), hyperlipidemia, hypertension, chronic kidney disease (CKD), metabolic syndrome, and psychological distress, have been suggested to contribute to the development of ED.[3–10] Osteoporosis has similar risk factors; hence, we postulate that osteoporosis and ED are related.
Osteoporosis is a systemic metabolic bone disease characterized by impaired bone strength caused by attenuated bone mineral density and compromised bone quality, which exposes patients to fragility fractures.[11] Previously, osteoporosis was generally considered to affect postmenopausal women; however, substantial bone loss occurs equally in men.[11] Osteoporosis and ED significantly affect the quality of life in men. The relationship between osteoporosis and ED has attracted attention since 2005. Keles et al[12] attempted to clarify a possible relationship between osteoporosis and ED; they reported that the frequencies of osteoporosis and ED increased with age; however, these conditions were independent of each other, and hormonal changes were not the major determinants for both conditions in elderly men. Recently, Dursun et al[13] investigated the relationship between ED and osteoporosis in 95 men with ED and 82 men with normal sexual function and reported that the men with ED had low bone mineral density and were at higher risk of osteoporosis than were their healthy counterparts. However, both these studies have a limited scope because they involved a small sample. Therefore, we conducted a nationwide population-based retrospective cohort study by using data from the Taiwan National Health Insurance (NHI) program database to clarify the relationship between ED and the subsequent risk of osteoporosis.
2. Methods
2.1. Database
The NHI is a mandatory single-payer health insurance program launched on March 1, 1995, by the Bureau of National Health Insurance (BNHI), covering approximately 99% of the 23.74 million residents of Taiwan. The National Health Research Institute is responsible for establishing the National Health Insurance Research Database (NHIRD), an encrypted secondary database, for medical research. This database contains administrative and health claims data collected through the NHI program, including comprehensive information on inpatient and ambulatory care and prescriptions dispensed at contracted pharmacies. The NHIRD provides researchers scrambled identification numbers associated with relevant claims information, including records of patient's sex, date of birth, registry of medical services, and medication prescriptions. In this study, we examined the ambulatory and inpatients care data in the Longitudinal Health Insurance Database 2010 (LHID 2010) between 1996 and 2010 for analysis and comparisons. The LHID 2010, a randomly sampled subset of the NHIRD, contains data on 1,000,000 beneficiaries during the period of January 1, 2010 to December 31, 2010. The database has a large sample size, thus facilitating the study of the risk of osteoporosis among ED patients. In this study, diseases were identified and classified according to the diagnostic codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
2.2. Ethical approval
The study was conducted in accordance with the Declaration of Helsinki guidelines and was evaluated and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT (I)-20150032).
2.3. Study population
Our ED (psychogenic and organic ED, ICD-9-CM 302.72 and 607.84)[14,15] identification criteria was similar to those of similar studies[16–19] and are hence valid. To ensure the accuracy of the diagnostic data, we included only those patients receiving ≥2 diagnoses during ambulatory visits or ≥1 diagnosis in inpatient care according to the ICD-9-CM codes assigned exclusively by urologists. The date of first clinical visit for ED was considered the index date. To maximize accuracy, we only included cases if they received ≥2 osteoporosis diagnoses for ambulatory visits or ≥1 diagnosis in inpatient care, and the ICD-9 code was assigned by orthopedists and receiving bone mineral density examination.[20,21] Patients with previous osteoporosis (ICD-9-CM 733),[20,21] female patients, those with missing information, and those aged <40 years were excluded. The ratio of ED to non-ED patients was maintained at 1:4 for enhancing the power of statistical tests and ensuring an adequate number of patients with osteoporosis for performing stratified analyses. The patients in the non-ED group were selected using a simple random sampling method, in which 4 insured NHI beneficiaries without ED were randomly selected and frequency matched for age and index year (year of ED diagnosis) with each patient diagnosed with ED in the same period; thus, 17,480 non-ED patients were identified.
