Abstract
Ageing-associated changes that affect articular tissues promote the development of osteoarthritis (OA). Although ageing and OA are closely linked, they are independent processes. Several potential mechanisms by which ageing contributes to OA have been elucidated. This Review focuses on the contributions of the following factors: age-related inflammation (also referred to as ‘inflammaging’); cellular senescence (including the senescence-associated secretory phenotype (SASP)); mitochondrial dysfunction and oxidative stress; dysfunction in energy metabolism due to reduced activity of 5′-AMP-activated protein kinase (AMPK), which is associated with reduced autophagy; and alterations in cell signalling due to age-related changes in the extracellular matrix. These various processes contribute to the development of OA by promoting a proinflammatory, catabolic state accompanied by increased susceptibility to cell death that together lead to increased joint tissue destruction and defective repair of damaged matrix. The majority of studies to date have focused on articular cartilage, and it will be important to determine whether similar mechanisms occur in other joint tissues. Improved understanding of ageing-related mechanisms that promote OA could lead to the discovery of new targets for therapies that aim to slow or stop the progression of this chronic and disabling condition.
Table of Contents
Although changes associated with ageing promote the development of osteoarthritis (OA), ageing and OA are independent processes. In this Review, the authors discuss the mechanisms by which age-related factors contribute to OA through effects on articular cartilage and propose that future improvements in our understanding of these mechanisms will inform new therapies to slow or stop the progression of OA.
A number of risk factors for the development of osteoarthritis (OA) exist, including prior joint injury, obesity, genetics, sex, and anatomical factors related to joint shape and alignment; however, the most prominent risk factor is increasing age1. A Spanish study published in 2014, which included more than 3 million individuals, examined the incidence of clinically diagnosed OA and reported that incident hand OA in women peaked between the ages of 60 and 64 years, whereas that of the hip and knee continued to increase with increasing age2. A US study using data from the National Health Interview Survey reported that incident symptomatic knee OA peaked between the ages of 55 and 64, whereas prevalent disease increased with age, such that at age 85 years and older, prevalence ranged from ~13% in nonobese men to 32% in obese women3.
Musculoskeletal conditions, including OA, are a major cause of disability worldwide4 and have a substantial contribution to health-care costs, accounting for an estimated 1.0–2.5% of the gross domestic product in the USA, Canada, the UK, France and Australia5. Advanced OA often requires joint replacement to reduce pain and disability, and the number of knee replacement surgeries has substantially increased over the past 20 years6. The ageing of our population will compound the number of older adults disabled by OA and in need of joint replacement. Improved understanding of how ageing contributes to the development of OA could lead to new therapies that slow or stop the progression of the disease, which would have a major impact on public health.
OA that occurs in young adults is most often caused by a prior joint injury, a process known as post-traumatic OA1, whereas in older adults a number of factors related to ageing can contribute to the development of OA (BOX 1). These ‘ageing factors’ probably work in concert with other OA risk factors. Important differences between joint ageing and OA demonstrate that they are distinct processes (BOX 2). Although OA is a condition that affects the entire joint7 and results in joint failure, the majority of research to date has focused on ageing-associated changes in the articular cartilage. However, studies on the meniscus8, anterior cruciate ligament9 and bone10 have shown age-related changes similar to those observed in articular cartilage — including loss of cellularity, and disruption and degeneration of the extracellular matrix — suggesting that common processes could be involved.
Box 1. Age-related factors that contribute to osteoarthritis development.
Reduced muscle mass and increased fat mass alter joint loading and are associated with an increase in adipokine and cytokine production, resulting in low-grade systemic inflammation109.
Changes in the extracellular matrix, including accumulation of advanced glycation end-products, reduced aggrecan size, reduced hydration, and increased collagen cleavage alter the mechanical properties of cartilage and make it more susceptible to degeneration31.
Extracellular matrix disruption and reduced cell density in the meniscus and ligaments promote degeneration and can potentially alter joint mechanics8,9.
Impairment in the function of subchondral bone due to reduced numbers of osteocytes and altered mineral composition10.
