Abstract
Deficits in prepulse inhibition (PPI), a measure of sensorimotor gating, are observed in neurodevelopmental and neuropsychiatric disorders. Despite the large PPI literature, the majority of studies characteristically employ tests with one interstimulus interval (ISI), of one modality, at one age. In the context of the auditory startle response (ASR), the present study examined (1) the profile for the ontogeny of PPI through adulthood in Long–Evans hooded rats with a reasonably comprehensive ISI function, (2) whether the ontogenetic profile for PPI is sensitive to modality of the prepulse stimulus, as a within-session variable, and (3) whether the maturation of PPI differs for males and females. Despite the basic effect of more pronounced PPI in adult relative to preweanling animals, each sensory modality displayed a unique ontogenetic profile for PPI, without any compelling evidence for major differences between males and females, in accordance with the known temporal course of peripheral and central maturational profiles of sensory systems in the rat. The context for assessing auditory PPI (auditory and tactile vs. auditory and visual prepulses) influenced the overall startle response, i.e., a shift in the height of the entire profile, but did not significantly impact the auditory PPI profile per se. The translational relevance of preclinical sensorimotor assessments to patients with neurodevelopmental and/or neuropsychiatric disorders depends partly on an understanding of the ontogeny of sensorimotor gating in different sensory systems, and can be strengthened with the use of a reasonably comprehensive number of ISIs to provide relatively precise and defined response functions.
Keywords: prepulse inhibition, sensorimotor gating, auditory startle response, preattentive process, neurodevelopmental disorders, neuropsychiatric disorders, Long–Evans hooded rat
INTRODUCTION
Sensorimotor gating defines the temporal regulation or filtering of incoming, irrelevant sensory information as indexed by a motor response. The ability to “gate” sensory information is putatively held to be common across vertebrate species, and even extends to invertebrate members of the animal kingdom (Riede, 1993). Sensorimotor gating may be measured through prepulse inhibition (PPI) of the startle response, which is demonstrated when a “prepulse”, that is a relatively weak sensory stimulus, is presented in close temporal proximity to the stronger “startling” stimulus. The degree to which the startle response is decreased by a preceding (auditory, tactile or visual) stimulus is fundamentally a function of the interstimulus interval (ISI) and provides a reliable measure of sensorimotor gating (Hoffman & Ison, 1980; Hoffman & Searle, 1965; Ison & Hammond, 1971). Individuals with neurodevelopmental disorders, such as schizophrenia and attention deficit hyperactivity disorder, as well as neuropsychiatric disorders, such as Tourette’s syndrome and Huntington’s disease, have shown disruptions in PPI, indicative of sensorimotor gating impairments (Braff et al., 1978; Castellanos et al., 1996; Smith & Lees, 1989; Valls-Sole, Munoz, & Valldeoriola, 2004).
Over the past two decades, a putative neural circuit map has been developed, based on lesion and electrical stimulation studies, for the mediation of PPI of the auditory startle response (ASR). Specifically, a serial circuit was proposed in which auditory input, processed via the ascending auditory pathway to the inferior colliculus (IC), activates the superior colliculus (SC), which in turn activates the pedunculopontine tegmental nucleus (PPTg), triggering a cholinergic projection to the caudal pontine reticular nucleus (PnC) to mediate PPI (Fendt, Koch, & Schnitzler, 1994; Fendt, Li, & Yeomans, 2001; Koch, Kungel, & Herbert, 1993; Koch & Schnitzler, 1997). The PnC is the obligatory nucleus in the primary startle pathway for integrating prepulse and startle stimuli. Further, this model suggested that visual and tactile prepulses are mediated via the SC, not the IC. In addition to this primary mediational circuit, the hierarchical regulation of PPI has also been firmly established. The “CSPP circuitry”, as it is often abbreviated, includes both sequential and parallel neural connections among the limbic cortex, ventral striatum, ventral pallidum, and pontine tegmentum, and is held to converge with the primary startle circuit at the level of the PnC (Davis, 1980, 1984; Davis, Gendelman, Tischler, & Gendelman, 1982; Fendt et al., 1994, 2001; Koch, 1999; Koch et al., 1993; Koch & Schnitzler, 1997; Li, Du, Li, Wu, & Wu, 2009; Yeomans, Lee, Yeomans, Steidl, & Li, 2006; Yeomans, Li, Scott, & Frankland, 2002). Thus, the general consensus on both the mediating and regulatory circuits underlying PPI provides the rationale for translational inference, albeit not without caveat (Davis, Antoniadis, Amaral, & Winslow, 2008; Wood, Beyer, & Cappon, 2003).
Sensorimotor gating indexed by PPI is evident at an early age in humans (Kisley, Polk, Ross, Levisohn, & Freedman, 2003) and rodents (Parisi & Ison, 1979); however, the nature of any characteristic change in response across development remains unclear. Furthermore, it is also uncertain whether there is any characteristic difference in response inhibition when utilizing sensory stimuli from different modalities. One seminal study in rodents noted a significant linear trend of PPI across preweaning development (PD12–18) that did not significantly vary across cutaneous (.5 or 1.0 mA), white noise (100–10,000 Hz) or pure tone (10 kHz) prepulses (Parisi & Ison, 1979), suggesting that the ontogenetic profile of PPI was invariant to modality of the prepulse stimulus. In contrast, response inhibition with a visual prepulse (68–171 cd/m2) was significantly less than with a white noise prepulse (56–70 dB) in preweanling and periadolescent (PD31–35) rats (Parisi & Ison, 1981). Additional variables that constrain the derivation of a general ontogenetic principal across the three prepulse sensory modalities examined include differences in duration of the prepulse stimuli (cutaneous .5 ms; auditory, 20–25 ms; visual, 50 ms), two of the three experiments in the former study were based on results from pups drawn from a single litter, and all of the prepulse modality comparisons were based on between-session, if not between-group, comparisons. Nevertheless, the extant data suggest that the ontogeny of PPI is contingent on development of central as well as peripheral sensory mechanisms.
The ontogenesis of sensory systems varies across species (Gottlieb, 1971). In the rodent, evidence for tactile stimulation sensitivity was found as early as gestational Day 15–17 (Gonzalez, 1932). By contrast, the auditory and visual sensory systems are not considered functional until after birth. Regarding audition, early studies demonstrated that rodents can respond with a primitive orienting reflex around postnatal Day (PD) 5, but do not respond with an orienting exploring reflex until PD 12 (Volokhov, 1968). Others confirmed that rodents are capable of their first overt auditory response around PD 10 (Crowley & Hepp-Reymond, 1966; Wada, 1923). Although the auditory sensory system displays sensitivity to stimuli before PD 10–12, it displays much more mature characteristics after opening of the external auditory meatus near the end of the second week of life. Similarly, the visual system of the rodent is capable of responding to a light stimulus before the eyes open. For example, neonatal rat pups (PD 6–8) showed avoidant behavior to a light stimulus (Detwiler, 1932; Routtenberg, Strop, & Jerdan, 1978), reflecting the significant retinal development noted in the first and second weeks of life (LeVere, 1978). Evidence for visual sensory function, however, is not noted with electrophysiological or histological measures until the eyes open at PD 10–12, and in some strains, PD 15–17 (Crozier & Pincus, 1937; Rose, 1968). The functional properties of primary rat visual cortex, such as selectivity for orientation and movement direction of visual stimuli, as well as visual acuity, achieve adult-like characteristics at approximately 45 days of age (Fagiolini, Pizzorusso, Berardi, Domenici, & Maffei, 1994; Fortin et al., 1999). Thus, the visual system is most aptly considered a later maturing system relative to the tactile and auditory sensory systems. The relatively delayed development of the visual system is consonant with the findings that suggest PPI may not be obtained with a visual prepulse until PD 21–23 (Parisi & Ison, 1981). The existing data, thus, beg the question of whether there are unique profiles of PPI for each of the sensory processing systems. The estimation of a relatively comprehensive ISI function was sought in answering this question.
