Abstract
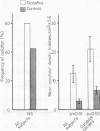
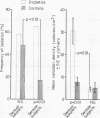
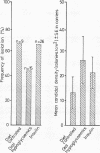
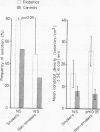
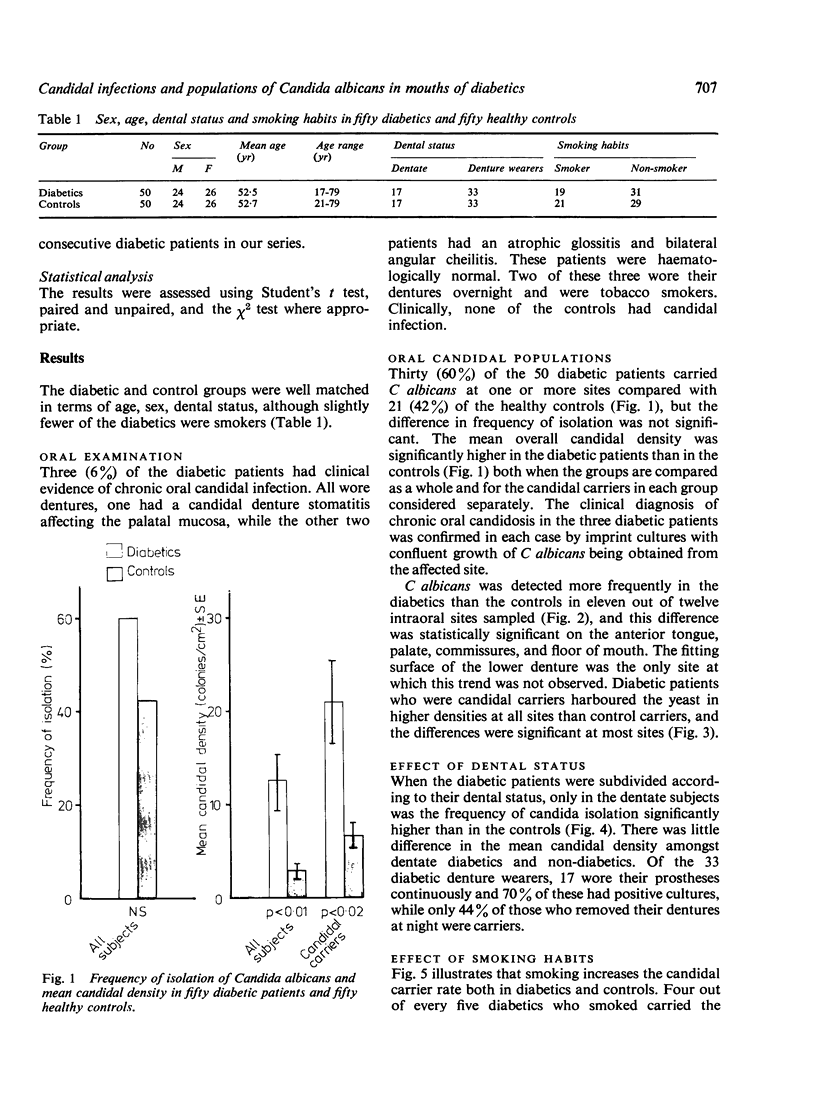
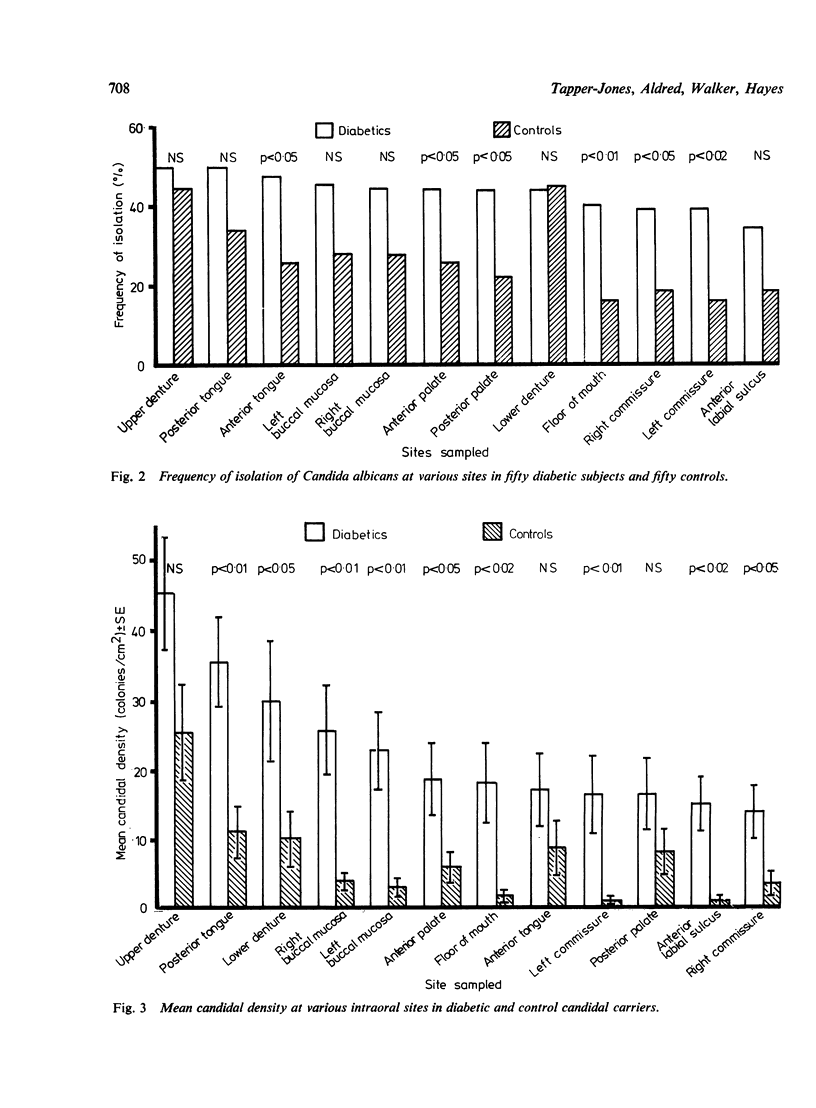
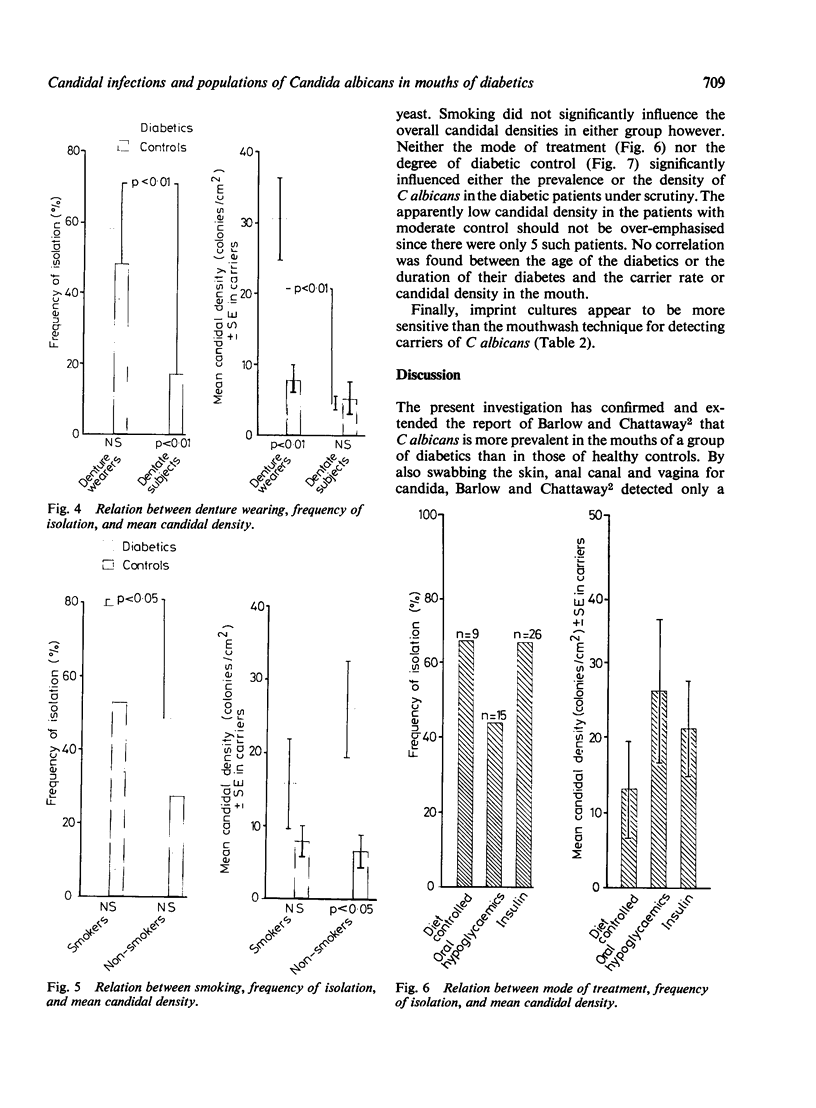
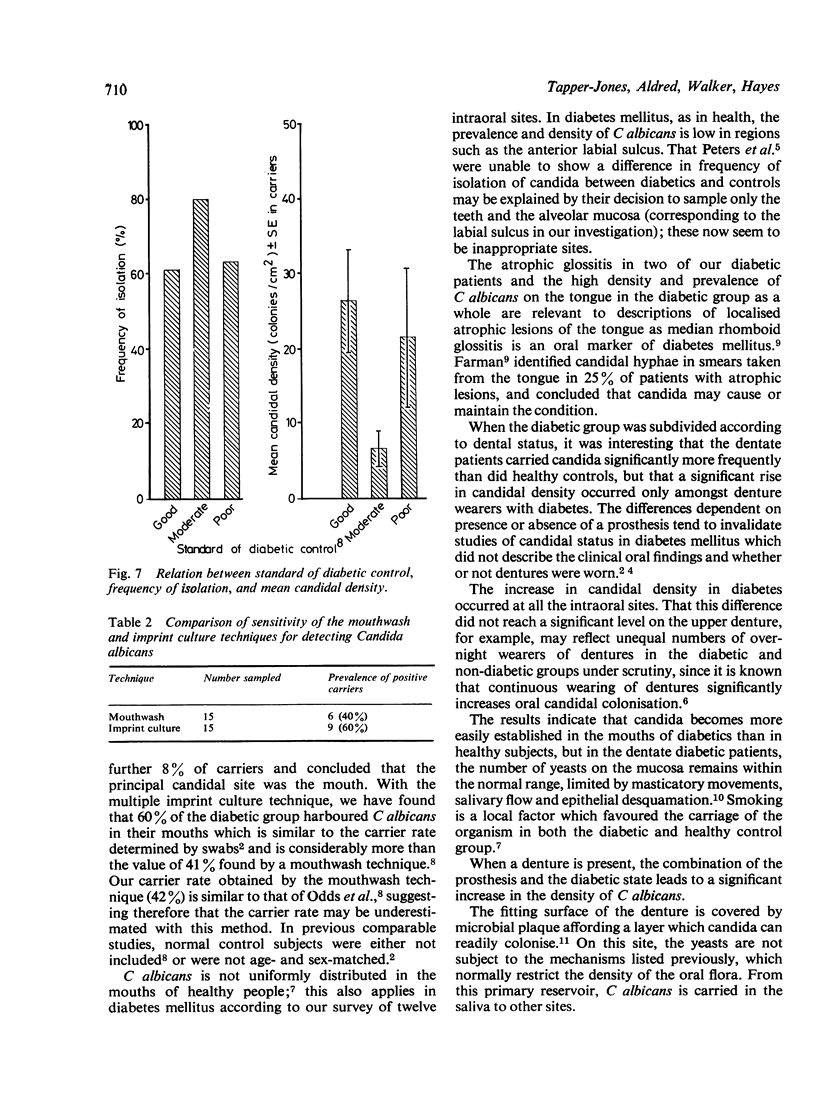
The prevalence of oral candidosis and the frequency of isolation of Candida albicans and its density and distribution have been determined in the mouths of 50 patients with diabetes mellitus and 50 healthy volunteers matched for age, sex, dental status and smoking habits. Three of the diabetic patients were found to have a chronic oral candidosis. According to an imprint culture technique, the oral carrier rate and density of C albicans were both higher in the diabetic group as a whole than in the control subjects. Smoking was associated with an increased prevalence of the yeast in diabetes mellitus. Diabetics wearing dentures had higher candidal density than those without a prosthesis. No differences in candidal status could be detected according to the degree of control of diabetes, mode of treatment, duration