Abstract
BACKGROUND
The survival benefit of a strategy of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, as compared with medical therapy alone, in patients with coronary artery disease, heart failure, and severe left ventricular systolic dysfunction remains unclear.
METHODS
From July 2002 to May 2007, a total of 1212 patients with an ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomly assigned to undergo CABG plus medical therapy (CABG group, 610 patients) or medical therapy alone (medical-therapy group, 602 patients). The primary outcome was death from any cause. Major secondary outcomes included death from cardiovascular causes and death from any cause or hospitalization for cardiovascular causes. The median duration of follow-up, including the current extended-follow-up study, was 9.8 years.
RESULTS
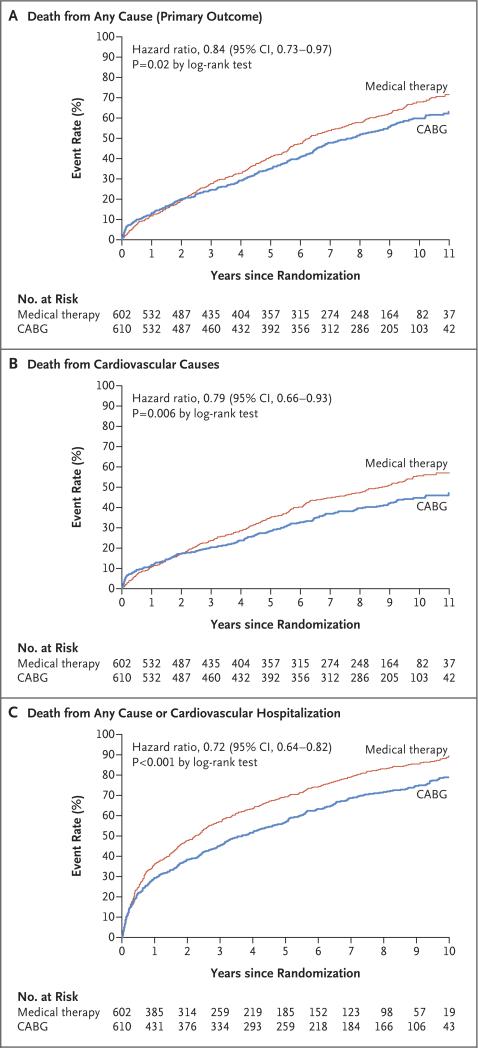
A primary outcome event occurred in 359 patients (58.9%) in the CABG group and in 398 patients (66.1%) in the medical-therapy group (hazard ratio with CABG vs. medical therapy, 0.84; 95% confidence interval [CI], 0.73 to 0.97; P = 0.02 by log-rank test). A total of 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical-therapy group died from cardiovascular causes (hazard ratio, 0.79; 95% CI, 0.66 to 0.93; P = 0.006 by log-rank test). Death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and in 524 patients (87.0%) in the medical-therapy group (hazard ratio, 0.72; 95% CI, 0.64 to 0.82; P<0.001 by log-rank test).
CONCLUSIONS
In a cohort of patients with ischemic cardiomyopathy, the rates of death from any cause, death from cardiovascular causes, and death from any cause or hospitalization for cardiovascular causes were significantly lower over 10 years among patients who underwent CABG in addition to receiving medical therapy than among those who received medical therapy alone. (Funded by the National Institutes of Health; STICH [and STICHES] ClinicalTrials.gov number, NCT00023595.)
Advances in the management of cardiovascular risk factors and acute coronary syndromes have increased survival among patients with coronary artery disease, transforming it into a chronic disease that affects 15.5 million U.S. patients; however, coronary artery disease still accounts for more than 538,000 deaths yearly in the United States alone.1 The major long-term manifestations of coronary artery disease, left ventricular dysfunction, and heart failure are projected to affect 8 million patients by 2030, which has enormous societal implications.1
Landmark clinical trials have established coronary-artery bypass grafting (CABG) as an effective treatment for patients with disabling angina symptoms.2-4 In these trials, CABG was associated with longer survival than was medical therapy alone among the subgroups with more extensive coronary artery disease and worse left ventricular dysfunction.5 However, the trials were conducted more than 40 years ago, before the availability of current guideline-based medical therapy for coronary artery disease and heart failure,6,7 and they did not include patients with severe left ventricular dysfunction; only 4% of participants had symptomatic heart failure.8 More recently, an increasing proportion of patients with heart failure and left ventricular dysfunction are referred for CABG.9
The Surgical Treatment for Ischemic Heart Failure (STICH) study consisted of two trials — a surgical revascularization component and a surgical ventricular reconstruction component. The surgical revascularization component was designed to test the hypothesis that CABG plus guideline-directed medical therapy for coronary artery disease, heart failure, and left ventricular dysfunction would improve survival over that with medical therapy alone. In the analysis of data from the surgical revascularization component of the STICH study at a median follow-up of 56 months, there was no significant difference between the CABG group and the medical-therapy group in the rate of death from any cause, although the rates of death from cardiovascular causes and of death from any cause or hospitalization for cardiovascular causes were lower among patients in the CABG group.10 We now report the results of the STICH Extension Study (STICHES), which was conducted to evaluate the long-term (10-year) effects of CABG in patients with ischemic cardiomyopathy.
