Abstract
The use of plant biomass as feedstock for biomaterial and biofuel production is relevant in the current bio-based economy scenario of valorizing renewable resources. Fungi, which degrade complex and recalcitrant plant polymers, secrete different enzymes that hydrolyze plant cell wall polysaccharides. The present review discusses the current research trends on fungal, as well as extremophilic cell wall hydrolases that can withstand extreme physico-chemical conditions required in efficient industrial processes. Secretomes of fungi from the phyla Ascomycota, Basidiomycota, Zygomycota and Neocalli-mastigomycota are presented along with metabolic cues (nutrient sensing, coordination of carbon and nitrogen metabolism) affecting their composition. We conclude the review by suggesting further research avenues focused on the one hand on a comprehensive analysis of the physiology and epigenetics underlying cell wall degrading enzyme production in fungi and on the other hand on the analysis of proteins with unknown function and metagenomics of extremophilic consortia. The current advances in consolidated bioprocessing, altered secretory pathways and creation of designer plants are also examined. Furthermore, recent developments in enhancing the activity, stability and reusability of enzymes based on synergistic, proximity and entropic effects, fusion enzymes, structure-guided recombination between homologous enzymes and magnetic enzymes are considered with a view to improving saccharification.
Keywords: Lignocellulosic biomass, Saccharification, Carbohydrate-active enzymes, Fungal secretome, Extremozymes, Synergistic activation
1. Introduction
Lignocellulosic biomass of plant secondary walls is the most abundant raw material on Earth, the richest potential source of renewable energy that provides biopolymers, as well as chemicals (e.g. glucose, furfural, xylitol). It is composed of cellulose fibres reinforced by a matrix of hemicelluloses (xylan, heteroxylans and glucomannans) impregnated with lignin. Despite its abundance, lignocellulose is extremely recalcitrant to degradation because of its chemical composition and structural organization. The chemical composition of plant biomass varies depending on genotype, environmental factors [1] and developmental stage. Cellulose crystallinity, degree of substitution and branching in xylan, together with lignin macromolecular heterogeneity and variability, are major bottlenecks to the development of cost-effective processes for lignocellulose pretreatment and subsequent enzymatic hydrolysis. Furthermore, the well-defined spatial organization of different wall layers (middle lamella; primary wall layer; S1, S2 and S3 secondary wall layers) increases the complexity of the structure, making plant biomass more difficult to dismantle. This hierarchical cell wall architecture confers mechanical strength and high stability to specific plant tissues, namely the conductive vessels that need to withstand high pressures.
In nature, however, there are organisms which have evolved the ability to overcome the recalcitrance of plant biomass and release nutrients from it. These are the natural biomass utilization systems (NBUS), such as white- and brown-rot fungi, animal rumens and termite guts [2]. These NBUS are an inspiration for the development of smarter ways of lignocellulosic biomass conversion and a model for reverse engineering approaches.
The integrated study using systems biology enables understanding NBUS at different levels of complexity (namely genomic, proteomic and metabolomic). This knowledge can then be used to devise engineering strategies via synthetic biology, where modular constructs are introduced into “chassis” organisms (i.e. organisms serving as “factories”).
Fungi occupy an important position among NBUS and are a target of intense study merging next generation sequencing with metabolomics and proteomics. The integrative omics analyses of fungal systems are currently providing excellent details about the coordination of steps in lignocellulose degradation and its regulation. The integrated use of high-throughput techniques covers the whole spectrum of the plant biomass conversion processes, from single molecules, to regulons (set of genes controlled by the same regulatory factor) [3], to interaction networks.
The enzymatic deconstruction of cell wall polysaccharides to fermentable sugars is a crucial step in lignocellulose conversion. The US Department of Agriculture predicted the utilization of 18 billion gallons per year of biofuel from cellulosic biomass [4]. However, the industrial production of these enzymes is still not cost-effective; therefore biotechnology is beginning to look at plant molecular farming as an attractive solution for the large-scale production of lignocellulolytic enzymes [5] along with isolation of extremozymes and strategies to enhance activity, stability and recycling of cellulolytic enzymes based on various genetic and chemical modification approaches.
2. The realm of fungi: an Eldorado for biofuel technology
Fungi play an essential role in the C cycle within the ecosystem and are an important group of plant cell wall degraders, either as saprophytes or as parasites. Most fungi are equipped with enzymes enabling them to use plant biomass as an energy source or to invade plant tissues. Many fungal taxa have been sequenced and their genomes compared [6,7], showing the richness of carbohydrate-active enzyme pools. The extracellular enzymes (that represent the “secretome”) of fungi grown under various conditions have been analyzed [8,9]. Fungi are among the most diversified NBUS known, due to the large number of species identified. They are able to colonize diverse ecological niches and can grow on different substrates, due to the secretion of a variety of cell wall degrading enzymes (CWDEs). Their genetic ability to secrete various CWDEs is exploited to engineer novel strains and to generate genetically modified proteins with the goal of enhancing their stability and enzymatic activity (reviewed in [10]).
Fungi are also used to improve the nutritive value and digestibility of lignocellulosic residues used for fodder. Although no case related to pathogenicity has so far been reported, potential hazards due to mycotoxin contamination need to be evaluated [11]. The addition of binding agents that sequester the mycotoxin in the gastrointestinal tract of animals is a possible way to overcome problems associated with poisoning [12].
The biotechnological and ecological importance of fungi is witnessed by specific databases and projects allowing wide cross-comparative studies of fungal genomes and carbohydrate-active enzymes (Supplementary Table 1 and references therein).
2.1. Cell wall hydrolases from ascomycetes
Filamentous ascomycetes (monophyletic group of fungi that produce spores in a structure known as “ascus”) are an important group of organisms from both an ecological and an industrial point of view. For example soil-dwelling saprotrophic Aspergillii that thrive on decaying plant matter have an important role in C and N recycling by degrading lignocellulosic biomass and show gene expression fine-tuning to adapt to their natural ecological niche. Some representatives (e.g. Aspergillus niger) are important heterologous expression systems for the industrial production of cell wall hydrolytic enzymes. Many studies are available in the literature on the physiology, transcriptomics and proteomics of ascomycetes grown on different plant wall polysaccharides [13,14]. These studies highlight the importance of combining data from various high-throughput techniques to achieve a unified understanding of the fungal enzymatic potential for its better exploitation.
In this section of the review three main genera of filamentous Ascomycota (Aspergillus, Trichoderma and Neurospora) will be surveyed, because of their application in plant biomass utilization.
The production of CWDEs in ascomycete fungi is under the control of transcriptional activators, some of which can regulate the expression of CWDEs acting on both xylan and cellulose (as XlnR in Aspergillus; [15]) and repressors (like CreA/Cre1, vide infra).
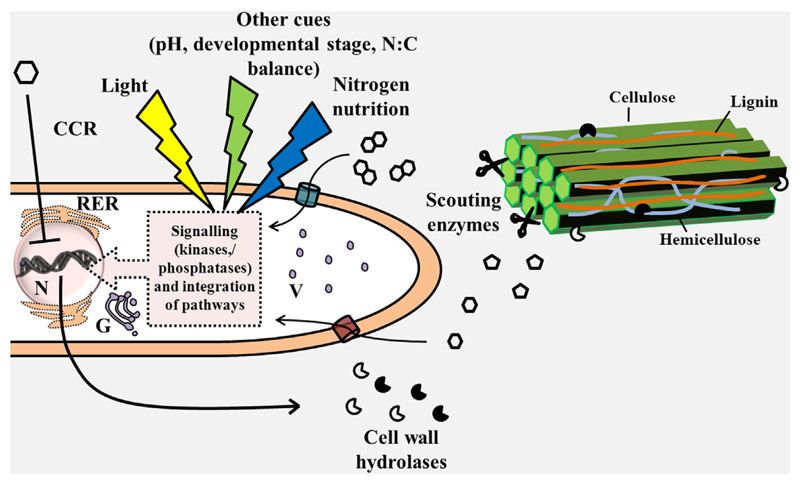
In Aspergillus, Neurospora and Trichoderma a general regulatory circuit, known as carbon catabolite repression, is present. This system is based on the simple principle that sugars such as glucose that can be promptly metabolized, are preferred over more complex substrates, such as lignocellulosic biomass components. Carbon catabolite repression relies on the zinc-finger transcription factor CreA/Cre1 that represses genes coding for xylanolytic, cellulolytic and pectinolytic enzymes, as well as genes encoding arabinanases and feruloyl esterases. CreA/Cre1 controls its own expression via glucose-6-phosphate levels [16]. Upon starvation, the constitutive expression of CWDEs degrades the substrate (they play a “scouting” role and look for available complex polysaccharide substrates) that triggers the release of monomeric sugars and oligosaccharides [15]. These function as signalling molecules and create a positive feedback resulting in increased expression of cell wall degrading genes (Fig. 1).
Fig. 1.
Schematic representation of the factors affecting plant cell wall hydrolase secretion in ascomycete filamentous fungi in the presence of simple (glucose) or complex (lignocellulosic biomass) substrates. CCR stands for carbon catabolite repression; the double hexagons represent cellobiose, the hexagon is glucose, the pentagons represent pentoses, the blue cylinder represents a transporter of cellobiose, the red cylinder a transporter of glucose. G stands for Golgi apparatus, N for nucleus, RER for rough endoplasmic reticulum, V for vesicles.
Additional regulatory mechanisms, both endogenous and environmental, exist in filamentous fungi, including epigenetics, natural antisense transcripts, coordination with secondary metabolism and light (Fig. 1; reviewed in [15]). The coordination of C and N metabolism is also important, given their role in fungal biomass production. However the details are not yet clear (see Section 4). Plausible candidates coordinating the signals arising from C and N nutrition are TOR (Target of Rapamycin) [17] and the transcription factor CpcA/Gcn4p acting in the cross-pathway control [18], because of their role in coordinating genes involved in different metabolic pathways. Both TOR kinase and CpcA/Ggn4p are regulators sensing nutrient signals and shunting them to the interior of the cell via modulation of transcription and translation. TOR modulates cell growth by coordinating mitogenic signals in response to N sources [19], while CpcA/Gcn4p regulates not only developmental programmes in response to amino acids starvation, but also the biosynthesis of secondary metabolites [20] and virulence [18].
The production of CWDEs by both Neurospora crassa and Trichoderma reesei relies on a polysaccharide sensing mechanism mediated by transporters. For example, in N. crassa the cellodextrin transporters CDT1 and CDT2 sense cellulose [21]. In Aspergillus nidulans the non-essential protein kinases SchA and SnfA sense the nutrient status (by transducing the exogenous signal via phosphorylation of proteins), control CreA intracellular re-localization (via nuclear export) and CWDEs production [17]. A transcriptomic study carried out on A. niger strain N402 grown on sugarcane bagasse subjected to steam-explosion (vide infra) revealed 3700 genes differentially expressed, that included numerous genes related to “carbohydrate metabolism” [22]. In particular, this study revealed that growth on sugarcane bagasse induced a decrease in genes involved in cell cycle progression and signalling, namely cyclin genes, cyclin-dependent kinases, G proteins and mitogen-activated protein kinases. These genes represent interesting targets for functional studies in order to understand the signalling mechanism during nutrient sensing in A. niger grown on complex substrates.
The most effective and economical pretreatment is steam-explosion [23], where lignocellulosic biomass is first heated (160–230 °C) for a short time (40–240 s) under high pressure (7–47 atm) steam and then subjected to explosive decompression. In addition to making biomass more accessible to cellulolytic enzymes, this treatment also favours hemicellulose hydrolysis [24]. It is noteworthy that addition of SO2 in steam explosion can reduce time and temperature, improve hydrolysis, and decrease the production of inhibitory compounds, with concomitant complete removal of hemicellulose [24]. SO2-steam explosion is very effective for softwoods that have a different lignocellulose chemistry (more condensed lignin) [25,26], as compared to hardwoods.