Mitochondrial dysfunction, oxidative stress and reduced autophagy in chondrocytes alters their function, promoting catabolic processes and cell death over anabolic processes31.
Box 2. Differences between normal joint ageing and osteoarthritis.
With normal joint ageing, articular cartilage remains intact but loses thickness and has a reduced glycosaminoglycan (GAG) content. With osteoarthritis (OA), fibrillation of the cartilage surface occurs in focal areas and can be associated with a complete loss of staining for GAGs31,110.
Non-enzymatic crosslinking of collagen by advanced glycation end-products (AGEs) increases in cartilage with age. A mouse model of injury-induced OA demonstrated that collagen crosslinking occurs through a distinct mechanism involving lysyl oxidase104.
The density of chondrocytes in cartilage decreases with age, but chondrocyte ‘clusters’ emerge during the development of OA near sites of tissue damage and may indicate attempted repair or altered cellular signals31,110.
Aged chondrocytes have reduced levels of extracellular matrix gene expression and synthesis, whereas during OA chondrocytes become highly active with increases in both anabolic processes (for example, matrix synthesis) and catabolic pathways (for example, those induced by inflammatory cytokines)31,110.
Synovial inflammation and hypertrophy occur in OA but have not been described in normal joint ageing7.
Bone mass and density decrease with ageing, whereas subchondral bone thickening is seen in patients with OA7.
In 2013, nine cellular and molecular hallmarks of ageing were proposed11 to highlight the underlying causes of age-related dysfunction and assist with research into potential therapeutic interventions. These hallmarks include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication. This Review covers selected aspects of ageing that are relevant to OA and, where possible, relates these studies to the hallmarks of ageing.
Ageing, inflammation and OA
Mounting evidence suggests that OA is associated with low-grade systemic and local inflammation12,13. Ageing has likewise been associated with chronic low-grade inflammation, sometimes referred to as ‘inflammaging’ (REF.14), which could promote OA, although studies to date have not identified the precise mechanisms. Several of the hallmarks of ageing could also have a role in OA, including epigenetic alterations, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence and altered intercellular communication. As discussed further below, increased production of proinflammatory mediators is a feature of the senescence-associated secretory phenotype (SASP) and could be an important mechanism in OA.
A key cytokine associated with ageing and age-related disease is IL-6. Levels of IL-6 in the systemic circulation increase with age15,16 and are strongly associated with the risk of OA progression17,18. IL-6 is important in the pathogenesis of rheumatoid arthritis, and IL-6 inhibition is an effective therapy approved for clinical use in this setting19. However, a causal role for IL-6 in age-related OA has not been established, as mice with deletion of the Il6 gene have more severe (rather than less severe) age-related OA20, suggesting that other mediators could be involved. In addition, the levels of multiple proinflammatory and anti-inflammatory mediators change with age16, and so it is unlikely that the association between age and OA is driven by a single factor.
Inflammaging is probably attributable, at least in part, to the age-related increase in visceral fat mass that is associated with a decline in muscle mass21. Obesity is a well-accepted risk factor for OA in all age-groups3. Reviews published elsewhere22,23 have detailed the potential mechanisms by which obesity leads to OA, and so here we will only summarize the key findings most relevant to age-related OA. The increase in fat mass with ageing is associated with increased numbers of adipocytes and proinflammatory macrophages in adipose tissue, and these cells produce a number of cytokines and adipokines that could contribute to OA24,25, although the importance of these mediators in OA remains unclear. Obesity and increased fat mass can also contribute to OA through metabolic alterations that are sometimes referred to as ‘meta-inflammation’ (REFS 22,23). In addition to increased levels of cytokines and adipokines, meta-inflammation is associated with increased levels of circulating free fatty acids, hyperglycaemia and oxidative stress, which can all negatively influence joint tissues to promote matrix destruction22,23. Furthermore, obesity associated with low muscle mass (that is, sarcopenic obesity) has been associated with knee OA26 and could contribute to joint instability and an increased risk of falls in older adults, particularly in women27.