Methods
Study Design
The design and enrollment characteristics of the STICH study have been published previously, as have the intermediate-term results of the surgical revascularization component and the final results of the surgical ventricular reconstruction component.10-13 The protocol (available with the full text of this article at NEJM.org) was approved by the principal investigator and by the ethics committee at each center. Before the treatment-group assignments were revealed or any intermediate-term results were reported, the protocol was amended to extend the follow-up period by an additional 5 years for all patients who were enrolled in the surgical revascularization component of the study. The Duke Clinical Research Institute coordinated all aspects of global trial operations, site management and monitoring, data collection, statistical analyses, and reporting. All the authors assume responsibility for the completeness and accuracy of the data and the analyses and for the fidelity of the trial to the protocol.
Patients
Patients were eligible for participation in the trial if they had coronary artery disease that was amenable to CABG and an ejection fraction of 35% or lower. Detailed enrollment criteria, including randomization strata criteria, have been published previously10 and are provided in Table S1 in the Supplementary Appendix, available at NEJM.org. Eligibility for participation was determined by site investigators after each patient underwent direct coronary angiography. Patients who did not have a left main coronary artery stenosis of 50% or more of the artery diameter or Canadian Cardiovascular Society class III or IV angina (with classes ranging from I to IV, and higher values indicating more disabling pain due to angina) while they were receiving medical therapy were eligible for random assignment to either the CABG group or the medical-therapy group. By design, in our trial, patients who met these criteria but did not meet the criteria for eligibility for surgical ventricular reconstruction (dominant anterior left ventricular akinesia or dyskinesia) were enrolled in stratum A, whereas patients who did meet the criteria for eligibility for surgical ventricular reconstruction were enrolled in stratum B; patients were included in the current analysis only if they were assigned to CABG or medical therapy. All patients provided written informed consent.
Trial Procedures
At the initial evaluation, a baseline physical examination was performed, and baseline demographic and clinical data (including information regarding current medications and previous diagnostic and other cardiovascular procedures) were obtained. Random assignment to either CABG or medical therapy was accomplished with the use of an interactive voice-response system.
Throughout the trial follow-up period, the use of guideline-recommended medications and devices for the treatment of heart failure and coronary artery disease was strongly emphasized for all patients. Patients assigned to CABG were to undergo the procedure within 14 days after randomization. CABG was performed by preapproved study surgeons who had provided documentation of an operative mortality of 5% or lower among patients whose risk of complications was similar to that of patients in our trial. During the enrollment period, a surgical therapy committee monitored the overall mortality and rates of complications associated with the CABG procedures.
All patients had follow-up evaluations at the time of discharge or at 30 days after randomization, then every 4 months for the first year and every 6 months thereafter. During the extended follow-up period, if a patient was unwilling or unable to return to the enrolling center, follow-up was maintained by the enrolling investigator through telephone contact or was transferred, for follow-up either in person or by telephone, to a lead regional investigator under the oversight of local ethics boards.
Outcomes
The primary outcome was death from any cause. The prespecified secondary outcomes included death from cardiovascular causes, death from any cause or hospitalization for cardiovascular causes, death from any cause or hospitalization for heart failure, death from any cause or hospitalization for any cause, and death from any cause or revascularization. The adjudication of the cause of death according to prespecified criteria was conducted by an independent clinical-events committee, the members of which were unaware of the treatment assignments (see the Supplementary Appendix).