The genome sequencing of the cellulase hyperproducing strains T. reesei QM9123, QM9414, NG14 and RUT-C30 showed mutations occurring in various genes that can be linked to a higher enzyme production. For instance, in RUT-C30, mutations in cre1 and a glucosidase II alpha subunit gene involved in protein secretion were reported, along with deletion of 29 genes coding for transporters, transcriptional regulators and primary metabolism enzymes ([27] and references therein). Interestingly, mutations in genes involved in cell wall formation, plasma membrane structuring and cytoskeleton function were identified in the hyperproducer QM9414. Such genes represent important candidates for the enhanced production/secretion of CWDEs, given their established role in protein secretion and vesicle trafficking.
N. crassa is a model organism and several biochemical and molecular genetic tools have been developed in this species. However, its potential in lignocellulosic biomass degradation has only been recently investigated (reviewed in [3]). The system of transcription factors of filamentous fungi responding to different C sources is complex and needs further investigation. Screening for growth deficiency on Avicel (an industrial preparation of crystalline cellulose obtained by removing the amorphous regions, via hydrolysis of diced wood pulp with mineral acid under heat and pressure and subsequent washing and filtering) of a N. crassa transcription factor deletion collection identified two transcriptional regulators, clr-1 and clr-2 that are required for growth on cellulose (these genes control 140 genes in the Avicel regulon) and cellulase secretion [28]. These regulators belong to a specific cellulose sensing mechanism in N. crassa. A further study on the transcriptional regulation of cellulose induction in N. crassa led to the identification of the transcription factors VIB1 and COL26. VIB1 regulates cellulose utilization by acting on clr-2, although it does not directly influence hydrolase expression; COL26 has a role in glucose sensing and negatively regulates the expression of the Cre1 repressor [29].
The comparison of N. crassa phosphoproteome (i.e. the proteome characterized by phosphorylation as post-translational modification) on Avicel vs no C source showed differential phosphorylation levels of the cellobionic acid transporter CBT1 and of the transcriptional regulator CLR-1 [30]. The subsequent validation study in Saccharomyces cerevisae showed that the transport activity of CBT1 was enhanced by phosphorylation of serine and threonine residues. These results imply that the response of N. crassa to cellulose involves not only transcriptional regulation, but also post-translational modifications.
Further efforts are needed to fully understand the transcriptional mechanisms leading to CWDEs production in filamentous fungi in order to identify key regulators whose expression can be altered for the creation of constitutively hyperproducing enzyme strains that do not show product-driven feedback suppression. In this context high-throughput transcriptomics coupled to comparative genomics can provide the necessary analytical depth to identify crucial participants in this process.
The deconstruction of lignocellulosic biomass requires the synergistic action of numerous oxidative, hydrolytic and non-hydrolytic enzymes. Oxidative enzymes include laccases (oxygen oxidoreductase, EC 1.10.3.2) and class II peroxidases, such as lignin-peroxidase (EC 1.11.1.14), manganese-peroxidase (EC 1.11.1.13) and hybrid lignin/manganese versatile-peroxidase (EC 1.11.1.16) that catalyze the oxidative cleavage of C—C and C—O—C bonds in a wide variety of organic compounds, including polyphenols and lignin, by generating free-radicals [31,32]. Hydrolytic enzymes degrade both cellulose and hemicellulose polymers and include endo-cellulases (endo-1,4-β-D-glucanase, EC 3.2.1.4), that act randomly on internal cellulose bonds and generate oligosaccharides, exo-cellulases (exo-1,4-β-D-glucanase, cellobiohydrolase EC 3.2.1.91), that only attack cellulose polymer from non-reducing ends to produce disaccharide cellobiose. The disaccharide is finally hydrolyzed by β-glucosidase (EC 3.2.1.21) to glucose units. Hydrolysis of hemicelluloses, complex heteropolysaccharides, involves many enzymes acting in a cooperative manner, e.g. endo-β-1,4-xylanases (EC 3.2.1.8), β-D-xylosidase (EC 3.2.1.37), that cleave bonds in xylan, 1,4-β-D-endo-mannanases (EC 3.2.1.78) and 1,4-β-D-mannosidases (EC 3.2.1.25) that hydrolyze mannan, as well as α-L-arabinosidases, β-glucosidases, β-galactosidases, α-glucuronidases, acetylxylan esterases, arabinofuranosidases, acetylmannan esterases, feruloyl esterases, galacto- and glucomannanases, p-coumaric acid- and acetylxylan esterases [33,34].
The non-hydrolytic proteins involved in the amorphogenesis (namely swelling, breaking of H-bonds, loosening) of cellulose include swollenins and loosenins that resemble plant expansins and can disrupt crystalline cellulose, cellulose induced protein-1 and -2 [35–37]. Cellulose induced protein-1 protein has synergistic activity with swollenins, cellulose induced protein-2 cleaves hemicellulose-lignin crosslinks. In addition to cellulose crystallinity, another factor affecting plant biomass recalcitrance is the chemical composition of hemicellulose and lignin that can vary depending on the species and growth conditions.
Various cellulases and xylanases have been characterized in Aspergillus (extensively reviewed in [16]) and currently strain modifications are tested for consolidated bioprocessing (i.e. the one-step conversion of lignocellulose into value-added products). Genetic modifications have been performed to boost lipid production from lignocellulose in the oleaginous strain Aspergillus oryzae A-4. In order to avoid glucose repression, a cellulase gene from A. oryzae ATCC 42149 under the hemolysin-like protein gene promoter was introduced via protoplast transformation [38]. In this way biodiesel can be produced in a single step by consuming lignocellulosic biomass via simultaneous saccharification and enhanced lipid production [39]. The biomass is used as feedstock, whereas the sugar-rich hydrolysate is used by the fungus as an energy source for lipid production.
Among Aspergillii it is worth mentioning Aspergillus japonicus that has the ability to degrade polysaccharide and phenolic components in lignocarbohydrate complexes by demethylating, hydroxylating and cleaving aromatic rings [40]. Strain improvement via genome shuffling (an engineering approach that aims at improving phenotypes via rounds of protoplast fusion) and cyclic mutagenesis (multiple rounds of mutagenesis) has proved to be an efficient way for the increased production and catalytic efficiency of CWDEs in Aspergillus [41]. Site-directed mutagenesis and directed evolution have also been successfully employed to improve the biochemical properties (such as higher pH optimum and thermostability) of cellulases (recently reviewed in [42]) and several other hydrolases from Aspergillus [43].
Ascomycete phytopathogenic fungi such as Fusarium species have the important natural ability to degrade components of secondary cell walls and therefore they can penetrate plant tissues. Exploring their hydrolytic potential can provide means to complement and enrich enzyme preparations from widely used industrial species, such as T. reesei. For example, the proteomic analysis of F. verticilloides secretome identified 166 proteins that included carbohydrate-active enzymes acting on pectin and hemicelluloses [44]. Proteomics-assisted analysis of F. graminearum in vitro and in planta secretomes (the so-called “secretomics”) revealed that while almost all the in vitro proteins contained predicted signal peptides, only ~60% of those in planta were secreted [45].
A bioinformatics-assisted study of the predicted F. graminearum secretome has led to the prediction of 574 extracellular proteins, 99% of which are supported by transcriptional data [46]. Interestingly, the chromosomal location of these genes shows preferential occurrence in sub-telomeric regions or zones with a high frequency of chromosomal recombination. These regions are under the epigenetic control mechanism called “telomere position effects“ [47]. Their location in hot-spots of recombination increases the diversity of the secretome, thereby conferring adaptive advantages following possible changes in the host plant constitution [46]. It is noteworthy that out of 574 secreted proteins, only 278 have a putative function, whereas 296 proteins have no known function. The elucidation of the function of unknown proteins will give further impetus to efforts for identifying enzymes involved in the synergistic degradation of lignocellulosic biomass. Out of 171 proteins that were predicted to be involved in plant cell wall degradation, 30, 46, 9 and 13 were implicated to act on cellulose, hemicellulose, callose and pectin, respectively. Nine enzymes (that included peroxidases, laccases and ferulic acid esterases) involved in the dissociation of lignin-hemicellulose crosslinks were also detected. Comparison of the predicted secretome with available proteomic data sets showed that 68% of the predicted proteins were detected by the proteomic study.
2.2. Plant biomass degradation: the strategy of basidiomycetes
Basidiomycetes (phylum of fungi that reproduce via the formation of a special spore-bearing structure called “basidium”) involved in wood decay have been divided into two main classes: brown- and white-rot fungi. While white-rot fungi can degrade all the components of secondary walls, including lignin, brown-rot fungi can degrade cellulose and hemicelluloses, but can only modify lignin, that is left as a residue in the decaying wood. This difference is due to the absence of class II peroxidases from the genomes of brown-rot fungi [7,48]. The main lignin modification carried out by brown-rot fungi is a demethylation reaction, where O-methyl groups are removed. The majority of brown-rot fungi are also devoid of processive exo-cellulases and carry out non-enzymatic Fenton-based reactions generating hydroxyl radicals (Fe2+ + H2O2 → Fe3+ + •OH + OH−) that depolymerize polysaccharides. For example, a study on the genome sequence from the brown-rot fungus Postia placenta revealed the absence of exo-cellulases and the abundance of genes involved in reactive oxygen species formation [49], implying that the depolymerization of cellulose is carried out via a combination of oxidative reactions and endo-cellulases [50]. One explanation for the lack of processive exo-cellulases in the genome of brown-rot fungi is provided by their inability to efficiently cleave linkages between sugar units modified by the free radicals [50]. Indeed, mixtures containing both endo- and exo-cellulases were shown to have a lower hydrolysis rate on cellulose modified by H2O2 and Fe2+ [51].
The sampling of more than 30 genomes has revealed that the ability of basidiomycetes to degrade wood should be revised and reclassified based on a continuum, rather than on two groups [7]. In particular, this study showed that the newly sequenced fungi Botryobasidium botryosum and Jaapia argillacea possess the cellulolytic system typical of white-rot fungi but lack peroxidases, a feature that puts them closer to brown-rot fungi. Moreover, these fungi were also shown to lack laccase activity in concentrated culture filtrates ([7] and reference therein). Despite their lack of peroxidases, these fungi still degrade lignin, implying the involvement of additional factors, such as alcohol oxidase, pyranose oxidase, benzoquinone reductase, vanillyl alcohol oxidase, glucose-methanol-choline oxidoreductase, cellobiose dehydrogenase. Experiments involving decay assays demonstrated that B. botryosum and J. argillacea are able to degrade all wood components, therefore showing white-rot decay ability [7]. It is noteworthy to mention that the genome of B. botryosum contains 3 putative cellobiose dehydrogenase genes, a number which is higher than the other set of fungi analyzed by Riley et al. [7]. Cellobiose dehydrogenase is an extracellular flavocytochrome [52] and it contributes to lignocellulose degradation via the formation of hydroxyl radicals (Fenton reaction). Moreover it stimulates copper-dependent lytic polysaccharide monooxygenases (Auxiliary Activity Family 9, formerly glycosyl hydrolase 61) [53,54]. Lytic polysaccharide monooxygenases perform the oxidative breakdown of cellulose in the presence of divalent metal ions and by using an external electron donor (as for instance gallic acid, ascorbic acid, reduced glutathione and also lignin [55,56]). They have the active site on a flat face, in contrast to the pockets/tunnels of cellulases, and they can take contact with crystalline regions of cellulose and subsequently oxidize them.
Enzymes involved in secondary metabolism, like terpene and polyketide synthases, also play an important role in wood decay, as they are linked to fungal survival in the specific ecological niche occupied. The involvement of secondary metabolites is very interesting if one considers their role in the synthesis of melanin, a pigment that in phytopathogenic fungi has been associated with virulence. The formation of melanized appressoria (fungal structures that generate high turgor pressure to penetrate plant tissues) is associated with the production of glycosyl hydrolases in Magnapothe oryzae [57].
The link between secondary metabolism and secretion of carbohydrate-active enzymes is not surprising, as this phenomenon was described in T. reesei where genomic regions rich in gene coding for carbohydrate-active enzymes also contain polyketide synthases. Polyketide synthases would confer the ability to compete with other organisms in the environment through the biosynthesis of secondary metabolites [27]. In T. reesei the regulator of secondary metabolism LaeA [58] (a methyltransferase acting at the level of heterochromatin structure) controls cellulose/hemicellulase expression in a manner that is not obviously correlated with histone methylation [27].
