Summary
We have been examining antigen presentation and the antigen presenting cells (APC) in the islets of Langerhans of the non-obese diabetic (NOD) mouse. The purpose is to identify the earliest events that initiate autoimmunity in this confined tissue. Islets normally have a population of macrophages that is distinct from those that inhabit the exocrine pancreas. Also found in NOD islets, is a minor population of dendritic cells (DC) that bear the CD103 integrin. We find close interactions between beta cells and the two APCs that result in the initiation of the autoimmunity. Even under non-inflammatory conditions, beta cells transfer insulin-containing vesicles to the APCs of the islet. This reaction requires live cells and intimate contact. The autoimmune process starts in islets with the entrance of CD4+ T cells and an increase in the CD103+ DCs. Mice deficient in the Batf3 transcription factor never develop diabetes due to the absence of the CD103/CD8α lineage of DCs. We hypothesize that the 12-20 peptide of the beta chain of insulin is responsible for activation of the initial CD4+ T cell response during diabetogenesis.
Keywords: Macrophages, CD103+ dendritic cells, autoimmune diabetes, islets of Langerhans, insulin autoreactivity
Introduction
In the past several years, we have been examining autoimmune diabetes in the non-obese diabetic (NOD) mouse focusing on antigen presentation events that transpire during the initiation of the process. This has led us to identify the early T cells that come into islets and, as importantly, the antigen presenting cells (APCs) that reside in them, as well as those that enter as the autoimmune process sets in (1-5). Our data shows a complex series of interactions, symbiotic to a great extent, among beta cells that donate the “diabetogenic” antigens, the APCs and the CD4+ T cells (6). Studying these interactions is not only important in the context of the autoimmune process per se but is also giving us new and important insights on how cell communication takes place between tissue cells and the innate and adaptive immune system cells. This review consists of a summary and discussion of our findings. Our previous reviews had a broader analysis with coverage of the extensive literature. Here our review of the literature is limited.
We start with a few comments on the NOD mouse. We then follow by reviewing insulin autoreactivity, an issue to which we have paid much attention. Then we review our studies on islet APCs and the early events taking place in the islets of Langerhans.
The NOD mouse
The NOD mouse develops spontaneous T cell-dependent autoimmune diabetes (7). The penetrance of the disease depends much on the state of the housing conditions and varies from 40% to close to 100%. The cleaner the housing conditions are, the higher the incidence, a point very evident in NOD mice raised in gnotobiotic conditions, where the incidence is high with somewhat accelerated time kinetics (8). In our specific pathogen-free colony, about 80-90% of mice become diabetic starting at about the 20th week of age.
The NOD mouse has a distinctive major histocompatibility complex class-II (MHC-II) molecule. The expression of this I-A allele, I-Ag7, is required for the development of the autoimmunity (9, 10). NOD mice that express a different allele do not become diabetic (11). Of great interest, the I-Ag7 molecule has structural features very much in common with the HLA/MHC-II molecules that confer the susceptibility for the human type 1 diabetes (T1D): HLA-DQ2 and HLA-DQ8 (12-15). All three of these MHC-II molecules have the distinctive feature of not having an aspartic acid at position 57 of the MHC-II beta chain. Most MHC molecules have an aspartic acid at this position, and it makes a salt bridge with an arginine at the MHC-II alpha 76 position thereby closing the binding site. A non-aspartic acid at beta 57 results in a very distinct MHC-II peptidome in which the most abundant peptides are those that have acidic residues at the carboxy end of the peptide (16-18). In our group, Anish Suri with Michael Gross made a comparison of the peptidome of I-Ag7 and HLA-DQ8 from a cell line that expressed either the human or the mouse MHC-II molecule and found a very similar spectrum of self-peptides displayed on the surface of the cell (16). Notably, many of the peptides had multiple acidic residues at their carboxy terminus. By mutating these acidic residues, it became clear to Suri that they were instrumental in the specificity of binding (19). Several structural analyses on these MHC-II molecules established that peptides having acidic residues at the carboxy-end established a salt bridge with the unpaired arginine at alpha 76.
Autoreactivity to insulin
Autoreactivity in the NOD mouse and in patients with T1D is broad. Several proteins have been shown to be targeted by T cells or by autoantibodies. Previous reviews have considered these, including a recent one of ours (20). The unresolved issue is whether there are immunoreactivities that start the process, while others follow as a result of “epitope spreading.” In NOD mice one can probe this by doing transfers of T cells into non-diabetic mice and determining whether there are stronger diabetogenic targets that readily cause diabetes in contrast to others that may add to the injury or not at all.
Several findings support insulin autoreactivity as having an important, if not the initiating role, in the diabetic process. The hypothesis that insulin autoreactivity is the major component of diabetes autoimmunity has been supported by many, but particularly by George Eisenbarth and his group (21-23). Briefly, we summarize four findings that we believe are critical in supporting the insulin-initiator hypothesis.
The first are the clinical studies in patients with T1D that showed the presence of autoantibodies to insulin (24-26). These antibodies are, in many instances, of the IgG isotype indicating class switch and, therefore, an involvement of CD4 helper T cells. They have prognostic value. In the NOD, autoantibodies to insulin are also found.
The second findings come from two studies in which an insulin transgene was engineered to have its expression driven by an MHC-II promoter expressed in all APCs. The broad expression of insulin epitopes by APCs resulted in a marked reduction in diabetes incidence (27, 28). Such was not the case when two other antigens were expressed as transgenes: glutamic acid decarboxylase and IA-2.
The third series of findings derive from genetic studies both in NOD and in humans in which the expression of insulin genes in thymic medullary epithelial cells (mTECs) and/or in beta cells were altered, thereby influencing the incidence of diabetes. The mouse expresses two insulin genes, Ins1 and Ins2; the latter is expressed in mTECs, while the beta cells expresse both genes. mTECs express insulin as well as other beta cell products most likely regulated by the Aire transcription factor (29). Ablation of Ins2 in the NOD accelerates diabetes, suggesting that the mTEC expression most likely controls T cell autoreactivity. In contrast, ablation of Ins1 decreased diabetes incidence (30, 31). In humans, the second gene variant that influences T1D incidence is the variable number of tandem repeats (VNTR) elements in the promoter region of insulin. Allelic variants of VNTRs affect the degree of expression of insulin in mTECs: those that result in lesser expression are the ones with higher susceptibility while the opposite results are found with those VNTR variants that correlate with higher thymic expression (32-34). Lastly, one notes a monogenic autoimmune disease, APECED for autoimmune polyendocrinopathy syndrome, that has mutations in the AIRE gene and includes multiple endocrine autoimmunities including diabetes (35).
Finally to consider are the studies examining T cells to insulin (1, 2, 6, 36-41). T cells of many different specificities have been identified in NOD diabetes since the initial isolation of T cell lines by Katie Haskins (42, 43). The capacity of these T cells to induce diabetes has varied depending on their specificities. The first studies on T cells to insulin identified a number of CD4+ T cells that reacted with segment 9-23 of the insulin B chain. These T cells induced diabetes when transferred into non-diabetic NOD mice (op cit).
Our findings with T cells to insulin
Studies in our laboratory combined binding analysis of insulin peptides to I-Ag7 molecules together with the characterization of the fine specificities of the insulin reactive CD4+ T cells (reviewed in 20, 44). Our peptide binding studies with INS B:9-23 revealed a surprising finding: this particular peptide could bind in two overlapping but distinct registers. The B:9-23 peptide contained epitopes that bound in either the 12-20 or the 13-21 register, a one amino acid shift in the I-Ag7 peptide binding groove (1, 2, 45) (Table 1). The B:13-21 segment bound at higher affinity than B:12-20 due to the influence of a glutamic acid at the P9 position of the core binding register. A structural analysis of the binding of insulin to HLA-DQ8 had shown insulin peptide binding via the B:13-21 register in which the P9Glu established an ion pair with the arginine at HLA-DQ8 alpha 76 (15). A similar interaction between an acidic residue at P9 and the Arg76 was found in the binding of the I-Ag7 molecule with a glutamic acid decarboxylase peptide (13). Overall, the binding of the insulin peptides to I-Ag7 is weak and changes throughout the nine amino acid core affected binding.
Table 1. Composition of human and mouse insulin.
| B chain | Sequence | ||||
|---|---|---|---|---|---|
|
| |||||
| 1 | 9 | 23 | 30 | ||
| Human Ins | FVNQHLCG | SHLVEALYLVCGERG | FFYTPKT | rr | |
| Mouse Ins-1 | FVKQHLCG | PHLVEALYLVCGERG | FFYTPKS | rr | |
| Mouse Ins-2 | FVKQHLCG | SHLVEALYLVCGERG | FFYTPMS | rr | |
| Register 1:12-20 | VEALYLVCG | ||||
| Register 2:13-21 | EALYLVCGE | ||||
|
| |||||
| C-peptide | |||||
|
| |||||
| 33 | 63 | ||||
| Human Ins | EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ | kr | |||
| Mouse Ins-1 | EVEDPQVEQLELGGSP | GDLQTLALEVARQ | kr | ||
| Mouse Ins-2 | EVEDPQVAQLELGGGPGAGDLQTLALEVAQQ | kr | |||
|
| |||||
| B chain | Sequence | ||||
|
| |||||
| 66 | 86 | ||||
| Human Ins | GIVEQCCTSICSLYQLENYCN | ||||
| Mouse Ins-1 | GIVDQCCTSICSLYQLENYCN | ||||
| Mouse Ins-2 | GIVDQCCTSICSLYQLENYCN | ||||
Indicated are the amino acid sequences of human and mouse insulin. In bold are the residues that differ among the three. The peptide segments from the B chain involved in T cell autoreactivity are indicated. The B chain segment 9-23 is a hot spot for generating autoimmune epitopes (1,2,36-38,45). Others have identified T cells to other segments of the B chain and to C-peptide (reviewed in references 20 and 22).