Statistical Analyses
The statistical methods used for comparative treatment analyses that included data from the extended follow-up period were similar to those used in the original STICH study.10 All major comparisons were performed according to the intention-to-treat principle — that is, treatment groups were defined according to the original randomization. Two-sided significance testing was used for all statistical tests. The cumulative event rates were calculated according to the Kaplan–Meier method,14 with event or censoring times calculated from the date of randomization. The significance of the differences in outcomes between the treatment groups was assessed with the use of the log-rank test, with adjustment for randomization stratum (A or B, as described previously).10 Relative risks were expressed as hazard ratios with associated confidence intervals and were calculated with the Cox proportional-hazards model.15 The consistency of treatment effects across a number of prespecified subgroups, including those defined according to age, sex, race and ethnic background, geographic region, randomization stratum, heart failure class, left ventricular ejection fraction, angina class, and number of diseased vessels, was examined by testing for interactions between treatment and these baseline characteristics with the use of the Cox model. To assess the robustness of the log-rank results with crossing of hazard functions, post hoc analyses without an assumption of constant relative risks were also performed.16,17
To assess the effect of early crossovers between treatment groups (within the first year), secondary as-treated and per-protocol analyses were also performed. The as-treated comparison was performed with the use of a Cox model in which CABG was incorporated as a time-dependent covariate.
The final clinical assessment for surviving patients was performed during the 6-month period before November 30, 2015, which was the cutoff date for the extended follow-up. Patients who provided documentation declining further participation at any point were classified as having withdrawn, whereas patients whose last contact occurred before June 1, 2015, were classified as lost to follow-up.
Throughout the extended follow-up period, an independent data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute met yearly to review the progress of the trial, ensure the safety of the participants, and assess the overall integrity of the follow-up data. Formal interim efficacy analyses were not planned during the extended follow-up period. For the final analysis, a P value of 0.05 or less was considered to indicate statistical significance. All analyses were performed with the use of SAS software, version 9.3 or higher (SAS Institute). The final statistical analysis plan was approved by the trial executive committee before the database lock (see the Supplementary Appendix).
Results
Study Population
Between July 24, 2002, and May 5, 2007, a total of 1212 patients across 99 sites in 22 countries were randomly assigned to receive CABG (610 patients) or medical therapy (602 patients) (Table S2 in the Supplementary Appendix). The characteristics of the patients at baseline, including ventricular function and coronary anatomy, were similar in the two groups (Table 1, and Table S3 in the Supplementary Appendix).
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | CABG Group (N = 610) | Medical-Therapy Group (N = 602) |
|---|---|---|
| Median age (IQR) — yr | 60 (54–68) | 59 (53–67) |
| Female sex — no. (%) | 73 (12) | 75 (12) |
| Race or ethnic group — no. (%)† | ||
| Hispanic, Latino, or nonwhite | 221 (36) | 200 (33) |
| White | 389 (64) | 402 (67) |
| Median body-mass index (IQR)‡ | 27 (24–30) | 27 (24–30) |
| Medical history — no. (%) | ||
| Previous myocardial infarction | 462 (76) | 472 (78) |
| Hyperlipidemia | 360 (59) | 370 (62)§ |
| Hypertension | 358 (59) | 370 (61) |
| Diabetes | 240 (39) | 238 (40) |
| Previous stroke | 51 (8) | 41 (7) |
| Chronic renal insufficiency | 49 (8) | 45 (7) |
| Previous percutaneous coronary intervention | 82 (13) | 74 (12) |
| Previous CABG | 22 (4) | 14 (2) |
| Current smoker — no. (%) | 130 (21) | 122 (20) |
| CCS angina class — no. (%)¶ | ||
| No angina | 217 (36) | 225 (37) |
| I | 96 (16) | 91 (15) |
| II | 265 (43) | 260 (43) |
| III | 25 (4) | 23 (4) |
| IV | 7 (1) | 3 (<1) |
| NYHA heart failure class — no. (%)¶ | ||
| I | 65 (11) | 74 (12) |
| II | 319 (52) | 307 (51) |
| III | 207 (34) | 205 (34) |
| IV | 19 (3) | 16 (3) |
| Median systolic blood pressure (IQR) — mm Hg | 120 (110–130) | 120 (110–130) |
| Median pulse rate (IQR) — beats/min | 74 (66–82) | 72 (65–80) |
| Median 6-min walk distance (IQR) — ft∥ | 1145 (863–1320) | 1115 (840–1345) |
There were no significant differences in baseline characteristics between the treatment groups. CABG denotes coronary-artery bypass grafting, and IQR interquartile range.