Traditionally, enzymes involved in plant cell wall polysaccharide degradation in basidiomycetes have been given less importance than those of ascomycetes, since the latter are more exploited from the industrial point of view [48]. Nevertheless, the polysaccharide degrading machinery of the basidiomycete Flammulina velutipes and its utility in the production of bioethanol has recently emerged [59]. Similar to ascomycetes, the secretome of basidiomycetes has been widely studied (recently reviewed in [8]) and can be used as a source of catalysts to enrich enzyme cocktails currently used for efficient plant biomass deconstruction. Quantitative proteomics studies on Phanerochaete chrysosporium have revealed that the production of lignocellulolytic enzymes not only depends on the C source, but is also influenced by the particle size and complexity of lignocellulosic biomass [60]. Nitrogen limitation triggers the production of lignin degrading enzymes.
Basidiomycete plant pathogens are also studied for supplementing the enzymatic cocktail for plant biomass degradation. For example, the crop pathogen Ustilago maydis has mechanisms for recognizing plant-surface cues that regulate, among other genes, the expression of CWDEs required for the penetration of plant tissues [61]. The surface sensing mechanism is also used by A. nidulans: the extracellular mucin MsbA that influences biofilm formation and attachment to the substrate, regulates cellulase secretion and activity [62].
2.3. Cell wall degrading enzymes from other fungal phyla
CWDEs from other fungal phyla (Zygomycota and anaerobic chytridiomycete gut fungi) are also currently attracting attention and are being studied using high-throughput technologies [63,64].
The annotation of Rhizopus oryzae genome identified a total of 317 carbohydrate-active enzymes, a number that is lower than filamentous ascomycetes. Out of 317 carbohydrate-active enzymes, 116 and 130 were predicted to be glycosyl hydrolase- and glycosyltransferase-encoding genes respectively. The number of glycosyltransferases is higher than other fungi [63], a finding that can be explained by the whole-genome duplication of R. oryzae. This may have led to the expansion in certain gene families, such as the cell wall biosynthesis-related family (especially glycosyltransferases involved in chitin synthesis) [65]. Interestingly, R. oryzae genome contains a high number of endo-glucanases from family 45, but lacks Auxiliary Activity Family 9 (formerly glycosyl hydrolase 61). In addition, none of the glycosyl hydrolases from family 3 encodes predicted β-glucosidases. Three of the five glycosyl hydrolases from family 45 were previously shown to encode functional enzymes with a carbohydrate binding module ([63] and references therein). Since neither β-glucosidases nor genes coding for glycosyl hydrolases from family 61 were found in R. oryzae, it is plausible to assume that the cellulolytic enzymatic system in this fungus relies on glycosyl hydrolases from family 45 and that cellulose is not used as a nutrient source.
Compared to aerobic fungi, anaerobic gut fungi have received less attention despite their important role in plant biomass deconstruction, mainly because of the difficulties in isolation and cultivation. However, recent studies on novel isolates and the screening of their carbohydrate-active enzymes are offering valuable insights for further analysis and industrial applications. For example a new strain isolated from cattle rumen, Orpynomyces sp. Y102, produces an enzyme with exo-cellulase activity, CelC7, that contains a dockerin domain at the N-terminus and a C-terminal cellobiohydrolase catalytic domain [66]. Anaerobic fungi typically inhabit the digestive tract of ruminants and degrade plant particles of different sizes [67]. The ability of anaerobic gut fungi to degrade plant matter of various dimensions depends on their cellulolytic system and on the formation of a rhizomycelium (a system of rhizoids that resemble a mycelium) that envelops and penetrates the substrate. This system is characterized by extracellular enzymes forming high molecular weight complexes (cellulosomes) via dockerins (i.e. domains in cellulosomes mediating the interaction with a membrane-bound non-catalytic platform). The cellulosome can further interact with other secreted enzymes via dockerins, such as cellulases from the same organism, and this interaction creates high molecular mass cellulolytic systems not attached to the host cell membrane ([67] and references therein). The exact fungal cellulosome stoichiometry is still a target of investigation; however a recent study carried out on Neocallimastix patriciarum J11 has shown that at least three types of cellulases, i.e. one endo- and two exo-glucanases, are present in the cellulosome of this organism [68]. A previous study on the same fungus showed that it is unable to disrupt the ether bond of hydroxycinnamic acid bridges between lignin and polysaccharides. Therefore N. patriciarum solubilizes lignocarbohydrate complexes by degrading xylan, rather than by depolymerizing lignin [69].
The recent genome analysis of the anaerobic gut fungus Orpinomyces sp. strain C1A revealed features that are absent from other Dikarya (subkingdom of Fungi comprising Ascomycota and Basidiomycota) members and are shared instead by early-branching fungi and non-fungal opisthokonts [70]. This study also revealed that the glycosyl hydrolase set of Orpinomyces is very different from that of aerobic fungi: the gut fungus has glycosyl hydrolases from family 8 and 48 that are only rarely found in aerobic fungi.
Several cell wall hydrolases have already been characterized in anaerobic gut fungi from mammalian herbivores, as well as the reptilian herbivore iguana (reviewed in [67]) with interesting characteristics such as pH (80% of activity retained over a range of pH from 5 to 11) and heat stability [69]. The pH and temperature stability, along with their efficient hydrolytic performance (e.g. for a cellobiohydrolase from N. patriciarum J11 a high specific activity of 1809 U mg−1 protein was observed on barley β-glucan), make these enzymes interesting for biotechnological applications.
3. The potential of extremozymes for plant biomass deconstruction
The industrial conversion of lignocellulosic biomass requires a pretreatment step to enable the subsequent enzymatic saccharification. This is often characterized by a combination of extreme physico-chemical conditions involving high/low pH and temperatures, elevated pressure and high salt concentrations in order to remove lignin, reduce cellulose crystallinity and increase porosity, so that cellulose fibres become more amenable to hydrolysis by enzymes. Since lignocellulosic biomass pretreatment uses harsh conditions (high temperatures and pressures; extremes of pH), extremozymes (i.e. enzymes capable of performing catalysis under extreme conditions) are advantageous to perform catalysis during pretreatment steps to minimize the cost and complication of varying process conditions between pretreatment and enzymatic hydrolysis steps.
Archaea, bacteria and fungi synthesize extremozymes, some of which have polyextremophilic properties. Polyextremophilic enzymes can simultaneously withstand a combination of more than one harsh condition such as high temperature and pressure (thermopiezophilic), low temperature and high pressure (psychropiezophilic), or high temperature and low pH (thermoacidophilic) [71].
Thermophilic hydrolases from prokaryotes have been expressed in planta that achieve autohydrolysis of polysaccharide components of secondary cell walls (see Section 3.1). These enzymes were shown not only to be stable and expressed at high levels (without hindering the development of the plants), but also to decrease the biomass recalcitrance [72].
In the following sections thermally adapted (psychrophilic, thermophilic and hyper-thermophilic), pH-adapted (acidophilic and alkaliphilic), high pressure-adapted (piezophilic/barophilic), halophilic and polyextremophilic CWDEs of archaeal and bacterial origin will be discussed to stress their importance in the saccharification process. It is noteworthy that, compared to bacterial and archaeal species, fungi have generally been thought of producing only mildly thermally and pH-adapted enzymes. The main enzymatic properties regarding activity, pH, temperature and salt-tolerance of selected extremozymes, mesophilic and genetically-modified enzymes are given in Supplementary Table 2.
3.1. Thermally adapted (psychrophilic and thermophilic) extremozymes
Fungi, bacteria and archaea are an important source of thermally adapted hydrolytic enzymes useful for industrial processes related to plant biomass deconstruction. These organisms are receiving increasing interest because their enzymes enable saccharification at higher temperatures, a feature that decreases substrate viscosity in the reactors [73], shortens the reaction time (reaction rate approximately doubles for every 10 °C rise in temperature) and avoids contamination.
Structural factors responsible for higher protein stability have already been discussed in detail before [74].
The properties of CWDEs from thermophilic fungi have been extensively surveyed in [75]. Among thermophilic fungi, the ascomycete Myceliophothora thermophila has received considerable attention lately as a producer of thermophilic CWDEs [76].
Bacteria and archaea colonizing and thriving in extreme thermal environments (4–100 °C) have been in the spotlight of biotechnology for many years now. The need to find new carbohydrate-active enzymes with enhanced properties for large-scale applications in plant biomass conversion has driven the intensive research around hyperthermophiles.
Archaea have extreme thermophilic properties and members of the Thermococcales can even grow beyond 100 °C (reviewed in [77]). Despite the biotechnological relevance of archaeal extremozymes, their expression in heterologous hosts is often difficult, as misfolding and tendency to aggregate are typical [78]. One possible alternative to partially overcome this problem is represented by the creation of chimaeras (proteins created by fusing together 2 or more genes), which couple the extremophilic properties (low pH) of an insoluble enzyme with the solubility and extreme thermostability of another through in vitro recombination (N- and C-termini of Thermotoga maritima and intervening sequence from Sulfolobus solfataricus). Such an approach has resulted in the successful refolding of an active hybrid cellulase (Topt, 85 °C; pHopt, 3) [Supplementary Reference 27], that was designed by combining the thermoacidophilic properties of the cellulase from S. solfataricus (Topt, 80 °C; pHopt, 1.8) and the extreme thermophilic and partial solubility of the enzyme from T. maritima (Topt, 95 °C; pHopt, 5) (Supplementary Table 2). However, more work is needed to test additional combinations without disrupting the active-site region, as the chimaeric cellulase had significantly less activity. Mapping amino acid changes to the structure and function will reveal sites that are involved in the modulation of activity, stability and pH. The identification of “hotspot” regions in structures of industrially relevant cellulolytic enzymes will generate vital information that can help design more stable and active proteins by genetic modifications for efficient saccharification (see point # 9 under Section 4).
Despite the exploitation of most of the available C sources, members of archaea, especially hyperthermophiles, have rarely been found to grow on lignocellulose. There are only a handful of organisms that can deconstruct lignocellulosic biomass, especially crystalline cellulose around 90 °C. A study focused on the analysis of an archaeal consortium growing on crystalline cellulose at 90 °C led to the identification of a cellulase, EBI-244, with unique properties [79]. This glycosyl hydrolase from family 5 has an optimal activity at 109 °C on crystalline and soluble substrates, Tm of 113 °C, half-life of 4.5 h and 0.2 h at 100 and 108 °C, respectively, can withstand high salt concentrations and ionic liquids and is resistant to detergents. These properties translate into high productivity of 1241 and 8261 μmol glucose-equivalents per μmol enzyme per 15 h on Avicel and Avicel pretreated with ionic liquid, respectively (Supplementary Table 2). These results highlight the power of metagenomic studies on microorganism consortia and provide a way to identify enzymes with desired cellulolytic properties. The hyperthermophilic endo-cellulase described above is the most thermotolerant endo-glucanase reported to date and has been proposed for the efficient and cost-effective deconstruction of lignocellulosic biomass during pretreatment steps involving high temperatures and ionic liquids. However, in order to use this enzyme during the steam-explosion pretreatment step, it is necessary to obtain cellulolytic enzymes capable of simultaneously withstanding high temperature as well as high pressure (see Section 3.3).
An efficient and cost-effective way to convert lignocellulosic biomass is to clone extremophilic enzymes in plants where they are inactive during plant growth and are only expressed when induced. Recently a cellulase gene from the hyperthermophilic archaeon S. solfataricus was expressed in plants and subsequently activated at high temperatures (maximum activity at around 90 °C). The recombinant cellulase was able to degrade complex cell wall preparations under harsh pretreatment conditions (such as high salt concentration and low pH) at 90 °C in the presence of ionic liquids that help dissolve crystalline cellulose. This study nicely demonstrates the significance of using plants themselves as factories for producing CWDEs [80]. The enzyme is not active at the temperatures used for plant growth, but will be activated at the stage of pretreatment, after harvesting of the biomass for further processing. Additional work along these lines is needed to incorporate lignin-degrading enzymes, as well as β-glucosidase, without affecting plant growth (see Section 4).