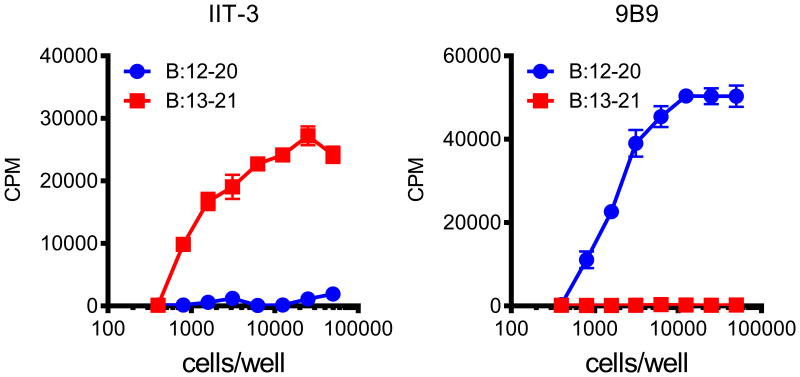
Peptides binding in either of the two binding registers induced specific insulin- reactive CD4+ T cells, a project that we started with Matteo Levisetti (45) and later was led by Jim Mohan (1,2,46) (Table 1, Fig 1). Initial experiments involved immunizing with insulin peptide and generating T cell hybridomas specific to the two registers. This technique revealed that the majority of insulin reactive CD4+ T cells recognized the B chain segment in “register 1” encompassing residues B:12-20. In contrast, a rarer set of T cells recognized “register 2”, the B:13-21 segment, the one amino acid shift in register. We established these reactivities either by testing the T cells with soluble peptides encompassing each segment or, more telling, by testing responses to APCs in which their I-Ag7 molecules were engineered to contain a peptide segment in either one or the other register (Fig. 1). Clonal T cell lines generated to either of the two registers were diabetogenic when transferred to NOD.Rag1-/- mice. Important to recall are the studies of Teyton's group showing the features of T cells to peptides not having an acidic residue at P9: their interaction with such peptides can be of high affinity as there is a rearrangement of the interactions of the receptor with the peptide (47).
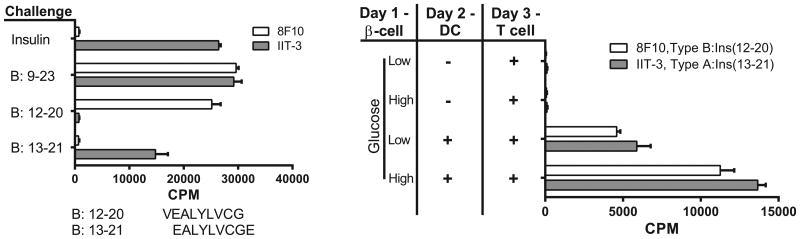
Figure 1.
CD4+ T cells recognize distinct registers of the B:9–23 peptide. Response of the hybridomas IIT-3 (Left Panel) and 9B9 (Right Panel) to the Register 1, B:12–20 and the Register 2, B:13–21 peptides covalently linked to I-Ag7 expressed on C3.G7 cells. T cells directed to Register 1, B:12-20 escape negative selection and can enter islets.
How to explain the presence and distribution of these two sets of T cells to insulin? The answer lies at three levels: (i) the processing of insulin or insulin peptides by APC; (ii) the nature of insulin presentation in the thymus; and (iii), the nature of insulin containing granules that are passed from beta cells to APC in the islets of Langerhans.
Different peptide-MHC (pMHC) complexes derive from the presentation of insulin or insulin peptides by an APC. Processing of intact mature insulin by APCs results in the presentation exclusively of the Register 2, B:13-21 peptide segment (1,2,6). T cells to Register 1, B:12-20 are simply not found after insulin processing. In striking contrast, treatment of APCs with denatured insulin, the entire B chain peptide, or the B:9-23 peptide results in presentation of either of the two peptides. The important finding is that the 12-20 segment is presented only when an APC handles the denatured insulin or peptide fragments.
Finding pMHC complexes from intracellular processing of a protein different from those derived from exogenous denatured proteins or peptides can be explained by the intracellular pathways for processing and presentation in APCs (1,48,49). Proteins are processed in deep vesicular compartments--the MIIC--and subjected to a series of biochemical events that result in the binding of peptides to MHC-II. Those peptides are subjected to an editing process in which H2-DM is involved: those pMHCs with stronger binding affinity are selected while those weaker binders are eliminated. Moreover the high binding pMHC survive the traffic to plasma membrane. When cells process peptide or denatured proteins, the compartments used are different and can, therefore, give rise to a different peptide repertoire. We believe this aberrant peptide presentation is important in the initiation of autoimmunity because it creates epitopes that are not correctly negatively selected in the thymus. See our reviews for our current understanding of the presentation of alternatively selected epitopes (44, 48).
Other findings that indicate a response to denatured self
Going back almost 100 years, there are indications of a different response to native versus denatured self-proteins that has been largely ignored. We first realized the differences in the pMHC repertoires of native versus denatured proteins while studying the model protein hen-egg white lysozyme (HEL) (49-51). In brief, we had found an epitope of the dominant segment 48-62 following native HEL processing--we called it a type A epitope. But a distinct pMHC conformer--the type B--was processed and presented after providing denatured HEL to APCs. The presentation of the type A epitope required processing in a late vesicular compartment of the APC while the type B-pMHC epitope was generated in early vesicles or by surface exchange at the plasma membrane (49). A series of manipulations indicated that the type B-pMHC was eliminated in the presence of H2-DM while the type A was not. H2-DM was not found in early vesicles and, thus, the type B-pMHC was presented. A major result was found when we generated HEL transgenic mice under the class II-MHC promoter and then immunized them with HEL, now a self-protein highly expressed by all APCs (52). In contrast to regular mice, most of the type A T cells were eliminated. But the type B T cells generated by peptide immunization were unaffected. This finding on HEL-transgenic mice raised the question whether it could apply to autoimmune reactions. The findings in NOD mice with insulin peptides answers this question.
Noteworthy are early observations made by many when immunizing with self- proteins. Immunization with autologous proteins was consistently negative; these are the findings that established the major immunology paradigm: we do not react to our own proteins. However, immunizing with the same protein in a denatured form elicited reactivity, i.e., we react against denatured self (53). On this issue, three papers are worth emphasizing. Weigle's group at Scripps reported that rabbits immunized with autologous thyroglobulin did not elicit antibodies or induced thyroiditis. In contrast, immunization with denatured protein elicited thyroid disease (54). Benacerraf's group reported that delayed-type hypersensitivity in guinea pigs could be elicited with denatured autologous immunoglobulin but not with the native protein (55). Finally, in some strains of mice or guinea pigs, denatured insulin, but not native insulin, elicited responses (56, 57). Denatured antigens are thus handled differently by the immune system than their native counterparts, and they elicit a broader repertoire of peptides.
Unresponsiveness to insulin but not to defined insulin peptides
We discussed before the importance of insulin expression in the thymus for preventing autoreactivity to insulin. mTECa that express the Aire transcription factor upregulate many tissue specific genes, including insulin. However, there will not be high level of antigen nor denatured or free peptide available. Therefore, because insulin protein is processed and presented as the Register 2, B:13-21 segment, it will result in the elimination of most of the T cells reactive to it. In contrast, the Register 1, B:12-20 segment will not be presented to significant levels. Direct evaluation of presentation of insulin epitopes by isolated thymic epithelial cells has not been examined. However, the current findings in toto suggest that NOD mice are tolerant to the Register 2, B:13-21 epitope while capable of responding to Register 1,B:12-20.
The main findings that support the idea of differential register expression in the thymus of NOD mice comes from our efforts immunizing with insulin or insulin-derived peptides. These results confirmed our T cell specificities studies and supported the statement on differential tolerance to the two insulin epitopes (2). Immunization to insulin or to the B:13-21 peptide showed no significant response over background in an ELISPOT assay. Immunization with the B:9-23 peptide elicited T cells that reacted to the B:12-20 peptide, but never to insulin or to B:13-21 peptide.
The experimentum crucis was to immunize with a non-immunogenic insulin, which Jim Mohan did by using a 9-23 peptide in which the Tyr16 had been substituted with an alanine. The T cells to B:12-20 do not recognize a peptide with Y16A, which is a TCR contact residue. The T cells to B:13-21 do not recognize it either. In Register 2, the Y16A is the P4 MHC contact residue. However, based on our results, we believe this mutation significantly alters the topology of the TCR contacts. When NOD mice were immunized with B:9-23 with the Y16A change, a response was elicited to wild-type insulin and the 13-21 peptide (2).
We followed this approach because of the important studies in the NOD.B16A mice generated by Nakayama in the Eisenbarth laboratory. This mouse strain is homozygous null for the Ins1 and Ins2 genes (58-60). Insulin production by the beta cells is driven by a mutated Ins2 transgene driven by the insulin promoter. The mutated insulin has the same amino acid change from alanine to tyrosine at position 16 of the B chain. These mice do not become diabetic presumably because most of the anti-insulin T cells that develop in the thymus are incapable of responding to the Y16A variant of the insulin peptide that is provided to APCs by the beta cells. It is important to note that the NOD.B16A mice still get sialitis, demonstrating that the effect of all the mutations in the mouse are specific to the islet autoimmunity and do not influence the general autoimmune predisposition of the NOD mouse strain.
Insulin epitopes are presented in islets
The pointed issue from these results is to explain the way in which the Register 1, B:12-20 pMHC complex is presented, since it does not derive from processing of native insulin. A CD4+ T cell receptor transgenic mouse named 8F10, that only recognizes the B:12-20 segment, provided us with important information on the biological outcomes of alternative peptide presentation (46). The 12-20 reactive CD4+ T cells entered islets without the need of the pancreatic lymph node (pLN). It is known that mice lacking pLNs do not develop diabetes (61,62). Yet, 8F10 T cells entered islets in the absence of pLN. From these findings, we have speculated that the important role of the pLN is to amplify the immunological process once it has been initiated, an issue that we are examining at this time. We believe the earliest T cells to participate in the autoimmune reaction are bloodborne B:12-20 T cells that enter through the vasculature, are activated in the islets, and then lead to a pLN-dependent amplification of the immune response. This idea is partly supported by our studies of antigen capture by APCs in islets.
