Abstract
Life-threatening refractory metabolic acidosis due to starvation ketoacidosis is rarely reported, even among nondiabetic pregnant women, and may be overlooked. Furthermore, stressful situations may increase the acidosis severity.
In the present case, a nondiabetic multiparous woman was admitted for a near-fatal asthma attack and vomiting during the third trimester of pregnancy. She was intubated and rapidly developed high anion gap metabolic acidosis. We diagnosed the patient with starvation ketoacidosis based on vomiting with concomitant periods of stress during pregnancy and the absence of other causes of high anion gap metabolic acidosis. She responded poorly to standard treatment, although the ketoacidosis and asthma promptly resolved after an emergency caesarean section. The patient and her baby were safely discharged.
Short-term starvation, if it occurs during periods of stress and medication, can result in life-threatening ketoacidosis, even among nondiabetic women during the third trimester of pregnancy. Awareness of this condition may facilitate prompt recognition and proactive treatment for dietary and stress control, and emergent interventions may also improve outcomes.
Keywords: accelerated starvation, emergent delivery, near-fatal asthma, nondiabetic ketoacidosis, pregnancy, short-term starvation
1. Introduction
Severe nondiabetic ketoacidosis due to acute starvation is a rare event, even among pregnant women, and occurrence in patients with near-fatal asthma as a comorbidity is even rarer. Therefore, we report a unique case of ketoacidosis that was induced by short-term starvation and a near-fatal asthma attack in a nondiabetic pregnant woman during her third trimester of pregnancy. Only 10 similar cases have been reported,[1–7] and the present case was the most challenging to treat due to life-threatening events (acidosis and asthma) that usually require potentially conflicting treatments. In this case, placenta-derived hormones, fuel homeostasis during starvation, stress during pregnancy, and pharmacological effects all likely contributed to this event.[4] Although the development of acidosis has adverse effects on the mother and infant, we achieved rapid acidosis reversal and a favorable outcome via timely caesarean delivery.
2. Case report
A 36-year-old woman presented to our emergency room at 34 weeks of gestation, due to worsening dyspnea and a 2-day history of severe vomiting with poor oral intake. She had been diagnosed with bronchial asthma at the age of 15 years and had experienced 2 episodes of near-fatal asthma. Due to a misperception regarding the effects of asthma medications during pregnancy, she had not taken her asthma medication since becoming pregnant. However, 1 week before this hospitalization, she experienced an increase in asthma attack frequency, resulting in frequent use of a rescue medication. She denied having a history of smoking, alcohol consumption, or illicit drug usage, and her outpatient labor surveillance, which included glucose tolerance testing, did not reveal any abnormalities.
At presentation, she was in clear consciousness but appeared agitated, with tachypnea, inspiratory and expiratory wheezing, and Kussmaul breathing. Her pulse was 138 beats/min, her respiratory rate was 25 breaths/min, her blood pressure was 126/78 mm Hg, and her temperature was 37°C. She was 1.65 m tall and weighed 72.2 kg. Initial arterial blood gas analysis (with an O2 mask) revealed mixed respiratory alkalosis, metabolic acidosis, and no hypoxia (Table 1). Based on our assumption of an asthma attack, we administered supplemental oxygen, a high-dose short-acting beta-agonist with ipratropium via a nebulizer, and intravenous steroids although her condition continued to deteriorate, and reassessment revealed a high respiratory rate and accessory muscle use. Thus, she was intubated 2 hours after admission to ER to prevent further maternal fatigue or deterioration of oxygenation. Initial postintubation ventilator settings were pressure-control ventilation mode, a pressure level of 18 cm H2O, a respiratory rate of 15 breaths/min, PEEP at 5 cm H2O, FiO2 at 80%, and Ti (inflation time) of 1 s. She was hydrated with 0.9% saline at 100 mL/h due to a history of severe vomiting. Fetal viability was monitored via cardiotocography. Blood tests revealed slightly elevated C-reactive protein levels (2.61 mg/L) and a leucocyte count of 15.85 × 109/L. Her renal function, liver function, and creatinine kinase results were normal, and her random capillary blood glucose levels were 152 mg/dL, with normal glycosylated hemoglobin levels (4.8%). Urinalysis revealed 2+ acetone and no glycosuria or proteinuria.
Table 1.
Laboratory parameters (acid–base results) and clinical course.