The hydrolysis of cellulose by endo- and exo-cellulases produces cellobiose that results in severe inhibition of both enzymes [81]. The addition of β-glucosidase will facilitate cellobiose conversion to glucose for simultaneous saccharification and fermentation (a process during which enzymatic hydrolysis and fermentation to ethanol are carried out simultaneously) for cost-effective biofuel production. An extremely thermophilic β-glucosidase was characterized from Pyrococcus furiosus: this enzyme has optimum activity at 102–105 °C and pH 5 [82].
A study using mesophilic bacterial enzymes successfully fused endo-glucanase and β-glucosidase attaining higher catalytic efficiencies and thermostabilities, with concomitant 2-fold enhanced glucose yield on alkali-treated rice straw as compared to the individual enzymes [83].
Most anaerobic bacteria (and anaerobic fungi, see Section 2.3) adopt a cellulolytic strategy based on the secretion of cellulosomes, while the majority of aerobic bacteria secrete free cellulases and hemicellulases (i.e. xylanases and β-xylosidases). The cellulosome architecture has been an inspiration for the design of a thermostable complex known as “rosettazyme” (a supramolecular structure with glucanase activity) [84]. This protein complex is formed via the interaction of “rosettasomes”, i.e. thermostable chaperonins (proteins that favour the correct folding of other proteins) from the extremophilic archaeon Sulfolobus shibatae, that assemble into an 18-subunit, double ring protein structure fused with cohesin domains (a module that, together with dockerin, is responsible for the organization of the multi-enzyme cellulosome complex) from Clostridium thermocellum. This structure binds dockerin-containing glucanases and acts like a cellulosome.
The thermophile Caldicellulosiruptor bescii has a cellulolytic strategy that depends on the secretion of many free cellulases with multiple catalytic domains. One of the cellulases secreted by this bacterium, CelA, is characterized by the occurrence of a glycosyl hydrolase family 9 and 48 catalytic domains together with three carbohydrate binding motifs. It was recently realized that beyond the surface ablation (i.e. erosion) mechanism shared by processive cellulases, CelA also burrows cavities into the surface of the substrate [85].
Psychrophilic enzymes that are active at very low temperatures have structural features opposite to that of thermophilic homologues and are characterized by a flexible structure with concomitant high activity and low stability near room temperature, in accordance with activity-stability trade-off. Although psychrophilic enzymes have huge potential for various biotechnological applications, they are yet to be exploited commercially [86]. Due to their high intrinsic activity (kcat) around room temperatures (20–40 °C), psychrophilic enzymes can potentially be applied before or after the biomass pretreatment step where substantial energy savings can be achieved and thermally generated side reactions minimized. Cellulases that allow simultaneous saccharification and fermentation are industrially desirable, but their applications are limited by different temperature optima for enzyme activity and microbial fermentation. Psychrophilic cellulases may provide an ideal solution to couple both processes around room temperature. Several psychrophilic hydrolases have already been identified and described (Supplementary Table 2). In a recent study, a cocktail of lignin-depolymerizing (laccase and manganese-independent peroxidases) enzymes from the cold-adapted fungus Cladosporium cladosporioides and a cheap commercial xylanase were shown to synergistically degrade lignocellulosic biomass (milled Jerusalem artichoke stalks) ~6-fold more efficiently and cost-effectively than mesophilic commercial cellulases alone, after 120 h around room temperature (28 °C). The reducing sugars yield (10 mg ml−1) achieved by the mixture of cold-adapted fungal lignin degrading enzymes and xylanase on milled Jerusalem artichoke stalks were ~3.5 times higher compared to a commercial fungal–bacterial cocktail of cellulases and xylanases within 4 days at 28 °C [87]. This study is a significant step forward towards achieving the goal of applying psychrophilic enzymes in a commercial setting for cell wall biomass conversion. It would be interesting to test if this process can be made even more efficient by taking advantage of hybrid fungal versatile peroxidase that show synergistic activation when both manganese- and lignin-peroxidases are simultaneously active. Whereas bulky lignin-versatile peroxidases depolymerize lignin by free-radical mechanism, manganese-peroxidases oxidize Mn2+ to Mn3+ which can diffuse inside the bulky lignin molecule and break bonds [32].
3.2. pH-adapted and halophilic cell wall hydrolases
The properties and applications of acidophilic and alkaliphilic enzymes have already been extensively reviewed [88]. The biochemical features of some pH-adapted and halophilic hydrolases are summarized in Supplementary Table 2. The majority of fungal cellulases cannot tolerate salts and ionic liquids (used as solvents during pretreatment and to decrease cellulose crystallinity); therefore, extensive washing steps have to be performed to achieve the right conditions for subsequent saccharification. However a recent study reported the existence of fungi that are able to tolerate very high concentrations of destabilizing salts (i.e. chaotropic salts, like MgCl2, and CaCl2) [89]. These fungi, that belong to the genus Wallemia, Cladosporium, Eurotium, Hortaea, can grow in environments with very low water activity.
In this section dedicated to pH-adapted and halophilic CWDEs it is worth reporting the results of a recent study focused on the secretome of the halotolerant mangrove fungus Pestalotiopsis sp. NCi6 (phylum Ascomycota). Proteomics showed that the lignocellulolytic secretome of this fungus changes when cultured with/without salt and that the addition of salt favours the secretion of (hemi)cellulolytic enzymes, while it decreases the production of oxidases for lignin deconstruction. In particular the increase in (hemi)cellulolytic activity is linked to the induction of genes encoding glycosyl hydrolases (xylanases) from families 10, 30 and 43 and Auxiliary Activity Family 9 (formerly glycosyl hydrolase 61) [90]. Genomics of acidophilic (e.g. Purpureocillium lilacinum recently isolated from Río Tinto [91]) and alkaliphilic (e.g. Sodiomyces alkalinus from soda soils [92]) fungi can lead to the further identification of superior catalysts withstanding low/high pH, that can be used to improve existing enzyme cocktails.
Enzymes capable of withstanding higher concentrations of salt and ionic liquids and higher pH make the washing steps less cumbersome and are consequently very useful in the industrial biomass conversion process. As discussed in Section 3.1, it is worth reiterating that the hyperthermostable archaeal endo-glucanase EBI244, in addition to showing activity on crystalline cellulose, also retained considerable activity (30–50%) in the presence of high concentrations of salt (2.5 M NaCl) and ionic liquid (25% (v/v) 1,3-dimethylimidazolium dimethyl phosphate) at 90 °C [79].
β-Glucosidases catalyzes the last reaction in the cellulose degradation pathway by hydrolyzing cellobiose to glucose thus relieving product inhibition of endo- and exo-glucanases and increasing the overall rate of saccharification [93]. Recently, an alkaliphilic β-glucosidase isolated by functional metagenomics showed not only broad pH optimum (5–10) and pH stability (8–12), but also an activation up to 250% in the presence of 1 M glucose. The lack of inhibition by high concentrations of glucose is a critical feature for efficient saccharification, as normally β-glucosidases show inhibition in the low concentration (mM) range [94].
3.3. Piezophilic and polyextremophilic extremozymes
Polyextremozymes are enzymes that can withstand any combination of more than one extreme condition, such as low and high temperatures, high pressure (piezophilic), high salt concentrations and extremes of pH. With an increase in water depth, hydrostatic pressure increases, whereas temperature drops rapidly. Therefore high-pressure adapted organisms are also adapted to low-temperatures (psychropiezophilic) [95]. However, exceptions exist in the case of organisms living near hydrothermal vents, such as black smokers. These organisms are simultaneously adapted to both high temperatures and pressures (thermopiezophilic) [96]. Due to logistic challenges of deep-sea exploration, very few enzymes from piezophilic organisms have been isolated. To the best of our knowledge, no high-pressure adapted CWDEs have been described, although enzymes degrading cellulose and/or hemicellulose have been identified in the genomes of piezophilic organisms [97] and [98]. One such thermopiezophilic archaeon (Thermococcus sibiricus), found at a depth of 2.3 km (~234 atm), can grow on cellulosic substrates at around 84 °C [99]. Poyextremozymes can be very valuable for applications in efficient and economical biomass conversion, especially during the pretreatment steps [24]. Since pretreatment (see section 3) can be carried out using either high-pressure steam-explosion (160–230 °C) or high-pressure CO2 explosion (35 °C), thermopiezophilic and psychropiezophilic CWDEs adapted to a range of pressures (4–47 atm) will be very valuable [24]. There are only a few examples where polyextremophilic cellulases or xylanases have been used for biomass conversion [100]. Although genomes of polyextremophilic organisms containing cellulases have been described [101], further research is needed to kinetically characterize piezophilic and polyextremophilic enzymes in detail and optimize their function under real biomass conversion conditions.
4. Future perspectives
The economic importance of plant lignocellulosic biomass as a source of chemicals and bioenergy continually drives the field towards improvements, despite technical challenges that include but are not limited to the slow turn-over and high cost of CWDEs. A multi-pronged approach is needed to efficiently and cost-effectively exploit lignocellulosic biomass. Hereafter is a list of the major targets that, if achieved concurrently, will be able to meet these challenges.
Fungal nutritional cues: A deeper investigation of the physiological factors affecting the secretion of CWDEs from fungi is desirable in order to engineer hyper-producing strains and improve fermentation procedures of filamentous fungi. The study of the impact that C and N sources have on the composition of fungal secretomes and on fermentation conversion efficiency is a good example. C and N sources affect both fungal biomass production, secretion of hydrolytic enzymes and protein production, by acting on regulators (e.g. transcription factors, see also Section 2.1) involved in metabolic pathways, as well as cell cycle progression, although the molecular mechanisms involved are not yet fully deciphered. Moreover, the interplay between C and N sources needs to be investigated using high-throughput approaches (proteomics, transcriptomics and metabolomics) to identify critical factors involved in the production and secretion of CWDEs.
Hyperproduction of enzymes: Concerning the heterologous production of CWDEs in filamentous fungi, modifications in cellular C fluxes via metabolic engineering of important pathways (for instance the oxidative pentose phosphate pathway for the production of NADPH) can increase the yield of the desired product. The deeper understanding of the vesicle trafficking mechanisms (responsible for the secretion of cell wall hydrolases) and the unfolded protein response (which is responsible for the misfolding of heterologous proteins in the endoplasmic reticulum) in fungi are likewise very important to improve the heterologous production of secreted CWDEs.
Fungal epigenetics: Epigenetic regulatory factors acting as a response to nutrient availability and environmental signals in fungi should be taken into account, especially when the genes are positioned near centromeres or telomeres (as these regions are often under the control of histone post-translational modifications and DNA methylation). The study of the impact of different environmental factors (such as light, temperature, pH and N/C sources) at the epigenetic level can help engineer novel strains with enhanced CWDEs production. The technique of chromatin immunoprecipitation-sequencing (termed ChIP-seq using next generation sequencing techniques) can for instance be used on wild-type/hyperproducer/mutant strains displaying, at a genome-wide scale, alterations in N/C response and thus allows the identification of regulators involved in CWDEs production. These strains can be grown under different nutritional and environmental conditions to study the alterations in the response to nutritional cues and the relationship with CWDEs production.
Organelle engineering: The modification of microbial strains using organelle engineering is offering promising results. For example “peroxicretion”, i.e. an artificial secretory pathway triggering fusion of peroxisomes with the plasma membrane [102], is an attractive approach for the expression of secreted hydrolases for plant biomass degradation. Hyperproducing fungal strains, like T. reesei RUT-C30, show a “pulsing” mode of secretion due to the endoplasmic reticulum subdomains reorganization following protein overload [103]. Peroxicretion could therefore represent an alternative secretion pathway limiting endoplasmic reticulum overload/stress.
Environmental metagenomics: More efforts should be devoted to the metagenomic study of microorganism consortia, especially those thriving in extreme environments, to identify novel candidates with potential industrial applications. Likewise, the symbiotic microbial consortia from the termite gut deserve further studies based on high-throughput sequencing and biochemical characterization. These consortia are composed of representatives from all three domains of life, namely Archaea, Bacteria and Eukarya, that make the termite gut an extremely efficient mini-bioreactor [104]. Mining genes from environmental samples is an added advantage, because a high proportion of these organisms are currently nonculturable.