Further studies indicated that intra-islet APCs presented insulin epitopes in the absence of any other inflammatory stimulus, i.e., even NOD.Rag1-/- islet APCs presented insulin antigens. Islet APCs from NOD or NOD.Rag1-/- mice were isolated and placed in T cell assays probing with the two sets of T cells, i.e., those against Register 1 or Register 2 (1-3). Both the B:13-21 peptide, derived from insulin processing, as well as the B:12-20 segment, derived from denatured insulin and insulin catabolic products, were detected in both groups of islet APCs--the macrophages and the CD103+ DC--which we discuss below (3).
To explain these findings, we decided to search within the secretory granules of beta cells for a distinct set that might contain insulin peptides (1, 6). There are at least two insulin-containing vesicle types in the beta cells. These were identified using monoclonal antibodies against conformationally intact insulin or insulin B:9-23 peptide. The insulin peptide specific antibodies were tested and found to have no reactivity to either insulin or proinsulin proteins (3, 23). We analyzed whole islets by confocal microscopy and found a population of vesicles that were only bound by the anti-insulin antibody. We found a second population of vesicles that were bound by the anti-INS-B:9-23 peptide. This reactivity could be competed by a molar excess of free peptide, confirming the specificity of the reaction. The generation of these peptide specific vesicles was not dependent on inflammation, as non-diabetic strains of mice also contained them (6). Beside the beta cells, insulin peptide containing vesicles could also be detected in APC isolated from islets, albeit to a lower level than in beta cells (3). An evaluation of the cell biology literature of beta cells suggests to us that the insulin peptide-containing vesicles are compatible with the crinophagic vesicles described in islets as well as other endocrine organs (63-65).
The crinophagy pathway appears to be a mechanism that the endocrine cells evolved to remove excess hormone and secretory granule material that is constantly being synthesized (65). However, it seems that an unexpected consequence of this homeostatic pathway is the passage of aberrant immunogenic peptides to the tissue resident APC population. The potential role of this passage in other endocrine tissues, which are all notoriously sensitive to autoimmune insults, remains unexplored. We discuss the interaction between the beta cells and the APC that result in passage of the granules in a later section.
In summary, our findings, first with HEL, but more importantly in the spontaneous autoimmunity of the NOD, plus the reports of others, some of which we discussed above, indicate that there is a repertoire of pMHC-II complexes from denatured proteins or peptides that is evident when exogenous denatured proteins or peptides are taken up and presented by APC. This self-repertoire can become central in autoimmunity, provided the tissue can generate denatured self-proteins or peptides and has a way of passing these to the APCs of the immune system. This situation is evident in NOD diabetes, but whether it applies to other autoimmune diseases needs to be examined.
Having discussed the CD4+ T cells, we now proceed to discuss how presentation takes place in the islet during the initial stage of autoimmune diabetes. We start by describing the APCs that normally reside in islets, which is a resident macrophage found in all strains. We follow by examining the islets in NOD where an additional APC, the CD11c+ CD103+ DC, is a major protagonist. Finally, we discuss the interaction among the beta cells, the APCs and the CD4+ T cells.
The resident APC of the islets: the macrophage
The last few years have seen a very large number of studies on the macrophage lineage. Our views of this lineage have changed markedly from the early ones in which macrophages were perceived as a phagocytic cell derived from blood monocytes and involved in the cleaning of tissues. One issue to note with emphasis is that macrophages are effective APCs, a point that we first made when starting to examine antigen processing and presentation some years ago (66-68). By various criteria, our results pointed to macrophages and not to DCs. Moreover, the experimental data that defined antigen presentation and established antigen processing for T cell recognition were made studying the macrophage (69, 70). Also, examination of macrophages, in the classical studies by Rosenthal and Shevach, led to the discovery of the rules for MHC-II-restriction (71-72). This presenting function of macrophages tended to be forgotten or ignored as a result of the discovery of the excellent presenting role of the DC lineage (discussed in 73). More recently, from examining resident macrophages from different tissues, it is evident that many of the resident macrophages express MHC-II proteins at high levels and can present antigen (74). The point is to define for each immunological reaction and in the various tissues whether macrophages and DC interact and communicate to bring in a complete response (for examples: 75-77). This could be due, for example, to differences in cytokine or costimulatory molecule functions on the different APCs that can sculpt T cell responses, an emerging theme in APC/T cell biology.
Our interest in islet macrophages started when we examined APCs in isolated islets (78). Early reports examining tissue sections have shown the presence of F4/80+ cells in many endocrine tissues and also in the islets proper by conventional histological approaches. We were aware of studies done by Paul Lacy in our Department when his laboratory was transplanting isolated islets, a technique that he was instrumental in developing. He found that some culture conditions resulted in the escape of leukocytes from the islets. These were interpreted as blood “passenger” cells. The issue was that when islets were devoid of these passenger leukocytes, they poorly rejected in certain allogeneic combinations. The take home message was that these leukocytes were central in tissue rejection, a concept that was also supported by the studies of others. We examined these early studies in two review papers that have the major references (79, 80).
Recently we carried out a project led by Boris Calderon examining the islet phagocytes in C57BL/6 (B6) mice as a base to compare with those in the islets of NOD mice (74) (Table 2). Initial studies that we and others had reported in NOD mice had made a point of calling the intra-islet APC a DC based on their expression of CD11c (78, 81, 82). But, both in B6 and in NOD mice, the major intra islet phagocytes are macrophages, identified not only by their composite of different surface markers but also by their gene expression pattern (3, 74, 80) (Table 2). (It appears that a peculiarity of most organ resident macrophages is that they express CD11c.)
Table 2. Profiles of pancreatic macrophages.
| Islet macrophage | Stroma CD206+ macrophage | Stroma CD206- macrophage | |
|---|---|---|---|
| Properties | |||
| Derivation | Definitive* | Yolk-sac* | Monocytes |
| Exchange | No | No | With monocytes |
| Antigen presentation | + | Poor | + |
| Cell Surface Markers | |||
| F4/80 | + | + | + |
| CD11b | + | + | + |
| CD11c | + | + | + |
| MHC-II | + | +/- | + |
| CD64 | + | + | + |
| CD68 | + | + | + |
| LyzM | + | + | + |
| CX3CR1 | + | +/- | +/- |
| CD206 | - | + | - |
| CD301 | - | + | - |
| Gene Expression | |||
| II1b | + | - | - |
| Tnfa | + | - | - |
| Nos2 | - | - | - |
| Arg1 | - | +/- | +/- |
| II10 | - | + | - |
| Ym1 | - | - | + |
| Fizz1 | - | + | + |
All three macrophages were negative for Ly6C, CD103, Zbtb46.
Refers to definitive hematopoiesis or yolk-sac-primitive hematopoiesis.
+, high expression; +/-, low expression; -, negative expression
Several striking findings were made examining macrophages in B6 mice and comparing the islet macrophages to those in the intra-acinar stroma of the pancreas (74). The islet macrophages are represented by only one set based on flow cytometric evaluation (see Table 2). In striking contrast, the pancreatic stromal macrophages contain two sets based mainly on their expression of the mannose receptor, CD206, and CLEC10A, CD301. The islet macrophages are found in the islets since birth and are derived from definitive hematopoiesis. The CD206/CD301 negative stromal set derives from blood monocytes and is constantly dividing. In contrast, the CD206/CD301+ population derives from yolk-sac hematopoiesis and shows slow turnover. Both sets of stromal macrophages actually have a different anatomical representation. The pancreatic ducts are surrounded by the CD206/CD301+ macrophages while the CD206/CD301 negative macrophages are interspersed throughout the acinar stroma.
The turnover of these macrophages was examined by parabiosis using the two alleles of CD45 to distinguish each of the parabionts. After several weeks of blood exchange, islets and the stromal macrophages were examined. The islet macrophages and stromal yolk-sac derived macrophages showed no interchange between the two parabionts. That is, islets of CD45.1+ mice still had the CD45.1+ macrophages and very few, if any, CD45.2+ cells and vice versa. These findings are in contrast to monocytes and lymphocytes in the spleen that exchanged evenly between the two mice (74). Therefore, these resident macrophages, under steady state, do not exchange with monocytes but are maintained by a low level of replication.
We identified that the stromal and islet macrophages differed in their gene expression profiles and function. The islet-resident displayed an M1-like activation pattern as well as high levels of MHC-II on their surface. The stromal macrophages expressed an M2-like pattern of transcripts, and also high levels of MHC-II. Expression of different genes in macrophages is complex, so the M1/M2 pattern is an oversimplification as others have stated (83). We used it, nevertheless, being aware that more thorough examination of the islet macrophages needs to be evaluated, particularly among strains and during islet inflammation. Yet our point stands that the gene expression patterns of islets and stromal macrophages are distinct and compatible with the M1/M2 patterns, albeit with the qualification stated above.
The pattern of gene expressions in islet and stromal macrophages was maintained during adulthood and, importantly, after irradiation and replacement with stem cells. Macrophages were depleted by lethal irradiation and then reconstituted with bone marrow stem cells. The reconstituted macrophages found in the islets were M1 and those found in the stroma were M2 in their gene signatures, respectively. We attributed such difference to the particular anatomy of the pancreas. In unknown ways, the islet promotes the activated M1-like macrophage while the stroma favors the M2-like. We concluded that the “pancreas anatomy conditions the origin and properties of the resident macrophages” (74, 80). Future studies are required to determine how the tissue microenvironment conditions the biology of macrophages (84).
What is the physiological role of the islet macrophages? We believe that there is a symbiotic relationship between beta cells and macrophages and that both influence each other. Whether the vascular endothelium is involved is an issue to also consider. The islet macrophages have a homeostatic or trophic role first reported by Pollard's group's examination of the osteopetrotic op/op mouse (85). The op mutation inactivates the Csf1 gene leading to a reduction or absence of macrophages at many anatomical locations. The islets of the op/op mouse were diminished in size and number. This mouse also showed impaired glucose tolerance. Calderon in our group showed that most of the islets of the op/op mouse lacked macrophages or contained no more than two per islet. In normal mice, we saw an average of 10 macrophages per islet. The range was dependent on the size of the islets, with bigger islets containing up 20 macrophages, while smaller islets contained fewer (78, 80).
