Abstract
Apple proliferation (AP) represents a serious threat to several fruit-growing areas and is responsible for great economic losses. Several studies have highlighted the key role played by the cell wall in response to pathogen attack. The existence of a cell wall integrity signaling pathway which senses perturbations in the cell wall architecture upon abiotic/biotic stresses and activates specific defence responses has been widely demonstrated in plants. More recently a role played by cell wall-related genes has also been reported in plants infected by phytoplasmas. With the aim of shedding light on the cell wall response to AP disease in the economically relevant fruit-tree Malus × domestica Borkh., we investigated the expression of the cellulose (CesA) and callose synthase (CalS) genes in different organs (i.e., leaves, roots and branch phloem) of healthy and infected symptomatic outdoor-grown trees, sampled over the course of two time points (i.e., spring and autumn 2011), as well as in in vitro micropropagated control and infected plantlets. A strong up-regulation in the expression of cell wall biosynthetic genes was recorded in roots from infected trees. Secondary cell wall CesAs showed up-regulation in the phloem tissue from branches of infected plants, while either a down-regulation of some genes or no major changes were observed in the leaves. Micropropagated plantlets also showed an increase in cell wall-related genes and constitute a useful system for a general assessment of gene expression analysis upon phytoplasma infection. Finally, we also report the presence of several ‘knot’-like structures along the roots of infected apple trees and discuss the occurrence of this interesting phenotype in relation to the gene expression results and the modalities of phytoplasma diffusion.
Keywords: apple proliferation, callose synthase, cellulose synthase, Malus × domestica
Introduction
Phytoplasmas are cell wall-free phloem-restricted bacteria belonging to the class Mollicutes, which are able to manipulate the metabolism of insects and plants, since their life cycle involves replication steps which take place in organisms belonging to two different kingdoms (Hogenhout et al. 2008).
Among the diseases caused by phytoplasmas, apple proliferation (AP) certainly constitutes a serious concern for the economy of many apple-growing areas in Central and Southern Europe (Rekab et al. 2010, Baric et al. 2011a). It is caused by ‘Candidatus Phytoplasma mali’ (Ca P. mali) (Seemüller and Schneider 2007), a bacterium recommended for regulation as quarantine pest by EPPO (European and Mediterranean Plant Protection Organization; http://www.eppo.org/QUARANTINE/listA2.htm), which can be transmitted through grafting of infected propagation material (Kartte and Seemüller 1988) and by sap-feeding insects (Frisinghelli et al. 2000, Tedeschi and Alma 2004). In addition, transmission of Ca. P. mali through root anastomoses (i.e., root bridges) has also been reported, both experimentally and naturally (Ciccotti et al. 2007, Baric et al. 2008).
The specific symptoms at the outbreak of AP are characterized by the typical ‘witches’ brooms’, enlarged stipule phenotypes, accompanied by less specific signs, like foliar reddening and yellowing, growth suppression, vigor reduction, increased susceptibility to powdery mildew infections and development of undersized/unmarketable fruits (Kartte and Seemüller 1988, Lee et al. 2000, Bertaccini 2007).
Infected apple trees usually display a non-homogeneous distribution of the pathogen (Rekab et al. 2010, Baric et al. 2011b). Canopy infection indeed follows a seasonal fluctuation: during winter the bacteria persist in the roots and disappear from the aerial parts, because of the almost complete degeneration of the phloem in the trunk and the branches (Schaper and Seemüller 1984, Musetti et al. 2010, Baric et al. 2011b). The root system, where intact sieve tubes are present throughout the year, constitutes a ‘reservoir’ where the bacteria overwinter and under circumstances recolonize the newly formed phloem in the canopy during spring (Seemüller et al. 1984, Bisognin et al. 2008).
The infected trees can sometimes spontaneously show a transient or permanent remission (recovery) of the disease symptoms (Seemüller et al. 1984, Carraro et al. 2004). Although all the molecular events of this complex phenomenon are still not completely understood, it has been recently shown that the cell wall-related enzyme callose synthase (CalS) and the phloem-protein plugging of the sieve tubes play a key role in favoring this process (Musetti et al. 2010). Callose and phloem protein deposition are calcium (Ca2+)-dependent processes. Ca2+ plays a pivotal role in the plant–pathogen interaction: one of the promptest responses upon pathogen attack is indeed the increase in its cytosolic concentration and it has been demonstrated to act both upstream and downstream of reactive oxygen species (ROS) production (Torres et al. 2006). Ca2+ signaling has also been shown to play a key role in the establishment of recovery from AP disease in apple plants (Musetti et al. 2010): a higher concentration of Ca2+ has been observed in the leaves of recovered apple plants, which is consistent with the increase in the expression of CalS and PP2 genes in those leaves.
The plant cell wall is a complex structure and its basic structural component is cellulose, a polymer of glucose units arranged in a network of microfibrils embedded in a matrix of heteropolymers, i.e. pectins and hemicelluloses (Cosgrove 1997, 2005). The cell wall plays an important role in plant physiology: it indeed regulates cell growth, controls cell volume, mediates stress response signaling (Ellis et al. 2002, Joubert et al. 2011, Ramírez et al. 2011) and constitutes a barrier against pathogens and water loss (Sieber et al. 2000).
Recent studies carried out on grapevine, as well as on apple and apricot have contributed to shed light on the role of cell wall-related genes after phytoplasma infection: in particular, genes involved in cell wall degradation were repressed, while those responsible for cell wall reinforcement were induced to limit the spread and invasion sites of the pathogen (Musetti et al. 2005, 2010, 2011, Albertazzi et al. 2009).
The presence of a cell wall integrity (CWI) signaling pathway in plants, similar to that present in the yeast Saccharomyces cerevisiae (Levin 2005), has been demonstrated (Hématy et al. 2007, 2009, Humphrey et al. 2007, Ringli 2010, Hamann and Denness 2011): the cell wall acts as a sensor of its own structural and functional integrity, which is usually compromised in situations of exogenous stresses or genetic perturbations (Steinwand and Kieber 2010). Signaling cascades are then activated, which in their turn trigger hormone responses (Caño-Delgado et al. 2003, Hernández-Blanco et al. 2007, Duval and Beaudoin 2009, Zhang et al. 2011), ectopic lignin deposition (Vance et al. 1980, Caño-Delgado et al. 2000, 2003, Fagard et al. 2000, Xu et al. 2008, Denness et al. 2011) and/or the synthesis of antimicrobial secondary metabolites (Hernández-Blanco et al. 2007).
With the purpose of investigating the cell wall response of the recently sequenced fruit tree Malus × domestica Borkh. (Velasco et al. 2010) to Ca. P. mali infection, we studied the seasonal expression of apple cellulose and callose synthase genes (designated MdCesA and MdCalS), by analyzing three different organs from healthy and Ca. P. mali-infected trees, as well as in vitro micropropagated control and infected apple plantlets. This is to our knowledge the first study providing a comprehensive analysis of the expression of the CesA and CalS genes in different organs of a relevant fruit tree, in response to phytoplasma infection.
Materials and methods
Plant material
The experimental plants (Malus × domestica Borkh. ‘Golden Delicious’ Clone B on M9 rootstock) were obtained in March 2008 as knip-boom trees from a commercial nursery. The plants were potted during late March in Gramoflor substrate (Manna, Italy) in 3.5 l pots and grown outdoor in an insect-proof tunnel at the Research Centre for Agriculture and Forestry Laimburg (South Tyrol, northern Italy).
The following year, the trees were repotted in 9 l pots and in 25 l pots in 2011. Each year the plants were fertilized [(Plant Prod 20-5-30 + B + Cu + Zn) + N (total 0.7 g/plant)] with urea at intervals of ~2 weeks, from April to July. In order to inoculate the experimental plants with Ca. P. mali, shoots were cut from apple trees from a commercial orchard. The orchard (‘Golden Delicious’ on M7 rootstock) was located in Tramin/Termeno (South Tyrol) and comprised a high percentage of trees naturally infected by AP and showing pronounced disease symptoms. Shoots were also obtained from healthy apple trees to treat the control plants the same way as the infected ones. The presence/absence of the pathogen in the donor plants was confirmed by a highly sensitive real-time polymerase chain reaction (PCR) detection approach (Baric and Dalla Via 2004, Baric et al. 2006). Samples tested positive for the phytoplasma were further typed at genes encoding a rhodanese-like protein and the ribosomal protein L22, as described by Baric et al. (2011a). All the infected donor trees used for this study carried the phytoplasma subtype AT-2/rpX-A, which is most common in the study area of South Tyrol (Baric et al. 2011a).