Genomic “dark matter” exploration: Besides looking into the carbohydrate-active enzyme pool of microorganisms living in extreme environments, biotechnology has recently started looking into the so-called genomic “dark matter” that corresponds to the portions of genomes coding for proteins with unknown functions [105]. After screening more than 5000 microbial genomes, a recent study identified 17 genes coding for putative cellulases [105]. The subsequent heterologous expression in E. coli confirmed the cellulolytic activity of 11 enzymes. The identification and functional characterization of proteins annotated as “hypothetical” in the genomes of sequenced microorganisms holds great potential for the discovery of new biocatalysts for lignocellulosic biomass conversion [77]. It would be very interesting to apply a similar approach to the genomes of extremophilic organisms to mine for additional hydrolytic enzymes.
Designer plants: Further research is needed to construct “biomass designer plants” producing CWDEs that are inactive during the growth phase, but that can be induced. The inactivation can be because: (a) they are expressed as inactive precursors, (b) they are sequestered into cell organelles, (c) they are active only at very high temperatures (see Section 3.1), (d) they possess thermostable self-splicing inteins (i.e. a protein fragment capable of self-excision) [106].
Consolidated bioprocessing: Consolidated bioprocessing efforts are also promising for lowering the cost associated with plant biomass conversion. The engineering of thermophilic bacteria for the production of ethanol directly from plant biomass and without pretreatment (a technique known as single step bioprocessing) is an inspiration for future consolidated bioprocessing strategies. Such an approach has been recently applied to the thermophilic bacterium C. bescii, that was engineered to heterologously produce a bifunctional acetaldehyde and alcohol dehydrogenase from C. thermocellum with the inability to synthesize lactate (the lack of lactate synthesis triggers a diversion of the metabolic flux to the production of more acetate and ethanol via the Embden–Meyerhof–Parnas pathway) [107].
Genetically modified novel enzymes: More efforts should be devoted to improve the activity and thermal stability of cell wall hydrolytic enzymes. Both rational (protein engineering) and irrational (directed evolution) designs can be employed to increase the enzyme diversity. SCHEMA structure-guided recombination between homologous enzymes is proving a very powerful approach to expand natural diversity with artificial diversity for improvement in enzymatic properties [108]. It is noteworthy that a protein rarely combines all the suitable catalytic properties (such as thermal, kinetic, pH and inactivation against harsh additives and inhibitors) in a single enzyme required for the efficient and cost-effective deconstruction of biomass. Structure-guided recombination can be used to merge several beneficial catalytic properties from homologous enzymes into a single cellulolytic or xylanolytic enzyme. For example, Arnold’s group has generated a huge number of synthetic enzymes by recombining and exchanging various sequence blocks from five parental thermophilic fungal exo-cellulases, in order to formulate novel cellulolytic enzyme mixtures for efficient biomass conversion [108]. The synthetic chimeric cellulases were expressed in active form and showed better thermostability (t1/2 of inactivation) and thermoactivity (Topt) at higher temperatures compared to the most stable parental wild type enzyme. Protein engineering of chimeric enzymes further extended the thermostability by 4.7 °C, resulting in an exo-cellulase variant that is 9.2 ° C higher than the most stable native enzyme from Talaromyces emersonii. Importantly, the enhanced thermostability and Topt (Δ10 °C) were translated to a 50% increase in sugar production from crystalline cellulose relative to the wild-type enzyme from T. emersonii [109]. Readers are referred to excellent papers by Arnold’s group for detailed discussions on this topic, which holds great promise for increasing the repertoire of homologous cellulolytic enzymes with diverse sequences and improved catalytic properties. Although thermostable (Topt, 80 °C) laccases have been reported from Opuntia vulgaris [110], the majority of ligninolytic enzymes characterized to date (lignin peroxidases, manganese-peroxidases, versatile peroxidases) are from psychrophilic and mesophilic fungi, and likewise these enzymes suffer from low stability at high temperatures. It would be interesting to see if structure-guided recombination also succeeds in generating a collection of more thermostable variants of peroxidases and laccases that can be employed for pretreatment, as well as efficient saccharification.
Synergistic effects: Cellulolytic, xylanolytic and lignin degrading enzymes are known to show positive synergy when present in close proximity to each other and this synergistic effect can be exploited for the efficient biomass conversion [111]. Such coupled systems consist of two or more enzymes present together in a reaction vessel, natural or artificially synthesized fusion enzymes, naturally found scaffold complex such as the cellulosome and two or more enzymes displayed on a cell surface, or immobilized on a particle [111]. The fusion enzymes show improved catalytic activities compared to single enzymes acting together, either due to close proximity (see below, point # 11) of active sites that enables efficient substrate/intermediate channelling before diffusion occurs, or as a result of a conformational change [111]. Fusion enzymes tend to show enhanced stabilities and reduced protease susceptibility [111]. Importantly, production costs are reduced when two enzymes are fused together into a single enzyme. The yield of the enzyme that alone might be produced in lower amounts is enhanced in a fused form. The creation of multifunctional complexes comprising more than one cellulolytic and/or xylanolytic enzyme (such as endo-/exo-glucanases, xylanases and β-glucosidase) with improved catalytic properties holds great promise for the efficient and cost-effective hydrolysis of biomass [111]. The choice of enzymes for end-to-end gene fusions (i.e. fusions of two genes carried out with an Overlap Extension PCR that introduces an intergenic linker and restriction sites at the extremities) depends on the hydrolysis conditions (types of substrate, temperature, pH, pressure and presence of additives) and possible synergistic effects [111]. Moreover, the orientation of the fused enzymes is also critical for the activity and stability of the complex, due to improper folding and protein interaction. Although hybrid construct between xylanase and cellulase from a thermostable organism showed enhanced activities on birchwood xylan and β-glucan respectively, as compared to when both enzymes were separate, thermostabilty, Topt and hydrolysis rates were dependent on the relative orientation of both enzymes [112]. Similarly, end-to-end fusion of thermophilic (Tm, 70.5 °C) endo-cellulase and mesophilic β-glucosidase (Tm, 54.5 °C) produced a bifunctional complex that showed improved thermostability (Tm, 65.5 °C) with an enhanced activity of 92% on insoluble cellulose. The endocellulase/β-glucosidase hybrid released 2-fold more glucose and accumulated 3-fold less cellobiose as compared to the mixture of both enzymes. Further analysis showed that increased glucose yield in the hybrid is because cellobiose produced by endo-cellulase is directly used by β-glucosidase, without diffusing in the medium. Interestingly, fusion hybrids where enzyme orientation was reversed showed decreased activity [113]. Readers are referred to a recent review [111] for a thorough discussion about the fusion enzyme technology and a list of fusion cellulolytic enzymes created to date. However, to the best of our knowledge, it remains to be seen if more than two different enzymes can be successfully fused together with improved saccharification. Moreover, further research is required for integrating this technology with a complete set of lignocellulose degrading enzymes. Combining two advantageous characteristics from an extremophilic enzyme is another approach for the utilization of fusion technology. An example, combining the beneficial features of two cellulases in a hybrid protein has been discussed earlier (see Section 3.1).
Proximity effects: The conversion of cellulosic biomass, especially for the purpose of making biofuels, is still relatively expensive because of the involvement of multiple steps and slow turnover of cellulases, due to the complex threedimensional (crystalline) structure of cellulose. For example, hydrolysis of amorphous starch by amylases is hundred times faster than cellulose hydrolysis [114]. Ways to overcome both hurdles involves the concerted action of at least three enzymes (exo-processing cellobiohydrolase, endo-glucanase and β-glucosidase) for the hydrolysis of cellulose and other enzymes such as xylanase and oxidative enzymes (laccase, manganese-peroxidase and lignin-peroxidase) for the degradation of hemicellulose and lignin components respectively. The recent experiments reveal that for the degradation of cellulose, a mixture of exo- and endo-cellulases show strong synergistic activation (also see point # 10) due to the removal of obstacles (disordered amorphous cellulose) in their path by endo-cellulases, thus facilitating exo-cellulases in crystalline cellulose degradation [115]. It is noteworthy that both endo- and exo-cellulases benefit from the action of each other. For example, endo-cellulases remove obstacles that prevent the stalling of exo-cellulases, whereas exo-cellulases make the substrate more accessible to endo-cellulases. However, due to the inhibition of exo-cellulases by cellobiose, the product needs to be removed from the reaction mixture [115]. A step in this direction was recently taken when both cellulases (exo- and endo-cellulase) and β-glucosidase were displayed on “arming’ yeast (yeast budding cells with the cell surface covered by proteins) as fusion proteins with the C-terminal half of the outer surface α-agglutinin. All three glucanases, when displayed on the same cell surface (average distance between enzymes, 0.1 μm), showed significantly more efficient cellulose degradation as compared to when these enzymes were displayed on different yeast cells (average distance between enzymes, 65 μm). Importantly, the positive proximity effect was also demonstrated for crystalline cellulose (Avicel) as a substrate. A further advantage of this system is that ethanol can be directly produced from cellulosic biomass [116]. In this context, it is interesting that psychrophilic cellulolytic enzymes are worth considering for display on yeast surface, due to their higher intrinsic activity around room temperature (see Section 3.1). In nature, however, cellulose is always found in association with lignin and hemicellulose and this makes it more recalcitrant to hydrolysis. Recent work has shown that simply fusing an endo-cellulase with a xylanase (retrieved from a metagenomic survey of cow rumen) by removing a stop codon between them can increase their respective activities [117]. Further improvements in the process can be achieved with other accessory enzymes that can include versatile peroxidase (hybrid of manganese- and lignin-peroxidase), laccase and hemicellulases.
Activation entropy effects: One of the key reasons limiting the efficiency of biomass conversion is the low activity (kcat) of endo- and exo-cellulases (Supplementary Table 2). To date most of the protein engineering has been directed towards improving thermostability rather than kcat. Although substantial improvements in the kcat have been achieved on small and large soluble substrates (such as carboxymethylcellulose), a significant increase in kcat towards crystalline cellulose is yet to be achieved. Recently, protein engineering of endo-glucanases resulted in enhanced kcat without affecting their hyperthermostability, making these good candidates for further research and biomass conversion (Supplementary Table 2 and references therein). In view of the recent mechanistic discoveries regarding the endo-/exo-glucanase synergism, product inhibition and protein engineering, it may be possible to enhance cellulose hydrolysis by genetic modifications that include rational protein engineering [118]. Subtle changes in and around the catalytic site that increase product release can enhance thermoactivity, as recently shown by a single Tyr-Gly mutation in hyperthermophilic cellulase [119]. The cleaved product dissociates much faster from the active sub-site, due to weaker interactions in the Tyr-Gly mutant enzyme. This is a nice illustration of higher activity at all temperatures between 50 and 90 °C due to increased entropy (ΔS#) upon product release. Another strategy to increase the activity of cellulases is to “open up” the binding-site by introducing amino acids with smaller side chains. This can result in an enhanced substrate turn-over and can also help cellulose chains get better and faster access to the active-site [120]. The opening-up of the active-site may be even more valuable in the case of exo-processing cellobiohydrolase for faster access to the cellulose chain. Importantly, in both cases, thermostability was not compromised at the expense of higher activity. This implies that in principle the rule of activity-stability trade-off can be avoided [121].In the majority of cases, such as in cold-adapted enzymes, higher activity (kcat) is due to lower activation enthalpy (ΔH#), although there are examples where higher activity of thermophilic enzymes can be attributed to higher activation entropy (ΔS#) between the ground-state and the transition-state [121]. Therefore, improvement in kcat of enzymes including glucanases can be achieved either by decreasing ΔH# or increasing ΔS# in accordance with the relationship ΔG# = ΔH# − TΔS#. In reality, a decrease in ΔH# is accompanied by an equivalent decrease in ΔS# due to enthalpy–entropy compensation offering only a small net gain in activity. Conditions where a decrease in ΔH# is accompanied by an unchanged or increased ΔS# will result in a huge gain in kcat. For example, a reduction of only 20 kJ mol−1 in ΔH# without a corresponding decrease in ΔS# would result in a massive 50,000-fold increase in kcat at 15 °C ([121] and references therein). In the context of thermostable glucanases that have intrinsically low activity due to high ΔH#, a pragmatic approach for substantial gain in activity may be to decrease ΔH# while keeping ΔS# constant. Data indicate that it is possible to bypass enthalpy-entropy compensation by employing engineered enzymes and various experimental conditions that include super-critical CO2 and organic solvents [95]. This offers an opportunity to engineer enzymes whose transition-state has higher ΔS# relative to its ground-state. Fortuitously, the activesites of glucanases are carpeted with water molecules (low ΔS#), few of which are displaced to the surrounding medium when the substrate binds. Relative to substrate binding, if more water molecules are released when the transition-state binds to the active-site, then there will be considerable entropic benefit with concomitant higher activity ([95] and references therein). An intriguing possibility exists to significantly increase the kcat by replacing amino acid residues in and around binding pockets of cellulases and xylanases that can displace more water molecules to the surrounding medium upon transition-state binding. This can be achieved by amino acid substitutions that either slightly rigidify parts of the active-site or that snugly fit the transition-state.
