Abstract
Purpose
Near infrared (NIR) fluorescence imaging is widely used for tracking antibodies and biomolecules in vivo. Clinical and preclinical applications include intraoperative imaging, tracking therapeutics, and fluorescent labeling as a surrogate for subsequent radiolabeling. Despite their extensive use, one of the fundamental properties of NIR dyes, the residualization rate within cells following internalization, has not been systematically studied. This rate is required for the rational design of probes and proper interpretation of in vivo results.
Procedures
In this brief report, we measure the cellular residualization rate of eight commonly used dyes encompassing three core structures (cyanine, BODIPY, and oxazine/thiazine/carbopyronin).
Results
We identify residualizing (half-life > 24 hrs) and non-residualizing dyes (half-life < 24 hrs) in both the far red (~650-680 nm) and near infrared (~740-800 nm) regions.
Conclusions
This data will allow researchers to independently and rationally select the wavelength and residualizing nature of dyes for molecular imaging agent design.
Keywords: cyanine dyes, fluorescent antibodies, protein metabolism, receptor internalization, multi-modality imaging, fluorophore retention
Intro
Near infrared (NIR) imaging is increasingly being used during the development of novel imaging agents either in dual-labeling approaches or interchanging the labeling moiety during development[1-3]. One of the biggest factors in determining the in vivo distribution of the label is the fate of the metabolic product after internalization. Despite the importance of this parameter and the well-characterized literature on radioactive tags[4-7], data on the retention of NIR tags is quite limited. Understanding the behavior of the NIR tag following local metabolism is critical in selecting fluorophores that will be representative of the radiolabeled compounds in preclinical development and designing effective fluorescent imaging agents for intraoperative applications. This information is also necessary in predictive mechanistic models[8-9] used in drug and imaging agent design[10-11].
Radiolabels and fluorescent dyes are often grouped as ‘residualizing’ or ‘non-residualizing’ depending on whether metabolites are trapped within the cell or wash out, respectively. Although this classification is somewhat arbitrary since the half-life of signal decay is a continuous spectrum, often half-lives less than 24 hrs such as iodine are referred to as ‘non-residualizing,’ while half-lives greater than 24 hrs (e.g. In-111) are considered residualizing[4]. The physiochemical properties of metabolites (molecular weight, charge, pKa, lipophilicity, etc.) and any interactions with transporters all impact the residualization rate.
The increased use of NIR dyes during the development of molecular imaging agents stems from the high spatial and temporal resolution of fluorescence imaging. NIR labeled probes can be followed in real-time in vitro and in vivo[12], and imaging techniques exist to monitor distribution from the whole animal and organ level down to cellular and subcellular resolution[13-15]. The fluorophore can then be replaced by a radioactive tag with similar physiochemical properties, or the dual-labeled targeting agent can be loaded with a radioactive isotope. NIR probes have significant advantages over visible light dyes due to the drastically lower tissue autofluorescence and high tissue penetration of light in this region of the spectrum. The low background enables the detection of very low (nM) concentrations of dyes, and the high tissue penetration allows longitudinal whole animal imaging to follow the probe kinetics.
While there has been extensive research into organic dye NIR fluorophore development, three of the most common (and commercially available) structures are cyanine dyes, red-shifted BODIPY fluorophores, and smaller polycyclic dyes (e.g. oxazine[16], thiazine[17], or carbopyronine[18] structures). A diverse set of cyanine dyes are available with multiple conjugation chemistries and varying charge, and these dyes are commonly used due to their high absorption coefficient and reasonable quantum yield (resulting in excellent brightness). Given the diversity in physiochemical properties of these fluorophores including a wide range in molecular weight, charge, and lipophilicity, we sought to determine the cellular retention rate of fluorescently labeled antibodies (using NHS-ester chemistry) for a direct comparison between dyes.
