Abstract
CYP2C19 loss-of-function (LOF) alleles adversely affect clinical outcome of clopidogrel therapy. Recent introduction of a newer-generation drug-eluting stent (DES) has significantly reduced the occurrence of stent thrombosis.
The aim of this study was to evaluate the impact of CYP2C19 LOF alleles on clinical outcome in patients treated with the newer-generation DES.
The effects of CYP2C19 genotypes were evaluated on clinical outcome of clopidogrel therapy in 2062 patients treated with percutaneous coronary intervention using either first-generation DES (sirolimus- and paclitaxel-eluting stent, n = 1349) or newer-generation DES (everolimus- and zotarolimus-eluting stent, n = 713). The primary clinical outcome was major cardiac and cerebrovascular event (MACCE) including cardiac death, nonfatal myocardial infarction, stroke, and stent thrombosis during 1 year of follow-up.
CYP2C19 LOF alleles were significantly associated with a higher risk of MACCE in patients treated with first-generation DES (hazard ratio [HR] 2.599, 95% confidence interval [CI] 1.047–6.453; P = 0.034). In contrast, CYP2C19 LOF alleles were not associated with primary outcome in newer-generation DES (HR 0.716, 95% CI 0.316–1.622; P = 0.522). In the further multivariate analysis, CYP2C19 LOF alleles were not associated with MACCE in patients receiving newer-generation DES (adjusted HR 0.540, 95% CI 0.226–1.291; P = 0.166), whereas they were demonstrated to be an independent risk factor for MACCE in those implanted with first-generation DES (adjusted HR 3.501, 95% CI 1.194–10.262; P = 0.022).
In contradiction to their clinical impact in first-generation DES era, CYP2C19 LOF alleles may not affect clinical outcome of clopidogrel therapy in patients treated with newer-generation DES.
Keywords: clopidogrel, CYP2C19 genotype, drug-eluting stents, percutaneous coronary intervention
1. Introduction
Clopidogrel with aspirin significantly improves clinical outcomes in patients with acute coronary syndrome (ACS) and those undergoing percutaneous coronary intervention (PCI).[1] However, clopidogrel has interindividual response variability in its antiplatelet efficacy, in part because of polymorphisms in hepatic cytochrome P450 isoenzymes, especially CYP2C19, a critical enzyme to produce active metabolite of clopidogrel.[2,3] Indeed, carriers of loss-of-function (LOF) alleles of CYP2C19 genotype (∗2 and/or ∗3 alleles) in patients undergoing PCI have a higher rate of subsequent cardiovascular events than noncarriers.[4–6]
The first-generation drug-eluting stents (DES) such as sirolimus-eluting stent (SES) or paclitaxel-eluting stent (PES) in real-world practice have significantly reduced the restenosis associated with bare-metal stents, but also caused problems with stent thrombosis, with a risk that is unpredictable and increases with time.[7,8] Impairment in arterial healing characterized by delayed re-endothelialization and persistence of fibrin are mainly responsible for the occurrence of stent thrombosis.[9] Compared with the first-generation DESs, newer-generation DESs such as everolimus-eluting stent (EES) and zotarolimus-eluting stent (ZES) have been developed with the intention of better safety and efficacy to overcome the major drawbacks of first-generation DESs. They are designed with improved stent platforms of thinner stent strut and newer alloys, more biocompatible polymer, and meticulous drug-eluting kinetics. As expected, these newer-generation DESs significantly reduced the occurrence of target lesion failure and the occurrence of stent thrombosis, compared with first-generation DESs.[10,11] Imaging studies using optical coherence tomography (OCT) showed that newer-generation DES made less intracoronary thrombi than first-generation DES after stent implantation in patients undergoing elective PCI.[12]
Although CYP2C19 LOF alleles consistently affect clinical outcome of clopidogrel therapy, no data exist on their clinical impact in the era of newer-generation DES. We therefore hypothesized that the clinical impact of CYP2C19 genotype in patients who underwent DES implantation and received clopidogrel therapy might be different according to the DES types: first-generation DES versus newer-generation DES. To test this hypothesis, we measured the association of CYP2C19 LOF alleles with adverse clinical outcomes in patients receiving newer-generation DES and clopidogrel therapy compared with those implanted with first-generation DES using the CathOlic University of Korea—percutAneous Coronary inTervention registry.
