Abstract
A positive correlation between albuminuria and severity of obstructive sleep apnea syndrome (OSAS) has been demonstrated, as indexed by urine albumin-to-creatinine ratios (UACRs). However, the effect of continuous positive airway pressure (CPAP) treatment on albuminuria in OSAS patients has not been established.
Sixty subjects, with apnea-hypopnea indices >15 events per hour and no other diagnoses associated with albuminuria, underwent overnight polysomnography for sleep apnea and were examined for UACR at baseline and after 6 months of CPAP therapy. CPAP compliance rates were also recorded.
Significant improvement in UACR was found in OSAS patients with good compliance to CPAP treatment after 6 months of therapy (baseline vs 6-month follow-up, 32.0 ± 9.5 vs 19.2 ± 6.5 mg/g, respectively, P = 0.007), whereas slight worsening in UACRs was noted in patients with poor compliance to CPAP treatment (baseline vs 6-month follow-up, respectively, 16.7 ± 4.4 vs 19.1 ± 6.3 mg/g, respectively, P = 0.39). Change in UACR was significant between poor compliance versus good compliance groups (2.4 ± 2.7 vs −12.8 ± 4.4 mg/g, respectively, t = 2.9, P = 0.005). A significant correlation between improvement in UACR and CPAP compliance rates was also noted (Spearman's correlation coefficient: −0.37, P = 0.007). Baseline UACR, good CPAP compliance, and body mass index were independent predictors of changes in UACR.
Adequate CPAP treatment improves albuminuria in OSAS patients. In addition to monitoring CPAP adherence and subjective sleepiness, UACR may offer an objective physiological index of CPAP therapeutic effectiveness.
Keywords: albuminuria, continuous positive airway pressure, obstructive, sleep apnea
1. Introduction
Obstructive sleep apnea syndrome (OSAS) affects approximately 2% to 4% of the population in developed countries[1,2] and is an independent risk factor for hypertension, stroke, and myocardial infarction.[3–5] Continuous positive airway pressure (CPAP) treatment has been shown to reduce sleepiness and cardiovascular risk and remains the major therapy for OSAS.[6] The goal of CPAP therapy is to reduce the apnea-hypopnea index (AHI) to <5 events per hour during sleep.[6] However, simple and objective indicators to monitor the physiological effects of CPAP treatment are needed.
Albuminuria, as indexed by the urine albumin-to-creatinine ratio (UACR), is a sensitive indicator of endothelial injury and is a cardiovascular risk factor in individuals with diabetes and/or hypertension, and the general population.[7–11] Intermittent hypoxia, a characteristic of OSA patients, has been shown to be a stronger indicator of endothelial injury than even persistent hypoxemia.[12] Lowering albuminuria is associated with better health outcomes and has become an important goal in treating patients with chronic kidney disease with or without hypertension or diabetes.[7,9] Several studies have already demonstrated increased UACRs in OSAS patients.[13–15] However, the effect of CPAP treatment on UACR in OSAS patients has not been reported. The goal of this study was to demonstrate the effects of CPAP treatment on UACRs in moderate-to-severe OSAS patients.
2. Subjects and methods
The study protocol was approved by the research and ethics committee of the Chang Gung Memorial Hospital (CGMH) in Taiwan (CGMH IRB no. 97-0231C, no. 98-2149B, and no. 99-0940C). Every patient gave their informed consent prior to participation in the study.
Between 2008 and 2012, all adult patients (≥20 years of age) who visited 1 of 2 sleep clinics with complaints of snoring and sleepiness were prospectively enrolled in the study. Standard in-laboratory overnight polysomnography (Embla N7000; Medcare, Reykjavik, Iceland) was performed in each patient.
Variables recorded included 4 channels of electroencephalogram (C3/A2, C4/A1, O1/A2, O2/A1); bilateral electro-oculograms; chin, left, and right anterior tibial electromyogram; electrocardiogram; airflow as measured by pressure sensors and thermistors; chest and abdominal wall movement as measured by inductive respiratory plethysmographic bands; snoring as confirmed by wearing a neck microphone; and arterial oxygen saturation (SpO2) as measured by pulse oximetry. Video recording assessed the behavior of all subjects. All measurements were collected on a computerized sleep system (Somnologica Studio 3.0; Medcare).