The aims of the present study were to ascertain the following: (1) the profile for the ontogeny of PPI in a rodent when assessing a reasonably comprehensive ISI function, (2) whether PPI is sensitive to modality of the prepulse stimulus, and 3) whether the maturation of PPI differs for males and females. The present study examined the ontogeny of PPI across PDs 15, 18, 31, 35, and 90. Punctate prepulse stimuli represented the tactile, auditory, and visual modalities. A repeated-measures within-session design was employed to maximize statistical power of detecting an effect of modality. To control for potential litter bias, no more than one male or female pup was selected from any one litter at any one test age. To the extent that PPI primarily reflects central inhibitory processing, it is anticipated that the response data will be invariant to modality of the prepulse stimulus. Alternatively, it may very well be that each prepulse sensory modality confers a unique ontogenetic profile of response inhibition, reflecting a constraint of sensory system maturation on expression of central inhibitory processing.
METHODS
Subjects
Long–Evans rat dams with pups (ns = 12) as well as adult male and female Long–Evans rats (ns = 12) were obtained from Harlan Laboratories, Inc. (Indianapolis, IN) at 7 or 70 days of age, respectively. The pups were housed with their biological dam until 21 days of age, as which time they were weaned, separated by sex, and pair-housed throughout the experiment. The adults were pair-housed with same sex animals. Food (Pro-Lab Rat, Mouse Hamster Chow no. 3000, National Institutes of Health diet no. 31) and water were available ad libitum. The vivarium conditions were set at 21 ± 2°C, 50 ± 10% relative humidity and had a 12-hr light:12-hr dark cycle with lights on at 0700 hr (EST). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina (animal assurance number: A3049-01).
Experimental Design
A randomized-block design was used, with litter as the blocking factor, and 90-day-old adult animals as an added maturation control. The animals from each litter were randomly assigned to one of four testing days (PD 15, 18, 31, and 35), representing the preweaning or periadolescent period of development. In the context of the auditory startle response (ASR), a within-session design was used to assess the factor of prepulse modality. Animals selected on PDs 15, 31, and 90 were tested in a combined auditory and tactile PPI session; animals selected on PDs 18, 35, and 90 were tested in a combined auditory and visual PPI session. No animal was tested in more than one session, but each litter was represented at each of the preweanling and adolescent test ages. Animals tested on PD 90 were sampled from different litters by the supplier, with the stipulation of no more than one male/one female pair selected from any litter. Thus, within-session comparisons were restricted to auditory versus tactile prepulses, as well as auditory versus visual prepulses.
Apparatus
The startle apparatus (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA) was acoustically isolated (22 dB(A) sound level within the chamber) within a 10 cm thick double-walled, 81 × 81 × 116-cm isolation cabinet (external dimensions; Industrial Acoustic Company, Inc, Bronx, NY). Auditory stimuli were delivered with the high-frequency loudspeaker of the SR-Lab system (Radio Shack model#40-1278B, frequency range of 5–16 kHz), mounted 30 cm above the perspex test cylinder. Tactile stimuli (16 p.s.i. air-puff) were delivered 2 cm above the dorsal surface of the rat via a semirigid plastic tube (.64 mm diameter) connected to a compressed air tank via a dual-stage regulator (SR2500, Victor Equipment Company, Denton, TX). A white LED light (luminous emittance of 200 lux) was mounted inside the chamber, 45 cm from the perspex cylinder, to provide the visual stimuli. The rise/fall time of each stimulus was 2 ms. Each age group was tested in an appropriately sized perspex test cylinder: 3.8 cm internal diameter (ID) for the preweanling rats, 5.7 cm ID for the adolescent rats, and 8.9 cm ID for the adult rats. The animal’s whole body startle response to the auditory stimulus produced deflection of the test cylinder, which was converted into analog signals by a piezoelectric accelerometer integral to the bottom of the cylinder. The response signals were digitized (12 bit A–D) and saved to a hard disk. Acoustic and visual stimulus intensities were measured and calibrated with a sound level meter (Bruel & Kjaer Model #2203) and a light level meter (Light Meter Model# 840006, Sper Scientific Ltd., Scottsdale AZ), respectively, at the level of the perspex test cylinder. Response sensitivities were calibrated with the SR-LAB startle calibration system.
Testing Procedure
At the selected test ages, one male and one female pup from each litter were randomly selected and tested for the either auditory and tactile PPI or auditory and visual PPI of the ASR.
Auditory and Tactile
The first protocol addressed the ontogeny of auditory PPI relative to that of tactile PPI, the latter representing a relatively earlier developing sensory system. All rats were tested for approximately 30 min. The testing procedure for the integral auditory and tactile PPI began with a 5-min acclimation period with background white noise (70 dB(A)), followed by six startle trials for adaptation/habituation (white noise stimuli (100 dB(A))) with a 10 s intertrial interval (ITI), and 72 PPI trials with a pulse-only stimulus, or 8, 40, 80, 120, or 4,000 ms interstimulus interval (ISI) between prepulse and pulse stimuli, assigned by a Latin-square design. The ISI represented the time from the offset of the prepulse stimulus to the onset of the startle stimulus. The incorporation of a range of ISIs was fundamental to establishment of a relatively precise and defined response function, and consequently, a more accurate assessment of response inhibition, as has been employed to examine alterations in the development of PPI as a function of developmental neurotoxin exposure (Fitting, Booze, Mactutus, 2006a,b,c; Ison, 1984; Moran, Fitting, Booze, Webb, & Mactutus, 2014). The auditory and tactile PPI trials were each presented in six-trial block sets (each set including each ISI) in a counterbalanced order throughout the session, with a variable 20 s ITI (range: 15–25 s). The pulse-only and 4,000 ms ISI trials were used to provide the reference ASR within the PPI test. The startle stimulus was a 100 dB(A) white noise (20 ms in duration) measured inside the test cylinder. The auditory prepulse was an 85 dB(A) white noise, 20 ms in duration; the tactile prepulse was a 16 p.s.i. air puff, 20 ms in duration. Stimulus parameters were chosen in accordance with recommendations in Current Protocols in Neuroscience (Geyer & Swerdlow, 2001) and pilot observations. The sound of the air puff prepulse was measured, in the absence of background white noise, as 70 dB(A) inside the tube, 2.5 cm from the end of the test cylinder. Thus, the air puff was effectively a pure tactile stimulus. All white noise stimuli were passed as broadband through the range possible by the horn tweeter (5–16 kHz), a range which spans the peak 8 kHz sensitivity of the audiogram of the Long–Evans rat (Heffner, Heffner, Contos, & Ott, 1994). The dependent measures collected were peak response amplitude (the highest amplitude during the 100 ms window following the onset of the startle tone) and response latency (the time in millisecond from the onset of the startle stimulus to the peak response).