2. Methods
2.1. Study population
We consecutively enrolled 2188 patients undergoing PCI with DES for ischemic heart disease in 2 affiliated hospitals (Seoul St. Mary's Hospital and Yeouido St. Mary's Hospital) of the Catholic University of Korea between January 2004 and December 2010. These hospitals perform high-volume PCI of >500 procedures per year. All patients received dual antiplatelet therapy of aspirin and clopidogrel after PCI for at least 1 year and gave an informed consent for genotyping. We excluded patients who had contraindications to aspirin or clopidogrel treatment, prior treatment with glycoprotein IIb/IIIa inhibitors during 10 days before PCI, and implantation of heterogeneous stents in their coronary arteries. Finally, 2062 patients who received the same kind of DESs in their coronary arteries and received dual antiplatelet therapy for at least 1 year were included in this study. Patients were divided into 2 groups according to the generation of stents: the first-generation DES (SES including Cypher and Cypher Select [Cordis, Johnson & Johnson, New Brunswick, NJ] and PES including Taxus Express and Taxus Liberte [Boston Scientific, Natick, MA]) or the newer-generation DES (ZES including Endeavor Resolute [Medtronic, Santa Rosa, CA] and EES including Xience V, Xience Prime [Abbott Vascular, Santa Clara, CA], and Promus Element [Boston Scientific]). Patients were started on a loading dose of 600 mg clopidogrel on the first day of clopidogrel treatment. The recommended pretreatment interval was >2 hours before elective PCI, whereas <1 hour before primary PCI in cases of acute myocardial infarction (AMI). Aspirin of 250–325 mg was given as a loading dose unless already chronically administered. Postprocedural antiplatelet therapy consisted of 100 mg aspirin once daily indefinitely and 75 mg clopidogrel once daily for at least 1 year. Intravenous anticoagulation treatment with unfractionated heparin was given to all patients during the procedure. The treatment strategy and selection of the type of DES were left to the discretion of the individual operator. The study complied with the Declaration of Helsinki regarding investigation in humans, and written informed consent was obtained from each patient before enrollment. There was no industry involvement in the design, conduct, or analysis of the study. The study protocol was approved by Institutional Review Board at each participating hospital. All outcomes of interest were confirmed by source documents and were centrally adjudicated by a local events committee at the Cardiovascular Center of Seoul St. Mary's Hospital whose members were unaware of patient status. For validation of complete follow-up data, information on censored survival data (death or survival) and the cause of death (cardiac or noncardiac death) was obtained up to December 31, 2010, from the Office of Statistics, Korea, with the use of unique personal identification numbers. This study is registered with ClinicalTrials.gov, number NCT01239914, available online at http://clinicaltrials.gov.
2.2. Genotyping
Genomic DNA was extracted from peripheral blood cells and tested for the presence of single nucleotide polymorphisms (SNPs). Genotypes for the presence of major Korean CYP2C19 alleles including CYP2C19∗2 (rs4244285), CYP2C19∗3 (rs4986893), and CYP2C19∗17 (rs12248560) were determined by single-base extension methods according to the manufacturer's protocol using an ABI PRISM genetic analyzer (Applied Biosystems, Foster City, CA) and its mounted GeneMapper software. All tested genotypes satisfied Hardy–Weinberg equilibrium and showed no statistical significance for all SNPs tested (P > 0.05) in this study. Primers 5′-AATTACAACCAGAGCTTGGC-3′ and 5′-TATCACTTTCCATAAAAGAAG-3′; 5′-CCAATCATTTAGCTTCACCC-3′ and 5′-ACTTCAGGGCTTGGTCAATA-3′; and 5′-GCCCTTAGCACCAAATTCTCT-3′ and 5′-CACCTTTACCATTTAACCCCC-3′ were used to amplify the DNA fragment containing CYP2C19∗2 19154G>A (rs4244285), CYP2C19∗3 17948G>A (rs4986893), and CYP2C19∗17 −806C>T (rs12248560) polymorphisms, respectively. CYP2C19 genotypes were classified into the following 3 phenotypes:) extensive metabolizers carrying normal function alleles (CYP2C19∗1/∗1), intermediate metabolizers carrying 1 LOF allele (∗1/∗2, ∗1/∗3), and poor metabolizers carrying 2 LOF alleles (∗2/∗2, ∗2/∗3, ∗3/∗3). All genotyping was performed with the validated genotyping technology platform established at the PharmacoGenomics Research Center, Inje University College of Medicine, Busan, Republic of Korea.