The patient was admitted to the intensive care unit (ICU) ∼8 hours after intubation and initial management in the ER. We further adjusted the ventilator settings to pressure-support ventilation, with a pressure level of 16 cm H2O, PEEP at 8 cm H2O, and FiO2 at 50%, due to relatively acceptable oxygenation and clear consciousness. We continued to administer an inhaled short-acting beta-agonist, an inhaled corticosteroid (ICS; budesonide, 1 mg twice per day), and intravenous magnesium sulfate. However, the patient reported feeling breathless and still presented with deep and labored breathing. The ventilator flow waveform revealed dynamic hyperinflation with evidence of intrinsic end-expiratory pressure. Therefore, we tried to adjust ventilator settings to ameliorate dynamic hyperinflation by lowering tidal volumes (TV: 6–8 mL/kg) indexed to the patient's ideal body weight (IBW: 55.8 kg), per Devine's formulas[9] and sedated the patient with propofol to prevent rapid respiratory rates. Intermittent high TV (800–1000 mL) and high minute ventilation (10–12 L/min) were detected. However, we could only partially sedate the patient because she did not respond well to high-dose propofol, and the patient could not tolerate lower pressure support, as evidenced by labored breathing. We did not attempt to increase the dosage further because we were not sure of the safety of continuous high-dose propofol administration in pregnant patients.[10] Subsequent chest radiography revealed subcutaneous emphysema throughout the neck region and suspected pneumomediastum (Fig. 2).
Figure 2.
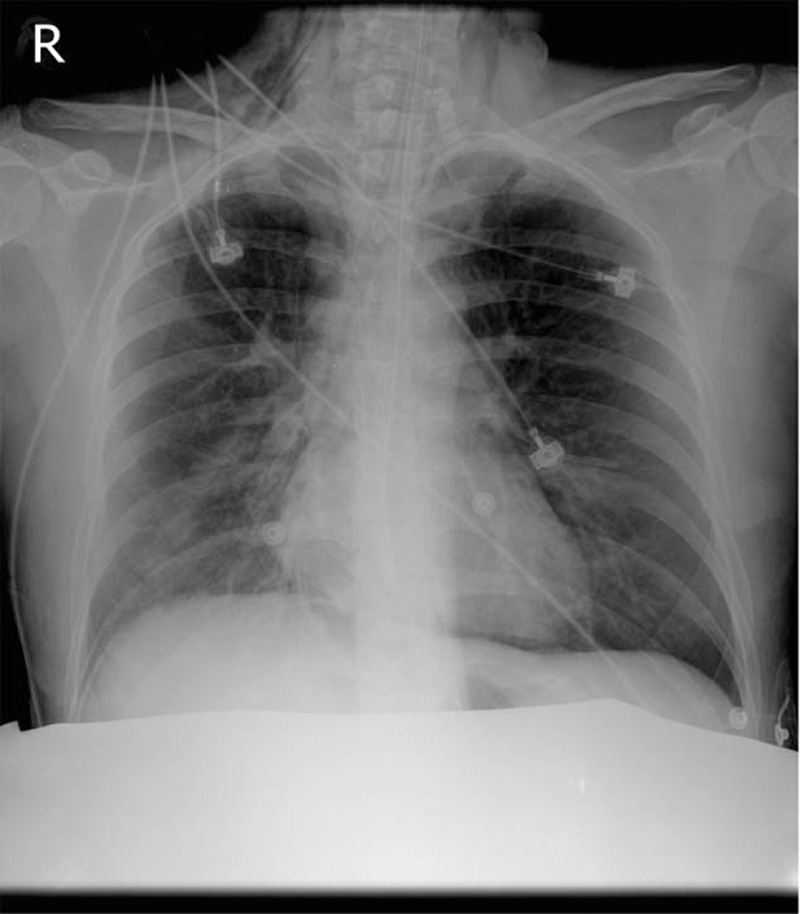
Plain chest radiography in the intensive care unit revealed subcutaneous emphysema over the neck region and suspected pneumomediastinum.
Follow-up arterial blood gas analysis revealed a rapid decrease in bicarbonate levels and an increased anion gap (AG) (Table 1, Fig. 1). We assumed that respiratory compensation for the worsening metabolic acidosis may have exacerbated her labored and deep breathing, which made it more difficult to control her asthma. Therefore, we tried to determine the source of acidosis. We concluded that lactic acidosis was unlikely, due to her normal serum lactate levels, and a sepsis work-up revealed no evidence of infection. Her serum ketone levels (3-β-hydroxybutyrate), pH, and bicarbonate levels were 3.7 mmol/L, 7.216, and 11.6 mmol/L, respectively at 29 hours (Table 1). Therefore, after excluding other possible explanations for the high-AG metabolic acidosis,[11] we diagnosed the patient with starvation ketoacidosis based on vomiting with concomitant periods of stress during her third trimester of pregnancy.