Auditory and Visual
The second protocol addressed the ontogeny of auditory PPI relative to that of visual PPI, the latter representing a relatively later developing sensory system. As with the previous protocol, all rats were tested for approximately 30 min. The testing procedure for the integral auditory and visual PPI was identical to the first protocol, with the exception that visual PPI trials were substituted for the tactile PPI trials. The illuminance of the visual prepulse was 50 lux, at the level of the perspex test cylinder, with a duration of 20 ms. Again, the dependent measures collected were peak response amplitude and response latency.
Data Analysis
Data were analyzed with analysis of variance (ANOVA) techniques (Winer, 1971; IBM SPSS Statistics for Windows, Version 22.0; Armonk, NY: IBM Corp.). Four-factor mixed-model ANOVAs (3, age × 2, sex × 6, ISI × 2, modality), with the first two factors representing between-subjects terms and the latter factors representing within-subjects terms, were conducted for each response measure (peak response amplitude and response latency) for both the auditory vs. tactile PPI protocol and for the auditory vs. visual PPI protocol. A comparison of the ontogenetic profiles of auditory PPI between the two protocols was also conducted to ascertain any potential difference that might exist as a function of the choice of our experimental design. For the within-subjects terms (e.g., ISI, modality), potential violations of sphericity (Winer, 1971) were preferentially handled by the use of planned orthogonal components analyses, which also characterized the shape of the response curves for each measure, or, if necessary, were handled indirectly by the Greenhouse–Geisser degrees of freedom correction factor (Greenhouse & Geisser, 1959). An α level of p ≤ .05 was considered statistically significant.
RESULTS
PDs 15, 31, and 90, Auditory and Tactile PPI
Within the context of the ASR, the ontogeny of PPI with a tactile prepulse stimulus, relative to that with an auditory prepulse stimulus, is illustrated in Figure 1A and C. The overall ANOVA on peak response amplitude revealed significant interactions for age × ISI [F (10, 330) = 4.9, pGG ≤ .001], and for modality × ISI [F (5, 330) = 27.0, pGG ≤ .001], which qualified the main effects of modality [F (1, 66) = 10.14, pGG ≤ .005], age [F (2, 66) = 4.25, p ≤ .05], and ISI [F (5, 330) = 255.07, pGG ≤ .001]. There was no significant effect of sex. Power analysis estimates for a small (.1) effect size were in excess of .94 for detecting a significant effect, indicating that if sex had a true effect on peak response amplitude, it should have been detected.
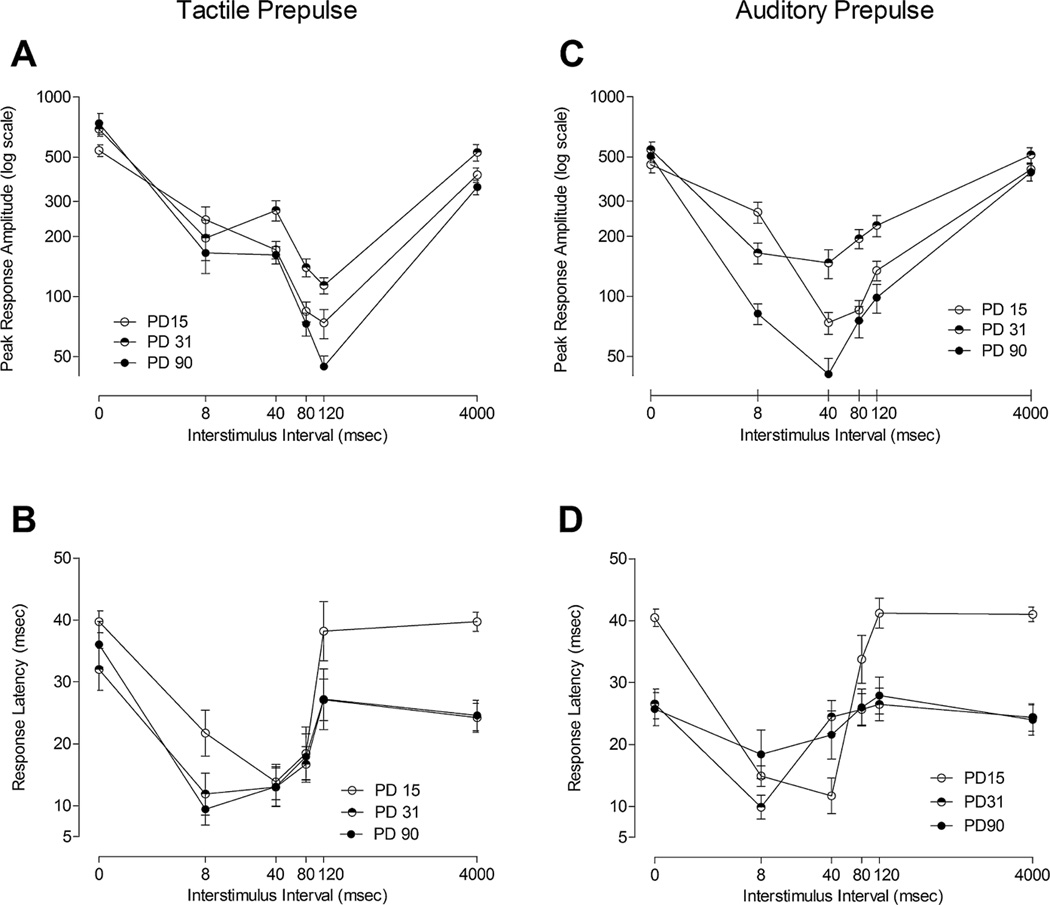
FIGURE 1.
Mean (±SEM) peak response amplitude across all ISIs (0, 8, 40, 80, 120, and 4,000 ms) for (A) the tactile prepulse condition and (C) the auditory prepulse condition across preweanling, adolescent, and adult animals. A significant effect of modality and a modality × ISI interaction suggested that maximal inhibition was focal to different ISIs, independent of the subject’s test age. Mean (±SEM) response latency across all ISIs (0, 8, 40, 80, 120, and 4,000 ms) for (B) the tactile prepulse condition and (D) the auditory prepulse condition across preweanling, adolescent, and adult animals. A significant age × modality × ISI interaction suggested a greater effect of age on the modulation of response latency by ISI for the broadband white noise prepulse latency curves than for the tactile prepulse latency curves.