2.3. Platelet function testing
Platelet aggregation was assessed by the VerifyNow P2Y12 assay (Accumetrics Inc, San Diego, CA) on day 2 in patients with stable angina and on day 4 or 5 in those with AMI. The results were reported as P2Y12 reaction units (PRU). A total of 662 patients had accessible test results because of the availability of the VerifyNow P2Y12 assay from June 2008 at each institution.
2.4. Study end points and definitions
The primary end point was the cumulative event rate of the composite of major adverse cardiac and cerebrovascular event (MACCE), defined as cardiac death, nonfatal myocardial infarction (MI), stroke, and stent thrombosis. The diagnosis of MI was based on an increase in the creatinine kinase-MB isoenzyme or troponin value to ≥2 times the normal upper limit and either symptoms consistent with acute myocardial ischemia or electrocardiogram changes in at least 2 contiguous leads (pathological Q waves, persistent ST-segment elevation, or ST-segment depression >0.1 mV). Stroke was defined as a new focal neurological deficit of vascular origin lasting >24 hours. Stent thrombosis was the cumulative event rate of combined definite and probable stent thrombosis according to the Academic Research Consortium criteria. Definite stent thrombosis was defined as the occurrence of an ACS with either angiographic or pathological confirmation of thrombosis, and probable stent thrombosis was defined as any unexplained sudden death within 30 days or as MI involving target vessel territory without angiographic confirmation of thrombosis. Each primary end point was evaluated only during the period of clopidogrel exposure. All of the events were adjudicated by an event adjudication committee blinded to the genotype and platelet function measurements of the patients.
2.5. Statistical methods
Variables are presented as mean ± standard deviation or frequencies (percentage). Continuous variables were compared using Student t test, and categorical variables were compared using a χ2 test or Fisher's exact test. We compared the median duration between groups using Mood's median test. SNPs evaluated in our study were tested for deviation from Hardy–Weinberg equilibrium using the Pearson goodness-of-fit χ2 test. Follow-up of patients was censored at the date of the first cardiovascular event corresponding to the primary end point occurring during clopidogrel treatment. In patients without an event, the outcomes were censored at a fixed point of 1 year (365 days) to avoid any bias caused by different follow-up duration or duration of clopidogrel treatment. Unadjusted estimates of the event rates for clinical outcomes at 1 year following PCI were estimated by the Kaplan–Meier method according to the presence of the CYP2C19∗2 or ∗3 alleles from first-generation DES and newer-generation DES groups, and compared by log-rank tests. Unadjusted estimates of hazard ratios (HRs) were calculated using a Cox proportional hazard model. We undertook multivariable Cox regression analyses to calculate HR and 95% confidence intervals (CIs) to achieve the first adverse clinical events according to the presence of the CYP2C19 LOF alleles. The HRs were adjusted for traditional risk factors of coronary artery disease for risk that had significant effects (P < 0.1) in the univariate analysis for clinical outcomes. For all analyses, 2-sided and P values of <0.05 were considered statistically significant. All data were processed with SAS software (version 9.2, SAS Institute, Cary, NC).
3. Results
3.1. Baseline characteristics
The patients were categorized into 2 groups: first-generation DES (n = 1349, 65.4% of total patients; 947 [70.2%] treated with SES, 402 [29.8%] with PES) and newer-generation DES (n = 713, 34.6% of total patients; 510 [71.5%] treated with EES, 203 [28.5%] with ZES). Because of the availability of newer-generation DES since 2008, patients treated with first-generation DES were mostly (95.6%) enrolled between 2004 and 2007, and those receiving newer-generation DES were all recruited from 2008. Among total patients, 1237 (60.0%) were carriers of CYP2C19∗2 or ∗3 alleles. The frequencies of CYP2C19 alleles in this study were consistent with previous reports done in Korean population.[12,13] The distribution of CYP2C19 genotype was similar between first-generation and newer-generation DES groups (carriers, n = 795 [58.9%] vs n = 442 [62.0%], P = 0.402). There were no significant differences of PRU between DES generation groups (the first-generation DES, 219.3 ± 83.7, vs the newer-generation DES, 226.7 ± 86.6; P = 0.267). Baseline characteristics of each group are shown in Table 1. Patient demographic characteristics were evenly distributed between groups. Carrier groups had a higher rate of P2Y12 reactivity unit (>240) compared with noncarrier group in both stent generation groups. As expected, carriers of CYP2C19 LOF alleles had the higher PRU than noncarriers regardless of DES generation groups (Fig. 1). There were no differences of PRU between carriers and noncarriers in each stent generation group.