Chips were cut from the shoots of infected and healthy donor plants and the inoculation of potted apple trees occurred in August 2008 (plants were 2 years old at the time of infection), using the chip-budding technique. Each plant was grafted with 2–3 chip buds. The first evaluation of symptoms was carried out in October 2009, the last in September 2011. The health status of the experimental plants was also tested on roots by real-time PCR (Baric and Dalla Via 2004, Baric et al. 2006). A total of 12 healthy and 12 symptomatic infected trees (3 biological replicates, each with a pool of 4 trees) were used for this study.
Approximately 5 g of tissues were collected (during mid-May and mid-October 2011; the plant material was always harvested between 9 and 11 a.m.), soil from roots was eliminated using a soft brush, then the harvested tissues were snap-frozen in liquid nitrogen and immediately brought to the laboratory for subsequent RNA extraction, or long-term storage at −80 °C.
In addition to the apple trees grown in pots in an insect-proof tunnel, in vitro micropropagated plantlets were analyzed in this study.
In vitro cultures of healthy (control) and infected ‘Golden Delicious’ were established and propagated according to the protocol of Jarausch et al. (1996) and Ciccotti et al. (2008). Cultures were incubated in a growth chamber at 23 ± 2 °C under 16 h photoperiod with cool-white fluorescent light (60 μE m−2 s−1). Diseased cultures were derived from apple plants infected experimentally with Ca. P. mali by Cacopsylla picta (Frisinghelli et al. 2000). The presence of subtype AT-2/rpX-A in the in vitro plantlets was confirmed using the procedure of Baric et al. (2011a).
A total of 30 homogeneously growing in vitro plantlets, healthy and infected respectively, sampled after 30 days of culture, were used in this study (3 biological replicates, each corresponding to 10 pooled plantlets).
Data mining and bioinformatics analysis
The identification of putative full-length CesA and CalS genes from M. × domestica was carried out first by performing BLASTp searches of the predicted apple CESA and CALS homologs (http://www.rosaceae.org/projects/apple_genome) against non-redundant protein databases of Populus balsamifera L. ssp. trichocarpa (Torr. & Gray ex Hook.) and Arabidopsis thaliana (L.) Heynh. from the National Centre for Biotechnology (NCBI; http://www.ncbi.nlm.nih.gov). The percentage of identities between M. × domestica/P. trichocarpa and M. × domestica/A. thaliana were then compared. Transmembrane domain prediction (Krogh et al. 2001) was performed using the license-free software program TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/) and the neighbor-joining phylogenetic tree was built using BIONJ (Dereeper et al. 2008), after aligning the conserved amino acid regions shown in Figure S1 available as Supplementary Data at Tree Physiology Online using ClustalW (Larkin et al. 2007).
RNA extraction, cDNA synthesis and real-time PCR
Total RNA was extracted from 100 mg of apple tissue, by using the RNeasy Plant Mini Kit (Qiagen, Milan, Italy), coupled with the on-column DNaseI digestion. Leaves (including the midribs, from the middle part of the canopy) and roots (~6–8 mm thick and 8 cm long, from which bark was removed with a sterile razor blade) were pulverized in liquid nitrogen (to help the pulverization process, the whole root was ‘shaved’ with a blade while keeping it frozen during processing), using a mortar and a pestle. Phloem shavings from branches were prepared using a sterile blade, after removal of the outer bark. RNA from in vitro micropropagated plantlets was extracted as described by Gambino et al. (2008), after having thoroughly rinsed the plants to remove agar and pulverized the stem and leaves in liquid nitrogen.
The quality of the extracted RNA was checked by electrophoresis and the concentration measured using a NanoDrop ND-1000 spectrophotometer.
Two hundred nanograms of extracted RNA were retro-transcribed using the Superscript Vilo cDNA Synthesis Kit (Invitrogen, Milan, Italy), following the manufacturer’s instructions.
The primers used to perform real-time PCR analyses on the CesA genes are the same as reported in Guerriero et al. (2012). The CalS primers are reported in Table 1 and their specificity was tested in preliminary PCRs. The PCR products obtained were cloned using the pGEM-T Easy Vector System (Promega, Milan, Italy) and then sequenced at GATC (Konstanz, Germany) with the universal primers M13 Fwd and M13 Rev.
Table 1.
Primer names and sequences used for the real-time PCR of M. × domestica CalS genes.
| Primer name | Sequence (5′ → 3′) |
|---|---|
| MdCalS1 Fwd | CGGTCGCCTTTATCTTGTTC |
| MdCalS1 Rev | TAACCATAGGCAAGGCCATC |
| MdCalS2 Fwd | ACCTAATCCAGTGGCCTCCT G |
| MdCalS2 Rev | CGATCTCCAAGGACCAAAAA |
| MdCalS3-A Fwd | TCTTGCTTTCATGCCAACTG |
| MdCalS3-A Rev | TGCTGAATGCTTGGTTGAAG |
| MdCalS3-B Fwd | TGAGATTCATGCCAGAGTGC |
| MdCalS3-B Rev | AAGCAACCAGAAACCACAGG |
| MdCalS5-A Fwd | TAATTCAGTGGCCACCCTTC |
| MdCalS5-A Rev | ATCTGCACATATCCGCTTCC |
| MdCalS5-B Fwd | CTTTGAAGATCCGCTTCCAC |
| MdCalS5-B Rev | TGTCCTCGCTCAGATTGATG |
| MdCalS7-A Fwd | TCTACGGGCAATCCTTTGAC |
| MdCalS7-A Rev | CCAATACCACCACGATTTCC |
| MdCalS7-B Fwd | TGGAAGTGCTGATTCTGCTG |
| MdCalS7-B Rev | AAGCAACCAGAAACCACAGG |
| MdCalS8 Fwd | AATCCTCGTTGTTGGTCGTC |
| MdCalS8 Rev | GACCTTGCGATGCTTTCTTC |
| MdCalS9 Fwd | TGTGTACGGCTTCTGCATTC |
| MdCalS9 Rev | TTGAGCACTGTGCAAACCTC |
| MdCalS11 Fwd | TGTTCAATGAAGCGTTCAGC |
| MdCalS11 Rev | CGAAAAGATTCGGAGACTCG |
| MdCalS12 Fwd | GCTTTTGGCATTTTTGTGGT |
| MdCalS12 Rev | ACAGGGCCGAAATACTTGTG |
| Ubq Fwd | TGATCTTCGCTGGAAAACAG |
| Ubq Rev | CCTGAATTTTTGCCTTGACG |
| GAPDH Fwd | GTTCGTTGTTGGTGTGAACG |
| GAPDH Rev | GTCTTCTGGGTGGCAGTGAT |
For quantitative real-time PCR analysis, ~20 ng cDNA were used as template. The cDNA was amplified using the SYBR GreenER qPCR SuperMix Universal (Invitrogen, Milan, Italy) on a 7500 Fast Real-time PCR System (Applied Biosystems, Milan, Italy), with the ROX Reference Dye.
The reactions were performed in triplicate and repeated on three biological independent replicates. The PCR conditions consisted of an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing/extension at 57°C for 60 s. A dissociation kinetics analysis was performed at the end of the experiment to check the specificity of annealing.
The results were analyzed with Q-gene software (Muller et al. 2002) and normalized against the housekeeping genes Ubiquitin and GAPDH (accession numbers EB109811 and CN929227, respectively). The values are expressed as log MNE (mean normalized expression), to facilitate visualization and comparison of the graphs. Following a Kolmogorov–Smirnov test, which confirmed normal distribution of data, the analysis of variance two-factor with replication test, as implemented in the QI Macros SPC Software for Excel, was performed.
Microscopic observation of apple roots
Thin cross sections of roots from control and infected trees were prepared by hand, using a sterile surgical blade. The sections were subsequently mounted on a microscope slide and observed by bright-field microscopy (N Plan ×40/0.65 objective, Leica DMRB, Leica AG, Heerbrug, Switzerland).
Results and discussion
Domesticated apple has 12 putative CalS genes
Data mining of the recently sequenced apple genome (Velasco et al. 2010) showed the presence of 13 putative CesA (Guerriero et al. 2012) and 12 CalS genes (this study). Taking into account the identity percentages with the orthologs from A. thaliana, we propose a nomenclature for M. × domestica CalS genes (Table 2 and Figure 1), The putative CalS genes code for proteins (hereafter referred to as CALS) displaying between 9 and 19 transmembrane helices and a deduced polypeptide length of 1478–2206 amino acids (Table 2).