These findings suggest that as age increases, the point of inflection indexing maximal PPI becomes more definable and greater inhibition is observed, independent of modality. The modality × ISI interaction suggests the response amplitude profile for the tactile prepulse stimulus displayed an adult-like pattern from PD15, whereas marked changes in the response amplitude profile were seen for the auditory prepulse stimulus. Moreover, maximal inhibition was focal to different ISIs, independent of the subject’s test age. For the tactile modality, the point of inflection fell at 120 ms, whereas for the auditory modality the point of inflection is obtained at 40 ms. Planned orthogonal component analyses indicated a significant quadratic trend best characterized the shape of the response amplitude curves [F (1, 66) = 423.57, p ≤ .001].
Within the context of the ASR, the ontogeny of PPI response latency with a tactile prepulse stimulus, relative to that with an auditory prepulse stimulus, is illustrated in Figure 1B and D. The overall ANOVA on response latency revealed significant interactions of age × ISI [F (10, 330) = 20.69, pGG ≤ .001], modality × ISI [F (5, 330) = 24.97, pGG ≤ .001], and age × modality × ISI [F (10, 330) = 9.3, pGG ≤ .001], qualifying the significant main effects of modality [F (1, 66) = 22.0, pGG ≤ .001], age [F(2,66) = 27.64, p ≤ .001], and ISI [F (5, 66) = 210.85, pGG ≤ .001]. Again, the effect of sex was not significant, despite sufficient power (>.94) to detect a small effect (.1).
As age increased, the modulation of response latency by ISI was much less pronounced, independent of prepulse modality. The interactions of modality × ISI and age × modality × ISI reflect a significantly greater effect of age on the modulation of response latency by ISI for the auditory prepulse trials than for the tactile prepulse trials. The fundamental characteristic reflected in the illustration, and confirmed by significant interactions, was a flattening of the response curves across ISI with increasing age, which occurs for both prepulse modalities for the longer ISIs at the earlier age transition (PD15–PD31), but which is greater for the auditory prepulse stimulus than for the tactile prepulse stimulus. Orthogonal components analyses revealed a prominent quadratic trend best characterized the shape of response latency curves [F (1, 66) = 438.11, p ≤ .001].
PDs 18, 35, and 90, Auditory and Visual PPI
Within the context of the ASR, the ontogeny of PPI with a visual prepulse stimulus, relative to that with an auditory prepulse stimulus, is illustrated in Figure 2A and C. The overall ANOVA for peak response amplitude revealed significant interactions of age × ISI [F (10, 330) = 26.1, p ≤ .001], modality × ISI [F (5, 330) = 59.1, p ≤ .001], and age × modality × ISI [F (10, 330) = 10.9, p ≤.001], which qualified the main effects of modality [F (1, 66) = 150.8, p ≤.001], age [F (2, 66) = 17.3, p ≤ .001], and ISI [F (5, 330) = 84.7, p ≤ .001]. A significant effect of sex was not revealed, despite sufficient power (>.94) to detect a small effect of sex on peak response amplitude (.1).
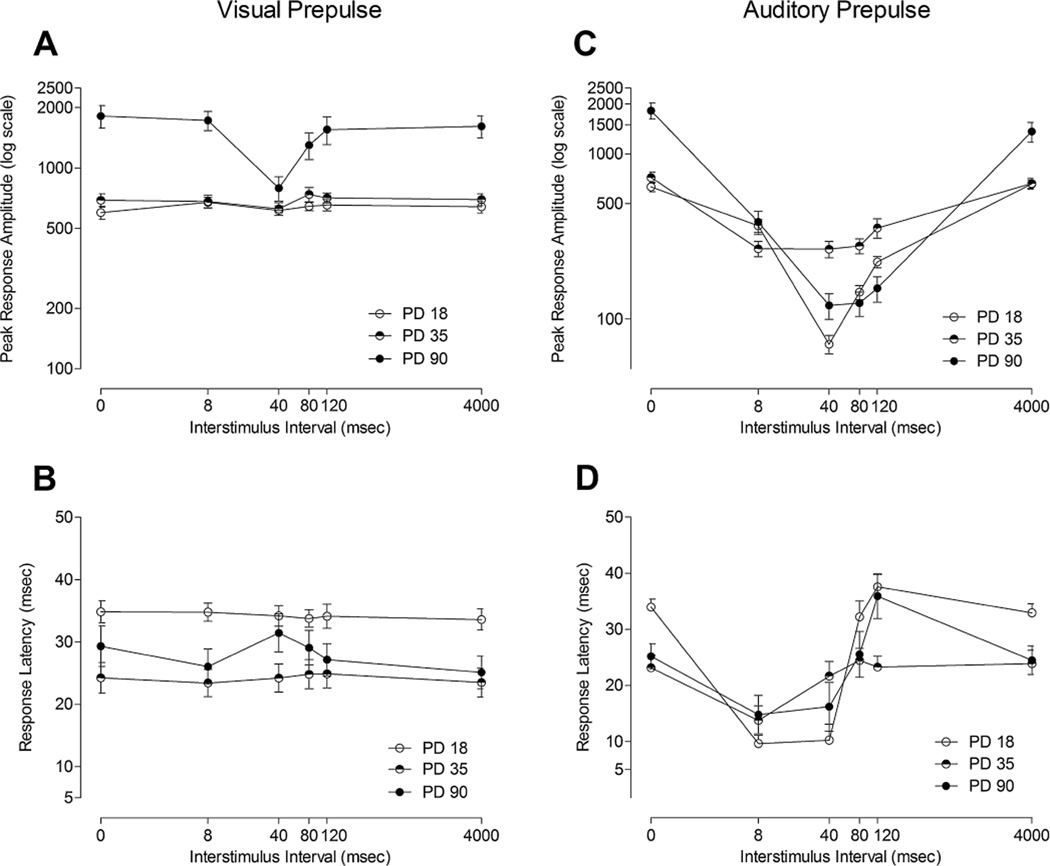
FIGURE 2.
Mean (±SEM) peak response amplitude across all ISIs (0, 8, 40, 80, 120, and 4,000 ms) for (A) the visual prepulse condition and (C) the auditory prepulse condition across preweaning, adolescent, and adult animals. A significant age × modality × ISI interaction suggested the relative age effect was differentially expressed in the response amplitude curves as a function of modality, with a highly focused effect of ISI with the visual prepulse stimulus. Mean (±SEM) response latency across all ISIs (0, 8, 40, 80, 120, and 4,000 ms) for (B) the visual prepulse condition and (D) the auditory prepulse condition across preweanling, adolescent, and adult animals. A significant age × modality × ISI interaction again suggested a greater effect of age on the modulation of response latency by ISI for the broadband white noise prepulse latency curves than for the visual prepulse latency curves.