Table 1.
Baseline characteristics of patients according to stent generation and CYP2C19 polymorphism.
Figure 1.
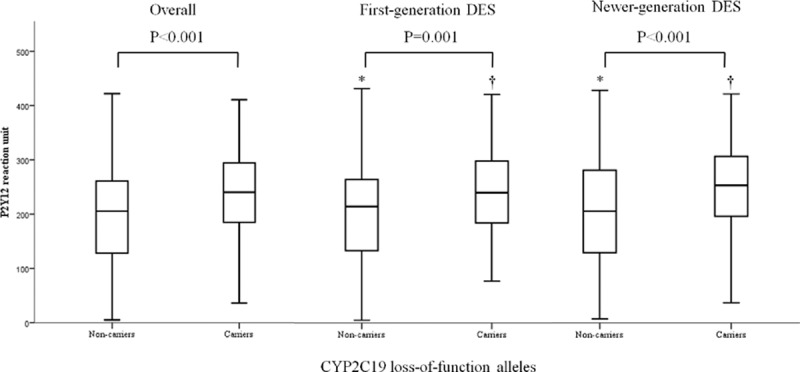
Platelet reaction unit according to the presence of CYP2C19 loss-of-function alleles. (∗) Comparisons between first- and newer-generation DES among noncarriers, P = 0.816; (†) comparisons between first- and newer-generation DES among carriers, P = 0.383. DES = drug-eluting stent.
3.2. CYP2C19 polymorphism and clinical outcomes
Cumulative rates of MACCE including cardiac death, nonfatal MI, stroke, and stent thrombosis during 1 year of clopidogrel therapy are shown according to CYP2C19∗2 or ∗3 alleles in Table 2. In the first-generation DES group, carriers of CYP2C19 LOF alleles tended to have an increased risk of stent thrombosis (unadjusted HR 5.605, 95% CI 0.701–44.815, P = 0.104) but did not reach clinical significance. However, the composite of cardiac death, nonfatal MI, stroke, and stent thrombosis, the primary end point, in first-generation DES group was significantly higher in carriers of CYP2C19∗2 or ∗3 alleles than in noncarriers (unadjusted HR 2.300, 95% CI 1.041–5.080, P = 0.039). In contrast, the cumulative rate of MACCE in the newer-generation DES group did not differ between CYP2C19 LOF allele carries and noncarriers (unadjusted HR 0.930, 95% CI 0.380–2.274, P = 0.873).
Table 2.
Clinical outcomes up to 1 year according to CYP2C19 loss-of-function allele.
Fig. 2 shows the cumulative event rate of MACCE in each DES generation group according to CYP2C19 polymorphism during 1 year of follow-up. Patients with CYP2C19 LOF alleles showed higher cumulative rates of MACCE during 1 year after PCI than noncarriers of CYP2C19 LOF alleles in first-generation DES group (log rank, P = 0.034). However, there was no significant difference in cumulative event rate of clinical outcome by CYP2C19 polymorphism in newer-generation DES group (log rank, P = 0.873). Among patients with stable angina, CYP2C19 LOF alleles were not associated with clinical outcome in those treated with the first-generation DES and the newer-generation DES. However, the same analysis in AMI patients group yielded different results. Among patients with AMI, CYP2C19 LOF alleles were associated with increased adverse clinical events in those treated with the first-generation DES, but not the newer-generation (Fig. 3).
Figure 2.