Materials and Methods
Materials
A-431 cells were obtained from ATCC (Manassas, Virginia). Cetuximab (Bristol-Myers Squibb, Princeton, New Jersey) was conjugated according to the manufacturer’s instructions with each of the following dyes: CellTraceTM Far Red DDAO-SE (DDAO) (Life Technologies, Eugene, Oregon), IRDye® 800CW NHS Ester (IRDye)(LI-COR, Lincoln, Nebraska), Alexa Fluor® 680 NHS Ester (AF680) (Life Technologies, Eugene, Oregon), Alexa Fluor® 750 NHS Ester (AF750) (Life Technologies, Eugene, Oregon), Sulfo-Cyanine7 (SulfoCy7) (Lumiprobe Corporation, Hallandale Beach, Florida), Cy5.5 (GE Healthcare Bio-Sciences, Pittsburgh, Pennslyvania), Bodipy® 650/660-X (BODIPY-650) (Life Technologies, Eugene, Oregon), Atto 740 NHS Ester (Atto 740) (Sigma-Aldrich Corp., St. Louis, Missouri). Dyes were reacted in 10% sodium bicarbonate and antibody solution (2mg/mL) for 2 hours at room temperature. A molar ratio of 1.0 was used for all dyes. The antibody-dye conjugates were purified using 800uL of 5g/50mL water of Biogel P-6, Fine (Bio-Rad, Hercules, California) in Spin-X centrifuge filter tubes (Corning, Corning, New York). The final degree of labeling was determined by the absorption at 280nm corrected for the fluorophore and the max absorption wavelength of each dye using a NanoDrop 1000 spectrophotometer. A protein gel was run to ensure that there was no free dye remaining.
Residualizing Dye Plate Assay
The rates of cellular dye loss were measured over a period of eight days with the Odyssey Imaging System (LI-COR). Cells were plated in 96-well plates overnight at between 90-100% confluency. The cells were labeled with cetuximab-dye conjugates at 40 nM for 30 minutes at 37°C, then subsequently washed three times to remove unbound probe. The cells were washed daily with media (DMEM supplemented with 10% fetal bovine serum, 2% sodium bicarbonate, and 1% penicillin/streptomycin) and then scanned with the Odyssey Imaging System. Signal was obtained from the background subtracted sum of pixel intensities using Licor Image Studio software. The fluorescent intensities for each dye were normalized to the maximum signal achieved after internalization and unquenching (see supplementary data).
Confocal Microscopy
Falcon™ Culture Slides (Fisher Scientific, Pittsburgh, Pennsylvania) were imaged with an upright Olympus FV1200 confocal microscope using 405, 633, and 750 nm lasers and a 60x objective. Cells were plated overnight at 90-100% confluency. Cells were labeled with each antibody-dye conjugates at 0, 24, and 48 hours under the same conditions described above and subsequently were washed twice with media. After 48 hours cells were incubated with Hoechst 33342 for 5 min at room temperature, washed with media, and then imaged.
PAMPA assay
Membrane permeability was measured using the BD Gentest™ Pre-coated PAMPA Plate System. Briefly, NHS ester derivatives of all dyes were mixed into aqueous solution overnight at room temperature to allow dyes to hydrolyze into the unreactive carboxylic acid form. 1uM hydrolyzed dye was used in the donor wells and was incubated for 5 hours at room temperature. Donor and acceptor concentrations were obtained using the Odyssey Imaging System and the permeability was then calculated according to manufacturer’s instructions.
Results
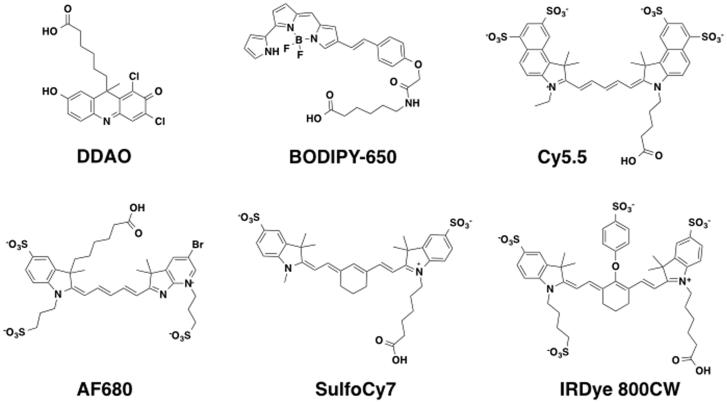
Eight commercially available NIR dyes available as NHS esters were chosen for this study. They represent three different classes of molecules with varying optical and physiochemical properties (Table 1). The structures include several cyanine dyes with varying numbers and positions of sulfate groups (Fig. 1). The structures for AF750 and Atto 740 are proprietary and are not included.