Table 2.
Proposed nomenclature (in bold and italics) of M. × domestica CalS genes (Gene IDs reported in the Genome Database for Rosaceae GDR are indicated) based on amino acid identities with the orthologous proteins from A. thaliana (GenBank accession numbers are indicated) and P. trichocarpa (identified by locus names). The percentage of amino acid identities between Arabidopsis/apple and poplar/apple are specified, as well as the number of transmembrane helices (TMHs) and the apple protein deduced amino acids length. GSL stands for glucan synthase-like and accession numbers in parentheses refer to the sequences reported in Musetti et al. (2010).
| M. × domestica | Deduced polypeptide length | TMHs | A. thaliana | % identity Arabidopsis–Malus | P. trichocarpa | % identity Populus–Malus |
|---|---|---|---|---|---|---|
| MdCalS1 MDP0000321472 | 2106 | 18 | AtCalS1/GSL06 At1g05570 | 74 | POPTR_0001s23710 | 73 |
| MdCalS2 MDP0000177875 (FN395072; FN397075) | 2206 | 19 | AtCalS2/GSL03 At2g31960 | 73 | POPTR_0001s04970 | 74 |
| MdCalS3-A MDP0000229527 (FN395071) | 1932 | 14 | AtCalS3/GSL12 At5g13000 | 81 | POPTR_0001s04970 | 83 |
| MdCalS3-B MDP0000230907 | 2124 | 16 | 76 | 80 | ||
| MdCalS5-A MDP0000286691 | 1781 | 12 | AtCalS5/GSL02 At2g13680 | 79 | POPTR_0005s05970 | 79 |
| MdCalS5-B MDP0000174437 | 1893 | 11 | 72 | 75 | ||
| MdCalS7-A MDP0000308574 | 1747 | 9 | AtCalS7/GSL07 At1g06490 | 73 | POPTR_0002s05970 | 73 |
| MdCalS7-B MDP0000203763 | 1777 | 15 | 70 | 73 | ||
| MdCalS8 MDP0000230621 | 1937 | 9 | AtCalS8/GSL04 At3g14570 | 67 | POPTR_0011s09740 | 71 |
| MdCalS9 MDP0000218486 | 1478 | 11 | AtCalS9/GSL10 At3g07160 | 66 | POPTR_0015s10090 | 56 |
| MdCalS11 MDP0000915307 (FN395073) | 1772 | 17 | AtCalS11/GSL01 At4g04970 | 71 | POPTR_0005s05970 | 46 |
| MdCalS12 MDP0000275961 (FN395074) | 1939 | 11 | AtCalS12/GSL05 At4g03550 | 75 | POPTR_0013s13490 | 86 |
Figure 1.
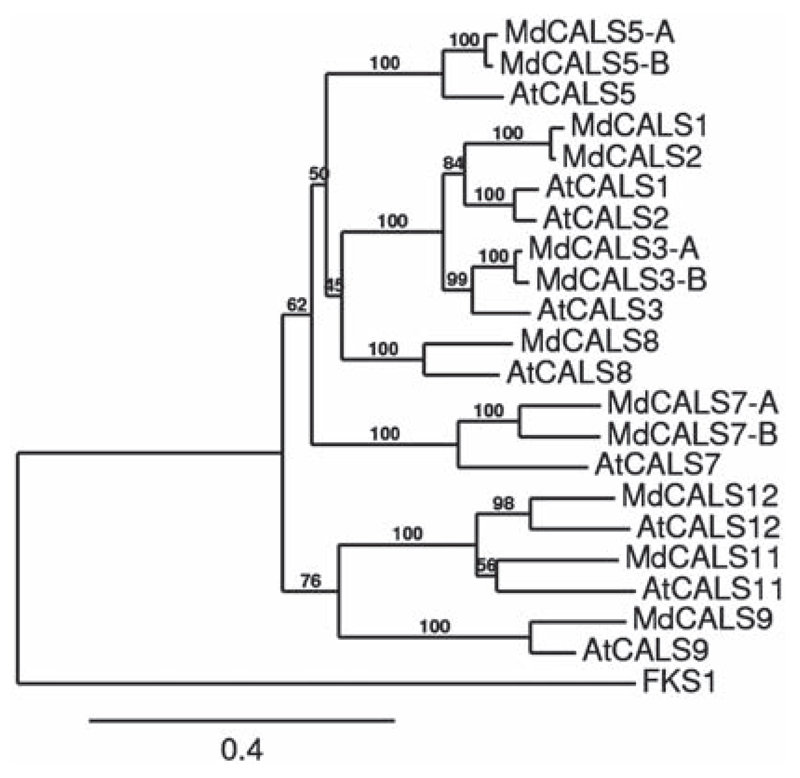
Neighbor-joining phylogenetic tree of M. × domestica and A. thaliana CALSs. The tree was rooted with Saccharomyces cerevisiae FKS1 (accession number NP_013446). Bootstrap = 1000. Numbers refer to % of branch support value. The scale bar refers to the number of amino acid substitutions per site.
The alignment included 22 protein sequences and was performed by aligning the conserved regions shown in Figure S1 available as Supplementary Data at Tree Physiology Online. The neighbor-joining phylogenetic tree of CALS from A. thaliana and M. × domestica shows well-supported branches for apple orthologs of AtCALS3, AtCALS5, AtCALS7, AtCALS8, AtCALS9 and AtCALS12 (Figure 1).
Interestingly, we found two apple orthologous genes of AtCalS7, shown to be involved in phloem transport (Barratt et al. 2011, Xie et al. 2011), which we named MdCalS7-A and B. This probably reflects a more complex transport system in the sieve tubes of M. × domestica which required the differentiation of two genes. We also found two orthologs of AtCALS3, an enzyme involved in callose deposition at the plasmodesmata (Vatén et al. 2011), as well as of AtCALS5, involved in exine formation in pollen wall (Dong et al. 2005). The differentiation of two CalS3 genes in M. × domestica could reflect a fine-tuning of callose deposition at the plasmodesmata during sieve tubes development and/or pathogen response. Functional studies are nevertheless necessary to investigate the role(s) of these genes and whether they have redundant functions.
MdCALS1 and MdCALS2, the orthologs of AtCALS1 and AtCALS2, cluster together (Figure 1) and this might reflect their functional redundant role in vivo in domesticated apple, as already previously reported in A. thaliana (Hong et al. 2001).