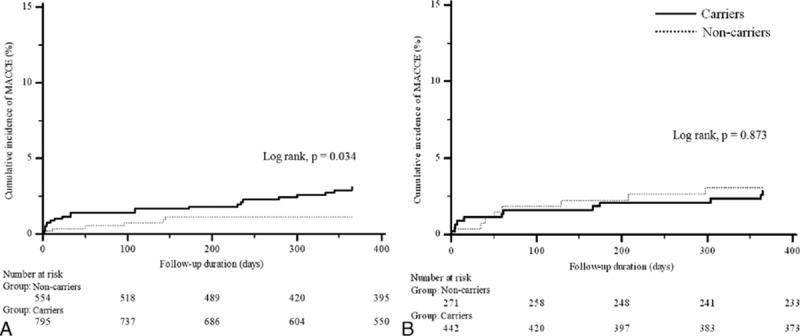
Kaplan–Meier cumulative event rates of the primary end points according to the presence of CYP2C19 loss-of-function alleles. (A) The first-generation drug-eluting stents group; (B) the newer-generation drug-eluting stents group. MACCE = major adverse cardiac and cerebrovascular event.
Figure 3.
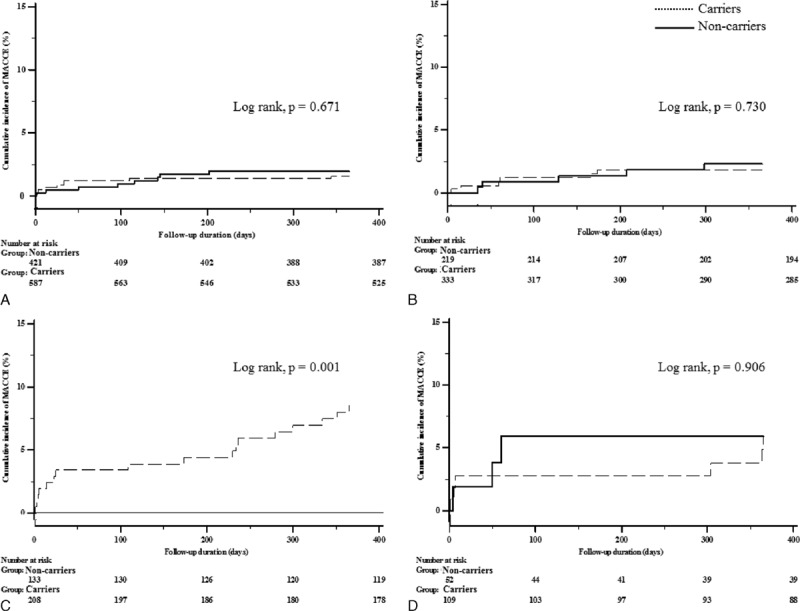
Kaplan–Meier cumulative event rates of the primary end points according to drug-eluting stent generation and the presence of CYP2C19 loss-of-function alleles. (A) The first-generation drug-eluting stents group among patients with stable angina; (B) the newer-generation drug-eluting stents group among patients with stable angina; (C) the first-generation drug-eluting stents group among patients with AMI; (D) the newer-generation drug-eluting stents group among patients with AMI. AMI = acute myocardial infarction, MACCE = major adverse cardiac and cerebrovascular event.
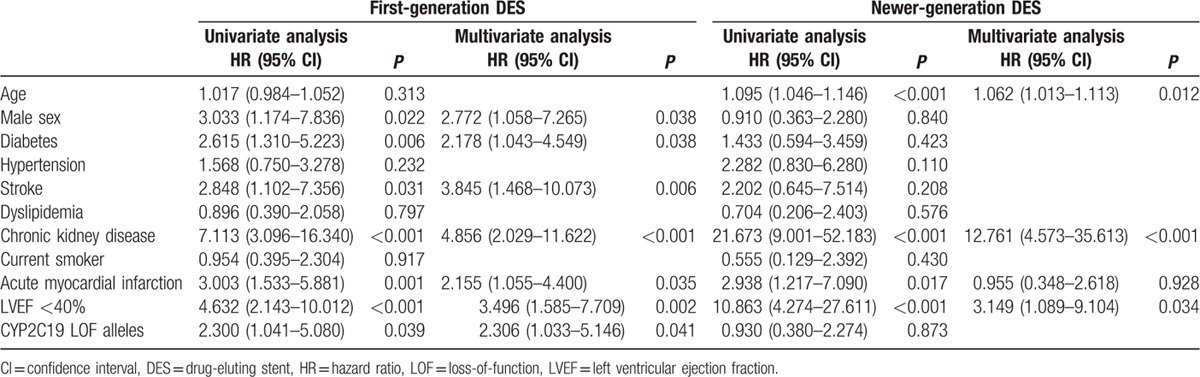
Multivariate Cox proportional analyses in each DES generation groups showed marked differences (Table 3). Although presence of CYP2C19 LOF alleles (adjusted HR 2.306, 95% CI 1.033–5.146, P = 0.041) was an independent predictor of MACCE in the first-generation DES group after multivariate adjusting, it was not associated with the clinical outcome of MACCE in the newer-generation DES group.