In addition to an age-related increase in visceral fat, local fat depots such as the infrapatellar fat pad increase with age28. Given that this fat pad is proximal to the knee joint and produces the adipokines adiponectin and leptin, as well as basic fibroblast growth factor (bFGF; also known as FGF-2), vascular endothelial growth factor, TNF and IL-6 (REF. 29), it is plausible that an age-related increase in fat pad volume could contribute to OA. However, in vitro studies of conditioned media from infrapatellar fat pads removed from patients with end-stage knee OA found a protective rather than catabolic effect on bovine cartilage explants30.
Cellular senescence and the SASP
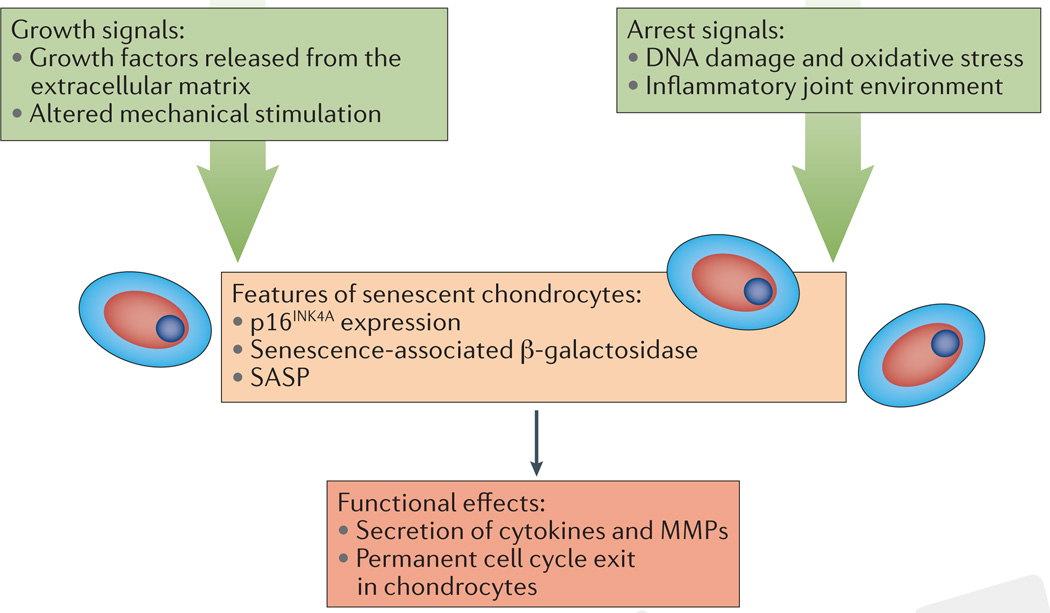
Cellular senescence is one of the hallmarks of ageing, and chondrocytes have many features that are characteristic of senescent cells during ageing and during OA31,32. The observation that OA catalyses the development of senescence indicates that cellular stresses probably have a key role in establishing this phenotypic state. A key challenge has been deciding how to best define chondrocyte senescence and determine the underlying mechanisms that might be targeted therapeutically (FIG. 1). Morphological changes and permanent replicative arrest after monolayer expansion have defined cellular senescence for in vitro settings, but defining cellular senescence in vivo has proved more difficult33.
Figure 1. Chondrocytes exhibit features of cellular senescence in the contexts of ageing and osteoarthritis.
Senescence is often caused by the combination of growth and arrest signals, conditions that can occur in cartilage during ageing. Features of senescence include expression of CDKN2A (encoding p16INK4A) and positive staining for senescence-associated β-galactosidase. The functional effects of chondrocyte senescence are challenging to measure because of the low proliferation rate of chondrocytes and the overlap between features of the senescence-associated secretory phenotype (SASP) and those associated with the development of osteoarthritis (OA). Understanding the mechanism of senescence could yield therapeutic interventions to prevent development of the SASP or specifically eliminate senescent cells from joint tissues. MMPs, matrix metalloproteinases.