2.4. Outcome and comorbidities
The patients in the ED and non-ED groups were followed until they were diagnosed with osteoporosis, withdrawal from insurance or end of follow-up. Baseline comorbidities (ICD-9-CM codes are provided in Supplementary Table S1)[15,22–25] were hypertension, DM, hyperlipidemia, CKD, chronic liver disease, chronic pulmonary disease, stroke, hyperthyroidism, and hyperparathyroidism before the index date. The Charlson comorbidity index (CCI) scores, categorized into 4 levels (0, 1–2, 3–4 and ≥5), were used for assessing the severity of comorbidities. Myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild, moderate, and severe liver disease, diabetes with and without chronic complications, hemiplegia and paraplegia, renal disease, malignancy (including lymphoma and leukemia, except skin malignancy), metastatic solid tumors, human immunodeficiency virus infection, and acquired immune deficiency syndrome were the comorbidities included in the analysis. In addition, the use of oral corticosteroids or testosterone was analyzed.
2.5. Statistical analyses
The distributions of categorical and clinical variables between the ED and non-ED groups were compared using the Chi-square test. The paired t test and Wilcoxon rank-sum test were used for examining the differences in the mean age and follow-up period (years) between the 2 cohorts, as appropriately. Kaplan–Meier curves were used for estimating the cumulative incidence, and the differences between the curves were tested using 2-tailed log-rank tests. The survival period was calculated for patients with ED until a hospitalization event, an ambulatory visit for osteoporosis, or the end of the study (December 31, 2010), whichever occurred first. Osteoporosis incidence rates were estimated (in 1000 person-years) for both groups and compared. Univariable and multivariable Cox proportional hazard regression models were used for estimating the hazard ratios (HRs) and 95% confidence intervals (CIs) for osteoporosis if the proportional hazards assumption was satisfied. The multivariable Cox models were employed after controlling for age, CCI scores, and relevant comorbidities. A 2-tailed P < 0.05 was considered statistically significant. All data processing and statistical analyses were performed using Statistical Analysis Soft ware version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Baseline characteristics of patients with and without ED
We enrolled 4460 men aged ≥40 years diagnosed with ED between 1996 and 2010 in our study cohort (Fig. 1).
Figure 1.
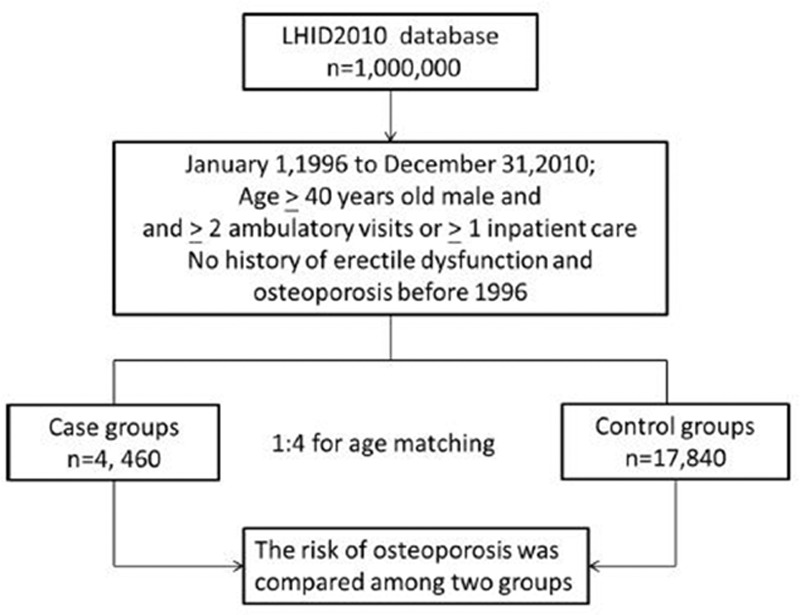
Flow diagram of the present study from the National Health Insurance Research Database. LHID = Longitudinal Health Insurance Database.