of diabetes or the patient's age. Local factors such as smoking and the presence of dentures, particularly when worn continuously, interact with diabetes mellitus in promoting candidal colonisation of the mouth. Attention to these predisposing factors could reduce the incidence of thrush in diabetics.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendorf T. M., Walker D. M. Oral candidal populations in health and disease. Br Dent J. 1979 Nov 20;147(10):267–272. doi: 10.1038/sj.bdj.4804344. [DOI] [PubMed] [Google Scholar]
- Arendorf T. M., Walker D. M. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol. 1980;25(1):1–10. doi: 10.1016/0003-9969(80)90147-8. [DOI] [PubMed] [Google Scholar]
- Barlow A. J., Chattaway F. W. Observations on the carriage of Candida albicans in man. Br J Dermatol. 1969 Feb;81(2):103–106. doi: 10.1111/j.1365-2133.1969.tb15988.x. [DOI] [PubMed] [Google Scholar]
- Budtz-Jörgensen E. Hibitane in the treatment of oral candidiasis. J Clin Periodontol. 1977 Dec;4(5):117–128. doi: 10.1111/j.1600-051x.1977.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Farman A. G. Atrophic lesions of the tongue: a prevalence study among 175 diabetic patients. J Oral Pathol. 1976 Sep;5(5):255–264. doi: 10.1111/j.1600-0714.1976.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Knight L., Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J Infect Dis. 1971 Apr;123(4):371–377. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- MEHNERT B., MEHNERT H. Yeasts in urine and saliva of diabetic and nondiabetic patients. Diabetes. 1958 Jul-Aug;7(4):293–297. doi: 10.2337/diab.7.4.293. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Evans E. G., Taylor M. A., Wales J. K. Prevalence of pathogenic yeasts and humoral antibodies to candida in diabetic patients. J Clin Pathol. 1978 Sep;31(9):840–844. doi: 10.1136/jcp.31.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. B., Bahn A. N., Barens G. Candida albicans in the oral cavities of diabetics. J Dent Res. 1966 May-Jun;45(3):771–777. doi: 10.1177/00220345660450034601. [DOI] [PubMed] [Google Scholar]
- Wain W. H., Price M. F., Cawson R. A. Factors affecting plaque formation by Candida albicans infecting the chick chorio-allantoic membrane. Sabouraudia. 1976 Jul;14(2):149–151. [PubMed] [Google Scholar]