Gene expression analysis in different organs of potted apple trees
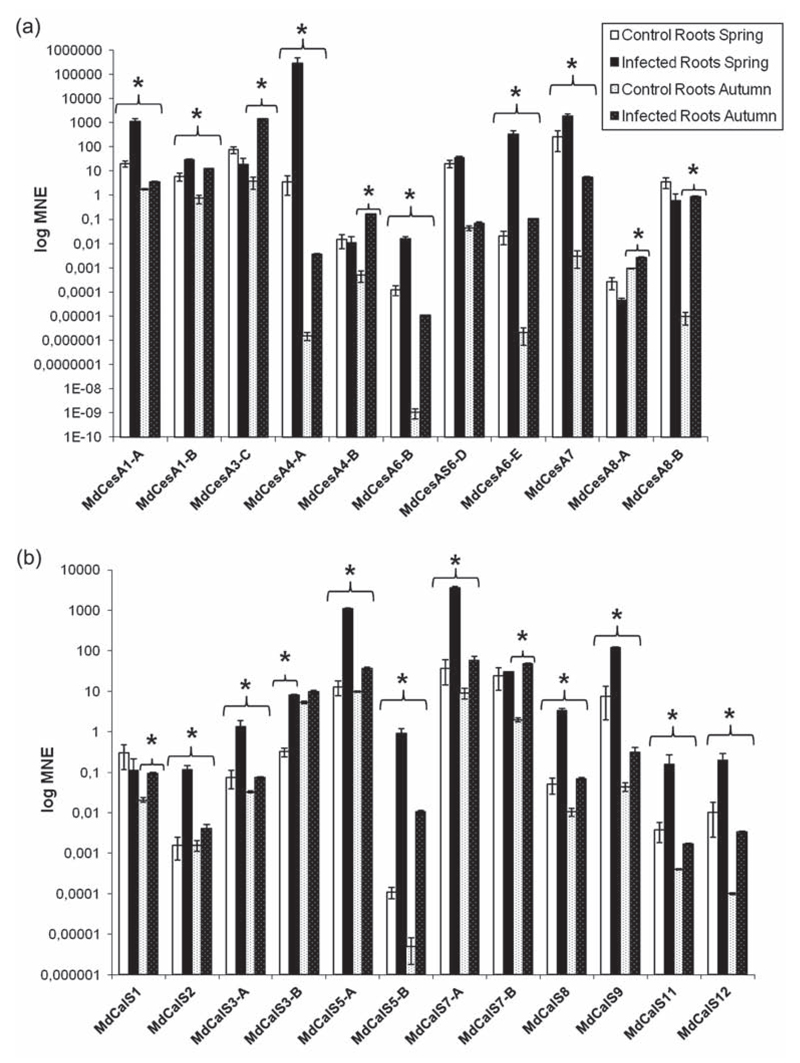
In our experimental system, the biggest changes in gene expression upon phytoplasma infection could be observed in the roots of apple trees (Figure 2a and b). In the infected roots collected during spring, it was possible to observe an increase in the expression of seven CesA genes: in particular there was a strong induction of MdCesA1-A (56-fold), MdCesA4-A (79600-fold), MdCesA6-B (121-fold) and MdCesA6-E (16500-fold) and a milder up-regulation of MdCesA1-B (5-fold), MdCesA7 (7-fold) and MdCesA6-D (1.8-fold) (Figure 2a). Ten of the CalS genes identified in domesticated apple were also induced in infected roots sampled in spring (Figure 2b). Among these, it is particularly noteworthy to mention MdCalS7-A (94-fold), one of the apple orthologs of AtCalS7, recently shown to be involved in phloem transport (Barratt et al. 2011, Xie et al. 2011), MdCalS3-A and B (17.5-fold and 77-fold respectively), orthologs of AtCalS3 involved in callose deposition at the plasmodesmata (Vatén et al. 2011), that are structures through which effector proteins can be transmitted from cell to cell (Sugio et al. 2011) and MdCalS12 (20-fold), the ortholog of AtCalS12, shown to be up-regulated in response to fungal and oomycete pathogens (Jacobs et al. 2003, Nishimura et al. 2003, Dong et al. 2008).
Figure 2.
Gene expression analysis of CesA (a) and CalS (b) in roots. Asterisks indicate statistically significant changes (P < 0.05) in gene expression among phytoplasma-infected and healthy control plants. MNE stands for mean normalized expression.
In infected roots collected during autumn an induction of almost all the cell wall genes analyzed was observed. Interestingly, the overall expression level of the genes investigated was lower than that in the spring (Figure 2a and b), both in control and infected roots. This behavior in the tissues from healthy trees might be explained by a seasonal effect on M. × domestica roots during winter. M. × domestica is a deciduous tree characterized by dormancy during winter, a season during which the growth of the roots is significantly reduced: it is therefore possible to relate the lower levels in gene expression observed in control roots in autumn to the changes in the metabolism of the tree entering the winter period. Similarly, the gene expression profile observed in infected roots might be due to the seasonal effect, but at the same time also reflect the difference in pathogen concentration in the two seasons analyzed. A recent study carried out using a TaqMan real-time PCR approach (Baric et al. 2011b) has indeed shed some light on the seasonal colonization of apple trees by Ca. P. mali, by monitoring the titer of the bacteria in roots and branches over two growing seasons in a commercial apple orchard. The authors showed that the concentration levels of bacteria in the roots were higher from December to May and that the appearance of disease symptoms was dependent on the titer of phytoplasma present in the above-ground organs of the tree and not on the pathogen occurrence alone. Therefore, the generally higher gene expression levels found in spring could be explained by the higher titer of pathogen present in the roots in spring. From these data it is possible to infer that the presence of the pathogen in apple roots could trigger specific responses, among which is remodeling of the cell walls, through the modulation in genes expression. It has indeed been shown that a cell wall response of Medicago truncatula L. roots is triggered after infection by Ralstonia solanacearum (Smith 1896) (Turner et al. 2009): the cell wall constitutes the first barrier encountered by invading pathogens and therefore the first site where defense mechanisms are triggered (Caño-Delgado et al. 2003, Turner et al. 2009). Cell wall reinforcement, through callose, cellulose and lignin deposition, helps in preventing further penetration and colonization of the pathogen and limits the extension of the damage (Caño-Delgado et al. 2003, Turner et al. 2009, Musetti et al. 2010).
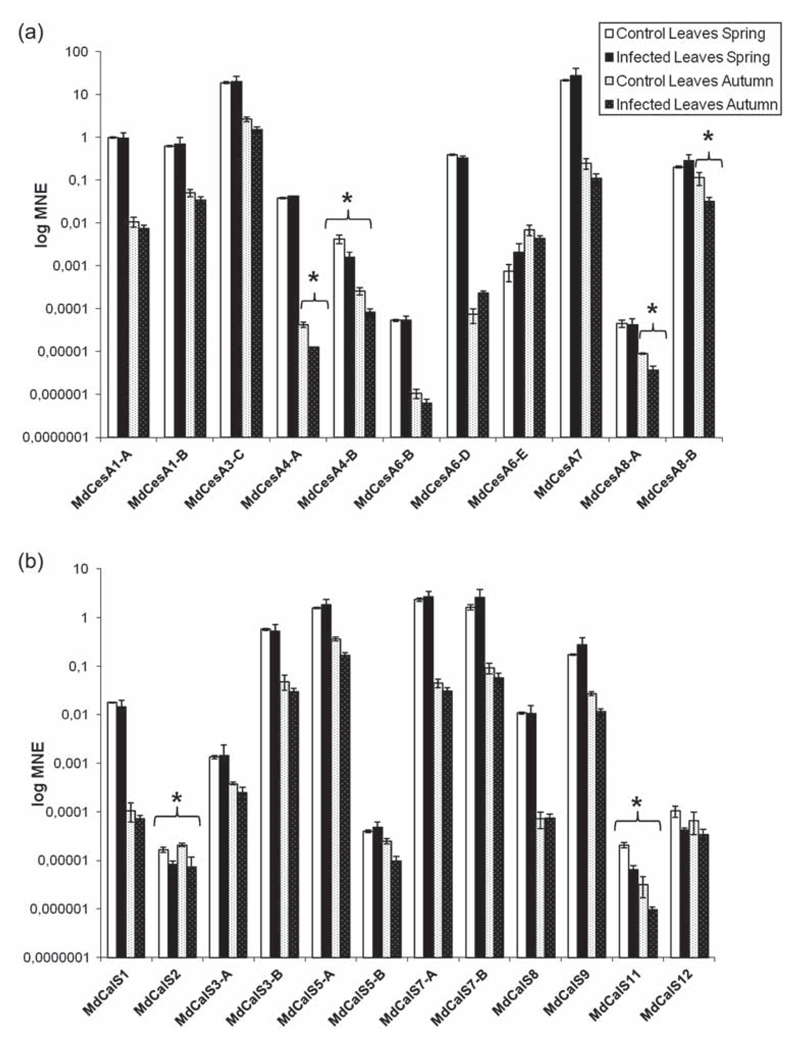
In contrast to roots, no significant increase in cell wall gene expression could be detected in leaves, either in spring or in autumn. On the contrary a mild down-regulation (between 2.5- and 4-fold) of secondary cell wall CesAs (MdCesA4-A and B, MdCesA8-A and B; Figure 3a) and a significant decrease in the expression of two CalS genes (namely MdCalS2 and MdCalS11; Figure 3b) were observed. This is in agreement with the data obtained by Musetti et al. (2010) for five CalS genes in infected apple leaves, where it was demonstrated that callose deposition is a process involved in the establishment of recovery of apple trees from AP.
Figure 3.
Gene expression analysis of CesA (a) and CalS (b) in leaves. Asterisks indicate significant changes (P < 0.05) in gene expression among phytoplasma-infected and healthy control plants. MNE stands for mean normalized expression.