As readily observed, the prominent modality × ISI interaction reflected the highly focused effect of ISI with the visual prepulse stimulus relative to the less precisely defined response amplitude function with the auditory prepulse stimulus. The age × ISI interaction suggested very pronounced PPI in the adult subjects relative to the younger ages, independent of modality. The age × modality × ISI interaction further suggested the relative age effect was differentially expressed in the response amplitude curves as a function of modality. PPI to the auditory prepulse stimulus was marked across many of the ISI intervals, rather than the focused inflection curve noted with the visual prepulse stimulus in adults (at the 40 ms ISI). The absence of any robust PPI detectable with the visual prepulse stimulus in PD18 or PD35 subjects stands in contrast to the presence of pronounced inhibition across multiple ISIs evident from PD18 with the auditory prepulse stimulus. Again, planned orthogonal contrasts indicated a significant quadratic trend best characterized the overall shape of the response amplitude curves [F (1, 66) = 130.8, p ≤ .001].
Within the context of the ASR, the ontogeny of PPI response latency with a visual prepulse stimulus, relative to that with an auditory prepulse stimulus, is illustrated in Figure 2B and D. The overall ANOVA on the PPI response latency curves revealed significant interactions for age × modality [F (2, 66) = 12.5, p ≤ .001], modality × ISI [F (5, 330) = 89.8, pGG ≤ .001], age × ISI [F (10, 330) = 13.1, p ≤ .001], age × sex [F(2, 66) = 3.5, p ≤ .05], and age × modality × ISI [F (10, 330) = 17.1, p ≤ .001], which qualified the significant main effects for modality [F (1, 66) = 113.3, p ≤ .001], ISI [F (5, 330) = 80.1, pGG ≤ .001], age [F (2, 66) = 20.5, p ≤ .001], and sex [F(1,66) = 8.3, p ≤ .005]. Separate analyses at each age revealed a significant effect of sex in the 90-day-old animals [F(1,22) = 18.5, p ≤ .001], but not in the younger animals. Male 90-day-old subjects showed significantly longer response latency compared to females during both visual and auditory prepulse trials.
The fundamental characteristic reflected in the illustration, and confirmed by the significant interactions, was a flattening of the response curves across ISI with increasing age, which was again prominently noted for the auditory prepulse stimulus. In stark contrast, there was a lack of modulation of response latency by ISI for the visual prepulse condition in any age group, reflected by the lack of an age × ISI interaction when the visual prepulse condition was analyzed separately. Orthogonal components analyses revealed a prominent cubic trend best characterized the shape of response latency curves [F (1,66) = 387.8, p ≤ .001], as particularly noted in the auditory prepulse condition.
PDs 15, 31, and 90 Auditory PPI Versus PDs 18, 35, and 90 Auditory PPI
Within the context of the ASR, the ontogeny of PPI with an auditory prepulse stimulus was compared across the two contemporaneously conducted protocols, i.e., when interdigitated with tactile prepulses versus visual prepulses. The overall ANOVA on peak response amplitude revealed significant interactions for age × replication [F(2,132) = 13.7, p ≤ .001], age × ISI [F(10,660) = 28.6, p ≤ .001], and replication × age × ISI [F(10,660) = 20.8, p ≤ .001], as well as significant main effects for replication, [F(1,132) = 53.7, p ≤ .001], age [F(2,132) = 6.5, p ≤ .005], and ISI [F(5, 660) = 246.5, pGG ≤ .001], but not for sex.
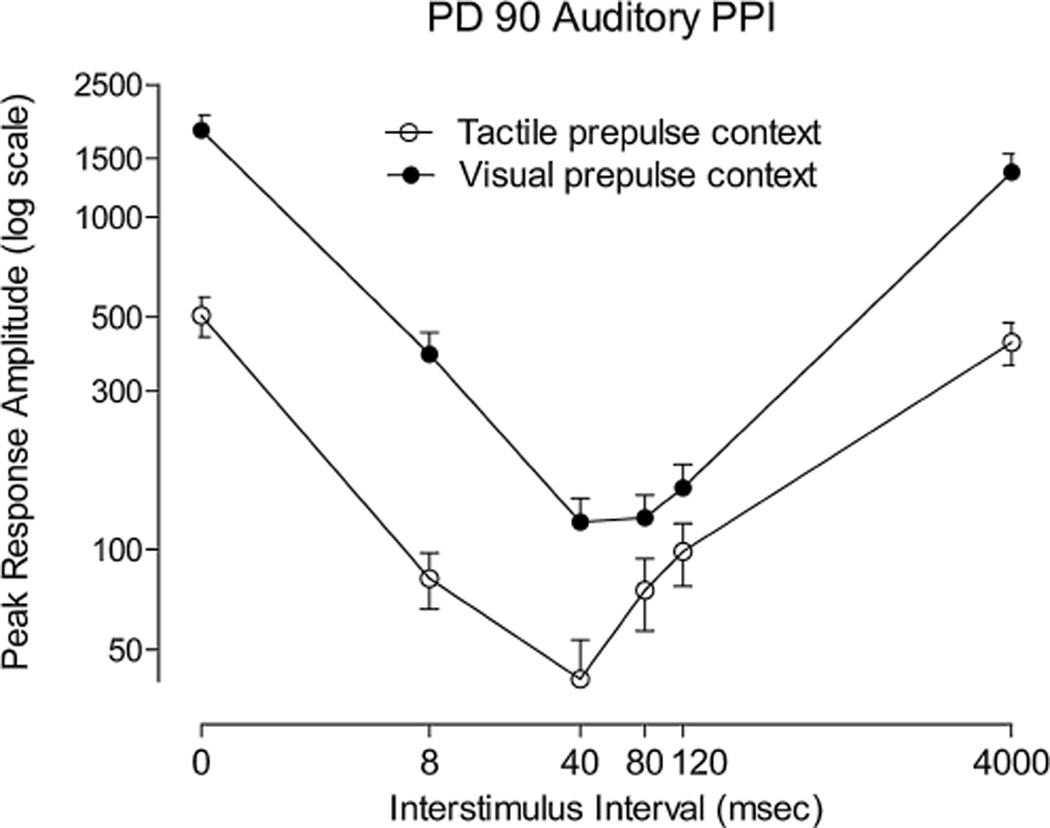
The most prominent effect of testing context (replication) may be most readily understood with the adult response amplitude curves for the two replications. As is depicted in Figure 3, the context for assessing auditory PPI (auditory-tactile vs. auditory-visual prepulse context) influenced the overall startle response, i.e., a shift in the height of the entire profile, but did not significantly impact the response amplitude profile per se. The startle responses during the initial six acclimation trials for the PD 90 auditory-tactile versus the auditory–visual groups did not differ significantly (mean+/−SEM: 1228+/−171 vs. 1363+/−199, respectively), and there was no significant effect for group [F(1,46) < 1.0)] or group by trial (F(5,230) = 1.73, p = .13). Thus, it appears that the effect of context, as opposed to any individual differences, is responsible for the sizable four-fold difference in startle response during PPI trials depicted in Figure 3.
FIGURE 3.