Magnetic enzymes and recycling: The cost of cellulolytic enzymes is another factor limiting their commercial application for biomass conversion. For example, saccharification with cellulase and amylase costs approximately US $5 and $0.75 per 100 L ethanol respectively, although cellulosic biomass has the advantage of being a non-food source [114]. A likely cost-effective way of biomass conversion is to immobilize lignocellulosic degrading enzymes (class II peroxidases, laccases and glucanases) on nano-magnetic particles. The benefits of magnetic enzymes for biomass conversion are numerous and are described below. For example, nano-magnetic enzyme particles offer easy separation from the reaction mixture due to their magnetic susceptibility. This can translate into lower operational costs due to ease of separation and repeated reusability and recycling of magnetic enzymes. Secondly, substrate and product inhibition that is a common feature in lignin degrading enzymes and cellulases can be avoided due to diffusion, separation of substrates and products from the magnetic enzymes. Additional advantages of nano-magnetic particle-linked glucanases and lignin degrading enzymes may include an increase in operational (thermal and pH) stability due to immobilization, lower protein aggregation, less fouling due to extended shelf-life with concomitant increased biotechnological productivity. Furthermore, hemeenzymes (such as peroxidases) catalyzing free-radical reactions can be further affected by the magnetic field of the magnetic particles at the nano-scale, thus modulating their activity. Immobilization of endo-, exo-cellulases and β-glucosidases on nano-magnetic particles has received limited interest [122], whereas to date none of class II peroxidases have been coupled to nano-magnetic particles. Historically, magnetically immobilized enzymes have been reported and used in the hydrolysis of pretreated lignocellulosic residues. In this pioneering study, a magnetically immobilized β-glucosidase was applied with T. reesei cellulases to saccharify steam-exploded Eucalyptus regnans. Subsequently, β-glucosidase was recovered by applying a magnetic field and recycled to re-hydrolyze fresh batches of pretreated Eucalyptus material. The recycled β-glucosidase sustained cellobiose (produced by cellulase) to glucose conversion with 80% yield over 24h [123].It was recently demonstrated that the cellulose degrading enzyme mixtures (endo-glucanase, exo-glucanase and β-glucosidase) immobilized on nano-magnetic particles were successfully recovered and recycled with a loss of only 16% of hydrolytic productivity relative to the 1st cycle at 50 °C using pretreated wheat straw [122]. It is noteworthy that in some cases enzymes have been recycled fifty times without any activity loss, implying that there is a large scope in improving the reusability kinetics of cellulases. Another study found that magnetic β-glucosidase added to free cellulases and pretreated lignocellulosic feedstock from spruce at 50 °C increased the yield of sugars by 21% relative to the reaction in the absence of β-glucosidase, with concomitant retention of hydrolytic potential for 4 × 24 h cycles. Further analysis revealed that retention of activity after 96 h exposure at 50 °C was due to the increased thermal stability of the immobilized β-glucosidase, as compared to the free enzyme [124].
Isothermal titration calorimetry and kinetic studies: Traditional methods of cellulolytic enzyme kinetics employ soluble cellulosic chromogenic and fluorogenic substrates and use endpoint measurements by determining reducing ends. The technique of isothermal titration calorimetry has recently been exploited to study the kinetics of plant biomass degrading enzymes employing complex and/or insoluble polymeric substrates such as celluloses, hemicelluloses and humic substances that reflect natural degradation conditions [125,126]. In these studies, the low hydrolytic enthalpy of insoluble crystalline cellulose and birch xylan/wheat arabinoxylan treated by exo-cellulase and xylanase respectively were overcome by amplification of the heat signal via coupled reactions using other enzymes, such as catalase and glucose oxidase. Recently, the technique of isothermal titration calorimetry was further extended to include complex polymeric humic substances by utilizing individual manganese-peroxidase and lignin-peroxidase enzymes, as well as both enzyme activities simultaneously [32]. A further advantage of using isothermal titration calorimetry is that it provides information about the product inhibition that is critical during cellulose hydrolysis [31,32]. It is envisaged that in the near future isothermal titration calorimetry will be further applied under different conditions to promote our understanding of the enzyme kinetics of lignocellulosic biomass degradation.
5. Conclusion
Collectively, the recent findings described in this review unequivocally suggest that: (1) a better understanding of the physiological/epigenetic cues affecting secretion of CWDEs in fungi will lead to an improved exploitation of their biotechnological potential, e.g. by engineering new hyperproducing strains, (2) efficient exploitation of the lignocellulosic biomass can be achieved via various means, such as synergistic effects and with novel enzymes showing improved catalytic properties (higher activity and stability and minimum product inhibition). These enzymes can be sourced either from extremophilic organisms, environmental samples or generated by structure-directed recombination, fusion (hybrid) enzymes, protein engineering and directed evolution. The enzymes with suitable catalytic properties can then be displayed on yeast cell-surface or on magnetic particles for cost-effective and efficient saccharification and fermentation.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.plantsci.2015.02.010.
Acknowledgements
GG gratefully acknowledges the Austrian Science Fund (FWF; http://www.fwf.ac.at/en/) Grant No. M1315. GG and JFH wish to thank the support by the Fonds National de la Recherche, Luxembourg, (Project CANCAN C13/SR/5774202 and project CADWALL INTER/FWO/12/14). JS acknowledges the support by the Lower Austria Science Fund NFB grant LS12-009. Personal assistance of KSS by KFUPM is acknowledged. Dr Lucia Silvestrini is thanked for providing the scanning electron microscope picture of A. nidulans appearing in the Graphical Abstract. The funders had no role in the preparation of the manuscript.
Abbreviations
- NBUS
natural biomass utilization systems
- CWDEs
cell wall degrading enzymes
References
- [1].Guerriero G, Sergeant K, Hausman JF. Wood biosynthesis and typologies: a molecular rhapsody. Tree Physiol. 2014;34:839–855. doi: 10.1093/treephys/tpu031. [DOI] [PubMed] [Google Scholar]
- [2].Xie S, Syrenne R, Sun S, Yuan JS. Exploration of Natural Biomass Utilization Systems (NBUS) for advanced biofuel—from systems biology to synthetic design. Curr Opin Biotechnol. 2014;27:195–203. doi: 10.1016/j.copbio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- [3].Znameroski EA, Glass NL. Using a model filamentous fungus to unravel mechanisms of lignocellulose deconstruction. Biotechnol Biofuels. 2013;6:6. doi: 10.1186/1754-6834-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].USDA. USDA Biofuels Strategic Production Report. U.S. Department of Agriculture; Washington, DC: 2010. pp. 1–23. [Google Scholar]
- [5].Jung S, Lee DS, Kim YO, Joshi CP, Bae HJ. Improved recombinant cellulase expression in chloroplast of tobacco through promoter engineering and 5′ amplification promoting sequence. Plant Mol Biol. 2013;83:317–328. doi: 10.1007/s11103-013-0088-2. [DOI] [PubMed] [Google Scholar]
- [6].Levasseur A, et al. The genome of the white-rot fungus Pycnoporus cinnabarinus: a basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genomics. 2014;15:486. doi: 10.1186/1471-2164-15-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Riley R, et al. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci USA. 2014;111:9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alfaro M, Oguiza JA, Ramírez L, Pisabarro AG. Comparative analysis of secretomes in basidiomycete fungi. J Proteomics. 2014;102:28–43. doi: 10.1016/j.jprot.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [9].Cortázar AR, Aransay AM, Alfaro M, Oguiza JA, Lavín JL. SECRETOOL: integrated secretome analysis tool for fungi. Amino Acids. 2014;46:471–473. doi: 10.1007/s00726-013-1649-z. [DOI] [PubMed] [Google Scholar]
- [10].Dashtban M, Schraft H, Qin W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Intl J Biol Sci. 2009;5:578–595. doi: 10.7150/ijbs.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sharma RK, Arora DS. Fungal degradation of lignocellulosic residues: an aspect of improved nutritive quality. Crit Rev Microbiol. 2015;41:52–60. doi: 10.3109/1040841X.2013.791247. [DOI] [PubMed] [Google Scholar]
- [12].Kong C, Shin SY, Kim BG. Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: an in vitro approach. SpringerPlus. 2014;3:346. doi: 10.1186/2193-1801-3-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Delmas S, et al. Uncovering the genome-wide transcriptional responses of the filamentous fungus Aspergillus niger to lignocellulose using RNA sequencing. PLoS Genet. 2012;8:e1002875. doi: 10.1371/journal.pgen.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martins I, et al. Investigating Aspergillus nidulans secretome during colonisation of cork cell walls. J Proteomics. 2014;98:175–188. doi: 10.1016/j.jprot.2013.11.023. [DOI] [PubMed] [Google Scholar]
- [15].Glass NL, Schmoll M, Cate JH, Coradetti S. Plant cell wall deconstruction by ascomycete fungi. Annu Rev Microbiol. 2013;67:477–498. doi: 10.1146/annurev-micro-092611-150044. [DOI] [PubMed] [Google Scholar]
- [16].de Vries RP, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev. 2001;65:497–522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brown NA, de Gouvea PF, Krohn NG, Savoldi M, Goldman GH. Functional characterisation of the non-essential protein kinases and phosphatases regulating Aspergillus nidulans hydrolytic enzyme production. Biotechnol Biofuels. 2013;6:91. doi: 10.1186/1754-6834-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Braus GH, Sasse C, Krappmann S. Amino acid acquisition, cross-pathway control, and virulence in A. fumigatus. Med Mycol. 2006;44:S91–S94. doi: 10.1080/13693780600898029. [DOI] [PubMed] [Google Scholar]
- [19].Rohde J, Heitman J, Cardenas ME. The TOR kinases link nutrient sensing to cell growth. J Biol Chem. 2001;276:9583–9586. doi: 10.1074/jbc.R000034200. [DOI] [PubMed] [Google Scholar]
- [20].Elliott CE, Fox EM, Jarvis RS, Howlett BJ. The cross-pathway control system regulates production of the secondary metabolite toxin, sirodesmin PL, in the ascomycete, Leptosphaeria maculans. BMC Microbiol. 2011;11:169. doi: 10.1186/1471-2180-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Znameroski EA, et al. Evidence for transceptor function of cellodextrin transporters in Neurospora crassa. J Biol Chem. 2014;289:2610–2619. doi: 10.1074/jbc.M113.533273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].de Souza WR, et al. Transcriptome analysis of Aspergillus niger grown on sugarcane bagasse. Biotechnol Biofuels. 2011;4:40. doi: 10.1186/1754-6834-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chandra RP, et al. Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Adv Biochem Eng/Biotechnol. 2007;108:67–93. doi: 10.1007/10_2007_064. [DOI] [PubMed] [Google Scholar]
- [24].Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 2009;48:3713–3729. [Google Scholar]