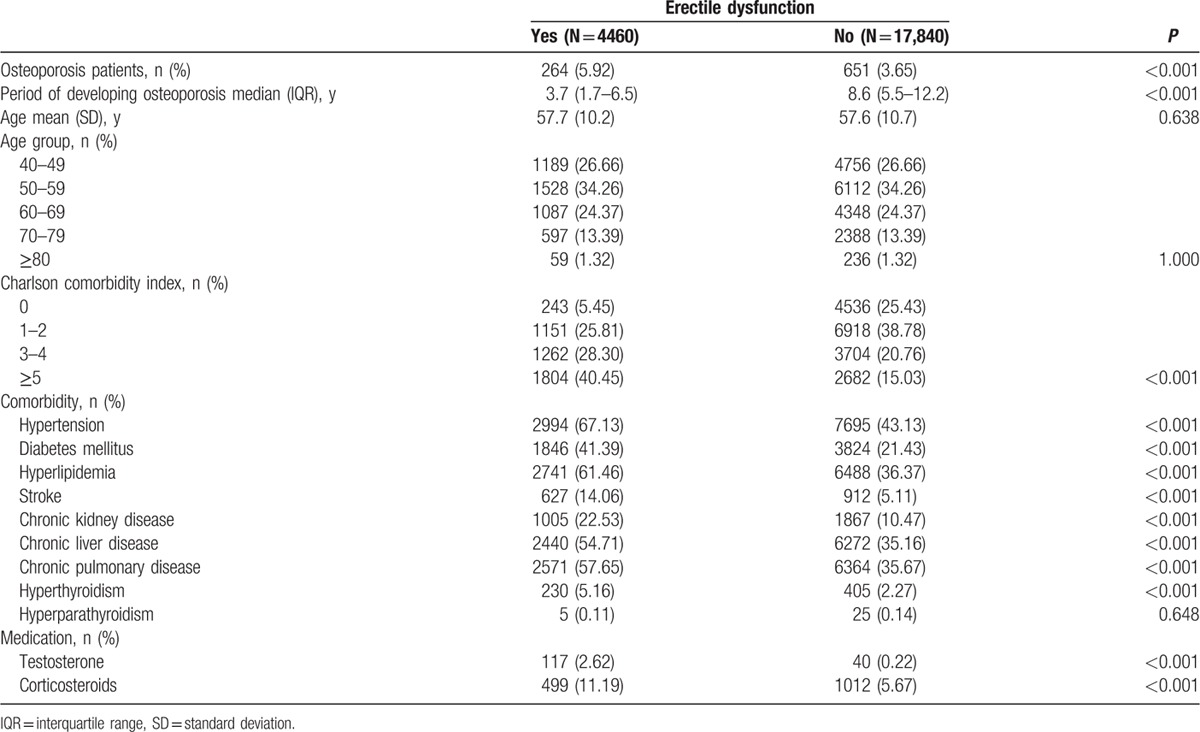
The baseline demographic characteristics and comorbidity statuses in the ED and non-ED groups are presented in Table 1. The mean age was57.6 ± 10.7 and 57.7 ± 10.2 years in the non-ED and ED cohorts, respectively. Most patients were in the age group of 50 to 59 years (34.26%) and 40 to 49 years (26.66%). The patients in the ED group were more likely to develop comorbidities than were those in the non-ED group. The comorbidities assessed were hypertension (67.13 vs 43.13, P < 0.001), DM (41.39 vs 21.43, P < 0.001), hyperlipidemia (61.46 vs 36.37, P < 0.001), stroke (14.06 vs 5.11, P < 0.001), CKD (22.53 vs 10.47, P < 0.001), chronic liver disease (54.71 vs 35.16, P < 0.001), chronic pulmonary disease (57.65 vs 35.67, P < 0.001), and hyperthyroidism (5.16 vs 2.27, P < 0.001). The CCI scores were higher in the ED group than in the non-ED group (40.5 vs 15.03, P < 0.001). Moreover, testosterone (2.62 vs 0.22, P < 0.001) and corticosteroid (11.19 vs 5.67, P < 0.001) use was higher in the ED group than in the non-ED group. A total 264 of 4460 patients with ED (5.92%) and 651 of 17,840 patients without ED (3.65%) were diagnosed with osteoporosis during a median observation time of 3.7 and 8.6 years, respectively (interquartile range = 1.7–6.5 and 5.5–12.2, respectively). Thus, the incidence of osteoporosis was significantly higher in the ED group than in the non-ED group (P < 0.001). Osteoporosis development was significantly faster in the ED group (3.7 years) than in the non-ED group (8.6 years) for the respective observation periods.