How the macrophage and the islet endocrine cells are interacting is unknown. We hypothesize that the endocrine cells secrete signals that make the macrophages convert to a trophic supportive role. Additionally, all the islet resident macrophages are closely associated with the vascular endothelium (78). Live images of islets carried out by Bernd Zinselmeyer showed macrophages (labeled with CX3CR1-GFP) extending long cytoplasmic projections in between beta cells (6). These cytoplasmic projections can be seen crossing to the lumen of the blood vessel (78). Calderon did an ingenious experiment injecting B10.BR or NOD mice with 1um latex bead coated with either anti I-Ak or anti I-Ag7 and then isolating the islets a few minutes later: in the islets, the specific antibody coated beads were localized to blood vessels, always next to a macrophage. It is a telling result that suggests the intra-islet macrophage communicates with the circulating blood cells.
Concerning antigen presentation, the islet resident macrophages take up beta cell secretory granules and can present diabetogenic antigens. In our first series of studies, we isolated the islet macrophages from NOD mice and found them to spontaneously present diabetogenic antigens including insulin (78). These studies are complex in the sense that NOD mice, as we discuss below, also contain a set of CD103+ DCs that can present antigen. For other strains, where the only cell in islet is a macrophage, the conclusion points to macrophages as the APC. For example, we tested macrophages isolated from B10.BR mice (H-2k) that expressed HEL as a transgene under the control of the insulin promoter. These islet resident macrophages presented antigen to HEL-specific CD4+ T cells. In contrast, the stromal macrophages did not present the HEL peptides indicating a clear compartmentalization. Thus, the islet macrophages took up the immunogens from beta cells, processed and presented them. In the pLN of such mice, there is proliferation of HEL-reactive CD4+ T cells as measured by CFSE dilution. This indicates that the islet feeds beta cell derived antigens to the pLN. In this case, the most likely transport from islets to pLN is via the resident macrophages. It is known that islets lack lymphatics at steady state; instead the drainage lies in the surrounding acinar stroma. If the transport is by way of the APC, as we believe, it indicates that these move out of islets into the stroma to be able to reach the lymphatics.
We have also tried to determine if the islet macrophages contain beta cell material that can be visualized and/or quantified. We used an antibody that only detects insulin peptides and not insulin protein to find out if the islet macrophage, the only islet APC at steady state, contained insulin catabolic products. On average, there were about 10 insulin-peptide positive granules per macrophage (6). Electron micrographs of islets from various strains of mice also disclosed the presence of granules in APC. The granules in the APCs had the same morphology as the dense core granules, the typical morphology of the insulin containing granule, an indication that such granules have been transferred. We discuss these issues in more detail in a later section, but suffice to say here that definitely the islet phagocytes captures and present beta cell antigens. It is likely that such transfer☹i) is responsible for the activated biology of the islet macrophages, but, (ii) importantly in the context of autoimmune diabetes, is the conduit of immunological information to the T cell system, which activates fully when all the genetic components responsible for autoimmunity are in place, as happens with the NOD mouse.
(Concerning the stromal macrophages, we examined their antigen presentation capabilities by culturing them with a defined antigen, the protein listeriolysin O, that we had experience with before. The stromal macrophages, in B6 mice, differed in their presentation when isolated and cultured with listeriolysin. The CD206/CD301 negative macrophages presented to T cells ex vivo, whereas the CD206/CD301+ macrophages presented it very weakly.)
The early events in islets: the interferon signature
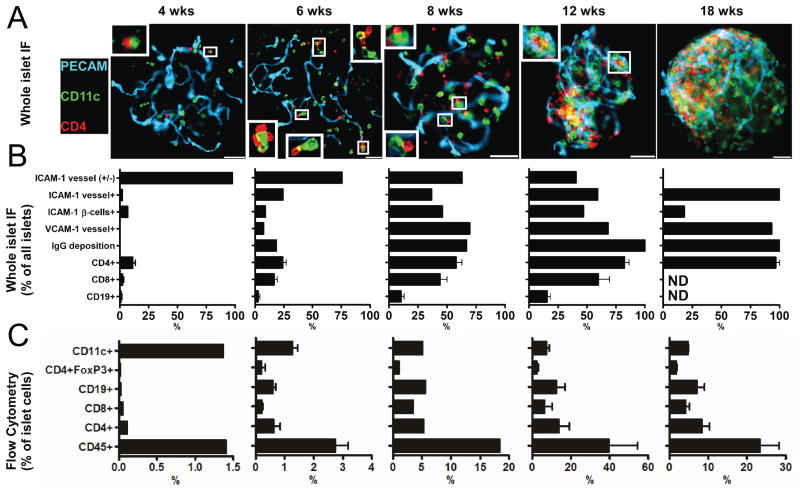
Several significant events transpire in the NOD at 3-6 weeks of age. In the islets, there is a discrete entrance of CD4+ T cells, a burst of CD103+ DCs, an increase in adhesion molecules in the vascular endothelium, and a new gene signature (4). Very shortly thereafter, CD8+ T cells start appearing, and the deposition of Ig within islets can be found (Fig 2).
Figure 2.
Examination of NOD islets throughout diabetogenesis. (A) Islets of Langerhans were isolated from NOD mice at the indicated ages and stained for blood vessels (PECAM-1), intra islet myeloid cells (CD11c), and T cell (CD4). Shown are representative images obtained from a pool of 6 mice per age from two independent experiments. Inserts show contacts between intraislet myeloid cells and T cells. White bars represent 50 μm. (B) Islets were isolated and stained for the indicated markers and then scored for presence or absence of staining. Bars represent mean+/−S.D. of the percentage of marker positive islets of Langerhans obtained from a pool of 6 mice per group from two independent experiments. (C) Islets of Langerhans were dispersed and cells were examined by flow cytometry for the indicated cell surface markers. Bars represent the mean+/−S.D. of the percentage of total islet cells identified in two independent experiments per age. Results were obtained from a pool of 8 to 10 mice per group. Taken from reference 4.
One of us (JAC) found that the earliest inflammatory signature in NOD diabetogenesis was an interferon (IFN)-inducible gene signature that took place by the 3rd to 4th week of life. The early interferon signature in NOD mice was identified by the increased expression of well-established interferon-inducible genes, such as the GTPases of the GBP and IRG families, and the chemokines Ccl5, Cxcl9 and Cxcl10. The signature was first detected by comparing female NOD mice at 2 and 4 weeks of age, and it increased in magnitude as the mice aged. There was no increase in the expression of interferon-signature genes in NOD.Rag1-/- mice, demonstrating the requirement for adaptive response in the process. Also, C57BL/6 and B6.g7 did not develop an interferon-signature, demonstrating the requirement for NOD background genes. In our colony, the first detectable upregulation of interferon genes was Ifnb1 at 4 weeks followed by Ifna at 6 weeks and Ifng at 8 weeks. Other groups have reported the upregulation of type I interferons in both the pancreatic lymph nodes and islets of NOD mice during the 3-4 week time period, although the timing seems to vary between colonies (86-87).
We are still trying to understand the importance of both type I and type II IFN in the progression of type 1 diabetes. Several major questions remain. How does interferon signaling modulate diabetes? This issue remains very confusing to the field. Neutralization of the type I interferon receptor with antibodies decreased diabetes incidence, but genetic ablation of the receptor had no effect (87). Removal of the type II interferon receptor had no effect in one study and led to a reduction of diabetes in females and an increase in diabetes in males in another (88, 89).
Do the housing conditions, including gut flora, influence the magnitude of interferon effects on diabetogenesis? We suspect that part of the disparate results obtained on NOD diabetogenesis regarding interferon may be due to the fact that these cytokines are pleiotropic and depending on context and magnitude of exposure can be both pro- and anti-inflammatory. In brief, the issues of environmental influence on interferon gene expression and their effects on diabetes development need to be addressed.
Finally, what are the sources of IFN during diabetes progression? It is easy to assume that any Ifng that is detected is being made by T cells, but which T cells? It is still unclear which pMHC specificities drive T cells to make IFN during diabetes. Is it the early insulin reactive CD4+ T cells or a second wave of amplifying CD4 or CD8+ T cells? The sources of type I interferon also remain elusive, and no group has definitively demonstrated its production by a specific cell type in either the pancreatic lymph node or the islets of Langerhans.
The early events in islets: the seminal role of the CD103+ DC
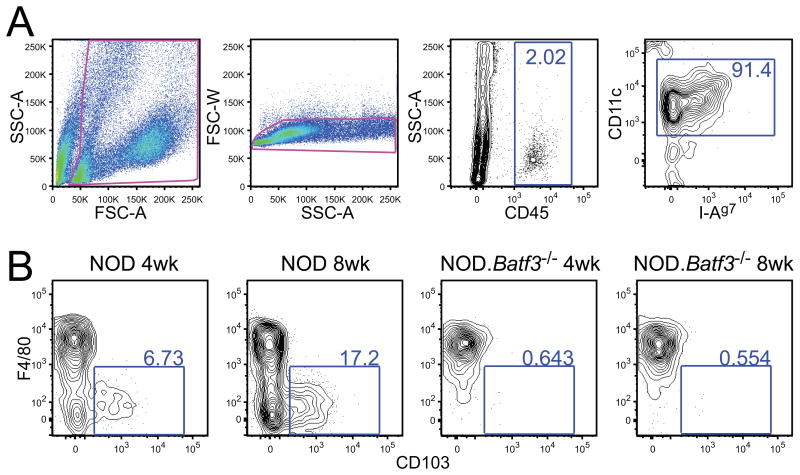
A striking finding in islets is the presence of the subset of DC characterized by expression of CD11c and CD103 (αE integrin) (3). In the NOD mice, CD103+ DCs are found in very small numbers or not at all at the 3-4 week period but increase shortly thereafter (Figs 3 and 4). A defining feature of this subset of DC is their high efficiency of cross presentation, i.e., processing soluble or particulate antigens and presenting them on MHC-I to CD8+ T cells. The CD103+ DC enters the islets at the same time that a few CD4+ T cells are also found. It is striking that most, if not all the T cells, are found in contact with the islet APCs. There is a tight correlation with the presence of CD4+ T cells, the CD103+ DC, and the appearance of a gene signature of inflammation (3). (We still do not know which cells enter the islet first, CD4+ T cell or CD103+ DC; and we feel this is an important issue to resolve in order to determine causality in the NOD diabetes model.)