Mean (±SEM) peak response amplitude across all ISIs (0, 8, 40, 80, 120, and 4,000 ms) for the broadband white noise prepulse conditions across contexts (replications) for PD 90 subjects. The influence of testing context for PD 90 subjects is suggested by the parallel shift in the response amplitude curves for the auditory prepulses interdigitated with tactile versus visual prepulses. The relative invariance of the shape of the response amplitude profiles to testing context was noted despite a four-fold difference in magnitude of the auditory startle response on the reference trials (0 and 4,000 ms) across replications.
DISCUSSION
Unique ontogenetic profiles for PPI for each sensory modality were demonstrated in the Long–Evans rat, using reasonably comprehensive ISI functions and assessments of both response amplitude and response latency. Analysis of the response amplitude and response latency curves showed interactions between age and ISI, as well as modality and ISI, suggesting that the PPI amplitude and latency profiles are sensitive to the variables of age and modality. Males and females generally displayed similar amplitude and latency profiles, without any compelling evidence for major differences despite using sample sizes chosen with the goal of sufficient statistical power (>.80) to detect a small effect. The results of the present study strongly support the hypothesis that the variance across modalities reflects the maturation of the particular sensory processing system. Furthermore, the overall amplitude of the startle response during auditory prepulse trials was dependent on whether the trials were interdigitated with tactile or visual prepulse trials, i.e., the testing context. However, the response inhibition profile was not altered as a function of context.
With broadband white noise stimuli (5–6 kHz), auditory response amplitude curves demonstrated significant PPI as early as PD15, as previously suggested using a 10 kHz pure tone stimulus (Parisi & Ison, 1979, 1981), with more pronounced PPI in adulthood relative to preweanling animals. The optimal ISI for auditory PPI, as most pronounced in adults, is approximately 40 ms with less inhibition at both shorter and longer intervals. Auditory response latency curves across ontogeny, which to the best of our knowledge have not been previously reported, suggested that the PPI response latency decreased with advancing age, but perhaps more importantly, was more invariant across the ISI function. These maturational changes are consistent with the developmental profile of the auditory system and auditory sensitivity, as described in the introduction (Crowley & Hepp-Reymond, 1966; Volokhov, 1968; Wada, 1923).
With a tactile prepulse (16 p.s.i. dorsal air puff), response amplitude curves displayed pronounced PPI at PD15, with adult-like characteristics, but continued to progress in magnitude of inhibition through to adulthood. The optimal ISI for tactile PPI, as most pronounced in adults, was approximately 120 ms with less inhibition at both shorter and longer intervals. The inflection of the response amplitude curves (where peak inhibition is obtained) was independent of age. Response latency curves across ontogeny for the tactile prepulse suggested marked variation in the ISI function, which persisted throughout development, albeit significant latency decreases at the longer ISIs were notable in the PD15–PD31 transition. As discussed in the introduction, the somatosensory system displays an early-developed sensitivity to tactile stimulation (Gonzalez, 1932; Gottlieb, 1971; Lane, 1917). Not surprisingly, a tactile prepulse stimulus permitted the expression of adult-like characteristics in PPI, even in preweanling animals.
With a prominent visual prepulse (50 lux), response amplitude curves failed to display any significant PPI until adulthood, and similarly failed to show any variation in response latency across ISI until adulthood. Because no definable inhibition or point of inflection was observed in the younger animals for peak amplitude or latency, it is reasonable to infer that these animals are not processing the prepulse light stimulus with the same efficiency as the adult animals. A clear, sharp response curve was not apparent for the visual PPI until adulthood, lending credence to the notion that the ontogeny of the PPI response is contingent on the development of the specific sensory system. Indeed, the visual sensory system is the latest maturing modality, and as the results show, does not produce prominent inhibition until adulthood. In a subsequent preliminary study with naïve adult rats, we found that increasing prepulse light intensity (200, 400, and 600 lux) did not appreciably alter prepulse inhibition at the 40 ms ISI (point of peak inhibition; 70, 72, 74% PPI). In this preliminary study as well as in a published study comparing 22 and 100 lux prepulses (Moran, Booze, & Mactutus, 2013), we also did not find any compelling evidence for alterations in the amplitude curves across prepulse intensity. For the present experiments, a single prepulse intensity, reflecting what has been suggested to be within the range for producing robust PPI, was employed for each prepulse modality.
The relatively late maturing peripheral and central components of the visual system likely account for much of the variance in the delayed ontogeny of PPI with visual prepulse stimuli. As has been well-established, the visual system is the last sensory system to develop in the rat, as is characteristic of numerous vertebrate species (Gottlieb, 1971). Nevertheless, different components of the visual system do not have a unitary ontogeny. For example, negative phototaxic reactions are observable in rodents as young as 5 days of age (Crozier & Pincus, 1937; Routtenberg et al., 1978), visual evoked potentials may be seen as early as 11 days of age (Rose, 1968), as the eyelids are not opaque in the immature rat, and the eyelids separate and open at approximately 14–16 days in the rat, dependent on the specific rat strain. At approximately 45 days of age, adult-like characteristics are seen in the functional properties of primary rat visual cortex, such as selectivity for orientation and movement direction of visual stimuli, as well as for visual acuity (Fagiolini et al., 1994; Fortin et al., 1999). Between 15 and 45 days of age, the superficial layers of the SC become thicker and more myelinated (Langer & Lund, 1974; Warton & Jones, 1985), while astrocytes and oligodendrocytes of the SC increase from 14 and 80 days of age (Virgili, Barnabei, & Contestabile., 1990). In the context of the circuit which mediated PPI, as described in the introduction (e.g., Fendt et al., 2001), visual and tactile prepulses are held to be mediated via the SC. Given that the superficial collicular layers are mostly devoted to visual functions, whereas the intermediate and deep strata contain multimodal neurons that display a wider range of activities in response to somatosensory, auditory, and visual stimuli (May, 2006), the present data are consistent with the hypothesis that these protracted developmental changes in the superficial layers of the SC contribute to, or perhaps mediate, the delayed ontogeny of PPI with visual prepulse stimuli.
One of the strengths of the present study was that it employed a repeated-measures within-session design to maximize statistical power of detecting an effect of modality. Thus, there was no contribution of between-subject and between-session variance to experimental error for assessing an effect of modality. A second strength was the control of litter variance, with each litter contributing one male and one female to each of the pre-adult test ages. Thus, any litter variance was orthogonal to detecting the ontogenetic profiles of PPI. Third, the use of a Latin-Square design for the presentation of ISIs and the counterbalancing of prepulse modality in six-trial blocks allowed for each ISI and prepulse modality to be balanced across the entire session, such that each ISI occurred once before any ISI could occur a second time. A fourth strength of the study was the incorporation of a reasonably comprehensive number of ISIs, which provided relatively precise and defined response functions, and consequently, more accurate determination and characterization of the response curves. A fifth strength was the determination of both response amplitude and response latency curves, each of which contributed to the characterization of the ontogenetic profiles and the effect of prepulse stimulus modality.