Table 1.
Baseline characteristics of patients with and without erectile dysfunction.
3.2. Incidence and risk of osteoporosis
The incidence densities and HRs of osteoporosis for different age groups and follow-up durations are presented in Table 2. During the follow-up period, 264 patients with ED (5.92%) and 651 patients without ED (3.65%) developed osteoporosis. The overall incidence of osteoporosis was 3.04-fold higher in the ED group than in the non-ED group (9.74 vs 2.47 per 1000 person-years, respectively) after controlling for age, CCI scores, related comorbidities of hypertension, DM, hyperlipidemia, stroke, CKD, chronic liver disease, chronic pulmonary disease, and hyperthyroidism, hyperparathyroidism, and testosterone and corticosteroid use.
Table 2.
Incidence and hazard ratios of osteoporosis among patients with or without erectile dysfunction stratified by age, and follow-up duration.
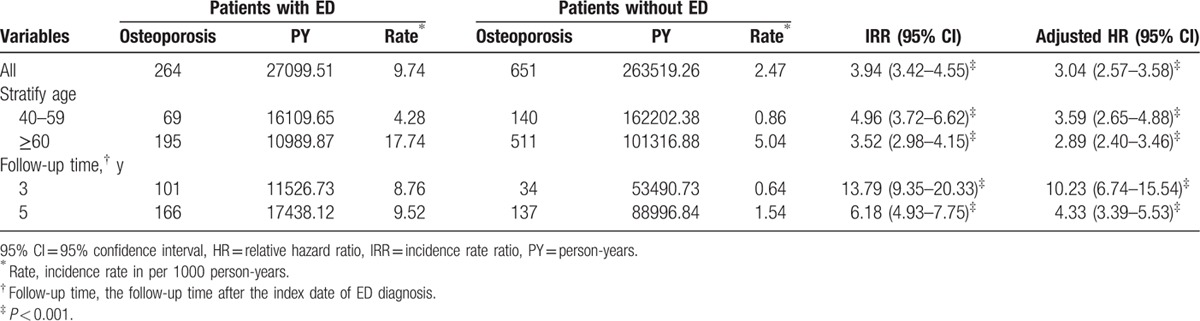
In addition, the incidence of osteoporosis was consistently higher in the ED group for all age groups, and the incidence rate increased with age. However, younger patients were at a significantly higher risk than were older patients (HR = 3.59, 95% CI = 2.65–4.88, P < 0.001).
The follow-up duration analysis revealed a significant relationship between the ED and non-ED groups. The incidence of osteoporosis in the ED groups remained increased compared to the non-ED groups in all the follow-up durations.
The Kaplan–Meier curve of the cumulative incidence of osteoporosis in patients with and without ED after a 15-year follow-up is shown in Fig. 2. The 1-, 5-, 10-, and 15-year actual osteoporosis rates were 0.915%, 4.670%, 8.170%, 12.030% and 0.006%, 0.768%, 2.210%, 3.650% in the ED and non-ED groups, respectively.
Figure 2.
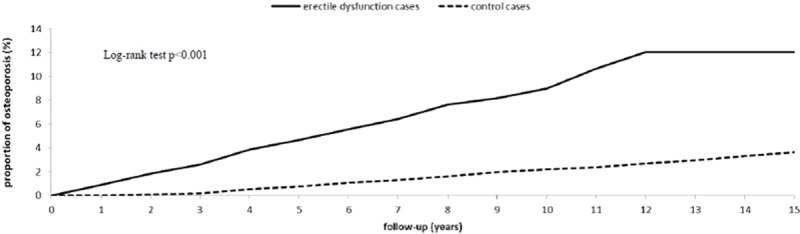
Cumulative incidence of osteoporosis among ED (solid line) and non-ED (dashed line) cohorts. ED = erectile dysfunction.