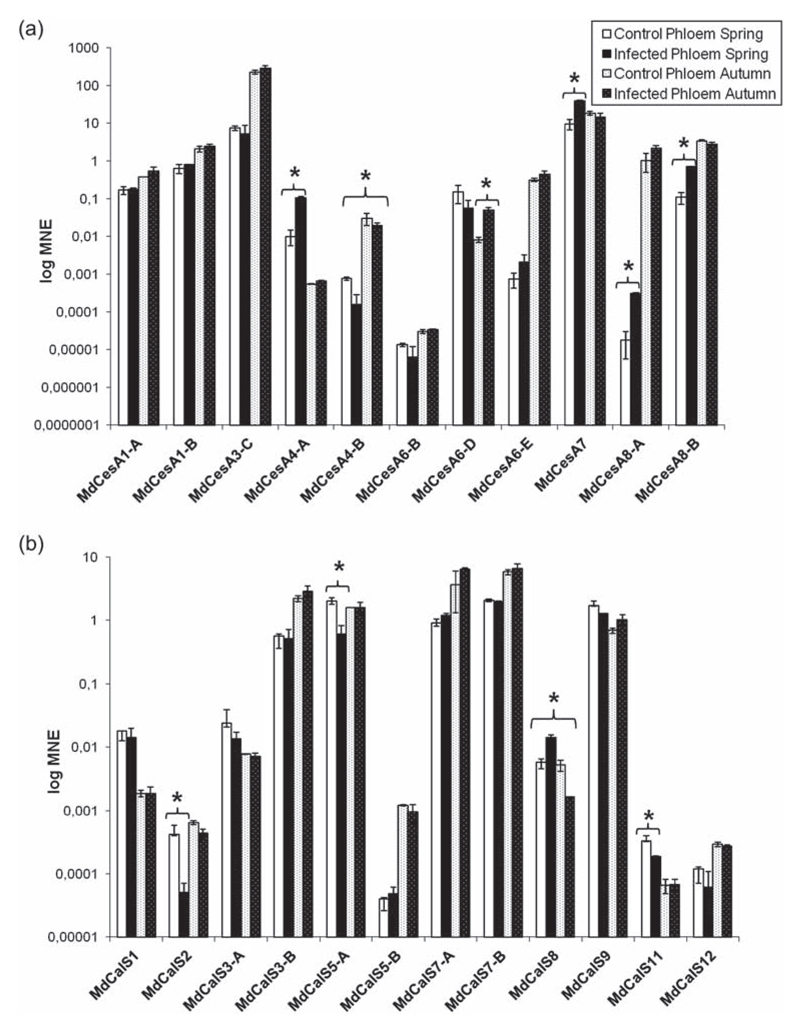
In the phloem from branches sampled during spring, it was possible to observe an up-regulation in gene expression of the secondary cell wall CesAs MdCes4-A (10.6-fold), MdCesA7 (4-fold), MdCesA8-A (17-fold) and B (7-fold) (Figure 4a), while in autumn no major differences in the expression of the cell wall-related genes were detected, apart from a down-regulation of MdCalS8 (3.2-fold; Figure 4b). The CalS genes identified in apple showed either no significant variations or down-regulation in leaves and branch phloem (Figures 3 and 4). These results indicate that in the leaves and branch phloem of symptomatic plants the infection of phytoplasma does not trigger an increase in callose deposition, in accordance with recent studies, which linked the induction in CalS gene expression to the process of recovery (Musetti et al. 2010, 2011). Recovery is a phenomenon whose physiological basis is still not fully understood. Several studies have suggested a connection between recovery from phytoplasma infection and production of H2O2 in the phloem (Musetti et al. 2004, 2005, 2007) and a connection between ROS and callose deposition has been documented (Sivaguru et al. 2000, Jongebloed et al. 2004, Luna et al. 2011). The cell wall response to pathogen attack is a versatile mechanism involving both short- and long-term mechanisms (Seifert and Blaukopf 2010): in this respect the recovery process might be seen as a long-term mechanism involving ROS production, signaling cascades, activation of transcription factors which in their turn activate specific metabolic pathways and resulting in phloem plugging mediated by callose, as well as PP2 and sieve element occlusion (SEO) proteins (Musetti et al. 2011).
Figure 4.
Gene expression analysis of CesA (a) and CalS (b) in branch phloem. Asterisks indicate significant changes (P < 0.05) in gene expression among phytoplasma-infected and healthy control plants. MNE stands for mean normalized expression.
The deposition of callose at plasmodesmata and sieve pores (Vatén et al. 2011, Barratt et al. 2011, Xie et al. 2011) may help establishing recovery, by inhibiting the spread of phytoplasma effectors (Sugio et al. 2011) and/or the diffusion of bacteria (Hogenhout et al. 2008, Xie and Hong 2011, Zavaliev et al. 2011).
Cultivated apple trees represent ‘chimeras’ of two different genotypes, the rootstock and the scion. The rootstock/scion combination of the trees analyzed in the present study was M9/‘Golden Delicious’. The differences in the relative expression levels of CesA and CalS genes between the rootstock and the above-ground organs of apple trees could thus be due to different responses of the two genotypes to phytoplasma infection. Based on the observation of symptoms, it was indeed shown that Malus taxa and hybrids differ in their susceptibility to Ca. P. mali (Kartte and Seemüller 1991). However, Bisognin et al. (2008) demonstrated a major influence of the rootstock genotype on the expression of symptoms and the pathogen titer in the canopy of the cultivar ‘Golden Delicious’. Rootstocks influence the growth and vigor of scions and they also affect the flowering precocity of the scion (Atkinson and Else 2001). The rootstock might also constitute the site of synthesis of effector proteins, which can then be transported to the scion and modulate the overall plant physiology to respond to the biotic stress through a modulation of cell wall biosynthesis. The presence of phytoplasma effector proteins inducing phenotypic changes in plants and even affecting the reproductive success of their insect vectors has been indeed proposed and shown (Sugio et al. 2011). Based on the results obtained in this study, it can be speculated that phytoplasma infection could modulate the interaction of the rootstock and the scion.
Gene expression analysis in in vitro micropropagated apple plantlets
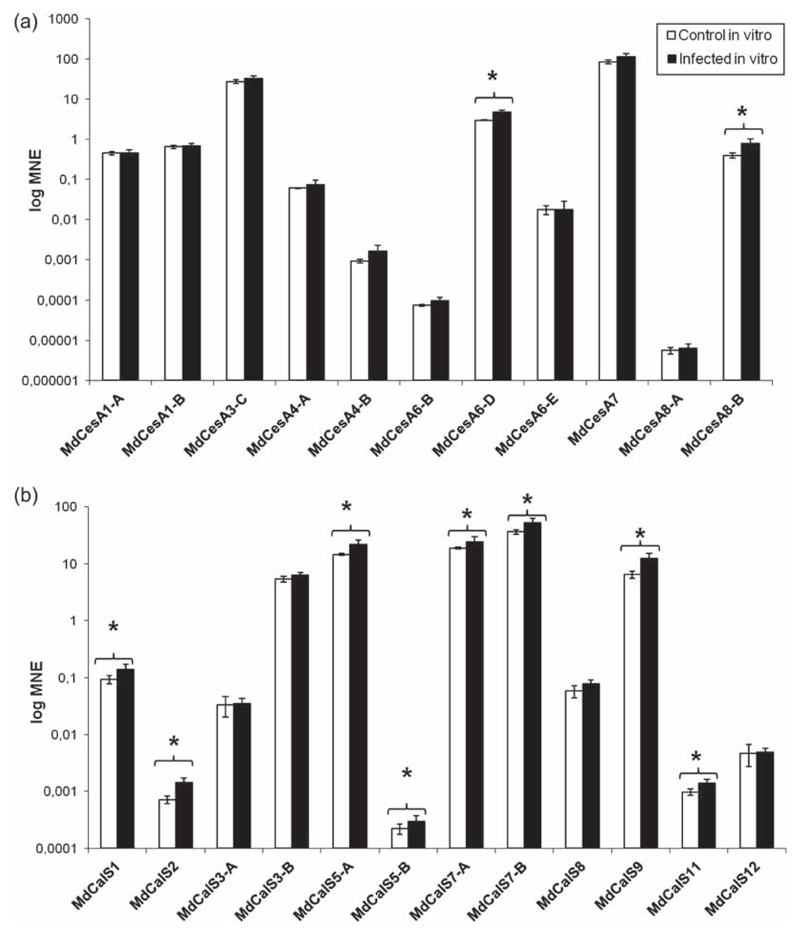
We decided to compare the gene expression values observed for potted apple trees (which were selected to reflect the situation in the field) with those of in vitro micropropagated apple plantlets, to check the response of cell wall biosynthetic genes to phytoplasma under different experimental growth conditions. In vitro grown plantlets displayed a mild up-regulation of the secondary cell wall MdCesA8-B (2-fold), together with the primary cell wall gene MdCesA6-D (1.6-fold) (Figure 5a). Interestingly, 8 of the 12 CalS genes showed a mild up-regulation too (1.3 to 2-fold) (Figure 5b), in particular both the apple AtCalS7 orthologs, MdCalS7-A and B. This might be due to a major role of callose with respect to cellulose in the in vitro infected plantlets: the physiology of the response to phytoplasma infection in the in vitro system could be primarily regulated by callose, which is a typical reaction polysaccharide, while in the actual trees, where a tissue differentiation together with lignifications and a seasonality of infection are present, cellulose is also involved in cell wall fortification. However, it is noteworthy to highlight that the typical ‘witches’ brooms’ phenotype is present in the in vitro system (Figure S2 available as Supplementary Data at Tree Physiology Online), a finding which implies a common response mechanism, at least in terms of phenotype appearance, between the micropropagated plantlets and the actual trees. From these data we can therefore conclude that the in vitro micropropagated plantlets constitute a useful system for a generalized assessment of gene expression following phytoplasma infection: it is possible to reproduce the ‘witches’ brooms’ phenotype, to easily increase both the number of biological replicates and that of pooled plantlets per biological replicate and to get information about variations in gene expression which, despite the differences in the growth parameters, are congruent with the results relative to the outdoor-grown trees. We must highlight, though, that the in vitro system does neither allow the selective analysis of different tissues, nor the seasonality of gene expression.