Race and ethnic group were self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Data on hyperlipidemia were missing for 1 patient.
The Canadian Cardiovascular Society (CCS) angina classes range from I to IV, with higher classes indicating more disabling pain due to angina. New York Heart Association (NYHA) heart failure classes range from I to IV, with higher values indicating greater disability.
To convert the values for the 6-minute walk distance to meters, multiply by 0.305.
Procedures
Among the 610 patients who were randomly assigned to the CABG group, 555 (91.0%) underwent CABG before completion of the trial; the median time from randomization to CABG was 10 days (interquartile range, 5 to 16), and the maximum was 177 days. Among the patients who were randomly assigned to the CABG group and underwent CABG, 505 (91.0%) received at least one arterial conduit, and 473 of the 553 patients for whom data were available (85.5%) received one or more venous conduits. Additional details of the surgical procedures have been published previously.18
Among the 602 patients who were randomly assigned to the medical-therapy group, 119 (19.8%) had CABG performed at any time before the completion of long-term follow-up; 66 patients (11.0%) underwent CABG within the first year of follow-up. The median time to CABG was 6.9 months (interquartile range, 1.2 to 33.6). The indications for crossovers between the treatment groups within the first year have been published previously.19
The frequency of the use of guideline-directed medication was high at baseline and throughout the study period. There were no significant differences between the treatment groups with regard to the frequency of the use of guideline-directed medication at baseline (Table S4 in the Supplementary Appendix).
Follow-up
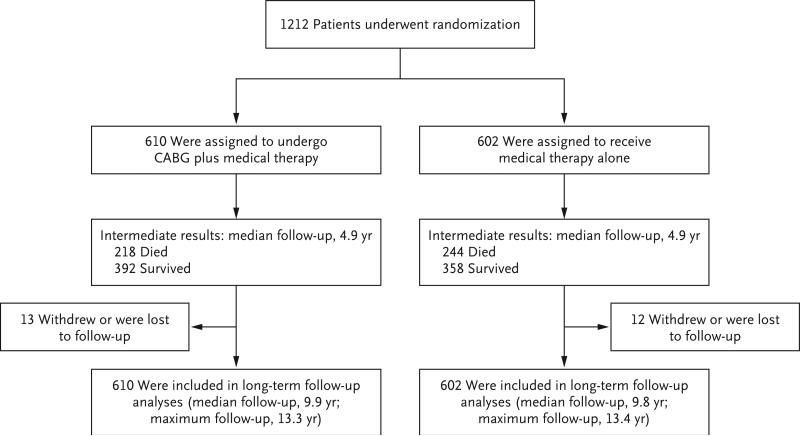
The median duration of follow-up among all patients was 9.8 years (interquartile range, 9.1 to 11.0); the minimum was 3.5 years, and the maximum was 13.4 years. Details regarding follow-up are provided in Figure 1.
Figure 1. Randomization and Follow-up.
CABG denotes coronary-artery bypass grafting.
The final follow-up status was ascertained for 1187 patients (97.9%) between June 1 and November 30, 2015. Among the 25 patients who could not be evaluated during the final follow-up period, 6 withdrew consent for further follow-up, and 19 could not be located by site investigators. The median time from randomization to the date of last contact for patients who withdrew from the trial or were lost to follow-up was 6.4 years (interquartile range, 5.9 to 8.1).
Outcomes
A primary outcome event (death from any cause) occurred in 359 of 610 patients (58.9%) in the CABG group and in 398 of 602 patients (66.1%) in the medical-therapy group (hazard ratio with CABG vs. medical therapy, 0.84; 95% confidence interval [CI], 0.73 to 0.97; P = 0.02 by log-rank test) (Table 2 and Fig. 2A, and Table S5 in the Supplementary Appendix). The median survival was 7.73 years among patients in the CABG group and 6.29 years among patients in the medical-therapy group; median survival was 1.44 years longer in the CABG group, and the number needed to treat to prevent one death was 14 patients (95% CI, 8 to 55). Post hoc analyses without an assumption of constant relative risks showed significance similar to the values in the prespecified log-rank test.
Table 2.