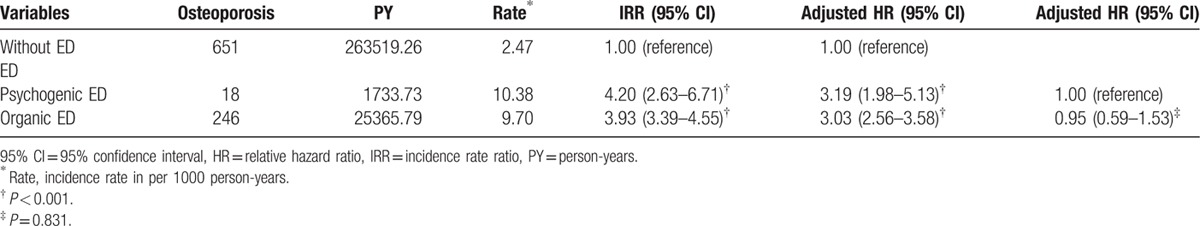
The relationships between the types of ED and the associated relative risks and HRs of osteoporosis are presented in Table 3. Compared with patients without ED, patients with psychogenic and organic ED were 3.19- and 3.03-fold more likely to develop osteoporosis (95% CI: 1.98–5.13 and 2.56–3.58, respectively); however, no significant differences were observed between the 2 groups.
Table 3.
Incidence and hazard ratios of osteoporosis in patients with different types of erectile dysfunction.
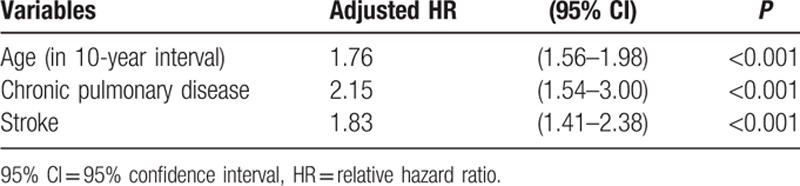
The multivariate Cox regression analysis presented in Table 4 revealed age, chronic pulmonary disease, and stroke as the 3 risk factors for osteoporosis in the ED group.
Table 4.
Cox regression model of significant predictors of osteoporosis in erectile dysfunction.
4. Discussion
In this study, we found a relationship between ED and osteoporosis which persisted even after adjustment for comorbidities. To the best of our understanding, this is the first nationwide population-based study investigating the relationship between ED and subsequent osteoporosis in an Asian population. During the follow-up period, we observed that 264 (5.92%) patients with ED and 651 (3.65%) patients without ED developed osteoporosis. After controlling for potential confounding factors, the risk of osteoporosis was 3.04-fold higher in the ED group than in the non-ED group. Psychogenic and organic ED were associated with osteoporosis when the relative risks and HRs were estimated. Patients with ED, particularly those in the age group of 40 to 59 years, exhibited a high risk of osteoporosis.