Table 1.
NIR dyes and their physiochemical properties. Ex/Em and extinction coefficient reported as listed by the manufacturer. MW and LogD calculated based on hydrolyzed form of NHS ester. LogD estimated using MarvinSketch (http://www.chemaxon.com/products/marvin/marvinsketch). t1/2 was calculated using best fit for one-phase decay in PRISM.
| Dye | Ex/Em (nm) |
Extinction Coefficient (cm−1 M−1) |
MW (kDa) | Net Charge |
LogD (pH 7.4) |
t1/2 (day) |
|---|---|---|---|---|---|---|
| DDAO | 648/656 | 42,000 | 408 | −1 (pKa = 5.0) |
2.04 | 0.99 (±0.4) |
| BODIPY-650 | 651/660 | 100,000 | 546 | 0 | 0.85 | 2.43 (±0.3) |
| Cy5.5 | 675/694 | 250,000 | 900 | −3 | −4.72 | 3.92 (±0.5) |
| AF680 | 684/707 | 183,000 | 857 | −2 | −6.39 | 2.43 (±0.1) |
| Atto740 | 740/764 | 120,000 | 451 | + 1 | NR | 0.85 (±0.1) |
| SulfoCy7 | 740/773 | 240,600 | 708 | −1 | 0.13 | 2.92 (±0.3) |
| AF750 | 753/782 | 290,000 | ~1200 | NR | NR | 2.55 (±0.2) |
| IRDye 800CW | 774/789 | 240,000 | 1000 | −3 | −4.71 | 2.33 (±0.1) |
Figure 1.
Published dye structures used in this study including cyanine-based structures, boron-dipyrromethene (BODIPY), and the acridine-based (7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridin-2-one)) dye DDAO. These structures represent the hydrolyzed NHS ester form of the dyes.
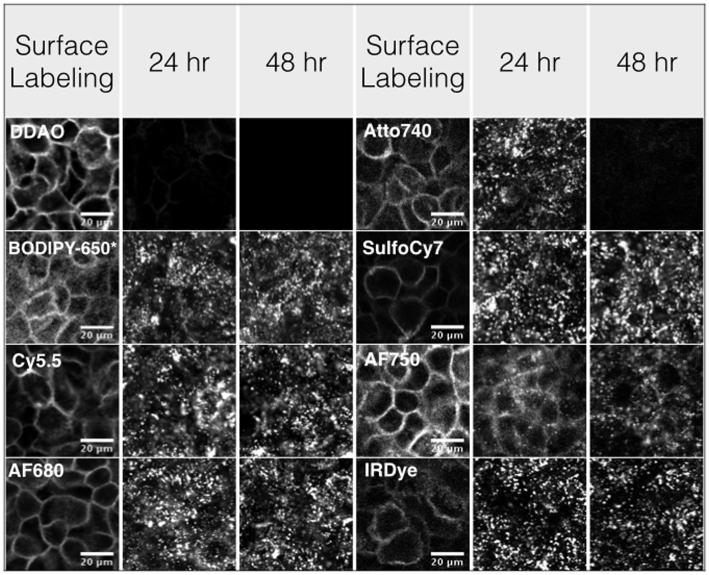
All the dyes were conjugated to cetuximab successfully, although the lipophilic BODIPY-650 dye could not be completely purified of free dye after the reaction (data not shown). The fluorescent cetuximab molecules specifically labeled the surface of A-431 cells following 30 minutes at 40 nM (Fig. 2). The labeled cetuximab molecules were internalized and trapped in punctate vesicles throughout the cell with varying degrees of protein-dye unquenching inside the cell (Fig S1). The cyanine dyes retained signal at 24 and 48 hrs. DDAO and Atto 740 signals were lower at 24 hrs and not visible at 48 hrs. A small amount of unquenching of Atto 740 after internalization results in the higher microscopy signal at 24 hrs but faster terminal half-life of clearance.