Figure 3.
Two sets of myeloid cells are identified in NOD mice islets. (A) Gating strategy for dispersed islets. (B) F4/80+ and CD103+ staining of myeloid cells from 4-week-old and 8-week-old NOD (left) and NOD.Batf3−/− (right) islets, as gated in (A). Adapted from reference 3 with permission.
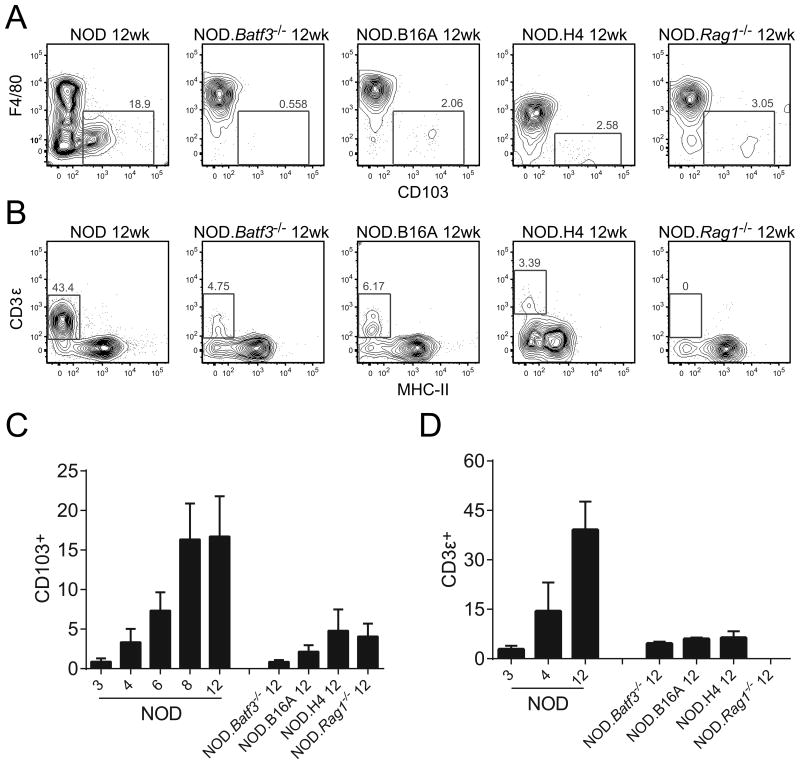
Figure 4.
Lymphocyte infiltration is absent in NOD.Batf3−/− mice islets. Flow cytometric analysis of (A) CD103+ myeloid cells gated as in Figure 2A and (B) CD3ε+ lymphocytes (gated on CD45+ cells) from 12 week NOD, NOD.Batf3−/−, NOD.B16A, NOD.H4, and NOD.Rag1−/− islets. (C) Representative graph of data from (A) including time course of NOD from 3 to 12 weeks of age. (D) Representative graph of data from (B) including NOD time course from 3 to 12 weeks of age. Representative flow cytometry plots and cumulative data from three or more independent experiments with each experiment pooling two or more mouse islets per sample (error bars, SD). Taken from reference 3 with permission.
The Batf3-deficient mice were generated by Dr. Kenneth Murphy. Murphy's group found that the Batf3 transcription factor is critical in the differentiation of the common DC precursor to the CD103+/CD8α+ lineage (90-92). In his studies, the Batf3-/- mice were poor at cross-presenting antigens to CD8+ T cells. The mice were susceptible to viral infections and did not reject methylcholanthrene-induced sarcomas. The role of the CD103+ DC in diabetogenesis was examined in the Batf3 gene knockout mouse in a project led by one of us (STF). We backcrossed the Batf3-/- allele onto the NOD background and ran microsatellite marker analysis to establish that our mice were fully backcrossed and contained all the IDD loci of NOD. The NOD.Batf3-/- lacked the CD103/CD8α DC lineage of DCs in all lymphoid tissues, confirming the findings by the Murphy laboratory (90).
We followed a large cohort of NOD.Batf3-/- mice for over a year in our colony and none of the mice became diabetic. Histological analysis showed intact islets without any detectable lymphocytic infiltration. This was confirmed by flow cytometry analysis of dispersed islet cells. Microarray analysis of the isolated islets did not show the signatures of inflammation that develop in the NOD by the 4th to 8th week of life. The gene expression analysis of NOD.Rag1-/- and the NOD.Batf3-/- were practically identical (3). Thus, the interruption of the autoimmune process is dramatic. We know of no other NOD gene knockout mice that have islets as clean as those found in the NOD.Batf3-/- mice, of course, omitting the NOD.SCID or NOD.Rag1-/- mice.
The defect in the Batf3-/- mice is clearly in the lack of the antigen presenting function of the CD103+ DC. We know this through an experiment in which we replaced the lineage in the NOD.Batf3-/- mice; these were lightly irradiated and reconstituted with NOD.Rag1-/- bone marrow cells. The transplanted bone marrow replaced the defective DC lineage in lymphoid tissues and in the islets. T cells entered the islets of such reconstituted mice resulting in diabetes. Reconstituting the NOD.Batf3-/- with bone marrow cells from a NOD.Rag1-/-xBatf3-/- did not replace the missing DC subset or lead to infiltration of islets by T cells. Moreover, the autoreactive T cells were still found in the secondary lymphoid organs of the NOD.Batf3-/-. T cells isolated from the spleen of the NOD.Batf3-/- transferred diabetes after injecting them into NOD.Rag1-/- mice, albeit with slower kinetics than transfer of NOD spleen T cells. We concluded that the absence of diabetes in the Batf3-deficient mice was caused by the lack of the CD103+ DCs, and that Batf3-deficient T cells were fully competent but unable to initiate diabetogenesis without this DC subset.
The T cells in the Batf3-/- mice
We have paid attention to T cells found in isolated islets. In dispersed islets from non-diabetic mice, including the NOD.Batf3-/-, we consistently find a small number of CD45+ myeloid cells, ∼2-5%. They are mostly represented by the F4/80+ macrophages described above. A very small percentage of these CD45+ cells are T cells, ranging from about 3-5%. If one considers that each mouse has ∼100-200 CD45+ cells in all their islets, this number of T cells is around 3-10 per mouse. We consider that this small number likely derives from blood in the islet vessels since, by microscopy, T cells are not detectable inside the islets (80). It is important in all these analyses to isolate islets free of stromal cells or the surrounding lymphoid aggregates that may contaminate the islet preparations (see Supplemental Figure 1 of reference 80).
The relationship between the presence of CD4+ T cells in islets and the presence and burst of CD103+ DC becomes very evident when one of us (STF) examined three congenic strains of mice: NOD.B16A (see above), NOD.H4, and NOD.Rag1-/-. The NOD.H4 mouse expresses I-Ak and not the diabetogenic allele I-Ag7. Therefore, this strain never becomes diabetic, despite the fact that they still get autoimmune thyroiditis. And, of course, the NOD.Rag1-/- mouse is deficient in adaptive T and B cells.
In normal NOD mice, there is a progressive increase in CD103+ DC in islets at the same time that CD4+ T cells also appear (3) (Fig 4). At 12 weeks of age, there are ∼20% CD103+ DCs within the CD11c+ MHCII+ gate. We used this time point as a reference to examine the presence of CD4+ T cells and of CD103+ DCs in the islets of NOD congenic strains. We found a basal level of about 5% CD103+ DC in the NOD.Rag1-/- mice. This same percentage was also found in wild-type NOD mice at 4-6 weeks of age. Hence T cells are dispensable for the CD103+ DC to seed the islets. However, T cells are essential for the increase during diabetogenesis. In the NOD.H4 mouse there are no diabetogenic T cells, but the rest of the genetics are NOD, so we used this mouse to verify that T cells are required for CD103+ DC entry. We found the same level of CD103+ DCs in islets of NOD.H4 as in NOD.Rag1-/- mice, strongly suggesting that diabetogenic antigen specific T cells are required for expansion of the islet CD103+ DC compartment. Interestingly, the examination of islets of the NOD.B16A mice revealed that at 12 weeks of age, the percentage of T cells was ∼3-4% of the CD45+ cells and the percentage of CD103+ DC was 5% of the APCs.
Therefore, these experiments indicate three clear requirements for initiation of the autoimmune diabetic process: CD103+ DCs, insulin reactive T cells, and the expression of the I-Ag7 molecule. The congenic mouse studies, especially the NOD.Rag1-/-, showed that T cells are unnecessary for the initial seeding of the islets by the Batf3-dependent DCs, but the increase of these DCs requires an autoreactive T cell population and, specifically, an insulin autoreactive T cell population.
In sum, in studies that are still in progress, we have identified three changes in the NOD.Batf3-/- mice. First, the absence of the CD103 resulted in poor presentation of beta cell epitopes in the pancreatic lymph node. Presentation was tested by the assay in which CFSE-labeled standard T cells are injected and their degree of proliferation is assayed in the pLN a few days later. Presentation of the islet specific glucose-6-phosphatase related protein (IGRP) MHC class I epitope was undetectable while presentation of the BDC2.5 MHC-II epitope was reduced by about half. Thus, the sensitization of the draining pLN, required for the progression of diabetes, is impaired. It is likely that the APCs from the islets serve to transport beta cell antigens to the pLN as we discussed above. A second defect was reduced presentation from islet APC. These presentation assays need to be expanded, but we are currently limited, as very few CD103+ DC can be isolated from the islets of young NOD mice.