Apnea was defined as the cessation of airflow for at least 10 seconds and hypopnea was defined as an abnormal respiratory event with at least 30% reduction of airflow, as compared to baseline, lasting at least 10 seconds, and with at least 3% oxygen desaturation and/or an arousal.
AHI was defined as the number of apnea along with hypopnea events per hour of total sleep time. Patients with AHIs ≥ 15/h were invited to participate in the study. Medical records were reviewed at baseline. Patients were excluded for following reasons: (1) if they were previously diagnosed with OSAS, (2) had a history of diabetes mellitus (defined as already under control using medication or a fasting blood glucose ≥126 mg/dL, or HbA1c ≥6.5% at baseline screening),[16] (3) abnormal renal function (defined as serum creatinine >1.4 mg/dL), (4) liver cirrhosis, (5) chronic obstructive pulmonary disease, (6) hematologic disease, (7) autoimmune disease, (8) cancer, or (9) recent infection. Patients who were taking angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, or diuretics were also excluded.
Attended CPAP titration studies were performed in all patients in order to detect the optimal therapeutic pressure. Compliance to CPAP treatment was recorded and then downloaded from the CPAP machine recorder. The compliance rate was defined as the number of nights in which CPAP was used for ≥4 hours divided by the total number of follow-up days. Morning blood and urine samples were collected from all patients at baseline and 6 months after CPAP treatment.
2.1. Statistical analysis
Independent samples t-test analysis was used to compare measurements between patients with poor versus good CPAP compliance. Repeat measures analysis of variance was used to analyze baseline and follow-up UACRs within- and between-compliance groups. Spearman's correlation test was used to analyze the correlation between CPAP compliance rate and changes in UACR. The effects of sex, age, AHI, oxygen desaturation index, creatinine, body mass index (BMI), baseline UACR, and compliance rate on changes in UACR were analyzed by multivariate stepwise linear regression. All values were expressed as mean ± standard error mean. All analyses were two-tailed, and a P-value <0.05 was considered statistically significant. Statistical analyses were performed using Statistical Package for Social Sciences 15.0 for Windows (SPSS Inc, Chicago, IL).
3. Results
Sixty-eight consecutive adult outpatients, who were diagnosed with OSA and agreed to use CPAP continuously, were enrolled in the study. Eight subjects were excluded for taking angiotensin converting enzyme inhibitors or angiotensin receptor blockade medications during the study (n = 5) or failing to comply with follow-up (n = 3). The remaining 60 patients were enrolled in the final analyses. The eating habit of these patients was normal without mainly protein style. No antioxidant, such as ascorbic acid (vitamin C) or acetylcysteine, was used in these patients during the study period.
UACRs correlated with severity of OSA at baseline (Fig. 1). Thirty-two (53%) patients with CPAP compliance rates higher than 50% were defined as the good compliance group. No significant differences in the demographic data were found between the poor compliance versus good compliance groups (Table 1). In both groups, the blood urea nitrogen levels were <20 mg/dL and the blood urea nitrogen-to-creatinine ratio were all <20. The data indicated that no high protein diet in our patients. No significant change of body weight was also found in both the groups during the treatment period.
Figure 1.