The mechanisms underlying the relationship between ED and osteoporosis are likely to be complex; some possible explanations are as follows. First, patients with ED have lower naturally available free testosterone than those without ED.[26] Androgens may play a critical role in the regulation of bone formation in men.[27] Reports have highlighted a marked increase in the risk of fragility fractures among patients with low testosterone levels.[28,29] In addition, androgen deprivation therapy and orchiectomy has been associated with an increased risk of osteoporosis and fractures.[30] Therefore, testosterone depletion might increase the risk of osteoporosis. ED has been highly associated with inflammation. Inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha, can substantially damage the endothelium in the systemic vascular circulation and in the peripheral vascular bed of organs, such as the penis, thus contributing to endothelial dysfunction, which subsequently leads to ED.[31] Furthermore, these cytokines may inhibit osteoblast growth, thus causing osteoporosis.[32,33]
Nitric oxide bioactivity is crucial to penile engorgement and is a major pathogenic mechanism underlying ED; therefore, nitric oxide is a possible explanation for the relationship between ED and osteoporosis. In addition, nitric oxide may affect bone metabolism through osteoblastic activity.[34,35] High concentrations of nitric oxide can directly inhibit osteoclast proliferation and bone resorption, suggesting its role in bone remodeling.[36,37]
The role of endothelial function in erectile physiology is well established. Any factor that contributes to endothelial dysfunction certainly contributes significantly to ED. Furthermore, penile vascular hemodynamics depend on the integrity of the vascular bed.[31] Moreover, endothelial dysfunction is indicative of early-stage atherosclerosis; however, this condition is also considered an essential regulator of vascular and bone health.[38] Patients with osteoporosis are more likely to have CVD and coronary microvascular endothelial dysfunction.[39–42] Endothelial dysfunction may be a common precursor for osteoporosis and ED, potentially explaining the relationship between the 2 conditions. Furthermore, ED has been highly associated with vitamin D deficiency. Low vitamin D levels might increase ED risk by promoting endothelial dysfunction. Endothelial dysfunction is critical in ED pathogenesis, and vitamin D deficiency is considered to promote endothelial dysfunctions.[43] Furthermore, vitamin D plays a major role in maintaining the bone health in people of all age groups. Lower vitamin D levels lead to substantial losses in the bone mass, eventually causing osteoporosis.[44] Another report with 267 patients with hip fractures (mean age, 80.3 years) reported that the serum vitamin D levels at admission of 67% of these patients were <25 ng/mL. These results assert the high frequency of vitamin D deficiency in men with osteoporosis.[45]
Traditional ED risk factors, such as DM, hypertension, and dyslipidemia, are known predictors of osteoporosis. ED and osteoporosis share similar risk factors; hence, unsurprisingly, an ED diagnosis increases the risk for osteoporosis, according to our results. The relationship between ED and osteoporosis may in part be caused by these comorbidities; however, these comorbidities do not account for the complete relationship between ED and osteoporosis.
The strength of our study lies in the use of a large population-based dataset, and our results confirm that ED is associated with an increased risk of osteoporosis. However, several limitations must be considered when interpreting these findings. The diagnoses were based on the ICD-9-CM codes, the accuracy of which depends on the performance of the clinical physicians; hence, verifying data could not be easily achieved. However, diagnostic accuracy was enhanced by limiting the study population to patients who had received medical care on ≥2 separate visits. Besides, medical experts of the BNHI conduct regular scrutinization to ensure the accuracy of diagnostic codes used in the dataset. Physicians are motivated to enter diagnostic codes accurately for that they are subject to large fines for incorrect entries. Furthermore, the NHIRD data have been used for various studies for several years.[14,15,20–25] Second, the NHIRD does not contain detailed information regarding such risk factors as body mass index, exercise capacity, dietary habits, alcohol consumption, and smoking, which potentially compromises our findings.[3,4] Discussion regarding impotence remains a sensitive issue in Taiwan. Patients with ED may not visit a specialist but may seek alternative medicine or receive treatment privately, possibly underestimating the real incidence of ED. In addition, imaging results, environmental exposure, and laboratory data are not documented in the database. Moreover, most inhabitants of Taiwan are of Chinese ethnicity; it is uncertain whether our results can be generalized to other ethnic populations. Finally, statistical significance does not always represent clinical significance. Without knowing detailed individual records of the aforementioned data, population-based studies cannot directly clarify the exact relationship between ED and osteoporosis. Additional clinical trials are necessary to confirm the underlying mechanisms of this relationship.
In conclusion, an increased risk of osteoporosis was observed among patients with ED, particularly among younger males. ED can be considered an early predictor of osteoporosis. Additional studies are required to gather in-depth information and explore the mechanisms underlying these relationships. Physicians should be aware of this relationship for early identifying such groups of patients. Because of the easy and noninvasive evaluation of osteoporosis, patients with ED should be examined for bone mineral density, and men with osteoporosis should be evaluated for ED.