Two distinguishing characteristics of senescent cells are stress-induced permanent proliferative arrest and resistance to both mitogenic and oncogenic stimuli33, both of which are challenging to assess in chondrocytes. Chondrocytes exhibit low proliferation rates, which hinders the measurement and interpretation of the functional impact of a further reduction in proliferation34. Lineage-tracing experiments in mice indicate that proliferation of particular chondrocyte subpopulations could help to maintain cartilage tissue35, suggesting, therefore, that the loss of proliferation in these cells due to senescence could contribute to OA progression. The other defining feature of senescence is a beneficial function of preventing the division of transformed cells36. Chondrosarcomas typically derive from the growth plate and perichondrium but not from articular cartilage37, suggesting that articular cartilage might be inherently unlikely to undergo uncontrolled growth. This characteristic presents a challenge for the identification of senescent chondrocytes on the basis of an increased resistance to oncogenic transformation.
Cellular senescence could have other functional roles aside from inhibition of proliferation, as the SASP is defined by the production of high levels of proinflammatory cytokines and matrix-degrading enzymes38. Indeed, the SASP seems to be regulated independently from cell cycle arrest39,40. Some of the most highly upregulated SASP-related factors, such as IL-1α, IL-6 and monocyte chemoattractant protein 1 (MCP-1, also known as CC chemokine ligand 2 (CCL2)) are found in OA cartilage41, hindering efforts to determine whether the level of a particular mediator is due to senescence or a result of OA. The cell type responsible for producing these cytokines is also difficult to determine. As these factors are also found in the synovial fluid, they could be produced by cells other than chondrocytes, such as cells in the meniscus, synovium or bone7. Future work is needed to determine whether any of these cell types display other features of senescence.
The induction of chondrocyte senescence
A detailed understanding of how chondrocytes enter the senescent state might enable the development of therapies designed to prevent such a phenotypic switch during OA. One model proposes that senescence occurs during conditions characterized by simultaneous signals for cell cycle arrest and cell growth7,42, and aged cartilage tissue has the potential to provide both these cues.
The cumulative DNA damage and oxidative stress that occurs with ageing and in the OA tissue microenvironment alters gene expression patterns in chondrocytes7,43 and could cause telomere attrition due to targeted DNA damage41,44. Notably, stem cells and postmitotic cells such as neurons and chondrocytes can be particularly susceptible to the accumulation of cellular damage during the long interval between replicative events45,46. One common consequence of accumulating DNA damage is the upregulation of cell cycle inhibitors, such as cyclin-dependent kinase inhibitor 2A (also known as p16INK4A) and cyclin-dependent kinase inhibitor 1 (also known as p21), which mediate the stable arrest that is associated with senescence33. Evidence correlating increasing age and OA with increased CDKN2A (p16INK4A) gene expression in chondrocytes has been complemented by mechanistic work showing that the suppression of p16INK4A expression by the microRNA miR-24 serves to prevent features of chondrocyte senescence47. These observations in chondrocytes seem to be aligned with a model explored in mouse muscle stem cells, in which increased p16INK4A expression with age made cells more likely to undergo senescence during subsequent proliferation after injury48.
The second signal required for establishing senescence, a strong growth signal, can be provided in the form of growth factors that are either directly released from damaged cartilage or synthesized at high levels by chondrocytes during OA. For example, bFGF is released from damaged cartilage tissue49, and can be detected in focal clusters of chondrocyte proliferation that develop near areas of cartilage damage50. As a downstream result of signalling by inflammatory cytokines associated with OA, expression of bone morphogenetic protein 2 (BMP-2) is increased,51 thereby stimulating enhanced turnover of extracellular matrix52. Although their competence for proliferation indicates that these chondrocytes are not senescent, cells that enter the cell cycle in the context of potentially damaging stimuli have an increased likelihood of entering senescence as opposed to returning to quiescence53,54. Observations that senescence occurs after monolayer expansion of chondrocytes (reviewed elsewhere55) provide further evidence that contexts of increased proliferation correlate with emergence of the senescent phenotype.