Primary and Secondary Outcomes.
| Outcomes | CABG Group (N = 610) | Medical-Therapy Group (N = 602) | Hazard Ratio (95% CI)* | P Value* |
|---|---|---|---|---|
| no. of patients (%) | ||||
| Primary outcome: death from any cause | 359 (58.9) | 398 (66.1) | 0.84 (0.73–0.97) | 0.02 |
| Secondary outcomes | ||||
| Death from cardiovascular causes | 247 (40.5) | 297 (49.3) | 0.79 (0.66–0.93) | 0.006 |
| Death from any cause or hospitalization for cardiovascular causes | 467 (76.6) | 524 (87.0) | 0.72 (0.64–0.82) | <0.001 |
| Death from any cause or hospitalization for heart failure | 404 (66.2) | 450 (74.8) | 0.81 (0.71–0.93) | 0.002 |
| Death from any cause or hospitalization for any cause | 506 (83.0) | 538 (89.4) | 0.81 (0.71–0.91) | 0.001 |
| Death from any cause or revascularization† | 388 (63.6) | 478 (79.4) | 0.63 (0.55–0.73) | <0.001 |
| Death from any cause or nonfatal myocardial infarction‡ | 376 (61.6) | 409 (67.9) | 0.86 (0.74–0.98) | 0.03 |
| Death from any cause or nonfatal stroke‡ | 367 (60.2) | 406 (67.4) | 0.85 (0.74–0.98) | 0.03 |
Hazard ratios (CABG vs. medical therapy) are based on the Cox model, and the associated P values are based on the log-rank test. All assessments were adjusted for patient stratum (A vs. B: patients who met the eligibility criteria for random assignment to the CABG group or medical-therapy group but did not meet the criteria for eligibility for surgical ventricular reconstruction were enrolled in stratum A; patients who did meet the criteria for eligibility for surgical ventricular reconstruction were enrolled in stratum B).
The method of revascularization was either percutaneous coronary intervention or CABG.
Death or nonfatal myocardial infarction and death or nonfatal stroke were not prespecified outcomes.
Figure 2.
Kaplan–Meier Estimates of the Rates of Death from Any Cause, Death from Cardiovascular Causes, and Death from Any Cause or Hospitalization for Cardiovascular Causes.
A total of 247 patients (40.5%) in the CABG group and 297 (49.3%) in the medical-therapy group died from cardiovascular causes (hazard ratio, 0.79; 95% CI, 0.66 to 0.93; P = 0.006 by log-rank test) (Table 2 and Fig. 2B). Death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and in 524 (87.0%) patients in the medical-therapy group (hazard ratio, 0.72; 95% CI, 0.64 to 0.82; P<0.001 by log-rank test) (Table 2 and Fig. 2C). The results for other prespecified secondary outcomes and additional outcomes are provided in Table 2. The results of the covariate-adjusted models, including those with CABG as a time-dependent covariate, are provided in Table S6 in the Supplementary Appendix. Among the patients in the CABG group, 2 had a repeat CABG during follow-up. A left ventricular assist device was inserted in 4 patients in the CABG group and in 2 patients in the medical-therapy group. Five patients underwent heart transplantation during follow-up: 1 patient in the CABG group and 4 patients in the medical-therapy group. During the entire follow-up period, 105 patients (17.2%) in the CABG group and 118 patients (19.6%) in the medical-therapy group received an implantable cardioverter–defibrillator (alone or in combination with cardiac resynchronization therapy). A percutaneous coronary intervention was performed in 43 patients (7.0%) in the CABG group and in 50 patients (8.3%) in the medical-therapy group. A list of all postrandomization adverse events is provided in Table S7 in the Supplementary Appendix.
Analysis of Crossovers
Among the 591 patients who did not undergo CABG within 1 year (55 in the CABG group and 536 in the medical-therapy group), 402 (68.0%) died during follow-up; among the 621 patients who underwent CABG either as randomly assigned or as a treatment crossover from the medical-therapy group within the first year after randomization, 355 (57.2%) died during follow-up (hazard ratio, 0.75; 95% CI, 0.65 to 0.87; P<0.001) (Fig. S1 in the Supplementary Appendix). We also performed a per-protocol analysis comparing the 536 patients in the medical-therapy group who did not cross over to CABG within the first year with the 555 patients in the CABG group who actually received CABG within the first year; the hazard ratio with CABG as compared with medical therapy alone was 0.77 (95% CI, 0.67 to 0.90; P = 0.001 by the log-rank test) (Fig. S2 in the Supplementary Appendix).