Figure 2.
Confocal microscopy of cells immediately after surface labeling or after 24 and 48 hrs incubation. Punctate spots after incubation show the substantial internalization, while the non-residualizing dyes DDAO and Atto740 have little signal at later times.
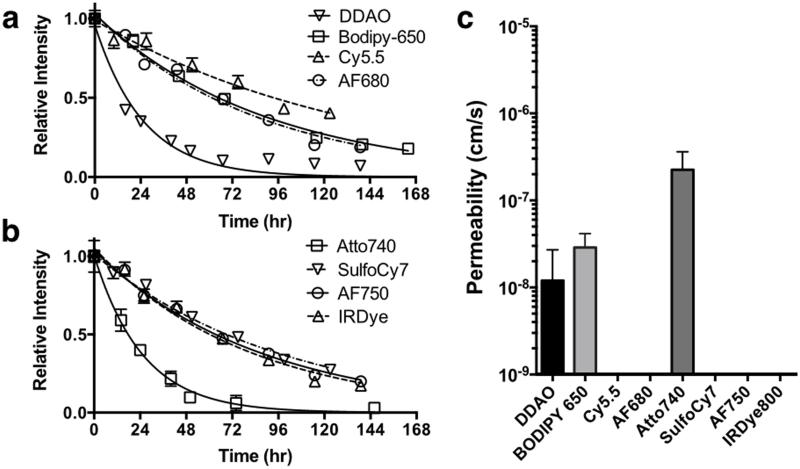
To quantify the overall retention over long times, a plate assay was used to measure the bulk fluorescence over 8 days (Fig. 3). To eliminate the effects of internalization, degradation, and un-quenching, the half-life was measured from the time of peak signal onward. Half-lives for each of the dyes were calculated using PRISM and are reported in Table 1. To check if passive diffusion through membranes could explain the more rapid loss of signal from internalized and degraded probe, a PAMPA assay was used to measure the membrane permeability of the free dye. The values were low (< 1×10−6 cm/s) for all the dyes, but only DDAO, Atto 740, and BODIPY-650 gave measurable signal in the acceptor well.
Figure 3.
The signal intensity for each labeled antibody is plotted from the time of peak signal. To avoid un-quenching effects on the half-life, only the later time points during signal decay were used (a,b). To determine if passive diffusion through membranes could explain the loss in signal, all the free dyes (carboxylic acid derivatives) were tested in a PAMPA assay (c).
Discussion
Understanding the cellular kinetics of NIR dyes is critical for designing imaging agents and predicting in vivo behavior. Whether used in direct applications for intraoperative imaging[12], in multi-modality imaging[1], or during preclinical development of radiolabeled probes, the rate at which the degraded probe diffuses out of cells is a major determinant of the time course and concentration of signal within the tissue. In this work, a wide range in the cellular residualization rate of NIR dyes was found following uptake by an NHS-ester labeled monoclonal antibody (cetuximab) based on the dye properties.
To quantify the cellular half-life, we selected the clinical anti-EGFR antibody cetuximab as the model targeting agent. This is a well-studied internalizing antibody[19-20], and our imaging results showed virtually complete internalization within 24 hrs. A-431 cells were selected because they express high numbers of EGFR, resulting in a strong signal that can be tracked over many days. They can also be maintained as a slower growing confluent monolayer, reducing the impact of repeated cell division. The clearance rates could be influenced by the cell line and probe, however, due to interactions with drug transporters and/or differences in internalization and degradation rates. The degree of labeling (DOL) was kept below 1 for most dyes to minimize the presence of multiple dyes on a single antibody. For in vivo work, this can have a strong impact on distribution [21].
At early times (within 24 to 48 hrs of cell surface labeling), the fluorescence signal is a combination of internalization, degradation, pH effects, and subcellular compartmentalization. Several dyes showed significant increases in signal as the covalently labeled antibody was degraded (Fig. S2), resulting in unquenching. The quenching is likely from dye-protein interactions, not dye-dye interactions, due to the low degree of labeling. At later times, however, the decrease in signal followed a single exponential decay that could be accurately and reproducibly quantified.