The role of circadian signals
Also of potential interest is the finding that chondrocytes have an intrinsic circadian clock which is disrupted in cartilage from aged mice56. A decline in expression of the circadian clock gene BMAL1 (also known as ARNTL) has been associated with stress-induced senescence in fibroblasts57 and has been noted to occur in human OA chondrocytes and in the cartilage of aged mice58. Mice with cartilage-specific deletion of Bmal1 developed premature knee cartilage lesions that appeared at 2 months of age and became progressively more severe in adult animals58. However, no other OA-associated changes in the bone, ligaments or synovium were evident in these mice, and the finding that the cartilage changes were detectable before the mice were skeletally mature suggests that the phenotype is the result of a developmental defect rather than OA. Further work is needed to determine the role of BMAL1 and the circadian clock in age-related human OA.
Therapeutic targeting of senescence
One emerging therapeutic strategy for age-related diseases is to specifically kill senescent cells in order to prevent detrimental secretion of SASP-related factors. Although this approach has not yet been explored in OA, the concept builds on studies that eliminated p16INK4A-positive cells in mouse models of premature59 and natural ageing60. Drugs that eliminate senescent cells in aged tissue would ideally mimic the efficient clearance of senescent cells that occurs after wound repair61. The ‘senolytic’ compounds dasatanib and quercetin target senescent cells by inhibiting the anti-apoptotic pathways that are upregulated in senescent cells62. One anti-ageing effect of this treatment was protection from proteoglycan loss in the intervertebral disc of Ercc1-mutant mice (a model of accelerated ageing based on compromised DNA damage repair). Although articular cartilage was not investigated in this study, other work has shown that ERCC1-mediated DNA repair helped to restrain senescence in human articular chondrocytes63. A separate study further supports the strategy of inhibiting anti-apoptotic pathways by identifying navitoclax as an inhibitor of B-cell lymphoma family proteins that specifically targets senescent human fibroblasts in vitro and mouse stem cells in vivo64.
Another therapeutic approach could be to interfere with inflammatory pathways active in senescent chondrocytes. Rapamycin inhibits the translation of SASP proteins in human fibroblasts through decreasing the rate of IL-1α translation, which is regulated by serine/threonine-protein kinase mTOR (also known as mammalian target of rapamycin)65. With increased progress in the understanding of how senescence is regulated in chondrocytes, particular cellular features could provide other novel drug targets for OA.
Oxidative stress and the mitochondria
Mitochondrial dysfunction is a hallmark of ageing that has attracted particular attention in the context of OA. The free radical theory proposes that cellular damage occurring as a result of excessive levels of reactive oxygen species (ROS) substantially contributes to the development of the ageing phenotype and to the progression of age-related diseases66. However, in addition to cellular damage, elevated levels of ROS produced as a result of age-associated oxidative stress also promote disease by disturbing homeostatic physiological cell signalling66–68.
Mitochondrial function has long been recognized to decline during ageing, and a causative link between mitochondrial dysfunction, oxidative stress and the ageing phenotype has been proposed66,69. Evidence from a 2015 study70 demonstrates that, compared with normal chondrocytes, chondrocytes from patients with OA have reduced mitochondrial mass and mitochondrial DNA content along with reduced levels of electron transport chain proteins and proteins involved in mitochondrial biogenesis. The researchers confirmed that some of these changes occurred during ageing and were independent of OA by analysing mouse cartilage tissue sections. Interestingly, protein levels of nuclear receptor erythroid 2-related factors 1 and 2 (also known as NFE2-related factor 1 and NFE2-related factor 2, respectively), which confer cellular protection through regulation of antioxidant gene expression, were also decreased in chondrocytes from patients with OA compared with those from healthy controls70.