Table 3.
Multivariate analysis for the prediction of cardiac death, myocardial infarction, stroke, or stent thrombosis according to stent generation.
4. Discussion
To our knowledge, this is the first study showing the impact of CYP2C19 LOF alleles on clinical outcome in newer-generation DES era. In this study, we observed that CYP2C19 LOF alleles adversely affect clinical outcome of clopidogrel therapy in patients treated with first-generation DES but were not associated with clinical outcome in those who were implanted with newer-generation DES and received clopidogrel therapy.
CYP2C19 LOF alleles are associated with higher on-clopidogrel platelet reactivity and increased risk of worse clinical outcome after coronary stent implantation.[4–6] Landmark studies demonstrating the association between CYP2C19 polymorphism and adverse cardiovascular outcomes have the following features in common: the study populations were mainly composed of patients with ACS[5,13] and they were treated with either bare-metal stents or first-generation DES, now withdrawn from the DES market. Along with these studies, some other studies observed adverse clinical impact of CYP2C19 LOF alleles in more stable populations such as elective PCI-treated patients, or in all-comers receiving DES.[14,15] The present study also confirmed the strong association between CYP2C19 LOF alleles and adverse clinical outcome in patients treated with first-generation DES and clopidogrel. In the meantime, a recent meta-analysis reported that carriers of CYP2C19 LOF alleles did not have an increased risk of cardiovascular events, but had an increased risk of stent thrombosis.[16,17] The authors questioned the relevance of the CYP2C19 LOF alleles to MACCE beyond stent thrombosis in coronary patients treated with clopidogrel.
The newer-generation DESs such as ZES and EES have rapidly expelled first-generation DES from the DES market because they were fabricated to overcome the major drawbacks of first-generation DES. Better stent platforms with newer alloys and thin-strut stent frame, improved biocompatibility of durable polymer coatings, and reduced toxicity of antiproliferative drugs have translated into better clinical outcome.[10,11,18] Animal experiments revealed that delayed arterial healing and re-endothelialization were greater after first-generation DES implantation than after newer-generation DES.[19,20] Clinically, the introduction of newer-generation DES in patients undergoing PCI has significantly reduced the risk of adverse cardiovascular events compared to that of first-generation DES. In the COMPARE study, EES group had further reduced annual rates of MI and stent thrombosis than PES in all-comer patients.[11] The SPIRIT IV study also revealed that the 1-year rates of MI and stent thrombosis were markedly lower with EES than with PES.[10] Recent study presented that more antiplatelet agents had no additional benefit in patients treated with the newer-generation DES.[21] Imaging studies using OCT found that EES showed a significantly lower incidence of uncovered stent struts and intracoronary thrombi than SES at 7 to 9 months after stent implantation in patients with ST-segment elevation MI and undergoing elective PCI.[12,22] These OCT findings support the fact that the newer-generation DES may have more favorable vascular response than the first-generation DES after stent implantation. In addition, a recent interesting study reported that intrastent thrombi in OCT findings were more frequently observed in carriers of CYP2C19 LOF alleles than in noncarriers in the first-generation DES, whereas this pattern was not obvious in the newer-generation DES group.[23] In the present study, we identified discordant impact of CYP2C19 LOF alleles on clinical outcome after DES implantation by DES types. Whereas carriers of CYP2C19 LOF alleles in the first-generation DES group had a higher event rate of adverse clinical outcome than noncarriers, those with CYP2C19 polymorphism in the newer-generation DES group did not differ in clinical outcome compared to noncarriers. Considering the findings in OCT studies and the present study, we speculate that CYP2C19 LOF alleles in the era of the newer-generation DES with better vascular healing properties may not strongly influence clinical outcome to such extent in the first-generation DES era, although CYP2C19 polymorphism still leads to reduced clopidogrel active metabolite formation and antiplatelet effect.