Figure 5.
Gene expression analysis of CesA (a) and CalS (b) in in vitro micropropagated apple plantlets. Asterisks indicate significant changes (P < 0.05) in gene expression among phytoplasma-infected and healthy control plants. MNE stands for mean normalized expression.
Infected apple roots show a distinctive phenotype
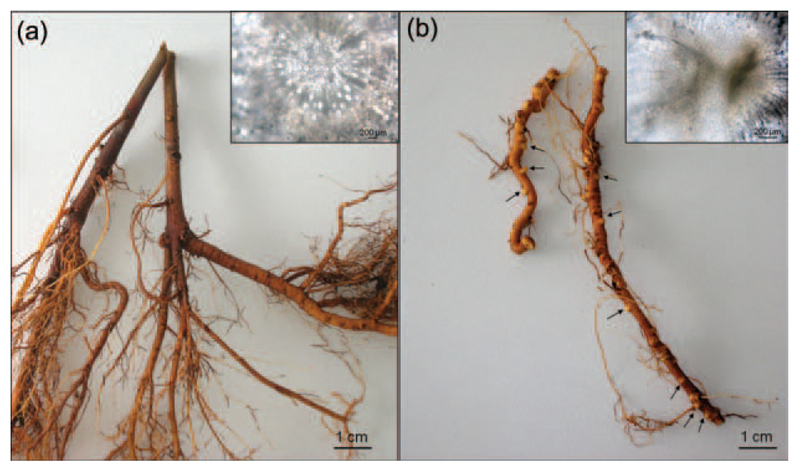
It was possible to observe the presence of a distinctive root phenotype in the infected trees analyzed, namely several bulges along the whole length of the roots (Figure 6). To understand the nature of this phenotype, cross sections coupled to bright field microscopy observations were carried out, which revealed the structures to be lateral root primordia (Figure 6 insets). Since we systematically observed this phenotype in the roots of all our symptomatic infected trees sampled during both the time points considered in the study, but not in any of our healthy controls, we propose that the infection by Ca. P. mali induces the formation of lateral root primordia in the infected young trees analyzed, which result in a more branched root system in older trees.
Figure 6.
Roots from healthy (a) and infected symptomatic (b) trees. Several bulges could be observed in the infected roots (arrows). Insets: cross sections of healthy (inset in a) and infected symptomatic (inset in b) roots, observed with bright field microscopy.
In our experimental system, the phenotype observed could be due to a higher sensitivity of the rootstock genotype to phytoplasma infection. However, a root system showing a higher number of lateral roots has been observed also in older self-rooting infected ‘Golden Delicious’ apple trees (age between 7 and 10 years old; Figure S3 available as Supplementary Data at Tree Physiology Online), a finding which suggests a correlation between infection and appearance of the phenotype. We hypothesize that the increase in cell wall biosynthetic genes observed in infected apple roots correlates with the increased branching of infected roots.
The presence of an increased number of lateral root primordia in young infected trees could be due to two reasons: (i) the infected plants try to extend the root apparatus through the formation of lateral roots which emerge from the ‘knot’-like structures, to be able to increase the nutrient uptake from the soil and therefore withstand biotic stress; (ii) the lateral roots originating from the ‘knots’ may represent an underground transmission system of phytoplasma to neighboring trees.
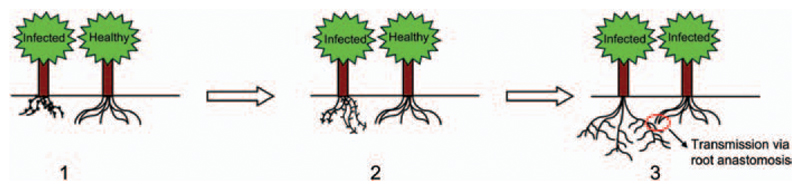
We propose a model for this last hypothesis (Figure 7) according to which, upon infection, the root system is induced to expand/branch out via emergence of lateral roots and eventually to form anastomoses when in contact with the roots of neighboring trees, to favor diffusion and spread of the pathogen. The ‘knots’ observed in the roots of infected trees develop into a more intricate root apparatus as the tree gets older, as can be seen in Figure S3 available as Supplementary Data at Tree Physiology Online. It is known that the emergence of lateral roots is a complex phenomenon which involves plant hormones and cell wall remodelling (Péret et al. 2009). An up-regulation of CesA and Csl (Cellulose synthase-like) genes in Populus during lateral roots emergence (Gou et al. 2010) has been described, similar to what we observed in infected roots of apple trees (Figure 6). To our knowledge, this is the first time such a phenotype is described.
Figure 7.
Proposed model for the underground transmission system of AP. (i) Roots of infected symptomatic trees show bulges (lateral root primordia); (ii) bulges progressively grow and (iii) branch out, until they are in contact with the roots of a healthy neighboring tree; the roots can form connections (root anastomoses), through which phytoplasmas can migrate and infect the neighboring tree.
The transmission of Ca. P. mali by psyllid vectors has been extensively studied (Frisinghelli et al. 2000, Jarausch et al. 2003, 2007, Tedeschi et al. 2003, Tedeschi and Alma 2004, Seemüller et al. 2004, Pedrazzoli et al. 2007, Carraro et al. 2008, Mayer et al. 2009, Malagnini et al. 2010). It was even shown that phytoplasma can manipulate the host plant in such a way that it emits a sesquiterpene (E-β-caryophyllene) which attracts the insect vector (Mayer et al. 2008a, 2008b). The pathogen can induce changes not only in the host plant, but also in the vector, to be efficiently transmitted. However, apart from insect vectors, a transmission of Ca. P. mali via natural/experimental root bridges (Ciccotti et al. 2007, Baric et al. 2008) has been already reported. We therefore propose that the apple root phenotype described here may be the result of an underground disease diffusion mechanism which ensures maximal diffusion and transmission of the pathogen, through an increased branching of the root system.
Conclusions
Here we have shown that the infection caused by Ca. P. mali induces a cell wall response which is particularly evident in the roots of infected trees. We have also shown that the in vitro system, despite the impossibility of analyzing different tissues and the seasonality of infection, is useful for a general assessment of gene expression following phytoplasma infection, since the results confirm an induction in the expression of cell wall-related genes. Moreover, this system allows an increase in the number of pooled material per biological replicate.
The cell wall response of infected apple trees likely provides a way to ensure turgor-driven expansion of the protoplast during biotic stress (Hèmaty et al. 2009) and at the same time might provide a way to stop the spread of the pathogen, by plugging the sieve elements and reinforcing the cell walls.
These results constitute a further step towards deciphering the intricate events which take place upon phytoplasma infection in an economically important fruit tree.
Supplementary data
Supplementary data for this article are available at Tree Physiology Online.
Acknowledgments
Dr Luis Lindner is gratefully acknowledged for help with the microscopic observations of apple roots.
Funding
G.G. was supported by the fellowship ‘Incoming researchers’ from the Autonomous Province of Bozen/Bolzano-South Tyrol (Promotion of Educational Policies, University and Research Department) and by the Austrian Science Fund (FWF): project number M1315. This study was funded by the Autonomous Province of Bozen/Bolzano, Italy (Departments 31 and 33). The authors acknowledge the South Tyrolean Fruit Growers’ Co-operatives, in particularly VOG and VIP, for co-financing the Strategic Project on Apple Proliferation—APPL.