Despite these aforementioned advantages, there remain a few caveats that must also be recognized. The commercially available software system used in the present study defines response latency as the time from stimulus onset to the peak of the startle response, rather than the more tradition definition of latency, which would be until the onset of the startle response. The error introduced by this protocol is estimated to be approximately 2–3 ms, accounting for roughly 10% of the error in response latency values. Another caveat arises in recognition of the strain differences that have been reported in PPI (Kinney, Wilkinson, Saywell, & Tricklebank, 1999). Thus, it may very well be that the characteristic shape of the response curves may vary as a function of strain. However, it would appear unlikely that differences in age of eye-opening of 1–2 days across strains would translate into any major change in the pronounced differences between PPI with visual versus auditory prepulse stimuli. A third caveat is that the ISIs were arbitrarily selected. However, similar ISIs were employed in other studies (Parisi & Ison, 1979, 1981), and these intervals produced clear, definable response amplitude and response latency curves. The replicability and utility of the approach utilizing response amplitude curves were highlighted in the third data figure in the present manuscript.
The ISI approach utilized herein deserves further consideration. Many published studies, as well as established protocols, employ and recommend the use of a single ISI for PPI studies (e.g., Curzon, Zhang, Radek, & Fox, 2009; Geyer & Swerdlow, 2001). The typical procedure is to express the amount of inhibition as a percent of the non-prepulse control trial (dividing the startle response magnitude on prepulse trials by that of non-prepulse trials × 100) or conversely the percent reduction of the inhibited response from the nonprepulse control trial (i.e., obtaining the difference between the startle response on prepulse vs. nonprepulse stimulus trials, dividing by the non-prepulse stimulus trial response × 100). The present data, expressed as percent PPI, as estimated for a 100 ms ISI (averaging the response amplitude at the 80 and 120 ms ISIs), are illustrated in Table 1. Despite the apparent simplicity and popularity of that approach, the frequent use of subjectively determined percentage data is not without potential consequence regarding validity of inferences made about meeting the assumptions of analysis of variance (Bliss, 1938). Unfortunately, percentage data have error variances that are a function of the mean and are not normally distributed (Bartlett, 1947); rather they are described by Poisson or bimodal distributions, depending on whether the data occur over a large portion of the percentage scale (bimodal) or are primarily grouped at either end (Poisson)(Cochran, 1940). The more classic tradition guided the present study, with the incorporation of a range of ISIs to determine the shape of the PPI response curves. The incorporation of a range of ISIs was fundamental to the establishment of a relatively precise and defined response function, and consequently, a more accurate assessment of response inhibition, as has been employed to examine alterations in the development of PPI as a function of developmental neurotoxin or drug exposure (Fitting et al., 2006a,b,c; Ison, 1984; Mactutus, Harrod, Hord, Moran, & Booze, 2011; Moran et al., 2014). The plotting of the raw startle amplitude scores, e.g., as in the log–log plot portrayed in Figure 3, graphically illustrates another advantage of the ISI function approach. The whole profile shift across replications clearly demonstrates the outcome of a change in overall startle response, but comparable relative inhibition. A decrease in PPI would be evident as a flattening of the response curve, e.g., as with the visual prepulse response amplitude curves displayed in Figure 2A. An increase in PPI would be evident in a sharper or greater overall inflection of the curve, as illustrated with the ontogenetic shifts in the auditory prepulse curves in Figure 1C. Given that PPI is a model of sensorimotor gating, the advantages of incorporating the temporal dimension in any assessment of PPI would appear undeniable.
Table 1.
Mean Percent Prepulse Inhibition at 100 ms ISI (±SEM)
| Tactile and Auditory PPI Test Session | ||
| Age | Tactile | Auditory |
| Day 15 | 83.3 (3.4) | 86.0 (15.4) |
| Day 31 | 79.2 (3.1) | 59.1 (4.2) |
| Day 90 | 83.7 (5.7) | 79.0 (3.4) |
| Visual and Auditory PPI Test Session | ||
| Age | Visual | Auditory |
| Day 18 | −4.55 (21.2) | 71.6 (5.6) |
| Day 35 | −4.46 (9.2) | 54.2 (6.1) |
| Day 90 | 16.76 (15.4) | 91.3 (2.4) |
There are a number of advantages of using the PPI paradigm, which make it an attractive preclinical model for neurodevelopmental and neuropsychiatric disorders. First, PPI is relatively easy and unambiguous to measure since it is the result of a sudden, intense stimulus, preceded by a weaker, less intense prestimulus. Second, PPI may be observed in all mammals, and even some invertebrates, allowing for comparative studies across different species and facilitating the difficult task of cross-species extrapolation. Third, the general consensus on both mediating and regulatory circuits underlying PPI facilitates the determination of how different drugs may affect PPI via habituation and sensitization. Finally, as illustrated in the present study, there is a characteristic ontogenetic profile of PPI for each sensory modality reflecting the constraints of maturation of the individual sensory systems.
In summary, sensorimotor gating, as assessed with PPI, is contingent on the development of individual sensory systems, demonstrated in the present study with unique, replicable profiles of response amplitude and latency. The results of this study also highlight the importance of the use of a comprehensive ISI function, as well as a within-session design for future PPI studies. The general consensus on both the mediating and regulatory circuits underlying PPI provides the rationale for translational relevance of preclinical sensorimotor assessments to patients with neurodevelopmental and/or neuropsychiatric disorders.