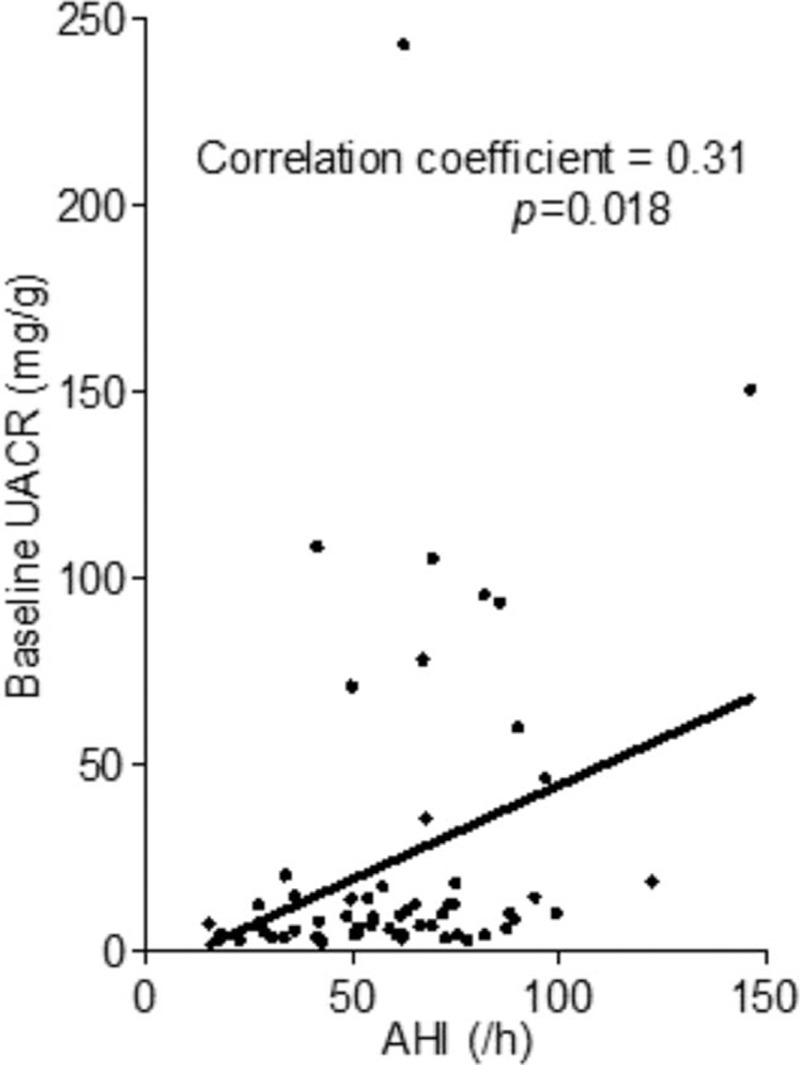
Correlation between AHI and baseline urine UACR. Each dot indicates an OSA patient. The black line shows the simple linear regression between AHI and baseline UACR in moderate-to-severe OSAS patients. AHI = apnea-hypopnea index; OSA = obstructive sleep apnea, OSAS = obstructive sleep apnea syndrome, UACR = urine albumin-to-creatinine ratio.
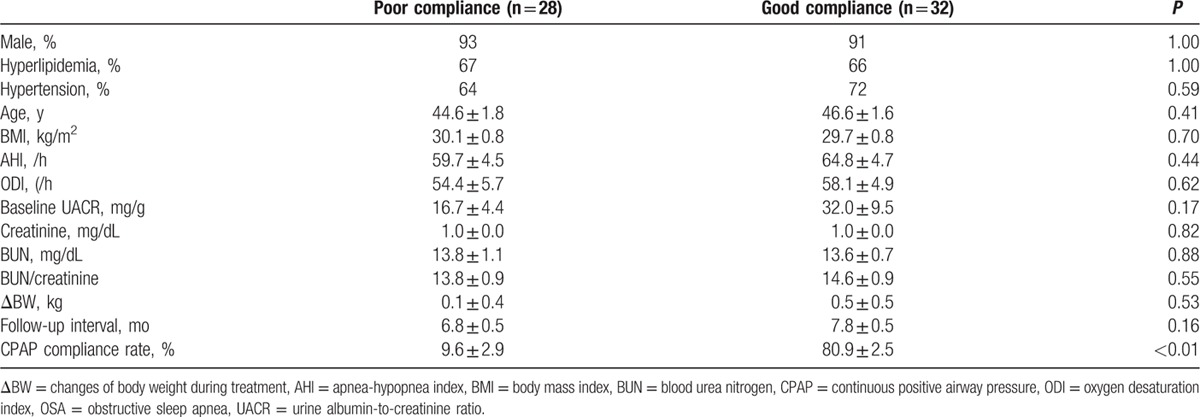
Table 1.