A third important defect in the NOD.Batf3-/- mice is the poor localization of the early CD4+ T cells into their islets. We stated before that the islets of the Batf3-/- lacked CD4+ T cells found in the regular NOD. Only a few percent were identified, a number similar to that found in non-diabetic strains and regarded as “passenger”. Confirming this finding are the results of other experiments in which insulin reactive T cells were transferred and their localization in islets was examined days later (3). Localization was absent in the Batf3-/- islets. The findings suggest that the CD103+ DC somehow conditions the islet to be receptive to the early influx of the insulin reactive T cells. How the CD103+ DC controls the entry as well as the priming of T cells is of seminal importance to determine.
Localization of diabetogenic CD4+ T cells into islets
Experiments transferring CD4+ T cells reactive to known antigens have given us important information on the mechanisms of entry that we now summarize, then discuss, the interpretation of the role of the CD103+ DCs. The experiments used two different models. In both cases, Calderon, with one of us (JAC), transferred activated CD4+ T cells, an important point to note (5, 93). One model was the transfer of BDC2.5 CD4+ T cells into NOD.SCID or NOD.Rag1-/- mice. The other was the transfer of a CD4+ TCR transgenic to reactive to HEL (3A9) into mice that expressed HEL in islets as a transgenic under the control of the insulin promoter, in B10.BR mice (H2k). The experiments consisted of activating the T cells ex vivo with the antigenic peptides, then injecting them and killing the mice a few hours to days later, isolating the islets and examining them. The results with both were identical. T cells localized specifically to islets, best shown in the HEL model where localization took place only in islets expressing HEL but not in those lacking it. Non-specifically activated T cells (such as with concanavalin A) did not enter islets. The entry of the specific activated T cells was not inhibited by pertussis toxin, suggesting that it did not involve chemokines. It was partially inhibited by monoclonal antibodies to MHC-II and to ICAM. ICAM is normally expressed in endothelial cells in islets. T cells did not localize in the pancreatic stroma. Most of the T cells in islets were in tight contact with the APC. The time of contact in live isolated islets was similar to that found in the T cells-APC interactions studied in lymph nodes.
Of particular interest is the profound series of changes in islets as soon as activated T cells enter. (All of the changes described below happened in islets during the normal diabetogenic process in the NOD mouse, albeit a slower pace than when induced with transfer of activated CD4+ T cells). Concurrent to T cell entry, there was the upregulation of IFN-signature genes described above. Almost simultaneously, there was increased class II expression in the APCs, and increased expression of the adhesion molecules ICAM-1 and VCAM-1. ICAM-1 was highly expressed throughout the vascular endothelium and, notably, also by beta cells. One can speculate that the elevated expression of ICAM-1 fosters the beta cell's interactions with T cells. VCAM expression is contained to the vascular endothelium. Experimentally, blocking ICAM-1 or VCAM-1 using monoclonal antibodies reduced the entry of the diabetogenic T cell clone BDC2.5 (5, 91). Similarly, blockade of ICAM-1 and LFA-1 with antibodies reduced diabetes incidence in the NOD mouse (94). Combination therapy with both anti-VCAM1 and anti-LFA-4 also prevented diabetes in NOD mice (95). We also observed the progressive upregulation of three other adhesion molecules: Glycam1, Amica1, and L1cam (4). However, there has been no assessment of the function of these molecules for diabetogenic T cell entry.
In addition to adhesion molecules, we detected the expression of T cell attractant chemokines as early as 4 weeks of age (4). The two strongest upregulated chemokines were Cxcl9 and Cxcl10, which are also among the most strongly upregulated genes in islets throughout all of diabetogenesis. In addition, we detected upregulation of Ccl19, albeit to a far lower level. It is still not clear which of these, if any, chemokines is responsible for the earliest T cell entry. Taken together, these observations show that very early in diabetogenesis the inflammatory cytokine milieu leads to upregulation of ICAM-1 and VCAM-1, which are major players in the amplification of T cell numbers or progression of diabetes in the NOD mouse.
An important finding is that nonspecifically activated CD4+ T cells entered the islets following the entrance of the diabetogenic T cells (93). This entrance, in contrast to that of the diabetogenic T cells, was inhibited by pertussis toxin and also inhibited by antibodies to VCAM. The non-specific CD4+ T cells did not contact the APCs, or if they did it was a transitory interaction. This last finding indicates that as soon as there is a specific entrance of diabetogenic T cells, the islet now becomes receptive to entrance of broader repertoire of T cells.
The interactions and passage of beta cells vesicles to APC
We believe that an important component driving the autoimmune process is the transfer of beta cell granules to the islet APC. This process appears to be constitutive, but it has important implications. It is the conduit of immunological information from beta cell to APC, the process that likely brings the CD4+ T cells into operation. We interpret these findings as follows: once these early, islet-intrinsic interactions take place, the pancreatic node enters into action resulting in a rapid amplification of the process that brings in regulatory cells as well as the CD8+ T cell component. Once the CD8+ T cells are primed, any MHC-I bearing beta cell will be eliminated. This will release more autoantigens that creates a feed-forward inflammatory loop that cannot be stopped. Studying the events before CD8+ T cells become primed has the most promise for both understanding human diabetes as well as developing interventions that may prevent disease. After CD8+ T cells become activated, we believe the process is “locked-in” and therapy will be essentially impossible. Therefore, the only way to prevent diabetes is to diagnose it well before hyperglycemia is detected.
How are the beta cells donating the diabetogenic antigens, i.e., insulin in our studies, to the resident APCs? Through various approaches, we can conclude that beta cells normally are in close contact with the resident APCs. In this close contact interaction, the secretory granules, as well as the peptide-bearing granules, are transferred to the macrophage and to the CD103+ DC. Indeed, we have firm evidence that macrophages as well as DC capture secretory granules. However, we need to evaluate which of the two APCs are more effective and whether there are interactions between the two, issues that are now under consideration.
We studied the passage of secretory granules from beta cells to APC in an assay in which primary beta cells are cultured with APC (either DC or macrophages), after which we probed the presentation of the insulin pMHC-II complex with either of the two T cells directed two epitopes of insulin (6) (Fig.5). Both T cells reacted, indicating that the APC was presenting both of the insulin peptide registers. The transfer required a close interaction between beta cells and APC, separating them by a cell impermeable membrane blocked presentation. The transfer also required viable beta cells. Killing beta cells with streptozotocin stopped the transfer completely. Beta cells from NOD.Rag1-/- mice, the non-diabetic C57BL/6 mouse or human all transferred the granules to APCs. In the case of the latter two, the APCs were from NOD. Extrapolating these results to the autoimmune situation suggests that active inflammation or cell death in islets is not a requirement for the initial presentation to take place.
Figure 5.
Beta cells transfer immunogenic insulin to phagocytes. Left Panel: Graph shows the characterization of the two CD4+ T cells to insulin (1). The FLT-3L DCs were incubated with insulin or with the B:9–23 peptide, each at 10 μM. 8F10 reacts with peptides B:9–23 or B:12–20 (sequence shown below the graph) but not with insulin or B:13–21. IIT-3 reacts with insulin and peptides B:9–23 and B:13–21. Right Panel: A representative assay (of n > 25). Indicated are the cells used in the assay. Beta cells were from 6-wk-old NOD.Rag1−/− mice; the APCs were DCs obtained from the spleen of mice previously injected with FLT-3L. Background response of the T cells never exceeded 150 cpm. Adapted from reference 6.
The process whereby the secretory granules are passed to beta cells is modulated by glucose concentration (6). Increasing glucose levels from the basal level of 2.5/5 mM, to 25 mM, resulted three times higher insulin presentation. We also found a requirement for intracellular calcium in the process. How the close cell interactions are taking place needs to be examined at depth, but imaging analysis as well as electron microscope studies of islets are confirming much of the results of the assay just described.
Electron microscopy of islets of non-diabetic mice and from NOD mice at the ∼6 week stage of diabetes revealed the presence of typical macrophages containing beta cell granules (Fig 6). Some of the vesicles were the typical dense core granules surrounded by only one membrane, an indication that the whole granule was internalized. However, a second type of granule within a double membrane vesicle containing a proteinaceous-like material was also seen. We need to distinguish the macrophages from the DC and substantiate whether the latter also captures granules in vivo.
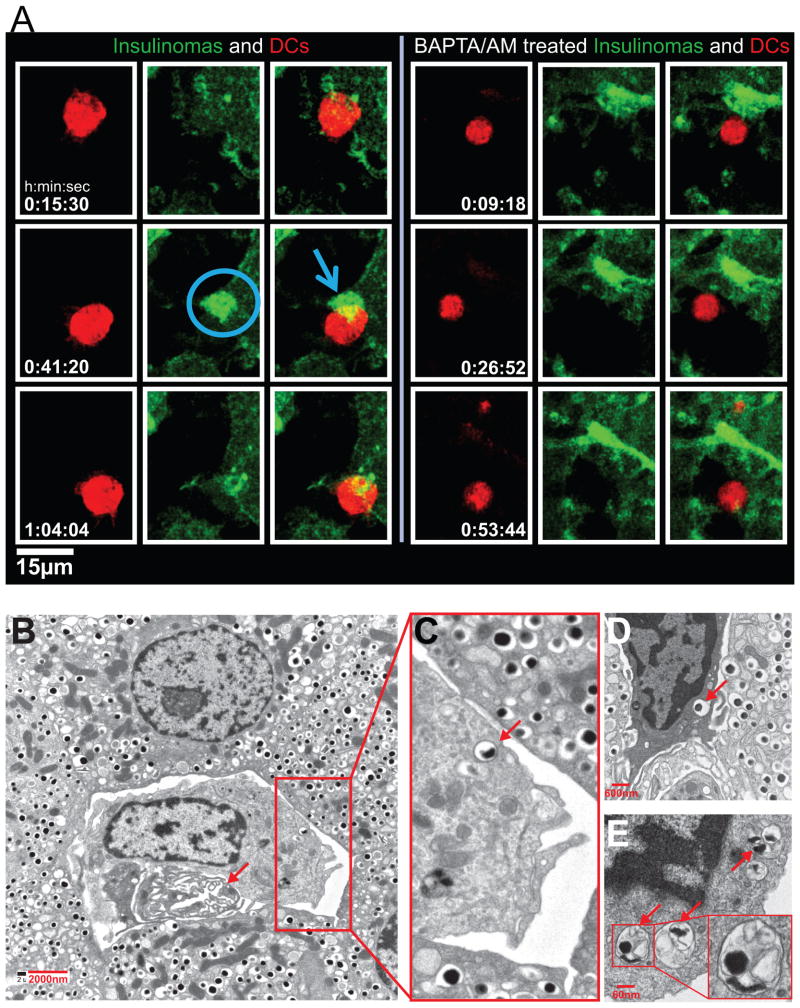
Figure 6.