- [25].Boussaid A, Robinson J, Cai YJ, Gregg DJ, Saddler JN. Fermentability of the hemicellulose-derived sugars from steam-exploded softwood (douglas fir) Biotechnol Bioeng. 1999;64:284–289. doi: 10.1002/(sici)1097-0290(19990805)64:3<284::aid-bit4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- [26].Mabee WE, et al. Updates on softwood-to-ethanol process development. Appl Biochem Biotechnol. 2006;129–132:55–70. doi: 10.1385/abab:129:1:55. [DOI] [PubMed] [Google Scholar]
- [27].Kubicek CP. Systems biological approaches towards understanding cellulase production by Trichoderma reesei. J Biotechnol. 2013;163:133–142. doi: 10.1016/j.jbiotec.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coradetti ST, et al. Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc Natl Acad Sci USA. 2012;109:7397–7402. doi: 10.1073/pnas.1200785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiong Y, Sun J, Glass NL. VIB1, a link between glucose signaling and carbon catabolite repression, is essential for plant cell wall degradation by Neurospora crassa. PLoS Genet. 2014;10:e1004500. doi: 10.1371/journal.pgen.1004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xiong Y, et al. The proteome and phosphoproteome of Neurospora crassa in response to cellulose, sucrose and carbon starvation. Fungal Genet Biol. 2014;72:21–33. doi: 10.1016/j.fgb.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ertan H, Siddiqui KS, Muenchhoff J, Charlton T, Cavicchioli R. Kinetic and thermodynamic characterization of the functional properties of a hybrid versatile peroxidase using isothermal titration calorimetry: insight into manganese peroxidase activation and lignin peroxidase inhibition. Biochimie. 2012;94:1221–1231. doi: 10.1016/j.biochi.2012.02.012. [DOI] [PubMed] [Google Scholar]
- [32].Siddiqui KS, et al. Versatile peroxidase degradation of humic substances: use of isothermal titration calorimetry to assess kinetics and applications to industrial wastes. J Biotechnol. 2014;178:1–11. doi: 10.1016/j.jbiotec.2014.03.002. [DOI] [PubMed] [Google Scholar]
- [33].Shallom D, Shoham Y. Microbial hemicellulases. Curr Opin Microbiol. 2003;6:219–228. doi: 10.1016/s1369-5274(03)00056-0. [DOI] [PubMed] [Google Scholar]
- [34].Kubicek CP. The Tools—Part 2: Enzymology of Hemicellulose Degradation in Fungi and Lignocellulosic Biomass. Wiley-Blackwell; Oxford, UK: 2012. [Google Scholar]
- [35].Arantes V, Saddler JN. Access to cellulose limits the efficiency of enzymatic hydrolysis: the role of amorphogenesis. Biotechnol Biofuels. 2010;3:4. doi: 10.1186/1754-6834-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gourlay K, Arantes V, Saddler JN. Use of substructure-specific carbohydrate binding modules to track changes in cellulose accessibility and surface morphology during the amorphogenesis step of enzymatic hydrolysis. Biotechnol Biofuels. 2012;5:51. doi: 10.1186/1754-6834-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Foreman PK, et al. Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J Biol Chem. 2003;278:31988–31997. doi: 10.1074/jbc.M304750200. [DOI] [PubMed] [Google Scholar]
- [38].Lin H, et al. Engineering Aspergillus oryzae A-4 through the chromosomal insertion of foreign cellulase expression cassette to improve conversion of cellulosic biomass into lipids. PLoS ONE. 2014;9:e108442. doi: 10.1371/journal.pone.0108442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gong Z, et al. Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol Biofuels. 2013;6:36. doi: 10.1186/1754-6834-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Milstein O, et al. Metabolism of lignin related aromatic compounds by Aspergillus japonicus. Arch Microbiol. 1983;135:147–154. [Google Scholar]
- [41].Zhao Y, Jiang C, Yu H, Fang F, Yang J. Genome shuffling of Aspergillus glaucus HGZ-2 for enhanced cellulase production. Appl Biochem Biotechnol. 2014;174:1246–1259. doi: 10.1007/s12010-014-1102-0. [DOI] [PubMed] [Google Scholar]
- [42].Bommarius AS, Sohn M, Kang Y, Lee JH, Realff MJ. Protein engineering of cellulases. Curr Opin Biotechnol. 2014;29:139–145. doi: 10.1016/j.copbio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- [43].Zhang SB, Pei XQ, Wu ZL. Multiple amino acid substitutions significantly improve the thermostability of feruloyl esterase A from Aspergillus niger. Bioresour Technol. 2012;117:140–147. doi: 10.1016/j.biortech.2012.04.042. [DOI] [PubMed] [Google Scholar]
- [44].Ravalason H, et al. Fusarium verticillioides secretome as a source of auxiliary enzymes to enhance saccharification of wheat straw. Bioresour Technol. 2012;114:589–596. doi: 10.1016/j.biortech.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [45].Paper JM, Scott-Craig JS, Adhikari ND, Cuomo CA, Walton JD. Comparative proteomics of extracellular proteins in vitro and in planta from the pathogenic fungus Fusarium graminearum. Proteomics. 2007;7:3171–3183. doi: 10.1002/pmic.200700184. [DOI] [PubMed] [Google Scholar]
- [46].Brown NA, Antoniw J, Hammond-Kosack KE. The predicted secretome of the plant pathogenic fungus Fusarium graminearum: a refined comparative analysis. PLoS ONE. 2012;7:e33731. doi: 10.1371/journal.pone.0033731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gacek A, Strauss J. The chromatin code of fungal secondary metabolite gene clusters. Appl Microbiol Biotechnol. 2012;95:1389–1404. doi: 10.1007/s00253-012-4208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mäkelä MR, Donofrio N, de Vries R. Plant biomass degradation by fungi. Fungal Genet Biol. 2014;72:2–9. doi: 10.1016/j.fgb.2014.08.010. [DOI] [PubMed] [Google Scholar]
- [49].Martinez D, et al. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci USA. 2009;106:1954–1959. doi: 10.1073/pnas.0809575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ryu JS, et al. Proteomic and functional analysis of the cellulase system expressed by Postia placenta during brown rot of solid wood. Appl Environ Microbiol. 2011;77:7933–7941. doi: 10.1128/AEM.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rättö M, Ritschkoff AC, Viikari L. The effect of oxidative pretreatment on cellulose degradation by Poria placenta and Trichoderma reesei cellulases. Appl Microbiol Biotechnol. 1997;48:53–57. [Google Scholar]
- [52].Zamocky M, et al. Cellobiose dehydrogenase – a flavocytochrome from wood-degrading, phytopathogenic and saprotropic fungi. Curr Protein Pept Sci. 2006;7:255–280. doi: 10.2174/138920306777452367. [DOI] [PubMed] [Google Scholar]
- [53].Langston JA, et al. Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl Environ Microbiol. 2011;77:7007–7015. doi: 10.1128/AEM.05815-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Phillips C, Beeson W, Cate J, Marletta M. Cellobiose dehydrogenase and a copper dependent polysaccharide monooxygenase potentiate fungal cellulose. ACS Chem Biol. 2011;6:1399–1406. doi: 10.1021/cb200351y. [DOI] [PubMed] [Google Scholar]
- [55].Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VG. Novel enzymes for the degradation of cellulose. Biotechnol Biofuels. 2012;5:45. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Levasseur A, Drula E, Lombard V, Coutinho PM, Henrissat B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels. 2013;6:41. doi: 10.1186/1754-6834-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Soanes DM, Chakrabarti A, Paszkiewicz KH, Dawe AL, Talbot NJ. Genome-wide transcriptional profiling of appressorium development by the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 2012;8:e1002514. doi: 10.1371/journal.ppat.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Seiboth B, et al. The putative protein methyltransferase LAE1 controls cellulase gene expression in Trichoderma reesei. Mol Microbiol. 2012;84:1150–1164. doi: 10.1111/j.1365-2958.2012.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Maehara T, et al. Ethanol production from high cellulose concentration by the basidiomycete fungus Flammulina velutipes. Fungal Biol. 2013;117:220–226. doi: 10.1016/j.funbio.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [60].Adav SS, Ravindran A, Sze SK. Quantitative proteomic analysis of lignocellulolytic enzymes by Phanerochaete chrysosporium on different lignocellulosic biomass. J Proteomics. 2012;75:1493–1504. doi: 10.1016/j.jprot.2011.11.020. [DOI] [PubMed] [Google Scholar]
- [61].Lanver D, et al. Plant surface cues prime Ustilago maydis for biotrophic development. PLoS Pathog. 2014;10:e1004272. doi: 10.1371/journal.ppat.1004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Brown NA, et al. The Aspergillus nidulans signalling mucin MsbA regulates starvation responses, adhesion and affects cellulase secretion in response to environmental cues. Mol Microbiol. 2014;94:1103–1120. doi: 10.1111/mmi.12820. [DOI] [PubMed] [Google Scholar]
- [63].Battaglia E, et al. Carbohydrate-active enzymes from the zygomycete fungus Rhizopus oryzae: a highly specialized approach to carbohydrate degradation depicted at genome level. BMC Genomics. 2011;12:38. doi: 10.1186/1471-2164-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou P, et al. Genome sequence and transcriptome analyses of the thermophilic zygomycete fungus Rhizomucor miehei. BMC Genomics. 2014;15:294. doi: 10.1186/1471-2164-15-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ma LJ, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen YC, Chen WT, Liu JC, Tsai LC, Cheng HL. A highly active betaglucanase from a new strain of rumen fungus Orpinomyces sp.Y102 exhibits cellobiohydrolase and cellotriohydrolase activities. Bioresour Technol. 2014;170:513–521. doi: 10.1016/j.biortech.2014.08.016. [DOI] [PubMed] [Google Scholar]
- [67].Haitjema CH, Solomon KV, Henske JK, Theodorou MK, O’Malley MA. Anaerobic gut fungi: advances in isolation, culture, and cellulolytic enzyme discovery for biofuel production. Biotechnol Bioeng. 2014;111:1471–1482. doi: 10.1002/bit.25264. [DOI] [PubMed] [Google Scholar]
- [68].Wang HC, Chen YC, Hseu RS. Purification and characterization of a cellulolytic multienzyme complex produced by Neocallimastix patriciarum J11. Biochem Biophys Res Commun. 2014;451:190–195. doi: 10.1016/j.bbrc.2014.07.088. [DOI] [PubMed] [Google Scholar]
- [69].Wang HC, Chen YC, Huang CT, Hseu RS. Cloning and characterization of a thermostable and pH-stable cellobiohydrolase from Neocallimastix patriciarum J11. Protein Expr Purif. 2013;90:153–159. doi: 10.1016/j.pep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- [70].Youssef NH, et al. The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl Environ Microbiol. 2013;79:4620–4634. doi: 10.1128/AEM.00821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Seckbach J, Oren A, Helga S-L. Polyextremophiles: Life Under Multiple Forms of Stress. Dordrecht, New York: Springer; 2013. [Google Scholar]
- [72].Mir BA, Mewalal R, Mizrachi E, Myburg AA, Cowan DA. Recombinant hyperthermophilic enzyme expression in plants: a novel approach for lignocellulose digestion. Trends Biotechnol. 2014;32:281–289. doi: 10.1016/j.tibtech.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [73].Blumer-Schuette SE, et al. Thermophilic lignocellulose deconstruction. FEMS Microbiol Rev. 2014;38:393–448. doi: 10.1111/1574-6976.12044. [DOI] [PubMed] [Google Scholar]