Demographic data and baseline parameters of OSA patients in poor compliance and good compliance groups.
Compared with baseline, UACR significantly improved after 6 months of CPAP treatment in the good compliance group (baseline vs 6 months of CPAP: 32.0 ± 9.5 vs 19.2 ± 6.5 mg/g, respectively, P = 0.007), whereas slight worsening in UACRs was noted in patients with poor compliance to CPAP treatment (baseline vs 6 months of CPAP: 16.7 ± 4.4 vs 19.1 ± 6.3 mg/g, respectively, P = 0.39). The changes in UACRs between the 2 groups were also significant (poor compliance vs good compliance groups: 2.4 ± 2.7 vs −12.8 ± 4.4 mg/g, respectively, t = 2.9, P = 0.005) (Fig. 2).
Figure 2.
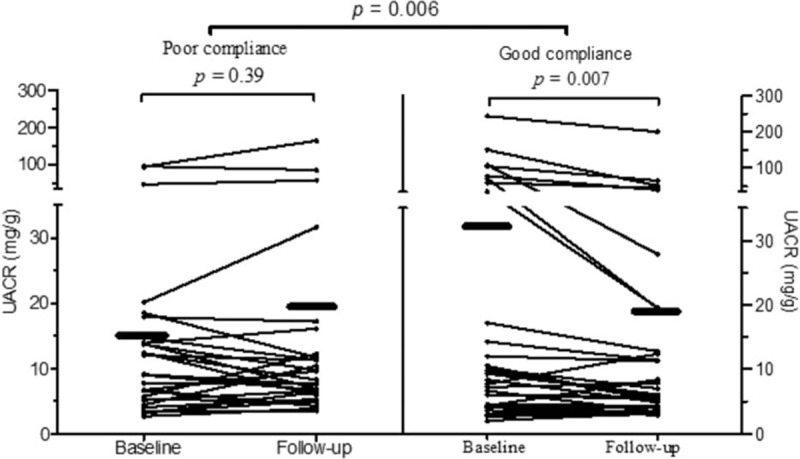
Mean and individual UACR before and after CPAP treatment in good versus poor CPAP compliance groups. Each dot indicates an UACR level in an OSA) patient and the black lines indicate the changes in UACR in individual patients before and after CPAP treatment. The black bars indicate the mean UACR before and after CPAP treatment in each group. CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea; UACR = urine albumin-to-creatinine ratio.
Higher compliance rates were associated with improvement in UACRs. There was a significant negative correlation between compliance rate and change in UACR (Spearman's correlation coefficient: −0.38, P = 0.003) (Fig. 3).
Figure 3.
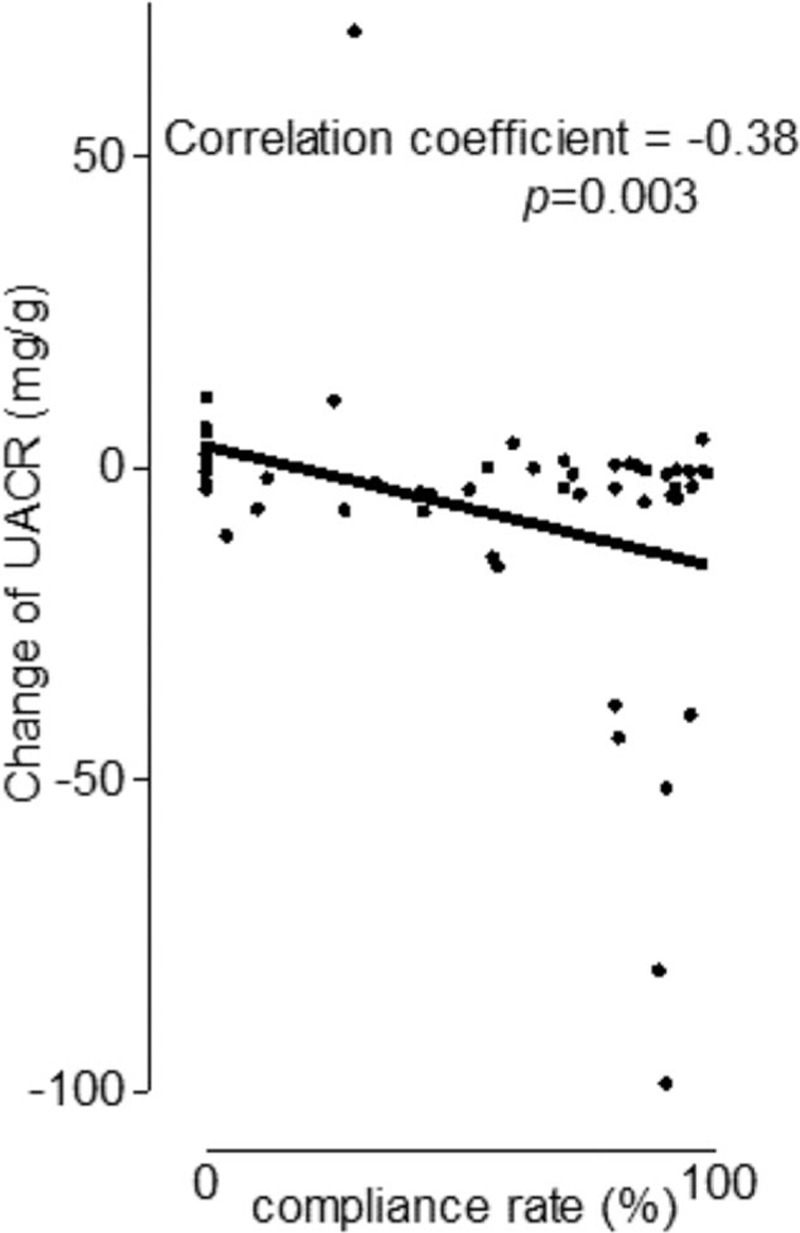
Correlation between compliance rate and change in UACR. Each dot indicates an OSA patient. The black line shows the simple linear regression between compliance rate and change in UACR in moderate-to-severe OSAS patients treated with CPAP. CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, OSAS = obstructive sleep apnea syndrome; UACR = urine albumin to creatinine ratio.