As mitochondria are an important source of ROS, age-related mitochondrial dysfunction that leads to an imbalance between the production of ROS and the antioxidant capacity of the cell has been identified as a contributing factor in the development of OA68,71,72 (FIG. 2). Mice with post-traumatic OA showed elevated intracellular and mitochondrial superoxide generation, which was associated with downregulation of mitochondrial superoxide dismutase 2 (SOD2) expression73. Furthermore, in this same model, SOD2 loss resulted in greatly increased age-related cartilage degeneration73. In vitro studies using paraquat to induce mitochondrial superoxide generation in primary mouse articular chondrocytes led to substantial mitochondrial dysfunction, along with reduced expression of both antioxidant genes (including Sod2) and anabolic genes in cartilage, whereas catabolic gene expression was upregulated73. These data are in accordance with other studies demonstrating downregulation of SOD2 expression at the mRNA and protein levels in cartilage from both humans74–76 and animals76 with OA, and support the hypothesis that mitochondrial dysfunction and the associated redox imbalance is a key mechanism contributing to cartilage degeneration and the pathogenesis of OA.
Figure 2. Mitochondrial dysfunction, oxidative stress and changes in normal cell signalling in ageing and osteoarthritis.
Mitochondrial dysfunction in both ageing and osteoarthritis (OA) is characterized by reduced mitochondrial integrity (mass, number and DNA content) and impaired electron transport chain (ETC) function. These features contribute to increased production of reactive oxygen species (ROS). Concomitant reductions in mitochondrial antioxidant capacity — including reduced mitochondrial superoxide dismutase 2 (SOD2) levels and peroxiredoxin (PRX) hyperoxidation — lead to enhanced oxidative stress and ROS-mediated damage in the mitochondria (indicated by red lightning bolts). Extra-mitochondrial antioxidant systems (namely, SOD1, catalase and glutathione synthetase; not shown) are also considerably less active in ageing and OA, which exacerbates oxidative stress. ROS cause damage to proteins, lipids and DNA, increase chondrocyte catabolism and, importantly, lead to disturbances in normal cell signalling. These disturbances can include inhibition of pro-survival IGF-1 signalling and increased catabolic mitogen-activated protein kinase (MAPK) signalling. Increased levels of ROS can also result in increased protein thiol oxidation, resulting in S-sulfenylation or, when excessive, hyperoxidation of reactive protein cysteines, which might contribute to altered cell signalling. mtDNA; mitochondrial DNA; ERK, extracellular signal-regulated kinase; IGFR, insulin-like growth factor receptor; IRS-1, insulin receptor substrate 1.
Consistent with the concept that age-related oxidative stress alters cell signalling, studies have demonstrated an age-related disruption in human chondrocyte insulin-like growth factor 1 (IGF-1) signalling that results in reduced extracellular matrix gene expression and protein synthesis77,78. In human chondrocytes from older adults, this effect was associated with an increased sensitivity to oxidative stress, which resulted in inhibition of IGF-1-mediated activation of RACα serine/threonine-protein kinase (AKT) and increased activation of catabolic mitogen-activated protein kinase (MAPK) signalling pathways78.
A key mechanism by which ROS regulate cell signalling is through oxidative post-translational modifications of specific thiol groups in proteins that contain reactive cysteines79. Cysteine oxidation initially results in the formation of a cysteine sulfenic acid (Cys-SOH) in a process known as S-sulfenylation. In a 2016 study, chondrocytes from patients with OA had an increased basal level of S-sulfenylation compared with those from healthy controls80. ROS induced sulfenylation of multiple chondrocyte proteins including the tyrosine kinase SRC, the activity of which promoted an increase in the production of matrix metalloproteinase 13 (MMP-13)80. These data suggest that ROS-induced sulfenylation of chondrocyte proteins can alter signalling pathways that can promote cartilage degradation.
In the presence of excessive levels of ROS, cysteine oxidation can proceed from cysteine sulfenic acid to sulfinic (Cys-SO2H) or sulfonic (Cys-SO3H) acid; this so-called hyperoxidation can lead to inactivation of redox-sensitive proteins. Hyperoxidation of the peroxiredoxin family of antioxidant enzymes has been demonstrated in cartilage samples from older adult humans and patients with OA81. Conditions of oxidative stress (induced in vitro with the ROS generator menadione) led to an age-related increase in peroxiredoxin hyperoxidation, which was associated with both inhibition of pro-survival cell signalling and p38 MAPK-induced chondrocyte cell death81. Importantly, reduction of ROS levels by mitochondrion-specific overexpression of the antioxidant enzyme catalase prevented peroxiredoxin hyperoxidation in vitro, and reduced the severity of age-related OA in mice in vivo81. Collectively, these studies highlight ROS as crucial secondary signalling molecules in chondrocytes that warrant further study in the context of ageing and OA.