Subgroup Analyses
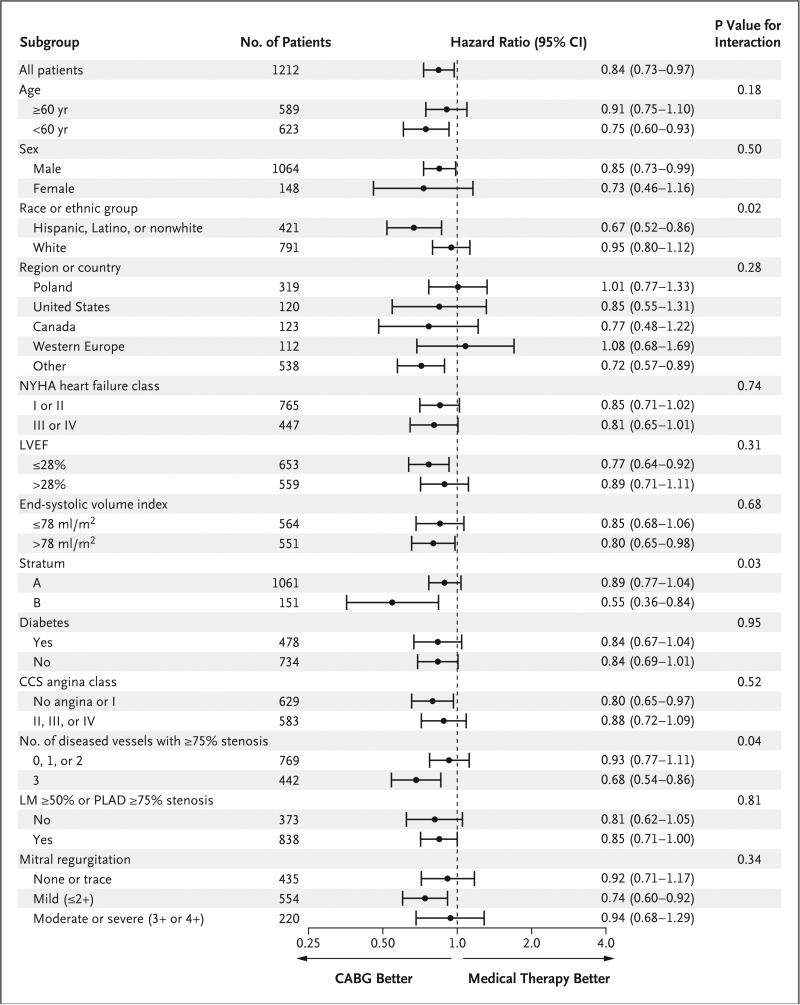
Subgroup analyses based on demographic and clinical characteristics of interest reflected the broad consistency of the effect of CABG on the primary outcome (Fig. 3). An exception was the nominally significant interactions of treatment with randomization stratum, race, and number of diseased vessels with 75% or greater stenosis.
Figure 3. Subgroup Analyses of Death from Any Cause.
Age, sex, race, region, New York Heart Association (NYHA) heart failure class, left ventricular ejection fraction (LVEF), stratum, Canadian Cardiovascular Society (CCS) angina class, and number of diseased vessels are prespecified subgroup factors. All other variables are post hoc subgroup factors. All subgroups are based on values measured at baseline. Data on ESVI were missing for 97 patients, data on the number of vessels with 75% or greater stenosis and on the degree of stenosis of the left main coronary artery (LM) and proximal left anterior descending artery (PLAD) were missing for 1 patient, and data on mitral regurgitation were missing for 3 patients. The Canadian Cardiovascular Society (CCS) angina classes range from I to IV, with higher classes indicating more disabling pain due to angina. New York Heart Association (NYHA) heart failure classes range from I to IV, with higher values indicating greater disability. The divisions between the LVEF and the end-systolic volume index subgroups were based on the median values. Patients who met the eligibility criteria for random assignment to the CABG group or medical-therapy group but did not meet the criteria for eligibility for surgical ventricular reconstruction were enrolled in stratum A; patients who did meet the criteria for eligibility for surgical ventricular reconstruction were enrolled in stratum B.
Discussion
In this randomized clinical trial involving patients with heart failure, left ventricular dysfunction, and coronary artery disease, the rate of death from any cause over 10 years was lower by 16% (an 8-percentage-point absolute difference in the 10-year Kaplan–Meier rates) among patients who underwent CABG in addition to receiving medical therapy than among those who received medical therapy alone. Overall, CABG was associated with an incremental median survival benefit of nearly 18 months and prevention of one death due to any cause for every 14 patients treated and of one death due to a cardiovascular cause for every 11 patients treated.