Our previous data demonstrated a different impact of CYP2C19 LOF alleles on clinical outcomes between patients with AMI and those with stable angina.[24] CYP2C19 poor metabolizers were associated with poor clinical outcomes in patients with AMI, not in patients with stable angina. This study showed patterns similar to our previous data. High incidence rate of MACCE in patients with CYP2C19 LOF alleles among patients with AMI undergoing PCI with the first-generation DES was seen in the present study. Also, patients of other groups had a relatively lower incidence rate of MACCE. It might affect results of the present study. Although East Asians have higher prevalence of CYP2C19 LOF alleles than Westerners, the previous large-scale studies presented a relatively lower rate of MACCE in East Asians.[25] Our data showed similar incidence rates of MACCE in this population. We identified a discordant impact of CYP2C19 LOF alleles on clinical outcomes according to DES generation and clinical situations. We speculate that CYP2C19 LOF alleles may not affect clinical outcomes in patients with AMI as well as stable angina in the newer-generation DES era.
4.1. Study limitations
First, because of the availability of newer-generation DES since 2008, patients treated with first-generation DES were mostly enrolled between 2004 and 2007, and those receiving newer-generation DES were all recruited from 2008. Therefore, we could not simultaneously evaluate clinical outcome of patients with first- and newer-generation DES, even though we obtained and analyzed complete data of clinical outcome in all patients. Second, because the proportion of patients with AMI was low (24.3%), the clinical impact of CYP2C19 LOF alleles may be underestimated. Third, the sample size of the newer-generation DES group in this study may limit our interpretation and discussion. However, several previous studies with a small sample size of even <300 patients in the first-generation DES era consistently reported that carriers of CYP2C19 LOF alleles had increased risks of adverse clinical outcomes.[26,27] Fourth, the incidence of nonfatal MI in patients undergoing DES implantation in the present study is relatively low, as in a recent report.[28] In the report analyzing 4056 patients receiving DES, the incidence of any MI during 1 year of follow-up was 0.5%. Although our registry data showed a similar trend, nonfatal MI and stroke may have been underestimated. Our findings should be confirmed through large randomized clinical trials with long-term follow-ups. Fifth, we did not collect data of a proportion of atrial fibrillation and use of anticoagulation.
5. Conclusions
CYP2C19 LOF alleles may not affect clinical outcome of clopidogrel therapy in patients treated with the newer-generation DES as much as in those receiving the first-generation DES. These findings suggest that the newer-generation DES may overcome the drawback of CYP2C19 LOF alleles following clopidogrel therapy. Future studies are needed to evaluate the impact of CYP2C19 LOF allele on long-term clinical outcome for patients in whom PCI was performed with the newer-generation DES.
Footnotes
Abbreviations: ACS = acute coronary syndrome, AMI = acute myocardial infarction, DES = drug-eluting stent, EES = everolimus-eluting stent, LOF = loss-of-function, MACCE = major adverse cardiac and cerebrovascular event, OCT = optical coherence tomography, PCI = percutaneous coronary intervention, PES = paclitaxel-eluting stent, PRU = P2Y12 reaction units, SES = sirolimus-eluting stent, ZES = zotarolimus-eluting stent.
Authorship: Ik Jun Choi and Yoon-Seok Koh contributed equally to this article.
Funding: This study was supported by grant A111218-11-PG02 from the National Project for Personalized Genomic Medicine, Korea Health 21 R&D Project, Ministry for Health & Welfare, by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Ministry of Education, Science and Engineering (MOEST) (No. R13-2007-023-00000-0), and partly supported by a grant from Sanofi-Aventis Korea to KC.
The authors have no conflicts of interest to disclose.