Acknowledgments
This research was supported by NIH grants DA013137, DA031604, DA021287, and HD043680. LMM is now a postdoctoral fellow at the National Institute on Drug Abuse, Clinical Pharmacology and Therapeutics Branch, Baltimore, MD 21224. Contract grant sponsor: NIH Contract grant numbers: DA013137, DA031604, DA021287, HD043680
REFERENCES
- Bartlett MS. The use of transformations. Biometrics. 1947;3:39–52. [PubMed] [Google Scholar]
- Bliss CI. The transformation of percentage for use in the analysis of variance. Ohio Journal of Science. 1938;38:9–12. [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen DL, Marsh WL, Rapoport JL, Hallet M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: Preliminary results. Biological Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The analysis of variance when experimental errors follow the poisson or bimodal laws. Annals of Mathematical Sciences. 1940;11:335–347. [Google Scholar]
- Crowley DE, Hepp-Reymond MC. Development of cochlear function in the ear of the infant rat. The Journal of Comparative and Physiological Psychology. 1966;62:427–432. [Google Scholar]
- Crozier WJ, Pincus G. Photic stimulation in young rats. The Journal of General Psychology. 1937;17:105–111. [Google Scholar]
- Curzon P, Zhang M, Radek RJ, Fox GB. The behavioral assessment of sensorimotor processes in the mouse: Acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. 2nd. Chapter 8. Boca Raton, FL: CRC Press; 2009. [PubMed] [Google Scholar]
- Davis M. Neurochemical modulation of sensory-motor reactivity: Acoustic and tactile startle reflexes. Neuroscience & Biobehavioral Reviews. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Davis M. The mammalian startle response. In: Eaton RC, editor. Neural mechanisms of startle behavior. New York: Plenum Press; 1984. pp. 287–351. [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT. Acoustic startle reflex in rhesus monkeys: A review. Reviews Neuroscience. 2008;19:171–185. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman D, Tischler M, Gendelman P. A primary acoustic startle circuit: Lesion and stimulation studies. The Journal of Neuroscience. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler SR. Experimental observations upon the developing rat retina. Journal of Comparative Neurology. 1932;55:473–492. [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vision Research. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Sensorimotor gating deficit after lesions of the superior colliculus. Neuroreport. 1994;5:1725–1728. doi: 10.1097/00001756-199409080-00009. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Intrahippocampal injections of Tat: Effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacology, Biochemistry, and Behavior. 2006a;84:189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: Developmental effects on prepulse inhibition (PPI) of the auditory startle response. International Journal of Developmental Neuroscience. 2006b;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: The role of dopaminergic alterations in prepulse inhibition in adult rats. The Journal of Pharmacology and Experimental Therapeutics. 2006c;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fortin S, Chabli A, Dumont I, Shumikhina S, Itaya SK, Molotchnikoff S. Maturation of visual receptive field properties in the rat superior colliculus. Brain Research Developmental Brain Research. 1999;112:55–64. doi: 10.1016/s0165-3806(98)00157-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Current Protocols In Neuroscience. 2001:8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Gonzalez AWA. The prenatal development of behavior in the albino rat. Journal of Comparative Neurology. 1932;55:395–442. [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson LR, Shaw E, editors. The biopsychology of development. New York: Academic Press; 1971. pp. 67–128. [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73:244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: Some empirical findings and their implications for how the nervous system processes sensory input. Psychological Review. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of the startle reaction in the rat. The Journal of Comparative and Physiological Psychology. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Ison JR. Reflex modification as an objective test for sensory processing following toxicant exposure. Neurobehavioral Toxicology and Teratology. 1984;6:437–445. [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of the startle reflex in the rat by changes in the auditory and visual environments. The Journal of Comparative and Physiological Psychology. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Wilkinson LO, Saywell KL, Tricklebank MD. Rat strain differences in the ability to disrupt sensorimotor gating are limited to the dopaminergic system, specific to prepulse inhibition, and unrelated to changes in startle amplitude or nucleus accumbens dopamine receptor sensitivity. The Journal of Neuroscience. 1999;19:5644–5653. doi: 10.1523/JNEUROSCI.19-13-05644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R. Early postnatal development of sensory gating. Neuroreport. 2003;14:693–697. doi: 10.1097/00001756-200304150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog. Neurobiology. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Experimental Brain Research. 1993;97:71–82. doi: 10.1007/BF00228818. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats: Circuits mediating evocation, inhibition and potentiation. Behavioural Brain Research. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Lane HH. The correlation between structure and function in the development of the special senses of the white rat. University of Oklahoma Bulletin, New Series 140, University Studies. 1917;8:1–88. [Google Scholar]
- Langer TP, Lund RD. The upper layers of the superior colliculus of the rat: A Golgi study. Journal of Comparative Neurology. 1974;158:418–435. doi: 10.1002/cne.901580404. [DOI] [PubMed] [Google Scholar]
- LeVere TE. The primary visual system of the rat: A primer of its anatomy. Physiological Psychology. 1978;6:142–169. [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience & Biobehavioral Reviews. 2009;33:1157–1167. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Harrod SB, Hord LL, Moran LM, Booze RM. Prenatal IV cocaine: Alterations in auditory information processing. Frontiers in Psychiatry. 2011;2:38. doi: 10.3389/fpsyt.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: Laminar structure and connections. Progress in Brain Research. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: Temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. Journal of Neuroimmune Pharmacology. 2013;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Fitting S, Booze RM, Webb KM, Mactutus CF. Neonatal intrahippocampal HIV-1 protein Tat 1–86 injection: Neurobehavioral alterations in the absence of increased inflammatory cytokine activation. International Journal of Developmental Neuroscience. 2014;38:195–203. doi: 10.1016/j.ijdevneu.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi T, Ison JR. Development of the acoustic startle response in the rat: Ontogenetic changes in the magnitude of inhibition by prepulse stimulation. Developmental Psychobiology. 1979;12:219–230. doi: 10.1002/dev.420120305. [DOI] [PubMed] [Google Scholar]
- Parisi T, Ison JR. Ontogeny of control over the acoustic startle reflex by visual prestimulation in the rat. Developmental Psychobiology. 1981;14:311–316. doi: 10.1002/dev.420140403. [DOI] [PubMed] [Google Scholar]
- Riede K. Prepulse inhibition of the startle reaction in the locust Locusta migratoria (Insecta: Orthoptera: Acidoidea) The Journal of Comparative Physiology A. 1993;172:351–358. [Google Scholar]
- Rose GH. The development of visually evoked electro-cortical responses in the rat. Developmental Psychobiology. 1968;1:35–40. [Google Scholar]
- Routtenberg A, Strop M, Jerdan J. Response of the infant rat to light prior to eyelid opening: Mediation by the superior colliculus. Developmental Psychobiology. 1978;11:469–478. doi: 10.1002/dev.420110510. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Lees AJ. Abnormalities of the blink reflex in Gilles de la Tourette syndrome. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52:895–898. doi: 10.1136/jnnp.52.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Muñoz JE, Valldeoriola F. Abnormalities of prepulse inhibition do not depend on blink reflex excitability: A study in Parkinson’s disease and Huntington’s disease. Clinical Neurophysiology. 2004;115:1527–1536. doi: 10.1016/j.clinph.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Virgili M, Barnabei O, Contestabile A. Regional maturation of neurotransmitter-related and glial markers during postnatal development in the rat. International Journal of Developmental Neuroscience. 1990;8:159–166. doi: 10.1016/0736-5748(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Volokhov AA. Comparative studies of the functional development of analyzer systems in animals in the process of ontogenesis. Progress in Brain Research. 1968;22:527–540. doi: 10.1016/S0079-6123(08)63531-1. [DOI] [PubMed] [Google Scholar]
- Wada T. Anatomical and physiological studies on the growth of the inner ear of the albino rat. American Anatomical Memoirs. 1923;10:1–174. [Google Scholar]
- Warton SS, Jones DG. Postnatal development of the superficial layers in the rat superior colliculus: A study with Golgi-Cox and Klüver-Barrera techniques. Experimental Brain Research. 1985;58:490–502. doi: 10.1007/BF00235865. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2nd. New York: McGraw-Hill; 1971. [Google Scholar]
- Wood SL, Beyer BK, Cappon GD. Species comparison of postnatal CNS development: Functional measures. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2003;68:391–407. doi: 10.1002/bdrb.10037. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Lee J, Yeomans MH, Steidl S, Li L. Midbrain pathways for prepulse inhibition and startle activation in rat. Neuroscience. 2006;142:921–929. doi: 10.1016/j.neuroscience.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neuroscience Biobehavioral Review. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]