CABG was associated with more favorable results than medical therapy alone across all clinically relevant long-term outcomes we evaluated. These findings were directionally similar to those reported earlier on the basis of a median follow-up period of 56 months.10 We believe that the further statistical separation between the groups that we now report resulted from a persistent and perhaps increasing effect size over time, coupled with the enhanced precision of estimates afforded by the greater number of events. We previously reported that CABG was associated with a risk of death within the initial 30 days after randomization that was triple the risk with medical therapy alone, with similar differences in risk up to the second year of follow-up, before a significant benefit began to accrue after 2 years. Thus, it appears that the operative risk associated with CABG is offset by a durable effect that translates into increasing clinical benefit to at least 10 years. The lack of convergence of the curves over this prolonged period of follow-up contrasts with other long-term follow-up studies involving patients with heart failure and severe left ventricular systolic dysfunction and underscores the lasting benefits of CABG.20,21 Furthermore, the analyses of the as-treated and per-protocol populations suggest that crossovers between the treatment groups diminished the effect of CABG observed when the data were analyzed according to the assigned group and that the mortality associated with CABG may be as much as 20 to 25% lower than that associated with medical therapy, under the assumption that the surgical mortality in routine clinical practice is similar to or lower than that reported in our trial.
Substantial declines in risk-adjusted mortality associated with CABG have occurred since the 1970s, when the landmark trials comparing CABG and medical therapy were performed. Improvements in myocardial protection techniques, surgical skill, and perioperative care, coupled with the near-universal use of the left internal mammary artery (LIMA) conduit are probably responsible. Among the patients randomly assigned to undergo CABG, 91.0% of patients in STICH received a LIMA graft, as compared with 9.9% of patients in the early CABG trials.8 Although there are limited data on the long-term patency of LIMA or saphenous vein grafts in patients at high risk for death or complications, like those enrolled in STICH, evidence from studies involving lower-risk patients supports the superior 1-year angiographic results with the LIMA.22 In addition, the high rate of use of statins, which have been shown to reduce the rate of vein-graft failure,23,24 is likely to have contributed to the durable effect of CABG and the low rates of repeat revascularization observed in this group.
Ischemic cardiomyopathy remains a high-risk and lethal condition, as indicated by an observed overall mortality of 62.5% with a median follow-up of 9.8 years, even on the background of guideline-directed medical therapy. Patients with heart failure and left ventricular dysfunction have abnormalities of coronary hemodynamics and myocardial energetics during rest, including an increase in myocardial oxygen consumption and altered myocardial lactate metabolism, even in the absence of epicardial coronary artery disease.25,26 Coronary disease compounds the already unfavorable myocardial conditions and limited cardiac reserve in these patients. The significant subgroup interaction we noted between treatment and the extent of coronary artery disease is consistent with previous analyses involving this trial population, which indicated a greater benefit of CABG in patients with three-vessel coronary artery disease than among patients with one-vessel or two-vessel disease27; it is also consistent with observations in studies involving cohorts of lower-risk patients with coronary artery disease who were treated before the current advances in medical therapy, which indicated that CABG may provide the greatest benefit to the patients who have the most extensive heart disease.8,28
By design, in this trial, both the enrolled patients and the site investigators were aware of the treatment-group assignments, and this lack of blinding may have affected the rates of revascularization procedures. We acknowledge this as a limitation of our trial, especially as it relates to the interpretation of nonfatal events. Unmeasured confounding owing to differences in subsequent care cannot be ruled out; however, we found high and similar rates of medical therapy and follow-up in both groups. It is not known whether percutaneous coronary revascularization as compared with medical therapy alone would result in benefits similar to those that we observed with CABG.
In summary, the results of the STICH Extension Study support a significant benefit of CABG plus medical therapy over medical therapy alone with respect to the rate of death from any cause among patients with ischemic cardiomyopathy.
Supplementary Material
Acknowledgments
This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
STICHES was supported by a grant (R01-HL105853/NCT NCT00023595) from the National Institutes of Health.