References
- 1.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671. [DOI] [PubMed] [Google Scholar]
- 2.Savi P, Combalbert J, Gaich C, et al. The antiaggregating activity of clopidogrel is due to a metabolic activation by the hepatic cytochrome P450-1A. Thromb Haemost 1994; 72:313–317. [PubMed] [Google Scholar]
- 3.Pereillo JM, Maftouh M, Andrieu A, et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos 2002; 30:1288–1295. [DOI] [PubMed] [Google Scholar]
- 4.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175. [DOI] [PubMed] [Google Scholar]
- 5.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362. [DOI] [PubMed] [Google Scholar]
- 6.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007; 369:667–678. [DOI] [PubMed] [Google Scholar]
- 8.Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029. [DOI] [PubMed] [Google Scholar]
- 9.Nakazawa G, Finn AV, Virmani R. Vascular pathology of drug-eluting stents. Herz 2007; 32:274–280. [DOI] [PubMed] [Google Scholar]
- 10.Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 2010; 375:201–209. [DOI] [PubMed] [Google Scholar]
- 11.Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674. [DOI] [PubMed] [Google Scholar]
- 12.Choi HH, Kim JS, Yoon DH, et al. Favorable neointimal coverage in everolimus-eluting stent at 9 months after stent implantation: comparison with sirolimus-eluting stent using optical coherence tomography. Int J Cardiovasc Imaging 2012; 28:491–497. [DOI] [PubMed] [Google Scholar]
- 13.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375. [DOI] [PubMed] [Google Scholar]
- 14.Trenk D, Hochholzer W, Fromm MF, et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol 2008; 51:1925–1934. [DOI] [PubMed] [Google Scholar]
- 15.Oh IY, Park KW, Kang SH, et al. Association of cytochrome P450 2C19∗2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart 2012; 98:139–144. [DOI] [PubMed] [Google Scholar]
- 16.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010; 304:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabalza M, Subirana I, Sala J, et al. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart 2012; 98:100–108. [DOI] [PubMed] [Google Scholar]
- 18.Hofma SH, Brouwer J, Velders MA, et al. Second-generation everolimus-eluting stents versus first-generation sirolimus-eluting stents in acute myocardial infarction. 1-Year results of the randomized XAMI (XienceV Stent vs. Cypher Stent in Primary PCI for Acute Myocardial Infarction) trial. J Am Coll Cardiol 2012; 60:381–387. [DOI] [PubMed] [Google Scholar]
- 19.Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 2008; 52:333–342. [DOI] [PubMed] [Google Scholar]
- 20.Nakazawa G, Nakano M, Otsuka F, et al. Evaluation of polymer-based comparator drug-eluting stents using a rabbit model of iliac artery atherosclerosis. Circ Cardiovasc Interv 2011; 4:38–46. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Choi YS, Kwon A, et al. It is not mandatory to use triple rather than dual anti-platelet therapy after a percutaneous coronary intervention with a second-generation drug-eluting stent. Medicine (Baltimore) 2015; 94:e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawada T, Shinke T, Otake H, et al. Comparisons of detailed arterial healing response at seven months following implantation of an everolimus- or sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction. Int J Cardiol 2013; 168:960–966. [DOI] [PubMed] [Google Scholar]
- 23.Konishi A, Shinke T, Otake H, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism on intra-stent thrombi and lesion outcome after everolimus-eluting stent implantation compared to that after first-generation drug-eluting stent implantation. Int J Cardiol 2015; 179:476–483. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Chang K, Koh YS, et al. CYP2C19 poor metabolizer is associated with clinical outcome of clopidogrel therapy in acute myocardial infarction but not stable angina. Circ Cardiovasc Genet 2013; 6:514–521. [DOI] [PubMed] [Google Scholar]
- 25.Kumar RS, Douglas PS, Peterson ED, et al. Effect of race and ethnicity on outcomes with drug-eluting and bare metal stents: results in 423 965 patients in the linked National Cardiovascular Data Registry and centers for Medicare & Medicaid services payer databases. Circulation 2013; 127:1395–1403. [DOI] [PubMed] [Google Scholar]
- 26.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317. [DOI] [PubMed] [Google Scholar]
- 27.Harmsze AM, van Werkum JW, Ten Berg JM, et al. CYP2C19∗2 and CYP2C9∗3 alleles are associated with stent thrombosis: a case–control study. Eur Heart J 2010; 31:3046–3053. [DOI] [PubMed] [Google Scholar]
- 28.Park KW, Lee JM, Kang SH, et al. Safety and efficacy of second-generation everolimus-eluting Xience V stents versus zotarolimus-eluting resolute stents in real-world practice: patient-related and stent-related outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korea registries. J Am Coll Cardiol 2013; 61:536–544. [DOI] [PubMed] [Google Scholar]