- [74].Vieille C, Zeikus GJ. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev. 2001;65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Maheshwari R, Bharadwaj G, Bhat MK. Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev. 2000;64:461–488. doi: 10.1128/mmbr.64.3.461-488.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Karnaouri A, Topakas E, Antonopoulou I, Christakopoulos P. Genomic insights into the fungal lignocellulolytic system of Myceliophthora thermophila. Front Microbiol. 2014;5:281. doi: 10.3389/fmicb.2014.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Blumer-Schuette SE, Kataeva I, Westpheling J, Adams MW, Kelly RM. Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr Opin Biotechnol. 2008;19:210–217. doi: 10.1016/j.copbio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [78].Hess M. Thermoacidophilic proteins for biofuel production. Trends Microbiol. 2008;16:414–419. doi: 10.1016/j.tim.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [79].Graham JE, et al. Identification and characterization of a multidomain hyperthermophilic cellulase from an archaeal enrichment. Nat Commun. 2011;2:375. doi: 10.1038/ncomms1373. [DOI] [PubMed] [Google Scholar]
- [80].Klose H, Röder J, Girfoglio M, Fischer R, Commandeur U. Hyperthermophilic endo-glucanase for in planta lignocellulose conversion. Biotechnol Biofuels. 2012;5:63. doi: 10.1186/1754-6834-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gusakov AV, Sinitsyn AP. A theoretical analysis of cellulose product inhibition: effect of cellulase binding constant, enzyme/substrate ratio, and β-glucosidase activity on the inhibition pattern. Biotechnol Bioeng. 1992;40:663–671. doi: 10.1002/bit.260400604. [DOI] [PubMed] [Google Scholar]
- [82].Kengen SW, Luesink EJ, Stams AJ, Zehnder AJ. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem. 1993;213:305–312. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- [83].Adlakha N, Sawant S, Anil A, Lali A, Yazdani SS. Specific fusion of β-1,4-endo-glucanase and β-1,4-glucosidase enhances cellulolytic activity and helps in channeling of intermediates. Appl Environ Microbiol. 2012;78:7447–7454. doi: 10.1128/AEM.01386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mitsuzawa S, et al. The rosettazyme: a synthetic cellulosome. J Biotechnol. 2009;143:139–144. doi: 10.1016/j.jbiotec.2009.06.019. [DOI] [PubMed] [Google Scholar]
- [85].Brunecky R, et al. Revealing nature’s cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science. 2013;342:1513–1516. doi: 10.1126/science.1244273. [DOI] [PubMed] [Google Scholar]
- [86].Cavicchioli R, Amils R, Wagner D, McGenity T. Life and applications of extremophiles. Environ Microbiol. 2011;13:1903–1907. doi: 10.1111/j.1462-2920.2011.02512.x. [DOI] [PubMed] [Google Scholar]
- [87].Ji L, et al. Synergy of crude enzyme cocktail from cold-adapted Cladosporium cladosporioides Ch2-2 with commercial xylanase achieving high sugars yield at low cost. Biotechnol Biofuels. 2014;7:130. doi: 10.1186/s13068-014-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sharma A, Kawarabayasi Y, Satyanarayana T. Acidophilic bacteria and archaea: acid stable biocatalysts and their potential applications. Extremophiles. 2012;16:1–19. doi: 10.1007/s00792-011-0402-3. [DOI] [PubMed] [Google Scholar]
- [89].Zajc J, et al. Chaophilic or chaotolerant fungi: a new category of extremophiles? Front Microbiol. 2014;5:708. doi: 10.3389/fmicb.2014.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Arfi Y, et al. Characterization of salt-adapted secreted lignocellulolytic enzymes from the mangrove fungus Pestalotiopsis sp. Nat Commun. 2013;4:1810. doi: 10.1038/ncomms2850. [DOI] [PubMed] [Google Scholar]
- [91].Oggerin M, et al. Specific jarosite biomineralization by Purpureocillium lilacinum, an acidophilic fungi isolated from Río Tinto. Environ Microbiol. 2013;15:2228–2237. doi: 10.1111/1462-2920.12094. [DOI] [PubMed] [Google Scholar]
- [92].Grum-Grzhimaylo AA, Debets AJ, van Diepeningen AD, Georgieva ML, Bilanenko EN. Sodiomyces alkalinus, a new holomorphic alkaliphilic ascomycete within the Plectosphaerellaceae. Persoonia. 2013;31:147–158. doi: 10.3767/003158513X673080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Singhania RR, Patel AK, Sukumaran RK, Larroche C, Pandey A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour Technol. 2013;127:500–507. doi: 10.1016/j.biortech.2012.09.012. [DOI] [PubMed] [Google Scholar]
- [94].Biver S, Stroobants A, Portetelle D, Vandenbol M. Two promising alkaline β-glucosidases isolated by functional metagenomics from agricultural soil including one showing high tolerance towards harsh detergents, oxidants and glucose. J Ind Microbiol Biotechnol. 2014;41:479–488. doi: 10.1007/s10295-014-1400-0. [DOI] [PubMed] [Google Scholar]
- [95].Siddiqui KS, et al. Psychrophiles. Annu Rev Earth Planet Sci. 2013;41:87–115. [Google Scholar]
- [96].Siddiqui KS, Thomas T. Protein Adaptations in Extremophiles. Nova Science Publishers Inc; Hauppauge, New York: 2008. [Google Scholar]
- [97].Qin QL, et al. The complete genome of Zunongwangia profunda SM-A87 reveals its adaptation to the deep-sea environment and ecological role in sedimentary organic nitrogen degradation. BMC Genomics. 2010;11:247. doi: 10.1186/1471-2164-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Aono E, et al. Complete genome sequence and comparative analysis of Shewanella violacea, a psychrophilic and piezophilic bacterium from deep sea floor sediments. Mol Biosyst. 2010;6:1216–1226. doi: 10.1039/c000396d. [DOI] [PubMed] [Google Scholar]
- [99].Mardanov AV, et al. Metabolic versatility and indigenous origin of the archaeon Thermococcus sibiricus, isolated from a siberian oil reservoir, as revealed by genome analysis. Appl Environ Microbiol. 2009;75:4580–4588. doi: 10.1128/AEM.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Anand A, Kumar V, Satyanarayana T. Characteristics of thermostable endo-xylanase and β-xylosidase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1 and its applicability in generating xylooligosaccharides and xylose from agro-residues. Extremophiles. 2013;17:357–366. doi: 10.1007/s00792-013-0524-x. [DOI] [PubMed] [Google Scholar]
- [101].Burguener GF, et al. Draft genome sequence of the polyextremophilic Halorubrum sp. strain AJ67, isolated from hyperarsenic lakes in the Argentinian Puna. Genome Announc. 2014;2:e01096–13. doi: 10.1128/genomeA.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sagt CM, et al. Peroxicretion: a novel secretion pathway in the eukaryotic cell. BMC Biotechnol. 2009;9:48. doi: 10.1186/1472-6750-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nevalainen H, Peterson R. Making recombinant proteins in filamentous fungi- are we expecting too much? Front Microbiol. 2014;5:75. doi: 10.3389/fmicb.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Brune A. Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol. 2014;12:168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- [105].Piao H, et al. Identification of novel biomass-degrading enzymes from genomic dark matter: populating genomic sequence space with functional annotation. Biotechnol Bioeng. 2014;111:1550–1565. doi: 10.1002/bit.25250. [DOI] [PubMed] [Google Scholar]
- [106].Shen B, et al. Engineering a thermoregulated intein-modified xylanase into maize for consolidated lignocellulosic biomass processing. Nat Biotechnol. 2012;30:1131–1136. doi: 10.1038/nbt.2402. [DOI] [PubMed] [Google Scholar]
- [107].Chung D, Cha M, Guss AM, Westpheling J. Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc Natl Acad Sci USA. 2014;111:8931–8936. doi: 10.1073/pnas.1402210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Smith MA, et al. A diverse set of family 48 bacterial glycoside hydrolase cellulases created by structure-guided recombination. FEBS J. 2012;279:4453–4465. doi: 10.1111/febs.12032. [DOI] [PubMed] [Google Scholar]
- [109].Komor RS, Romero PA, Xie CB, Arnold FH. Highly thermostable fungal cellobiohydrolase I (Cel7A) engineered using predictive methods. Protein Eng Des Sel. 2012;25:827–833. doi: 10.1093/protein/gzs058. [DOI] [PubMed] [Google Scholar]
- [110].Kumar GN, Srikumar K. Thermophilic laccase from xerophyte species Opuntia vulgaris. Biomed Chromatogr. 2011;25:707–711. doi: 10.1002/bmc.1506. [DOI] [PubMed] [Google Scholar]
- [111].Elleuche S. Bringing functions together with fusion enzymes-from nature’s inventions to biotechnological applications. Appl Microbiol Biotechnol. 2015;99:1545–1556. doi: 10.1007/s00253-014-6315-1. [DOI] [PubMed] [Google Scholar]
- [112].Rizk M, Elleuche S, Antranikian G. Generating bifunctional fusion enzymes composed of heat-active endo-glucanase (Cel5A) and endo-xylanase (XylT) Biotechnol Lett. 2015;37:139–145. doi: 10.1007/s10529-014-1654-7. [DOI] [PubMed] [Google Scholar]
- [113].Lee HL, Chang CK, Teng KH, Liang PH. Construction and characterization of different fusion proteins between cellulases and β-glucosidase to improve glucose production and thermostability. Bioresour Technol. 2011;102:3973–3976. doi: 10.1016/j.biortech.2010.11.114. [DOI] [PubMed] [Google Scholar]
- [114].Banerjee S, et al. Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels Bioprod Biorefining. 2010;4:77–93. [Google Scholar]
- [115].Jalak J, Kurašin M, Teugjas H, Väljamäe P. Endo-exo synergism in cellulose hydrolysis revisited. J Biol Chem. 2012;287:28802–28815. doi: 10.1074/jbc.M112.381624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bae J, Kuroda K, Ueda M. Proximity effect between cellulose-degrading enzymes displayed on the yeast cell surface. Appl Environ Microbiol. 2015;81:59–66. doi: 10.1128/AEM.02864-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kang YM, Kim MK, An JM, Haque MA, Cho KM. Metagenomics of un-culturable bacteria in cow rumen: construction of cel9E-xyn10A fusion gene by site-directed mutagenesis. J Mol Catal B: Enzym. 2015;114:29–38. [Google Scholar]
- [118].Trudeau DL, Lee TM, Arnold FH. Engineered thermostable fungal cellulases exhibit efficient synergistic cellulose hydrolysis at elevated temperatures. Biotechnol Bioeng. 2014;111:2390–2397. doi: 10.1002/bit.25308. [DOI] [PubMed] [Google Scholar]
- [119].Cheng YS, et al. Enhanced activity of Thermotoga maritima cellulase 12A by mutating a unique surface loop. Appl Microbiol Biotechnol. 2012;95:661–669. doi: 10.1007/s00253-011-3791-4. [DOI] [PubMed] [Google Scholar]
- [120].Srikrishnan S, Randall A, Baldi P, Da Silva NA. Rationally selected single-site mutants of the Thermoascus aurantiacus endo-glucanase increase hydrolytic activity on cellulosic substrates. Biotechnol Bioeng. 2012;109:1595–1599. doi: 10.1002/bit.24414. [DOI] [PubMed] [Google Scholar]
- [121].Siddiqui KS, Cavicchioli R. Cold-adapted enzymes. Annu Rev Biochem. 2006;75:403–433. doi: 10.1146/annurev.biochem.75.103004.142723. [DOI] [PubMed] [Google Scholar]
- [122].Alftrén J, Hobley TJ. Immobilization of cellulase mixtures on magnetic particles for hydrolysis of lignocellulose and ease of recycling. Biomass Bioenergy. 2014;65:72–78. [Google Scholar]
- [123].Dekker RFH. Application of a magnetic immobilized β-glucosidase in the enzymatic saccharification of steam-exploded lignocellulosic residues. Appl Biochem Biotechnol. 1990;23:25–39. [Google Scholar]
- [124].Alftrén J, Hobley TJ. Covalent immobilization of β-glucosidase on magnetic particles for lignocellulose hydrolysis. Appl Biochem Biotechnol. 2013;169:2076–2087. doi: 10.1007/s12010-013-0122-5. [DOI] [PubMed] [Google Scholar]
- [125].Murphy L, Baumann MJ, Borch K, Sweeney M, Westh P. An enzymatic signal amplification system for calorimetric studies of cellobiohydrolases. Anal Biochem. 2010;404:140–148. doi: 10.1016/j.ab.2010.04.020. [DOI] [PubMed] [Google Scholar]
- [126].Baumann MJ, et al. Advantages of isothermal titration calorimetry for xylanase kinetics in comparison to chemical-reducing-end assays. Anal Biochem. 2011;410:19–26. doi: 10.1016/j.ab.2010.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.