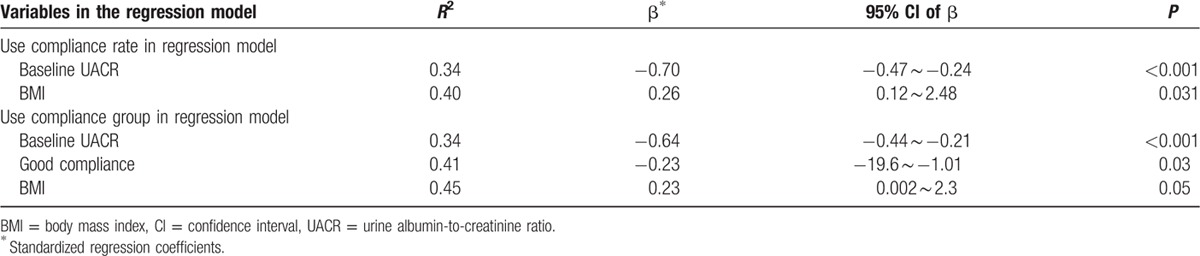
Multivariate linear regression models were used to determine the associations between changes in UACR and the patients’ demographic characteristics (including sex, age, BMI, AHI, oxygen desaturation index, creatinine, baseline UACR), and compliance rates. When considering compliance rate, stepwise linear regression analyses revealed that baseline UACR was the most important independent factor in predicting improvement in UACR whereas BMI also had some influence. Based on a stepwise linear regression model, the compliance rate was an additional factor that predicted improvement in UACR (Table 2).
Table 2.
Stepwise multivariate linear regression models for changes in UACR.
4. Discussion
Our study was designed to evaluate the effect of 6 months of CPAP treatment on albuminuria in adults with moderate-to-severe OSAS. As shown in previous studies, a significant correlation between albuminuria and OSA severity was noted.[13–15] However, we also found a significant improvement in UACRs in patients with good CPAP compliance and a slight worsening in UACRs was noted in patients with poor compliance to CPAP treatment. Our study also showed a dose-dependent correlation between CPAP compliance rates and improvement in UACR levels. Because elevated UACR is present in subjects with OSAS, even after excluding diabetes, hematological disorders, and other known causes of albuminuria, UACR may provide a complementary physiological index of CPAP treatment efficacy.