Dysfunctional energy metabolism
Another hallmark of ageing is dysregulated nutrient sensing. 5′-AMP activated protein kinase (AMPK) is a key regulator of cellular metabolism and energy balance that is activated by stressors that enhance the cellular AMP:ATP ratio82. The activity of AMPK and its regulatory upstream kinase, serine/threonine-protein kinase STK11 (also known as liver kinase B1 (LKB1)), is reduced in cartilage from aged mice and mice with OA, as well as in bovine chondrocytes after dynamic compression-induced biomechanical injury83. Similarly, OA-associated reductions in AMPK activity have been observed in human chondrocytes and cartilage84, and this reduced activity has been associated with substantially reduced mitochondrial biogenesis70.
Importantly, AMPK might also modulate key homeostatic signalling pathways through regulation of autophagy, a cellular process that removes damaged and dysfunctional organelles and proteins82,85. Autophagy is relevant to another hallmark of ageing: loss of proteostasis. Transgenic overexpression of AMPK delays the onset of the ageing phenotype through direct upregulation of autophagy in Drosophila melanogaster, which ultimately increases the lifespan of these organisms86. Consistent with this observation, the expression of key autophagy proteins was considerably reduced in cartilage from aged mice compared with that from young mice, and these changes were associated with increased levels of apoptosis and cartilage degeneration87. Similar results were also found in a mouse model of post-traumatic OA88, implicating dysfunctional autophagy as a key mechanism in both ageing and OA. Although AMPK levels were not measured in these post-traumatic OA studies, other work has demonstrated that AMPK signalling stimulates autophagy in chondrocytes89. As inhibition of mTOR signalling is a widely investigated strategy to prevent age-related disease90, the fact that cartilage-specific loss of mTOR increased AMPK levels and autophagy, and was sufficient to protect mice from OA following surgery, is of particular interest91. Taken together, a plausible interpretation of these findings is that age-related and OA-related reductions in AMPK signalling reduce autophagy and contribute to the cellular dysfunction observed in OA cartilage, a connection that is worthy of future investigation.
In addition to AMPK, many lines of evidence suggest that the evolutionarily conserved sirtuin (SIRT) family of NAD+-dependent deacetylase proteins also contributes to cellular homeostasis through regulation of energy balance. This regulation is achieved via direct nutrient sensing, which can contribute to increased lifespan in model organisms, such as worms and flies92. However, whether overexpression of sirtuins can have a direct longevity-promoting effect remains unclear93. SIRT6 has been identified as a crucial regulator of processes that are hypothesized to be directly involved in ageing, including metabolic homeostasis, genome stability and transcription94. Sirt6-knockout mice display reduced IGF-1 levels and accelerated ageing-like degenerative processes that ultimately lead to premature death, which suggests a role for SIRT6 in ageing95. Similarly, male transgenic mice overexpressing Sirt6 had an increased lifespan, in part due to regulation of IGF-1 signalling96, which suggests that age-related loss of SIRT6 activity could contribute to ageing by disturbing normal cell signalling pathways.
In cartilage, research has predominantly focused on the role of SIRT1 (reviewed elsewhere97). As such, there is a paucity of research on how other sirtuins regulate chondrocyte function. However, depletion of SIRT6 in human chondrocytes (achieved using RNA interference) resulted in substantially increased DNA damage and telomere dysfunction, which was associated with premature senescence98. SIRT6 depletion also increased expression of MMP1 and MMP13, which are implicated in the pathogenesis of OA. Compared with cells from normal human tissue, human OA chondrocytes show reduced expression of SIRT6 at both the mRNA and protein level, and lentivirus-induced overexpression of SIRT6 in the knee joint protects young mice from surgically induced OA99. These studies highlight a potential role for SIRT6 in cartilage homeostasis that warrants further investigation.