Figure 1.
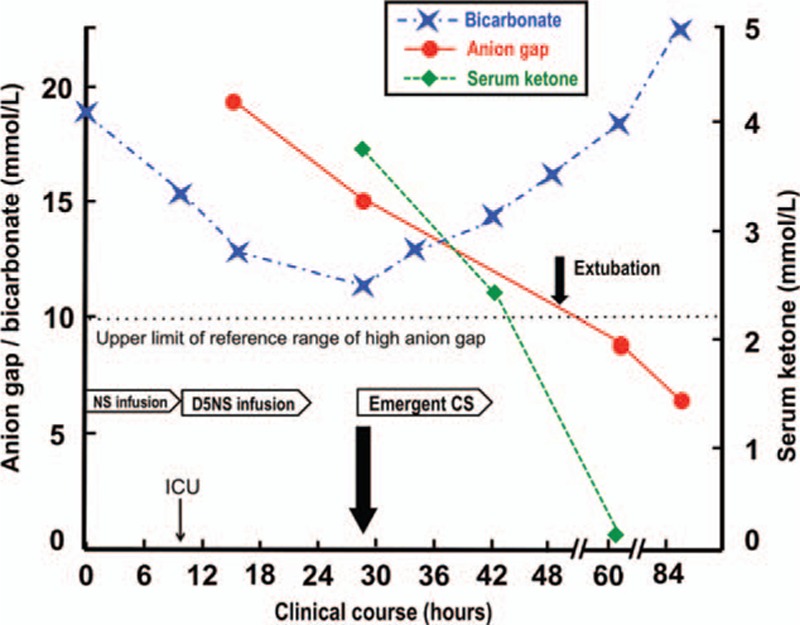
The relationship between the patient's clinical course and metabolic derangement. An emergency caesarean section kept the ketoacidosis (high anion gap metabolic acidosis with declining bicarbonate) from getting worse and made early extubation possible. D5NS: 5% dextrose in normal saline.
Although we hydrated the patient with 5% dextrose in normal saline [D5NS] at 125 mL/h after she was admitted to the ICU, it only partially resolved the starvation ketoacidosis. In addition, although aggressive treatment is indicated for an asthma attack, we were concerned with the possible pharmacologic acceleration effects that the beta-agonist and corticosteroid might have on ketoacidosis and the adverse effects of long-term high-dose propofol usage in pregnancy.[10] Due to the potential harmful effects of ketoacidosis and uncontrolled asthma, we performed an emergency caesarean section under general anesthesia after administering 2 doses of betamethasone (12 mg, 24 h apart) to prevent fetal respiratory distress syndrome. The patient delivered a 2.3-kg girl 29 h after admission. The umbilical cord arterial pH was 7.143, and the 5-min Apgar score was 9 although the child received intensive care for 2 days due to grade II respiratory distress syndrome. She was successfully extubated 20 hours post-delivery due to rapid resolution of the metabolic derangement (Table 1, Fig. 2). Subsequent panendoscopy revealed superficial gastritis although both the mother and the baby were ultimately discharged safely.
2.1. Ethical statement
Ethical approval was not necessary because the study was focused on the retrospective observation of a patient's hospital course, which in no way affected her treatment. Informed consent was obtained from the patient for publication of this case report.
3. Discussion
We describe a case of ketoacidosis that was induced by short-term starvation and a near-fatal asthma attack in a nondiabetic pregnant woman. We believe that this case is theoretically and practically relevant, as the woman initially did not respond to standard medical treatment although the ketoacidosis and asthma promptly resolved after an emergency caesarean section delivery.
The patient's initial presentation with uncontrolled asthma masked the severity of the development of high-AG metabolic derangement. Although we differentiated the possible causes of the high-AG metabolic acidosis based on the “GOLD MARK” mnemonic (glycols [ethylene and propylene], 5-oxoproline [pyroglutamic acid], l-lactate, d-lactate, methanol, aspirin, renal failure, rhabdomyolysis, and ketoacidosis).[11,12] The ultimate diagnosis of ketoacidosis was complicated by the patient being previously healthy, nonalcoholic, and nondiabetic, not having a history of precipitating factors (e.g., ketogenic diet or long-term fasting), not having previous ketoacidosis episodes prior to this pregnancy, and having normal levels of blood glucose and glycosylated hemoglobin during this episode. Furthermore, the initial arterial blood gas test pH appeared misleadingly normal, due to the concomitant metabolic alkalosis that results from pregnancy-related vomiting or respiratory alkalosis,[12] which obscured the severity of the acidosis.