To test our hypothesis that passive diffusion from the cell dictates the residualization rate, the membrane permeability of the dyes was measured using a parallel artificial membrane permeability assay (PAMPA). This eliminates any effect from drug transporters such as p-glycoprotein or organic anion transporters, which can shuttle dyes across membranes[22-23]. While the permeability of all the dyes was low, only measurable permeation through the membranes was detectable for DDAO, Atto 740, and BODIPY-650, providing evidence that these dyes can exit the cells by passive diffusion through membranes after antibody degradation.
The dye residualization rate has a major impact on imaging properties, but dye properties alone are not solely responsible for the rate of washout after cell labeling. The linker region, conjugation chemistry, and/or targeting molecule can have a major impact on the residualizing behavior of a dye[24], and properly designed linkers can increase cellular retention if desired. In this work, the intrinsic rate of several commercially available dyes containing a common NHS ester lysine linkage was quantified due to the extensive use of this labeling chemistry. Other labeling strategies would need to be tested individually.
There are several other steps to washout from tissues in vivo. After exiting the cell, the fluorescent degradation product must diffuse through the tissue to a capillary or lymph vessel and intravasate before exiting the tissue. These steps can occur within minutes[25], so the hours required for exiting the cell are often the rate-limiting step. The in vitro assay here does not capture all the complexity in vivo, however, so care must be taken when extrapolating to animal data.
Conclusions
Commonly used and commercially available NIR dyes exhibit varying rates of cellular retention after internalization. DDAO and Atto 740 antibodies diffused out of cells quickly following internalization and degradation, while the cyanine based probes had significant retention in cells over several days. Residualizing dyes are useful for studying protein metabolism, signal amplification of internalizing targets, and fluorescent surrogates for residualizing radioisotopes (supplementary data). For monitoring the distribution of intact protein therapeutics, reducing signal in clearance organs, and/or using fluorescent surrogates for non-residualizing radioisotopes such as iodine, the non-residualizing fluorophores are ideal. Fortunately, this study has identified both residualizing behaviors in far-red and near-infrared wavelength dyes, allowing investigators to select the wavelength and residualizing properties appropriate for their application. These rates should prove useful in experimental design, in vivo data interpretation, and molecular probe development.
Supplementary Material
Acknowledgements
We thank the University of Michigan Biointerfaces Institute for use of the plate reader. Funding was provided by NIH grant 1K01DK093766 (GMT).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Brand C, Abdel-Atti D, Zhang Y, et al. In vivo imaging of GLP-1R with a targeted bimodal PET/fluorescence imaging agent. Bioconjug Chem. 2014;25:1323–1330. doi: 10.1021/bc500178d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura RH, Cheng Z, Gambhir SS, Cochran JR. Engineered Knottin Peptides: A New Class of Agents for Imaging Integrin Expression in Living Subjects. Cancer Research. 2009;69:2435–2442. doi: 10.1158/0008-5472.CAN-08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibold U, Wangler B, Schirrmacher R, Wangler C. Bimodal imaging probes for combined PET and OI: recent developments and future directions for hybrid agent development. BioMed research international. 2014;2014:153741. doi: 10.1155/2014/153741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Press OW, Shan D, HowellClark J, et al. Comparative metabolism and retention of iodine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer Research. 1996;56:2123–2129. [PubMed] [Google Scholar]