Imaging and electron microscopy of beta cell granule transfer to APC. (A) DCs labeled with CellTrace Violet (red) were added to NIT GFP-ZnT8-Insulinomas. Left panel shows the contact area of NIT insulinomas in green with the DCs. Note the accumulation of GFP+ granules at the contact area. No movement of granules was observed in BAPTA/AM-treated NIT cells (Right panel). (B–E) Electron micrographs. B represents an islet from an 8-wk-old female NOD; C–E are islets taken from NOD.Rag1−/− mice at 14 wk of age. The arrow in B indicates a vessel. In the enlarged area, one of the dense-core granules is indicated by an arrow. In panel D a phagocyte is shown in between beta cells, and an arrow points to a dense-core granule. Panel E shows a portion of a phagocyte with endocytosed material in the form of vesicles containing an electron-dense core, with others containing amorphous content. Graph published in the Proc. Nat. Acad. Sci. (6).
In a project led by Bernd Zinselmeyer, we have live images of APC interacting with beta cells and capturing the entire granules (Fig 6). We made use of an insulinoma cell line bearing granules expressing the ZnT8 granule proteins fused with GFP. This allowed us to track the movement of the secretory granule in real-time. Soon after putting the ZnT8+ insulinoma with DC, we saw a polarization of the granules to the site of contact. Then, one can see the movement of the intact GFP+ material from the insulinoma to the DC (6).
We hypothesize that the transfer of the intact granules to the APC has several important features to the diabetogenic process. First, the granules contain potential modulatory substrates, such as ATP, which enhance the activation of pattern recognition receptors on the APC. This activation may be physiologically important to prevent infection of beta cells by circulating pathogens, but may have the unintended consequence of creating a “poised” APC that is ready to prime diabetogenic T cell responses. Second, the granules are a depot of very concentrated insulin protein or peptides, as is the case of the crinophagic vesicles. This material may be the substrate that may be presented to T cells by the APC leading to the initiation of autoimmune diabetes (fig 7).
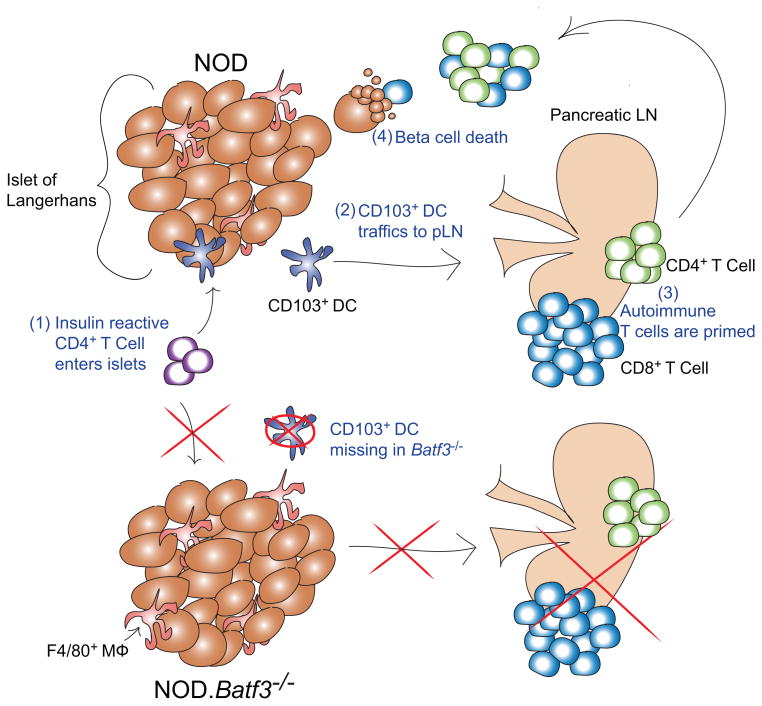
Figure 7.
Graph shows our current model of diabetes progression.
- Insulin autoreactive CD4+ T cells primarily directed to register 1, B:12-20 enter the islets. The entry of autoreactive CD4+ T cells requires the Batf3-dependent dendritic cells and, in their absence, the diabetogenic process is never established. Hence, the prisitine uninfiltrated islets evidenced in the NOD.Batf3-/- mouse.
- Either the macrophage or the CD103+ DC leads to the CD4+ T cells getting activated. Then, the trafficking DC, presumably the CD103+ DC, exits the islets.
- The draining pancreatic lymph node, upon receiving an activating and inflammatory signal from the trafficking DC, switches from a regulatory depot, to a priming and amplifying source for islet autoreactive T cells.
- Primed autoreactive T cells enter islets and destroy beta cells. (Taken from reference 3 with permission.)
Summary
In summary, a number of critical events transpire very rapidly in the NOD islets and pLN that set up the autoimmune process many weeks before overt diabetes. This is one of the great advantages of studying NOD in that the first stage of the autoimmune can be identified, a stage that sets this process forward. One can definitely state that in the islet there are important interactions between beta cells and the resident macrophages independent of the autoimmune program. These translate in healthy beta cells (note the islets in the op/op mouse) and in macrophages that have features of activation. It is likely that the beta cell:macrophage talk sets the scenario for the next two cellular protagonists to come into action, the CD103DC and the initial CD4+ T cell; the NOD is a strain that contains all the favorable genetic elements that allows the autoimmune process to develop.
A series of questions come immediately to mind and that need to be resolved first:
Are the initial CD4+ T cells to insulin peptides the only or the major initiating cells, and what is the explanation for their recognition of insulin catabolites by the I-Ag7 MHC-II molecules? Although our data is pointing to a unique presentation of the insulin catabolites, the range of insulin-specific cells, the presentation by mTEC and/or by thymic APCs, the interactions leading to anti-insulin antibodies and the role of the antibodies are part of the many issues that need immediate answers.
What is the nature of the interactions between beta cells and the two main APCs? It was surprising to note the close interactions between beta cells and APC pointing to a beta cell-APC synapse that is critically involved. Dissecting its different molecular elements is a priority as well as the mechanism of granule transfer that appears to be so distinct from the classical process of degranulation following glucose challenge.
Why is CD103+ DC such an important cell? The CD103DC is definitely having a role in presentation of MHC-I epitopes to the pLN leading to the subsequent stage that amplifies the process. How the transport process from islets to pLN takes place is likely via the pancreatic lymphatics. Based on our findings, the CD103Dc is also involved in MHC-II presentation, an issue that is ignored because of the exaggerated role given to its “cross presentation” function. For us, the important issue is to determine how the CD103+ DC is brought into islets, how it gets hold of insulin epitopes and whether there is cooperativity with the resident macrophage. This includes the presentation of both MHC-I and II epitopes. Unquestionably the major cell in islets containing insulin epitopes is the macrophage. It may not surprise us if the macrophage and DC are interacting and exchanging material, rather than being separate independent entities. Selective depletions of one or the other may give us an indication of their function.
As expected, there are many other issues to consider and follow that are as important as these. Among them are the programs of activation of the CD4+ T cells, the involvement of the regulatory pathways, and the biology of the CD8+ T cells. Other groups are prioritizing these questions. Hopefully all the pieces of the diabetes autoimmune puzzle may all fit together into a whole, but we should be prepared that some of the pieces of the puzzle may fall or not fit at all.
Acknowledgments
We thank all the members of our laboratory who participated in many of the studies discussed in this paper. In particular, we want to cite Jim Mohan, Boris Calderon and Tony Vomund who led the projects cited here on insulin reactive T cells, islet macrophages, and granule transfer, respectively. Research reported in this publication was supported by the Juvenile Diabetes Research Foundation 2-SRA-2014-293-Q-R and 17-2013-512, and the National Institutes of Health awards R01DK058177 and AI114551. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Juvenile Diabetes Research Foundation.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
Contributor Information
Emil R. Unanue, Email: unanue@pathology.wustl.edu.
Stephen T. Ferris, Email: ferrisstephen@wustl.edu.
Javier A. Carrero, Email: jcarrero@pathology.wustl.edu.