Supplementary Material
Footnotes
Abbreviations: BNHI = Bureau of National Health Insurance, CCI = Charlson comorbidity index, CI = confidence interval, CKD = chronic kidney disease, CVD = cardiovascular disease, DM = diabetes mellitus, ED = erectile dysfunction, HR = hazard ratio, ICD-9-CM = International Classification of Disease, Ninth Revision, Clinical Modification, IL = interleukin, LHID 2010 = Longitudinal Health Insurance Database 2010, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007; 52:54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consensus development conference statement. National Institutes of Health. Impotence. December 7–9, 1992. Int J Impot Res 1993; 5:181–284. [PubMed] [Google Scholar]
- 3.Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013; 381:153–165. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med 2010; 7:1598–1607. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JY, Ng EM, Chen RY, et al. Prevalence of erectile dysfunction in Asian populations: a meta-analysis. Int J Impot Res 2007; 19:229–244. [DOI] [PubMed] [Google Scholar]
- 6.Quek KF, Sallam AA, Ng CH, et al. Prevalence of sexual problems and its association with social, psychological and physical factors among men in a Malaysian population: a cross-sectional study. J Sex Med 2008; 5:70–76. [DOI] [PubMed] [Google Scholar]
- 7.Navaneethan SD, Vecchio M, Johnson DW, et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: a meta-analysis of observational studies. Am J Kidney Dis 2010; 56:670–685. [DOI] [PubMed] [Google Scholar]
- 8.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med 2009; 6:1232–1247. [DOI] [PubMed] [Google Scholar]
- 9.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol 2004; 171:2341–2345. [DOI] [PubMed] [Google Scholar]
- 10.Ryu JK, Zhang LW, Jin HR, et al. Derangements in endothelial cell-to-cell junctions involved in the pathogenesis of hypercholesterolemia-induced erectile dysfunction. J Sex Med 2009; 6:1893–1907. [DOI] [PubMed] [Google Scholar]
- 11.Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. Am J Kidney Dis 2014; 64:290–304. [DOI] [PubMed] [Google Scholar]
- 12.Keles I, Aydin G, Orkun S, et al. Two clinical problems in elderly men: osteoporosis and erectile dysfunction. Arch Androl 2005; 51:177–184. [DOI] [PubMed] [Google Scholar]
- 13.Dursun M, Ozbek E, Otunctemur A, et al. Possible association between erectile dysfunction and osteoporosis in men. Prague Med Rep 2015; 116:24–30. [DOI] [PubMed] [Google Scholar]
- 14.Shen TC, Chen WC, Lin CL, et al. The risk of erectile dysfunction in chronic obstructive pulmonary disease: a population-based cohort study in Taiwan. Medicine (Baltimore) 2015; 94:e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CC, Su JS, Yeh HZ, et al. Association between colonic diverticulosis and erectile dysfunction: a nationwide population-based study. Medicine (Baltimore) 2015; 94:e2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung SD, Chen YK, Lin HC, et al. Increased risk of stroke among men with erectile dysfunction: a nationwide population-based study. J Sex Med 2011; 8:240–246. [DOI] [PubMed] [Google Scholar]
- 17.Huang CY, Keller JJ, Sheu JJ, et al. Migraine and erectile dysfunction: evidence from a population-based case-control study. Cephalalgia 2012; 32:366–372. [DOI] [PubMed] [Google Scholar]
- 18.Keller JJ, Lin HC. A population-based study on the association between rheumatoid arthritis and erectile dysfunction. Ann Rheum Dis 2012; 71:1102–1103. [DOI] [PubMed] [Google Scholar]
- 19.Keller JJ, Chen YK, Lin HC. Varicocele is associated with erectile dysfunction: a population-based case-control study. J Sex Med 2012; 9:1745–1752. [DOI] [PubMed] [Google Scholar]