- 5.Ferl GZ, Kenanova V, Wu AM, DiStefano JJ. A two-tiered physiologically based model for dually labeled single-chain Fv-Fc antibody fragments. 2006;5:1550–1558. doi: 10.1158/1535-7163.MCT-06-0072. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths GL, Govindan SV, Sgouros G, Ong GL, Goldenberg DM, Mattes MJ. Cytotoxicity with Auger electron-emitting radionuclides delivered by antibodies. International journal of cancer Journal international du cancer. 1999;81:985–992. doi: 10.1002/(sici)1097-0215(19990611)81:6<985::aid-ijc23>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Knowles SM, Zettlitz KA, Tavare R, et al. Quantitative immunoPET of prostate cancer xenografts with 89Zr- and 124I-labeled anti-PSCA A11 minibody. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:452–459. doi: 10.2967/jnumed.113.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamzei N, Samkoe KS, Elliott JT, et al. Comparison of Kinetic Models for Dual-Tracer Receptor Concentration Imaging in Tumors. Austin journal of biomedical engineering. 2014:1. [PMC free article] [PubMed] [Google Scholar]
- 9.Thurber GM, Wittrup KD. A mechanistic compartmental model for total antibody uptake in tumors. Journal of Theoretical Biology. 2012;314:57–68. doi: 10.1016/j.jtbi.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams SP. Tissue distribution studies of protein therapeutics using molecular probes: molecular imaging. The AAPS journal. 2012;14:389–399. doi: 10.1208/s12248-012-9348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatnagar S, Deschenes E, Liao J, Cilliers C, Thurber GM. Multichannel Imaging to Quantify Four Classes of Pharmacokinetic Distribution in Tumors. J Pharm Sci. 2014;103:3276–3286. doi: 10.1002/jps.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 13.Laughney AM, Kim E, Sprachman MM, et al. Single-cell pharmacokinetic imaging reveals a therapeutic strategy to overcome drug resistance to the microtubule inhibitor eribulin. Sci Transl Med. 2014;6:261ra152. doi: 10.1126/scitranslmed.3009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurber GM, Yang KS, Reiner T, Kohler RH, Sorger P, Mitchison T, Weissleder R. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nature Communications. 2013;4:1504. doi: 10.1038/ncomms2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurber GM, Reiner T, Yang KS, Kohler RH, Weissleder R. Effect of Small-Molecule Modification on Single-Cell Pharmacokinetics of PARP Inhibitors. Mol Cancer Ther. 2014;13:986–995. doi: 10.1158/1535-7163.MCT-13-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jose J, Burgess K. Benzophenoxazine-based fluorescent dyes for labeling biomolecules. Tetrahedron. 2006;62:11021–11037. [Google Scholar]
- 17.Kessel D. Photodynamic Therapy of Neoplastic Disease. CRC Press, Inc; 1990. [Google Scholar]
- 18.Arden-Jacob J, Frantzeskos J, Kemnitzer NU, Zilles A, Drexhage KH. New fluorescent markers for the red region. Spectrochimica acta Part A, Molecular and biomolecular spectroscopy. 2001;57:2271–2283. doi: 10.1016/s1386-1425(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 19.Luo FR, Yang Z, Dong H, et al. Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft. Cancer Chemotherapy and Pharmacology. 2005;56:455–464. doi: 10.1007/s00280-005-1022-3. [DOI] [PubMed] [Google Scholar]
- 20.Devaraj NK, Upadhyay R, Hatin JB, Hilderbrand SA, Weissleder R. Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/trans-Cyclooctene Cycloaddition. Angewandte Chemie-International Edition. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conner KP, Rock BM, Kwon GK, et al. Evaluation of near infrared fluorescent labeling of monoclonal antibodies as a tool for tissue distribution. Drug metabolism and disposition: the biological fate of chemicals. 2014;42:1906–1913. doi: 10.1124/dmd.114.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Shi C, Tong R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2833–2844. doi: 10.1158/1078-0432.CCR-10-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro AB, Corder AB, Ling V. P-glycoprotein-mediated Hoechst 33342 transport out of the lipid bilayer. European journal of biochemistry / FEBS. 1997;250:115–121. doi: 10.1111/j.1432-1033.1997.00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Thorpe SR, Baynes JW, Chroneos ZC. The Design and Application of Residualizing Labels for Studies of Protein Catabolism. Faseb Journal. 1993;7:399–405. doi: 10.1096/fasebj.7.5.8462781. [DOI] [PubMed] [Google Scholar]
- 25.Thurber GM, Weissleder R. A Systems Approach for Tumor Pharmacokinetics. PLoS One. 2011:6. doi: 10.1371/journal.pone.0024696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.