In theory, short-term fasting only results in mild ketosis among healthy individuals, which reaches its maximum severity at ∼2 weeks. However, the effects of the ketosis can become more severe during pregnancy, due to pregnancy-related relative insulinopenia and insulin resistance,[4,13] which we initially overlooked. Therefore, we did not assess the metabolic derangement in a timely manner by checking chloride data and AG when she was initially admitted, and we merely focused on the management of asthma exacerbation. We knew that ketosis investigation is important in sick women with diabetes, including gestational diabetes, but we learned that it is also important in nondiabetic pregnant women who have ketonuria with concomitant periods of vomiting or stress, as in an asthma attack.
Our patient had severe vomiting and an inability to tolerate an oral diet; this resulted in a starvation state. Pregnant women experience “accelerated starvation,” particularly during their third trimesters. This state might indicate an adaptation to lipid metabolism because available glucose is directed toward the fetus.[4] This concept was initially described in 1970 by Felig and Lynch,[14] and it was further clarified by Metzger et al.[15] In addition, placenta-derived hormones augment ketoacidosis via the production of placental lactogen, cortisol, and glucagon, which increase gluconeogenesis, glycogenolysis, ketogenesis, and insulin resistance. As in the present case, levels of these glucose counter-regulatory hormones are increased during periods of stress,[4] which was likely the mechanism underlying this episode of ketoacidosis. Pharmacological effects also likely played an important role in the present case. Large systemic doses of β-agonists may increase levels of glucose, lactate, and free fatty acids in plasma to precipitate ketoacidosis. In addition, corticosteroids can also affect lipid metabolism and lead to ketogenesis.[16] Therefore, if tocolytics are necessary, β-adrenergic agonists should be avoided, and clinicians should consider ketoacidosis diagnoses for pregnant women who are treated using high-dose glucocorticoids and β-adrenergic agonists.
Among the 10 reported nondiabetic starvation ketoacidosis cases,[1–7] only 3 patients[5–7] experienced reversal of severe acidosis after being treated with a 5% or 10% dextrose infusion[6,7] or a fixed-dose insulin regimen,[5] and most patients required emergency delivery.[1–4] Those 3 cases focused on the ability of endogenous/exogenous insulin to suppress ketoacidosis, although dextrose infusion-related neonatal hyperinsulinemia and subsequent hypoglycemia were concerns.[6] Furthermore, the 10 reported cases did not involve severe comorbidities, which may indicate that early administration of dextrose-containing fluids or caloric supplementation may be beneficial in some cases, especially in the absence of severe comorbidities. However, we believe that most cases required emergency deliveries due to the counter-regulatory hormone effects dominating the effects of circulating insulin in these nondiabetic patients. Furthermore, there is no standard treatment for these patients. Thus, the proposed management strategy is early recognition of ketoacidosis by AG evaluation and ketone testing via reassessment in the critical care unit, regular assessment of fetal wellbeing, administration of dextrose-containing fluids, insulin therapy (if necessary), and delivery if the patient responds poorly or if fetal compromise is observed.[4] In this context, removal of the fetus and placenta via caesarean section eliminates the source of counter-regulatory hormones and blocks the vicious cycle of refractory ketoacidosis. Although a positive outcome was achieved in the present case, emergency delivery is associated with a high risk of adverse outcomes when the mother has severe ketoacidosis and the fetus is preterm. Therefore, prompt recognition of ketosis, followed by timely treatment (e.g., caloric supplementation), may help optimize the outcomes for both the mother and fetus. In addition, underlying causes of gastrointestinal symptoms, such as pancreatitis, contribute to nondiabetic ketoacidosis in pregnancy and thus should be identified and treated if possible.[17] Issues similar to nondiabetic ketoacidosis have also been noted for lactating women on ketogenic diets, which are low-carbohydrate and high-fat diets, or those who experience starvation.[18,19] Clinicians should be aware of these associated risks in high-risk patients.