Our results also showed that baseline UACR, CPAP compliance, and BMI were independent predictors of changes in UACR after CPAP treatment in OSA patients. The higher the baseline UACR (i.e., the larger the difference from normal levels), the greater the effect of CPAP treatment on UACR. But higher BMI was associated with less improvement in UACR under CPAP therapy.
The association between obesity and abnormal UACR has been reported in several studies.[17] Improvement in UACR after weight reduction was also noted while weight gain resulted in worsening UACR.[18,19] The possible mechanisms underlying obesity-induced deterioration in UACR include obesity-related structural and functional changes such as focal segmental glomerulosclerosis or glomerulomegaly with a progressive increase in renal blood flow and filtration rate in the early phase followed by a progressive reduction in filtration rate, with worsening histological lesions, in the late phase.[20] Because adiponectin is shown to have a protective effect against the development of albuminuria in the kidneys,[21] downregulation of adiponectin in obesity patients may also play a role in limiting the improvement in UACR after CPAP therapy in OSA patients with higher BMI. Thus, weight reduction is still needed in obese OSA patients to induce a better treatment response.
Our most important findings were that good CPAP compliance was an independent factor in improving albuminuria in OSA patients and that there was a dose-dependent correlation between CPAP compliance rate and improvement in UACR. UACR is a sensitive marker of endothelial injury.[7] OSA can induce endothelial injury via intermittent hypoxia, increasing sympathetic activity, and an increasing intrathoracic pressure surge.[3] CPAP therapy can improve UACR by eliminating these OSA-induced consequences. Previous studies have also shown that good CPAP compliance was associated with better outcomes in OSA patients when compared with poor CPAP compliance.[22–24] Similarly, our studies showed that UACR was improved in patients with good CPAP compliance, whereas UACR worsened in patients with poor CPAP compliance.
This study had several limitations. Although CPAP treatment was provided, patients were not strictly required to use it every day. Several of our patients did not turn on the machine and their compliance rate was 0%. Patients who had poor compliance with CPAP treatment might potentially take less care of their health status and, therefore, would be at higher risk for a worse outcome. In addition, the relatively small sample size could have resulted in type II statistical error. However, the power of our regression model was 99.9%. Our results might be criticized when applying to female population due to most of our subjects were male. Both epidemiological and sleep clinic-based studies indicate that sleep apnea syndromes are more common in men than in women, the sex difference in prevalence is more marked within the sleep clinic.[1,2,3] In addition to this, no different treatment guideline for albuminuria between male and female was reported. Finally, most of our patients did not have profound albuminuria (UACR > 30 mg/g). Studies have shown, however, that subjects with intermediate albuminuria (5 mg/g ≤ UACR < 30 mg/g) were already at an increased risk for hypertension, heart failure, and death from cardiovascular diseases, and the risk was higher with higher levels of albuminuria.[7,10,11,25] Our study provides evidence that even intermediate albuminuria (within a certain range) could be reversed by CPAP therapy.
In summary, treating OSAS with CPAP (for 6 months) can improve albuminuria and the improvement in albuminuria significantly correlated with CPAP compliance rate. Our findings suggested that measuring UACR can offer a simple screen to examine compliance to (and effectiveness of) CPAP therapy. Whether the improvement in UACR after CPAP treatment in OSAS patients is concomitant with reductions in other comorbidities requires further investigation.
Footnotes
Abbreviations: AHI = apnea-hypopnea index, BMI = body mass index, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, OSAS = obstructive sleep apnea syndrome, UACR = urine albumin-to-creatinine ratio.
This study was funded by National Institute of Science of Taiwan (NMRPG3E6251) and Chang Gung Memorial Hospital (CMRPG3D1011 and CMRPG391231-3).