Footnotes
Conflict of interest
None declared.
References
- Albertazzi G, Caffagni A, Mile JA, Francia E, Roncaglia E, Ferrari F, Tagliafico E, Stefani E, Secchioni N. Gene expression in grapevine cultivars in response to Bois Noir phytoplasma infection. Plant Sci. 2009;176:792–804. [Google Scholar]
- Atkinson CJ, Else MA. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree. 2001;34:46–49. [Google Scholar]
- Baric S, Dalla Via J. A new approach to apple proliferation detection: a highly sensitive real-time PCR assay. J Microbiol Methods. 2004;57:35–145. doi: 10.1016/j.mimet.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Baric S, Kerschbamer C, Dalla Via J. TaqMan real-time PCR versus four conventional PCR assays for detection of apple proliferation phytoplasma. Plant Mol Biol Rep. 2006;24:169–184. [Google Scholar]
- Baric S, Kerschbamer C, Vigl J, Dalla Via J. Translocation of apple proliferation phytoplasma via natural root grafts—a case study. Eur J Plant Pathol. 2008;121:207–211. [Google Scholar]
- Baric S, Berger J, Cainelli C, Kerschbamer C, Dalla Via J. Molecular typing of ‘Candidatus Phytoplasma mali’ and epidemic history tracing by a combined T-RFLP/VNTR analysis approach. Eur J Plant Pathol. 2011a;131:573–584. [Google Scholar]
- Baric S, Berger J, Cainelli C, Kerschbamer C, Letschka T, Dalla Via J. Seasonal colonisation of apple trees by ‘Candidatus Phytoplasma mali’ revealed by a new quantitative TaqMan real-time PCR approach. Eur J Plant Pathol. 2011b;129:455–467. [Google Scholar]
- Barratt DH, Kölling K, Graf A, Pike M, Calder G, Findlay K, Zeeman SC, Smith AM. Callose synthase GSL7 is necessary for normal phloem transport and inflorescence growth in Arabidopsis. Plant Physiol. 2011;155:328–341. doi: 10.1104/pp.110.166330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaccini A. Phytoplasmas: diversity, taxonomy, and epidemiology. Front Biosci. 2007;12:673–689. doi: 10.2741/2092. [DOI] [PubMed] [Google Scholar]
- Bisognin C, Schneider B, Salm H, Grando MS, Jarausch W, Moll E, Seemüller E. Apple proliferation resistance in apomictic rootstocks and its relationship to phytoplasma concentration and simple sequence repeat genotypes. Phytopathology. 2008;98:153–158. doi: 10.1094/PHYTO-98-2-0153. [DOI] [PubMed] [Google Scholar]
- Caño-Delgado Al, Metzlaff K, Bevan MW. The elil mutation reveals a link between cell expansion and secondary cell wall formation in Arabidopsis thaliana. Development. 2000;127:3395–3405. doi: 10.1242/dev.127.15.3395. [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A, Penfield S, Smith C, Catley M, Bevan M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 2003;34:351–362. doi: 10.1046/j.1365-313x.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- Carraro L, Ermacora P, Loi N, Osler R. The recovery phenomenon in apple proliferation-infected apple trees. J Plant Pathol. 2004;86:141–146. [Google Scholar]
- Carraro L, Ferrini F, Ermacora P, Loi N, Labonne G. Infectivity of Cacopsylla picta (Syn. Cacopsylla costalis), vector of ‘Canditatus Phytoplasma mali’ in North East Italy. Acta Horticult. 2008;781:403–407. [Google Scholar]
- Ciccotti AM, Bianchedi PL, Bragagna P, Deromedi M, Filippi M, Forno F, Mattedi L. Transmission of ‘Candidatus Phytoplasma mali’ by root bridges under natural and experimental conditions. Bull Insectol. 2007;60:387–388. [Google Scholar]
- Ciccotti AM, Bisognin C, Battocletti I, Salvatori A, Herdemertens M, Jarausch W. Micropropagation of Malus sieboldii hybrids resistant to apple proliferation disease. Agron Res. 2008;6:445–458. [Google Scholar]
- Cosgrove DJ. Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol. 1997;13:171–201. doi: 10.1146/annurev.cellbio.13.1.171. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005;42:315–328. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- Dong X, Hong Z, Chatterjee J, Kim S, Verma DPS. Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta. 2008;229:87–98. doi: 10.1007/s00425-008-0812-3. [DOI] [PubMed] [Google Scholar]
- Duval I, Beaudoin N. Transcriptional profiling in response to inhibition of cellulose synthesis by thaxtomin A and isoxaben in Arabidopsis thaliana suspension cells. Plant Cell Rep. 2009;28:811–830. doi: 10.1007/s00299-009-0670-x. [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–1566. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, et al. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell. 2000;12:2409–2424. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisinghelli C, Delaiti L, Grando M, Forti D, Vindimian M. Cacopsylla costalis (Flor 1861), as a vector of apple proliferation in Trentino. J Phytopathol. 2000;148:425–431. [Google Scholar]
- Gambino G, Perrone I, Gribaudo I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal. 2008;19:520–525. doi: 10.1002/pca.1078. [DOI] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X, Busov VB. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell. 2010;22:623–39. doi: 10.1105/tpc.109.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero G, Spadiut O, Kerschbamer C, Giorno F, Baric S, Ezcurra I. Analysis of cellulose synthase genes from domesticated apple identifies collinear genes WDR53 and CesA8A: partial coexpression, bicistronic mRNA and alternative splicing of CESA8A. J Exp Bot. 2012 doi: 10.1093/jxb/ers255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Denness L. Cell wall integrity maintenance in plants: lessons to be learned from yeast? Plant Signal Behav. 2011;6:1706–1709. doi: 10.4161/psb.6.11.17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Hématy K, Cherk C, Somerville S. Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol. 2009;12:406–413. doi: 10.1016/j.pbi.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Hernández-Blanco C, Feng DX, Hu J, et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenhout SA, Oshima K, Ammar E-D, Kakizawa S, Kingdom HN, Namba S. Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol. 2008;9:403–423. doi: 10.1111/j.1364-3703.2008.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Delauney AJ, Verma DP. A cell plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell. 2001;13:755–768. doi: 10.1105/tpc.13.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR. Sentinels at the wall: cell wall receptors and sensors. New Phytol. 2007;176:7–21. doi: 10.1111/j.1469-8137.2007.02192.x. [DOI] [PubMed] [Google Scholar]
- Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB. An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell. 2003;15:2503–2513. doi: 10.1105/tpc.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarausch B, Schwind N, Jarausch W, Krczal G. First report of Cacopsylla picta as a vector of apple proliferation phytoplasma in Germany. Plant Dis. 2003;87:101. doi: 10.1094/PDIS.2003.87.1.101A. [DOI] [PubMed] [Google Scholar]
- Jarausch B, Fuchs A, Schwind N, Krczal G, Jarausch W. Cacopsylla picta as most important vector for ‘Candidatus Phytoplasma mali’ in Germany and neighbouring regions. Bull Insectol. 2007;60:189–190. [Google Scholar]
- Jarausch W, Lansac M, Dosba F. Long-term maintenance of nonculturable apple proliferation phytoplasmas in their micropropagated natural host plant. Plant Pathol. 1996;45:778–786. [Google Scholar]
- Jongebloed U, Szederkényi J, Hartig K, Schobert C, Komor E. Sequence of morphological and physiological events during natural ageing and senescence of a castor bean leaf: sieve tube occlusion and carbohydrate back-up precede chlorophyll degradation. Physiol Plant. 2004;120:338–346. doi: 10.1111/j.0031-9317.2004.0245.x. [DOI] [PubMed] [Google Scholar]
- Joubert A, Bataille-Simoneau N, Campion C. Cell wall integrity and high osmolarity glycerol pathways are required for adaptation of Alternaria brassicicola to cell wall stress caused by brassicaceous indolic phytoalexins. Cell Microbiol. 2011;13:62–80. doi: 10.1111/j.1462-5822.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Kartte S, Seemüller E. Variable response within the genus Malus to the apple proliferation disease. J Plant Dis Protect. 1988;95:25–34. [Google Scholar]
- Kartte S, Seemüller E. Susceptibility of grafted Malus taxa and hybrids to apple proliferation disease. J Phytopathol. 1991;131:137–148. [Google Scholar]