- 20.Lin TH, Lung CC, Su HP, et al. Association between periodontal disease and osteoporosis by gender: a nationwide population-based cohort study. Medicine (Baltimore) 2015; 94:e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CH, Lin CL, Kao CH. Relation between hepatitis C virus exposure and risk of osteoporosis: a nationwide population-based study. Medicine (Baltimore) 2015; 94:e2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CI, Li TC, Liu CS, et al. Extreme values of hemoglobin a1c are associated with increased risks of chronic obstructive pulmonary disease in patients with type 2 diabetes: a competing risk analysis in national cohort of Taiwan diabetes study. Medicine (Baltimore) 2015; 94:e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang TY, Kuo HT, Chen HJ, et al. Increased risk of chronic fatigue syndrome following atopy: a population-based study. Medicine (Baltimore) 2015; 94:e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai SW, Lin CL, Liao KF. Atorvastatin use associated with acute pancreatitis: a case-control study in Taiwan. Medicine (Baltimore) 2016; 95:e2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun LM, Kuo HT, Jeng LB, et al. Hypertension and subsequent genitourinary and gynecologic cancers risk: a population-based cohort study. Medicine (Baltimore) 2015; 94:e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor D, Clarke S, Channer KS, et al. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl 2007; 30:500–507. [DOI] [PubMed] [Google Scholar]
- 27.Leder BZ, LeBlanc KM, Schoenfeld DA, et al. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab 2003; 88:204–210. [DOI] [PubMed] [Google Scholar]
- 28.Abbasi AA, Rudman D, Wilson CR, et al. Observations on nursing home residents with a history of hip fracture. Am J Med Sci 1995; 310:229–234. [PubMed] [Google Scholar]
- 29.Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med 2008; 168:47–54. [DOI] [PubMed] [Google Scholar]
- 30.Wu CT, Yang YH, Chen PC, et al. Androgen deprivation increases the risk of fracture in prostate cancer patients: a population-based study in Chinese patients. Osteoporos Int 2015; 26:2281–2290. [DOI] [PubMed] [Google Scholar]
- 31.Traish AM, Feeley RJ, Guay A. Mechanisms of obesity and related pathologies: androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J 2009; 276:5755–5767. [DOI] [PubMed] [Google Scholar]
- 32.Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 1992; 257:88–91. [DOI] [PubMed] [Google Scholar]
- 33.Ammann P, Rizzoli R, Bonjour JP, et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest 1997; 99:1699–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastell R, Newman C, Crossman DC. Cardiovascular disease and bone. Arch Biochem Biophys 2010; 503:78–83. [DOI] [PubMed] [Google Scholar]
- 35.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 2010; 31:266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacIntyre I, Zaidi M, Alam AS, et al. Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A 1991; 88:2936–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralston SH, Todd D, Helfrich M, et al. Human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology 1994; 135:330–336. [DOI] [PubMed] [Google Scholar]
- 38.Gossl M, Khosla S, Zhang X, et al. Role of circulating osteogenic progenitor cells in calcific aortic stenosis. J Am Coll Cardiol 2012; 60:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt MT, Cauley JA, Kuller LH, et al. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res 1997; 12:283–289. [DOI] [PubMed] [Google Scholar]
- 40.Frye MA, Melton LJ, Bryant SC, III, et al. Osteoporosis and calcification of the aorta. Bone Miner 1992; 19:185–194. [DOI] [PubMed] [Google Scholar]
- 41.Warburton DE, Nicol CW, Gatto SN, et al. Cardiovascular disease and osteoporosis: balancing risk management. Vasc Health Risk Manag 2007; 3:673–689. [PMC free article] [PubMed] [Google Scholar]
- 42.Sinnott B, Syed I, Sevrukov A, et al. Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int 2006; 78:195–202. [DOI] [PubMed] [Google Scholar]
- 43.Barassi A, Pezzilli R, Colpi GM, et al. Vitamin D and erectile dysfunction. J Sex Med 2014; 11:2792–2800. [DOI] [PubMed] [Google Scholar]
- 44.Peterlik M, Boonen S, Cross HS, et al. Vitamin D and calcium insufficiency-related chronic diseases: an emerging world-wide public health problem. Int J Environ Res Public Health 2009; 6:2585–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrera A, Lobo-Escolar A, Mateo J, et al. Male osteoporosis: a review. World J Orthop 2012; 3:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