Authors’ contributions: Conception and design of the work: CNH, CYT, LSW, CLP; data acquisition: CNH, CYT, LPH, LSW, CLP, YCT; analysis and interpretation of data for the work: CNH, CYT, LSW, CLP, LPH, YCT; drafting the work or revising it critically for important intellectual content: CNH, CYT, LPH; and final approval of the version submitted for publication: CNH, YCT.
The authors declared no conflicts of interest.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2.Chuang LP, Hsu SC, Lin SW, et al. Prevalence of snoring and witnessed apnea in Taiwanese adults. Chang Gung Med J 2008; 31:175–181. [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009; 373:82–93. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 6.Phillips B, Kryger MH. Kryger MH, Roth T, Dement WC. Management of obstructive sleep apnea-hypopnea syndrome. Overview Principles, Practice of Sleep Medicine 4th ed.Philadelphia, PA: Elsevier Saunders; 2005. 1109–1121. [Google Scholar]
- 7.Jarraya F, Lakhdar R, Kammoun K, et al. Microalbuminuria: a useful marker of cardiovascular disease. Iran J Kidney Dis 2013; 7:178–186. [PubMed] [Google Scholar]
- 8.Mauer M, Fioretto P, Woredekal Y. Schrier RW. Diabetic nephropathy. Diseases of the Kidney and Urinary Tract. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. 2083–2127. [Google Scholar]
- 9.KDIGO. 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [DOI] [PubMed] [Google Scholar]
- 10.Romundstad S, Holmen J, Kvenild K, et al. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Tr⊘ndelag Health Study (HUNT), Norway. Am J Kidney Dis 2003; 42:466–473. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Evans JC, Meigs JB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 2005; 111:1370–1376. [DOI] [PubMed] [Google Scholar]
- 12.Gozal E, Sachleben LR, Jr, Rane MJ, et al. Mild sustained and intermittent hypoxia induce apoptosis in PC-12 cells via different mechanisms. Am J Physiol Cell Physiol 2005; 288:C535–C542. [DOI] [PubMed] [Google Scholar]
- 13.Chou YT, Lee PH, Yang CT, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant 2011; 26:2244–2250. [DOI] [PubMed] [Google Scholar]
- 14.Faulx MD, Storfer-Isser A, Kirchner HL, et al. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep 2007; 30:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ursavas A, Karadag M, Gullulu M, et al. Low-grade urinary albumin excretion in normotensive/non-diabetic obstructive sleep apnea patients. Sleep Breath 2008; 12:217–222. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34 suppl 1:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf P, Baker J, Scott A, et al. Albuminuria in people at least 40 years old: effect of obesity, hypertension, and hyperlipidemia. Clin Chem 1992; 38:1802–1808. [PubMed] [Google Scholar]
- 18.Amor A, Jiménez A, Moizé V, et al. Weight loss independently predicts urinary albumin excretion normalization in morbidly obese type 2 diabetic patients undergoing bariatric surgery. Surg Endosc 2013; 27:2046–2051. [DOI] [PubMed] [Google Scholar]
- 19.Bello AK, de Zeeuw D, El Nahas M, et al. Impact of weight change on albuminuria in the general population. Nephrol Dial Transplant 2007; 22:1619–1627. [DOI] [PubMed] [Google Scholar]
- 20.Kiortsis DN, Christou MA. Management of obesity-induced kidney disease: a critical review of the literature. Obes Facts 2012; 5:821–832. [DOI] [PubMed] [Google Scholar]
- 21.Christou GA, Kiortsis DN. The role of adiponectin in renal physiology and development of albuminuria. J Endocrinol 2014; 221:R49–R61. [DOI] [PubMed] [Google Scholar]
- 22.Dorkova Z, Petrasova D, Molcanyiova A, et al. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134:686–692. [DOI] [PubMed] [Google Scholar]
- 23.Engleman HM, Kingshott RN, Wraith PK, et al. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med 1999; 159:461–467. [DOI] [PubMed] [Google Scholar]
- 24.Stanchina ML, Welicky LM, Donat W, et al. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013; 9:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blecker S, Matsushita K, Kottgen A, et al. High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis 2011; 58:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