Near-fatal asthma attacks present another risk to the mother and fetus, due to both hypoxemia and pregnancy-related stress. Optimal outcomes will only be achieved via appropriate medication use and patient education regarding drug adherence, associated risk factors, and self-management skills. ICS treatment can prevent asthma exacerbation during pregnancy,[20,21] although acute exacerbations should be aggressively managed using systemic corticosteroids to avoid fetal hypoxia. Furthermore, the discontinuation or reduction of asthma medications (especially ICS), due to misunderstanding the effects of asthma drug safety during pregnancy, is a major contributor to exacerbations during pregnancy.[20,21] The Global Initiative for Asthma highlights the fact that poorly controlled asthma poses a much greater risk to the fetus than current asthma treatments, and the potential risks associated with systemic glucocorticoid use are relatively small (e.g., cleft palate, preeclampsia, and low birth weight).[20,21] Therefore, it is preferable to maintain adequate asthma control based on the current guidelines when treating pregnant patients.
In summary, short-term starvation, if it occurs during periods of stress and medication (e.g., steroid use), can result in life-threatening ketoacidosis, even among nondiabetic women during the third trimester of pregnancy. This finding is particularly relevant to clinicians who care for pregnant women, especially those with asthma or pregnancy-related stress. Furthermore, early recognition and differential diagnosis of high-AG metabolic acidosis, prompt intervention, and additional preventive control are essential steps for achieving favorable maternal and fetal outcomes.
Footnotes
Abbreviations: AG = anion gap, ICS = inhaled corticosteroid.
The authors have no conflicts of interest to disclose.
References
- 1.Chico M, Levine SN, Lewis DF. Normoglycemic diabetic ketoacidosis in pregnancy. J Perinatol 2008; 28:310–312. [DOI] [PubMed] [Google Scholar]
- 2.Patel A, Felstead D, Doraiswami M, et al. Acute starvation in pregnancy: a cause of severe metabolic acidosis. Int J Obstet Anesth 2011; 20:253–256. [DOI] [PubMed] [Google Scholar]
- 3.Scholte JB, Boer WE. A case of nondiabetic ketoacidosis in third term twin pregnancy. J Clin Endocrinol Metab 2012; 97:3021–3024. [DOI] [PubMed] [Google Scholar]
- 4.Frise CJ, Mackillop L, Joash K, et al. Starvation ketoacidosis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2013; 167:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Karpate SJ, Morsi H, Shehmar M, et al. Euglycemic ketoacidosis in pregnancy and its management: case report and review of literature. Eur J Obstet Gynecol Reprod Biol 2013; 171:386–387. [DOI] [PubMed] [Google Scholar]
- 6.Sinha N, Venkatram S, Diaz-Fuentes G. Starvation ketoacidosis: a cause of severe anion gap metabolic acidosis in pregnancy. Case Rep Crit Care 2014; 2014:906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbos N, Shiner AM, Morris E. Severe metabolic acidosis as a consequence of acute starvation in pregnancy. Arch Gynecol Obstet 2009; 279:399–400. [DOI] [PubMed] [Google Scholar]
- 8.Figge J, Jabor A, Kazda A, et al. Anion gap and hypoalbuminemia. Crit Care Med 1998; 26:1807–1810. [DOI] [PubMed] [Google Scholar]
- 9.Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm 1974; 8:650–655. [Google Scholar]
- 10.Chan AL, Juarez MM, Gidwani N, et al. Management of critical asthma syndrome during pregnancy. Clin Rev Allerg Immunol 2015; 48:45–53. [DOI] [PubMed] [Google Scholar]
- 11.Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st century. Lancet 2008; 372:892. [DOI] [PubMed] [Google Scholar]
- 12.Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med 2014; 371:1434–1445. [DOI] [PubMed] [Google Scholar]
- 13.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 2006; 26:1–22. [DOI] [PubMed] [Google Scholar]
- 14.Felig P, Lynch V. Starvation in human pregnancy: hypoglycemia, hypoinsulinemia, and hyperketonemia. Science 1970; 170:990–992. [DOI] [PubMed] [Google Scholar]
- 15.Metzger BE, Ravnikar V, Vileisis RA, et al. “Accelerated starvation” and the skipped breakfast in late normal pregnancy. Lancet 1982; 1:588–592. [DOI] [PubMed] [Google Scholar]
- 16.Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed2011; New York: McGraw-Hill, Chapter 42. [Google Scholar]
- 17.Frise C, Ashcroft A, Jones BA, et al. Pregnancy and ketoacidosis: is pancreatitis a missing link? Obstet Med 2016; 9:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep 2015; 9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J 2015; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Chapter 3, part D, page 51-52. Available: http://www.ginasthma.org Accessed November 25, 2015. [Google Scholar]
- 21.Murphy VE, Gibson PG. Asthma in pregnancy. Clin Chest Med 2011; 32:93–110. [DOI] [PubMed] [Google Scholar]