- Krogh A, Larsso B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lee IM, Davis RE, Gundersen-Rindal DE. Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol. 2000;54:221–255. doi: 10.1146/annurev.micro.54.1.221. [DOI] [PubMed] [Google Scholar]
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact. 2011;24:183–193. doi: 10.1094/MPMI-07-10-0149. [DOI] [PubMed] [Google Scholar]
- Malagnini V, Pedrazzoli F, Gualandri V, Zasso R, Bozza E, Fiamingo F, Pozzebon A, Mori N, loriatti C. Detection of ‘Candidatus Phytoplasma mali’ in different populations of Cacopsylla melanoneura in Italy. Bull Insectol. 2010;63:59–63. [Google Scholar]
- Mayer CJ, Vilcinskas A, Gross J. Pathogen-induced release of plant allomone manipulates vector insect behavior. J Chem Ecol. 2008a;34:1518–1522. doi: 10.1007/s10886-008-9564-6. [DOI] [PubMed] [Google Scholar]
- Mayer CJ, Vilcinskas A, Gross J. Phytopathogen lures its insect vector by altering host plant odor. J Chem Ecol. 2008b;34:1045–1049. doi: 10.1007/s10886-008-9516-1. [DOI] [PubMed] [Google Scholar]
- Mayer CJ, Jarausch B, Jarausch W, Jelkmann W, Vilcinskas A, Gross J. Cacopsylla melanoneura has no relevance as vector of apple proliferation in Germany. Phytopathology. 2009;99:729–738. doi: 10.1094/PHYTO-99-6-0729. [DOI] [PubMed] [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32:1372–1374, 1376, 1378–1379. Biotechniques. 2002;33:514. Erratum in. [PubMed] [Google Scholar]
- Musetti R, Sanità di Toppi L, Ermacora P, Favali MA. Recovery in apple trees infected with the apple proliferation phytoplasma: an ultrastructural and biochemical study. Phytopathology. 2004;94:203–208. doi: 10.1094/PHYTO.2004.94.2.203. [DOI] [PubMed] [Google Scholar]
- Musetti R, Sanità di Toppi L, Martini M, Ferrini F, Loschi A, Favali MA, Osler R. Hydrogen peroxide localization and antioxidant status in the recovery of apricot plants from European stone fruit yellows. Eur J Plant Pathol. 2005;112:53–61. [Google Scholar]
- Musetti R, Marabottini R, Badiani M, Martini M, Sanità di Toppi L, Borselli S, Borgo M, Osler R. On the role of H2O2 in the recovery of grapevine (Vitis vinifera, cv. Prosecco) from Flavescence dorée disease. Funct Plant Biol. 2007;34:750–758. doi: 10.1071/FP06308. [DOI] [PubMed] [Google Scholar]
- Musetti R, Paolacci A, Caffi M, et al. Phloem cytochemical modification and gene expression following the recovery of apple plants from apple proliferation disease. Phytopathology. 2010;4:390–399. doi: 10.1094/PHYTO-100-4-0390. [DOI] [PubMed] [Google Scholar]
- Musetti R, De Marco F, Farhan K, Polizzotto R, Santi S, Ermacora P, Osler R. Phloem-specific protein expression patterns in apple and grapevine during phytoplasma infection and recovery. Bull Insectol. 2011;64:S211–S212. [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science. 2003;301:969–972. doi: 10.1126/science.1086716. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli F, Gualandri V, Forno F, Mattedi L, Malagnini V, Salvadori A, Stoppa G, Ioriatti C. Acquisition capacities of the overwintering adults of the psyllid vectors of ‘Candidatus Phytoplasma mali’. Bull Insectol. 2007;60:195–196. [Google Scholar]
- Péret B, Larrieu A, Bennett MJ. Lateral root emergence: a difficult birth. J Exp Bot. 2009;60:3637–3643. doi: 10.1093/jxb/erp232. [DOI] [PubMed] [Google Scholar]
- Ramírez V, García-Andrade J, Vera P. Enhanced disease resistance to Botrytis cinerea in myb46 Arabidopsis plants is associated to an early down-regulation of CesA genes. Plant Signal Behav. 2011;6:911–913. doi: 10.4161/psb.6.6.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekab D, Pirajno G, Cettul E, De Salvador FR, Firrao G. On the apple proliferation symptom display and the canopy colonization pattern of ‘Candidatus Phytoplasma mali’ in apple trees. Eur J Plant Pathol. 2010;127:7–12. [Google Scholar]
- Ringli C. Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 2010;153:1445–1452. doi: 10.1104/pp.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper U, Seemüller E. Recolonization of the stem of apple proliferation and pear decline-diseased trees by the causal organisms in spring. J Plant Dis Protect. 1984;91:608–613. [Google Scholar]
- Seemüller E, Schneider B. Differences In virulence and genomic features of strains of ‘Candidatus Phytoplasma mali’, the apple proliferation agent. Phytopathology. 2007;97:964–70. doi: 10.1094/PHYTO-97-8-0964. [DOI] [PubMed] [Google Scholar]
- Seemüller E, Kunze L, Schaper U. Colonization behaviour of MLO, and symptom expression of proliferation-diseased apple trees and decline-diseased pear trees over a period of several years. J Plant Dis Protect. 1984;91:525–532. [Google Scholar]
- Seemüller E, Dickler E, Berwarth C, Jelkmann W. Occurrence of psyllids in apple orchards and transmission of apple proliferation by Cacopsylla picta (syn. C. costalis) in Germany. Acta Hortic. 2004;657:533–537. [Google Scholar]
- Seifert GJ, Blaukopf C. Irritable walls: the plant extracellular matrix and signaling. Plant Physiol. 2010;153:467–478. doi: 10.1104/pp.110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Métraux JP, Nawrath C. Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell. 2000;12:721–738. doi: 10.1105/tpc.12.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Fujiwara T, Samaj J, et al. Aluminum-induced 1→3-beta-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 2000;124:991–1006. doi: 10.1104/pp.124.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwand BJ, Kieber JJ. The role of receptor-like kinases in regulating cell wall function. Plant Physiol. 2010;153:479–484. doi: 10.1104/pp.110.155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio A, Kingdom HN, MacLean AM, Grieve VM, Hogenhout SA. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci USA. 2011;108:E1254–E1263. doi: 10.1073/pnas.1105664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi R, Alma A. Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae) J Econ Entomol. 2004;97:8–13. doi: 10.1093/jee/97.1.8. [DOI] [PubMed] [Google Scholar]
- Tedeschi R, Visentin C, Alma A, Bosco D. Epidemiology of apple proliferation (AP) in northwestern Italy: evaluation of the frequency of AP-positive psyllids in naturally infected populations of Cacopsylla melanoneura (Homoptera: Psyllidae) Ann Appl Biol. 2003;142:285–290. [Google Scholar]
- Torres MA, Jones JD, Dangl JL. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Jauneau A, Genin S, Tavella MJ, Vailleau F, Gentzbittel L, Jardinaud MF. Dissection of bacterial Wilt on Medicago truncatula revealed two type III secretion system effectors acting on root infection process and disease development. Plant Physiol. 2009;150:1713–1722. doi: 10.1104/pp.109.141523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Kirk TK, Sherwood RT. Lignification as a defence mechanism of disease resistence. Annu Rev Phytopathol. 1980;18:259–288. [Google Scholar]
- Vatén A, Dettmer J, Wu S, et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21:1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Velasco R, Zharkikh A, Affourtit J, et al. The genome of the domesticated apple (Malus × domestica Borkh.) Nat Genet. 2010;42:833–839. doi: 10.1038/ng.654. [DOI] [PubMed] [Google Scholar]
- Xie B, Hong Z. Unplugging the callose plug from sieve pores. Plant Signal Behav. 2011;6:491–493. doi: 10.4161/psb.6.4.14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B, Wang X, Zhu M, Zhang Z, Hong Z. CalS7 encodes a callose synthase responsible for callose deposition in the phloem. Plant J. 2011;65:1–14. doi: 10.1111/j.1365-313X.2010.04399.x. [DOI] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell. 2008;20:3065–3079. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaliev R, Ueki S, Epel BL, Citovsky V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma. 2011;248:117–130. doi: 10.1007/s00709-010-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pettolino FA, Dhugga KS, et al. Cell wall modifications in maize pulvini in response to gravitational stress. Plant Physiol. 2011;156:2155–2171. doi: 10.1104/pp.111.179606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.