References
- 1.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an autoimmune insulin peptide-MHC II complex allows for the escape of diabetogenic T cells from negative selection. J Exp Med. 2011;208:2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41:657–669. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER. Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One. 2013;8:e59701. doi: 10.1371/journal.pone.0059701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci USA. 2011;108:1561–1566. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vomund AN, et al. Beta cell transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc Natl Acad Sci USA. 2015;112:5496–5502. doi: 10.1073/pnas.1515954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 8.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori M, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–735. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- 10.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci USA. 1987;84:2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund T, et al. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990;345:727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- 12.Todd JA, Bell JI, McDevitt HO. HLA-DQ β gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 13.Corper AL, et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 14.Latek RR, et al. Structural basis of peptide binding and presentation by the type 1 diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12:699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee KH, Wucherpfenning KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 16.Suri A, Walters JJ, Gross M, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-Ag7 molecules show common sequence specificity. J Clin Invest. 2005;115:2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang KY, Unanue ER. Prediction of HLA-DQ8β cell peptidome using a computational program and its relationship to autoreactive T cells. Int Immunol. 2009;21:705–713. doi: 10.1093/intimm/dxp039. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godkin A, et al. Use of eluted peptide sequence data to identify the binding characteristics of peptides to the insulin-dependent diabetes susceptibility allele HLA-DQ8 (DQ 3.2) Int Immunol. 1997;9:905–911. doi: 10.1093/intimm/9.6.905. 1997. [DOI] [PubMed] [Google Scholar]
- 19.Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER. In APC, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J Immunol. 2002;168:1235–1243. doi: 10.4049/jimmunol.168.3.1235. [DOI] [PubMed] [Google Scholar]
- 20.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 21.Wegmann DR, Eisenbarth GS. It's insulin. J Autoimmun. 2000;15:286–291. doi: 10.1006/jaut.2000.0444. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brezar V, Carel JC, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 2011;32:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- 24.Verge CF, et al. Prediction of type I diabetes mellitus in first degree relatives using a combination of insulin, glutamic acid decarboxylase and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 25.Pietropaolo M, Towns R, Eisenbarth GS. Humoral autoimmunity in type 1 diabetes: Prediction, significance, and detection of distinct disease subtypes. Cold Spring Harb Perspect Med. 2012;2:1–18. doi: 10.1101/cshperspect.a012831. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegler AG, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.French MB, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46:34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 28.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5:1028–1035. doi: 10.1038/ni1120. 2004. [DOI] [PubMed] [Google Scholar]
- 29.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nature Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 30.Thebault-Baumont K, et al. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111:851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriyama H, et al. Evidence for a primary islet autoantigen (preproinsulin1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci USA. 2003;100:10376–10381. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–183. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- 33.Lucassen AM, et al. Susceptibility to insulin dependent diabetes mellitus maps to a 4.1 kb segment of DNA spanning the insulin gene and associated VNTR. Nat Genet. 1993;4:305–310. doi: 10.1038/ng0793-305. [DOI] [PubMed] [Google Scholar]
- 34.Pugliese A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 35.Husebye ES, Anderson MS. Autoimmune polyendocrine syndromes: clues to type 1 diabetes pathogenesis. Immunity. 2010;32:479–487. doi: 10.1016/j.immuni.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 37.Wegmann DR, Gill RG, Norbury-Glaser M, Schloot N, Daniel D. Analysis of the spontaneous T cell response to insulin in NOD mice. J Autoimmun. 1994;7:833–843. doi: 10.1006/jaut.1994.1066. [DOI] [PubMed] [Google Scholar]
- 38.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 39.Heath VL, Hutchings P, Fowell DJ, Cooke A, Mason DW. Peptides derived from murine insulin are diabetogenic in both rats and mice, but the disease-inducting epitopes are different: evidence against a common environmental cross-reactivity in the pathogenicity of type 1 diabetes. Diabetes. 1999;48:2157–2165. doi: 10.2337/diabetes.48.11.2157. [DOI] [PubMed] [Google Scholar]
- 40.Halbout P, Briand JP, Becourt C, Muller S, Boitard C. T cell response to preproinsulin I and II in the nonobese diabetic mouse. J Immunol. 2002;169:2436–2443. doi: 10.4049/jimmunol.169.5.2436. [DOI] [PubMed] [Google Scholar]
- 41.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 43.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci USA. 1989;86:8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohan JF, Unanue ER. Unconventional recognition of peptides by T cells and the implications for autoimmunity. Nat Rev Immunol. 2012;12:721–728. doi: 10.1038/nri3294. [DOI] [PubMed] [Google Scholar]
- 45.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of NOD mice recognize a weak MHC-binding segment in more than one register. J Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 46.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4+ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med. 2013;210:2403–2414. doi: 10.1084/jem.20130582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida K, Corper AL, Herro R, Jabri B, Wilson IA, Teyton L. The diabetogenic mouse MHC class II moleculeI-Ag7 is endowed with a switch that modulates TCR affinity. J Clin Invest. 2010;120:1578–1590. doi: 10.1172/JCI41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unanue ER, Turk V, Neefjes J. Variations in MHC class II antigen processing and presentation in health and disease. Ann Rev Immunol. 2016 doi: 10.1146/annurev-immunol-041015-055420. in press. [DOI] [PubMed] [Google Scholar]
- 49.Pu Z, Carrero JA, Unanue ER. Distinct recognition by two subsets of T cells of an MHC class II--peptide complex. PNAS. 2002;99:8844–8849. doi: 10.1073/pnas.092260499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity. 2004;20:467–476. doi: 10.1016/s1074-7613(04)00073-1. [DOI] [PubMed] [Google Scholar]
- 51.Viner NJ, Nelson CA, Deck B, Unanue ER. Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J Immunol. 1996;156:2365–2368. [PubMed] [Google Scholar]
- 52.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 53.Landsteiner K. The specificity of serological reactions. New York: Dover Publications; 1962. [Google Scholar]
- 54.Weigle WO. The induction of autoimmunity in rabbits following injection of heterologous or altered homologous thyroglobulin. J Exp Med. 1965;121:289–308. doi: 10.1084/jem.121.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCluskey RT, Miller F, Benacerraf B. Sensitization to denatured autologous globulin. J Exp Med. 1962;115:253–273. doi: 10.1084/jem.115.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenthal AS, Barcinski MA, Blake JT. Determinant selection is a macrophage dependent immune response gene function. Nature. 1977;267:156–158. doi: 10.1038/267156a0. [DOI] [PubMed] [Google Scholar]
- 57.Thomas JW, George-Gattner H, Danho W. T cells recognize both conformational and cryptic determinants on the insulin molecule. Eur J Immunol. 1989;19:557–558. doi: 10.1002/eji.1830190323. [DOI] [PubMed] [Google Scholar]
- 58.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakayama M, Babaya N, Miao D, Sikora K, Elliott JF, Eisenbarth GS. Thymic expression of mutated B16: A preproinsulin messenger RNA does not reverse acceleration of NOD diabetes associated with insulin 2 (thymic expression insulin) knockout. J Autoimmun. 2005;25:193–198. doi: 10.1016/j.jaut.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Nakayama M, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of β cell reactive T cells in NOD mice. J Exp Med. 2001;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levisetti MG, Suri A, Frederick K, Unanue ER. Absence of lymph nodes in NOD mice treated with lymphotoxin-β receptor immunoglobulin protects from diabetes. Diabetes. 2004;53:3115–3119. doi: 10.2337/diabetes.53.12.3115. [DOI] [PubMed] [Google Scholar]
- 63.Halban PA, Wollheim CB. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980;255:6003–6006. [PubMed] [Google Scholar]
- 64.Orci L, et al. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol. 1984;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arvan P, Halban PA. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 66.Unanue ER, Askonas BA. Persistence of immunogenicity of antigen after uptake by macrophages. J Exp Med. 1968;127:915–926. doi: 10.1084/jem.127.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Unanue ER, Askonas BA. The immune response of mice to antigen in macrophages. Immunol. 1968;15:287–296. [PMC free article] [PubMed] [Google Scholar]
- 68.Unanue ER, Cerottini JC, Bedford M. Persistence of antigen on the surface of macrophages. Nature (London) 1969;222:1193–1195. doi: 10.1038/2221193a0. [DOI] [PubMed] [Google Scholar]
- 69.Ziegler K, Unanue ER. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to lymphocytes. J Immunol. 1981;127:1869–1875. [PubMed] [Google Scholar]
- 70.Ziegler K, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;78:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenthal AS, Shevach EM. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973;138:1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shevach EM, Rosenthal AS. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973;138:1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hume DA. Macrophages as APC and the dendritic cell myth. J immunol. 181:5829–5353. doi: 10.4049/jimmunol.181.9.5829. http://www.ncbi.nlm.nih.gov/pubmed/189411702008. [DOI] [PubMed] [Google Scholar]
- 74.Calderon B, et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med. 2015;212:1497–1512. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asano K, et al. D169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 76.Backer R, et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc Natl Acad Sci USA. 2010;107:216–221. doi: 10.1073/pnas.0909541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nikbakht N, Shen S, Manser T. Macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J Immunol. 2013;190:4923–4927. doi: 10.4049/jimmunol.1300350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calderon B, Suri A, Miller M, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci USA. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calderon B, Unanue ER. Antigen presentation events in autoimmune diabetes. Curr Opin Immunol. 2012;24:119–128. doi: 10.1016/j.coi.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol. 2014;26C:32–40. doi: 10.1016/j.coi.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melli K, et al. Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol. 2009;182:2590–2600. doi: 10.4049/jimmunol.0803543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banaei-Bouchareb L, et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukocyte Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 86.Li Q, Xu B, Michie SA, Rubins KH, Schreiber RD, McDevitt HO. Interfon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quah HS, et al. Deficiency in type I interferon signaling prevents the early interferon-induced gene signature in pancreatic islets but not type 1 diabetes in NOD mice. Diabetes. 2014;63:1032–40. doi: 10.2337/db13-1210. [DOI] [PubMed] [Google Scholar]
- 88.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes. 2000;49:2007–2011. doi: 10.2337/diabetes.49.12.2007. [DOI] [PubMed] [Google Scholar]
- 89.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J Immunol. 2000;164:a3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 90.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grajales-Reyes GE, et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α(+) conventional DC clonogenic progenitor. Nat Immunol. 2015;16:708–717. doi: 10.1038/ni.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calderon B, Carrero JA, Miller MJ, Unanue ER. Entry of diabetogenic T cells into islets induces changes that lead to amplification of the cellular response. Proc Natl Acad Sci USA. 2011;108:567–572. doi: 10.1073/pnas.1018975108. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hasegawa Y, et al. Prevention of autoimmune insulin-dependent diabetes in non-obese diabetic mice by anti-LFA-1 and anti-ICAM-1 mAb. Int Immunol. 1994;6:831–838. doi: 10.1093/intimm/6.6.831. [DOI] [PubMed] [Google Scholar]
- 95.Tsukamoto K, et al. Administration of monoclonal antibodies against vascular cell adhesion molecule-1/very late antigen-4 abrogates predisposing autoimmune diabetes in NOD mice. Cell Immunol. 1995;165:193–201. doi: 10.1006/cimm.1995.1205. [DOI] [PubMed] [Google Scholar]