Dr. Velazquez reports receiving consulting or advisory board fees from Amgen, Merck, and Novartis, lecture fees from Novartis and Spire Learning, and grant support from Abbott, Medtronic, Alnylam, Amgen, Pfizer, and Novartis; and Dr. Rouleau, receiving consulting fees from Novartis. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Elizabeth Cook and Seanna Horan of the Duke Clinical Research Institute for their help in manuscript preparation, and all the patients for their participation in the trial.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics — 2016 update: a report from the American Heart Association. Circulation 2016. 133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Coronary Artery Surgery Study (CASS): a randomized trial of coronary artery bypass surgery: survival data. Circulation. 1983;68:939–50. doi: 10.1161/01.cir.68.5.939. [DOI] [PubMed] [Google Scholar]
- 3.The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable an-gina. N Engl J Med. 1984;311:1333–9. doi: 10.1056/NEJM198411223112102. [DOI] [PubMed] [Google Scholar]
- 4.Varnauskas E, the European Coronary Surgery Study Group Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319:332–7. doi: 10.1056/NEJM198808113190603. [DOI] [PubMed] [Google Scholar]
- 5.Passamani E, Davis KB, Gillespie MJ, Killip T, the CASS Principal Investigators and Their Associates A randomized trial of coronary artery bypass surgery: survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–71. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/ STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012. 60(24):e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013. 62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–70. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 9.Topkara VK, Cheema FH, Kesavaramanujam S, et al. Coronary artery bypass grafting in patients with low ejection fraction. Circulation. 2005;112(Suppl):I344–50. doi: 10.1161/CIRCULATIONAHA.104.526277. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velazquez EJ, Lee KL, O'Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RH, White H, Velazquez EJ, et al. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol. 2010;56:490–8. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–17. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 16.Qiu P, Sheng J. A two-stage procedure for comparing hazard rate functions. J R Stat Soc Series B Stat Methodol. 2008;70:191–208. [Google Scholar]
- 17.Yang S, Prentice RL. Semiparametric analysis of short-term and long-term hazard ratios with two-sample survival data. Biometrika. 2005;92:1–17. [Google Scholar]
- 18.Wrobel K, Stevens SR, Jones RH, et al. Influence of baseline characteristics, operative conduct, and postoperative course on 30-day outcomes of coronary artery bypass grafting among patients with left ventricular dysfunction: results from the Surgical Treatment for Ischemic Heart Failure (STICH) trial. Circulation. 2015;132:720–30. doi: 10.1161/CIRCULATIONAHA.114.014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doenst T, Cleland JG, Rouleau JL, et al. Influence of crossover on mortality in a randomized study of revascularization in patients with systolic heart failure and coronary artery disease. Circ Heart Fail. 2013;6:443–50. doi: 10.1161/CIRCHEARTFAILURE.112.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swedberg K, Kjekshus J, Snapinn S. Long-term survival in severe heart failure in patients treated with enalapril: ten year follow-up of CONSENSUS I. Eur Heart J. 1999;20:136–9. doi: 10.1053/euhj.1998.1098. [DOI] [PubMed] [Google Scholar]
- 21.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dys-function: a follow-up study. Lancet. 2003;361:1843–8. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 23.The Post Coronary Artery Bypass Graft Trial Investigators The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153–62. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 24.Kulik A, Voisine P, Mathieu P, et al. Statin therapy and saphenous vein graft disease after coronary bypass surgery: analysis from the CASCADE randomized trial. Ann Thorac Surg. 2011;92:1284–90. doi: 10.1016/j.athoracsur.2011.04.107. [DOI] [PubMed] [Google Scholar]
- 25.De Marco T, Chatterjee K, Rouleau JL, Parmley WW. Abnormal coronary hemo-dynamics and myocardial energetics in patients with chronic heart failure caused by ischemic heart disease and dilated cardiomyopathy. Am Heart J. 1988;115:809–15. doi: 10.1016/0002-8703(88)90883-6. [DOI] [PubMed] [Google Scholar]
- 26.White M, Rouleau JL, Ruddy TD, De Marco T, Moher D, Chatterjee K. Decreased coronary sinus oxygen content: a predictor of adverse prognosis in patients with severe congestive heart failure. J Am Coll Cardiol. 1991;18:1631–7. doi: 10.1016/0735-1097(91)90495-u. [DOI] [PubMed] [Google Scholar]
- 27.Panza JA, Velazquez EJ, She L, et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in ischemic LV dysfunction. J Am Coll Cardiol. 2014;64:553–61. doi: 10.1016/j.jacc.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlbaier LH, Pryor DB, Rankin JS, et al. Observational comparison of event-free survival with medical and surgical therapy in patients with coronary artery disease: 20 years of follow-up. Circulation. 1992;86(Suppl):II198–204. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.