Changes in the extracellular matrix
Articular cartilage is a load-bearing tissue, and the ability of chondrocytes to sense and respond to mechanical signals is essential for maintaining joint homeostasis (reviewed elsewhere100). Accumulation of advanced glycation end-products (AGEs) with ageing causes non-enzymatic collagen crosslinking that directly alters the mechanical properties of the extracellular matrix101. However, a canine model using ribose and threose injections to increase AGE levels in young animals to match those of aged controls was insufficient to initiate OA102. Nevertheless, chondrocytes did synthesize fewer proteoglycans in the highly crosslinked tissue, which might indicate that compromised cellular function would prevent sufficient tissue maintenance in the context of ageing or injury. This possibility is supported by a study in rabbits, in which forced exercise in combination with ribose injections did induce OA, through AGE-mediated suppression of protective chondrocyte signalling103.
Whether limiting AGE accumulation would be sufficient to prevent age-related OA remains unclear, but genetic deletion of lysyl oxidase (an enzymatic collagen crosslinker that is upregulated by joint injury) protected mice from injury-induced OA104. The potential relevance of this finding to AGEs and thus ageing was suggested by the finding that collagen crosslinking induced by AGEs and by lysyl oxidase had similar effects on chondrocyte signalling in collagen gels and on the development of OA after injury.
Chondrocytes integrate signals from mechanotransduction pathways that sense changes in the extracellular matrix and those initiated by soluble factors, and both types of signals are affected by ageing. Mechanical compression of bovine cartilage stimulates increased expression of the gene encoding transforming growth factor-β (TGF-β) and the subsequent phosphorylation of SMAD2 and SMAD3 through TGFR-1 (TGF-β receptor type-1), which increases matrix synthesis and limits catabolic signalling105. However, ageing causes a significant loss in expression of TGFR-1, resulting in a shift that favours TGF-β signalling through serine/threonine-protein kinase receptor R3 (SKR3, also known as TGF-β superfamily receptor type I). SKR3-mediated signalling leads to phosphorylation of SMAD1, SMAD5 and SMAD8, which is associated with an increase in catabolic signalling and increased production of MMP-13 (REF. 106). In a 2016 study of bovine cartilage explants subjected to anabolic dynamic compression, tissues from aged animals had deficient phosphorylation of SMAD2 and SMAD3 in response to loading compared with explants from young animals105. The exact mechanism responsible for this effect is difficult to deduce because, compared with young explants subjected to the same load, aged tissue showed less deformation, exhibited less upregulation of growth factors and also had lower levels of TGFR-1 on the cell surface. However, previous work using a hydrogel system with varied stiffness revealed that mouse chondrocytes show a maximal response to exogenous TGF-β at a physiologically relevant stiffness of 500 kPa107, indicating that the age-related increase in matrix stiffness probably contributes to the reduced phosphorylation of SMAD2 and SMAD3 in aged tissue.
Conclusions
Researchers are becoming increasingly interested in elucidating the mechanisms by which ageing promotes the development and progression of OA. Studies in the early 2000s detailed age-related changes in the extracellular matrix, such as the accumulation of AGEs, which could promote OA by altering the mechanical properties of joint tissues. Subsequent research has focused on age-related cellular changes that include not only an accumulation of damaged proteins, lipids and DNA, but also alterations in mitochondrial function, levels of ROS and energy metabolism that disrupt signalling and associated cell functions. These changes promote catabolic activity over anabolic activity and eventually result in cell death. We can easily envision how these changes could result in joint tissue degeneration and matrix loss, but the precise mechanisms need to be clarified. The concept of hallmarks of ageing suggests that common mechanisms will drive dysfunction in various tissues and organ systems that are most affected by ageing. These hallmarks represent areas for further research into elucidating the connections between ageing and OA. Although the majority of research into the effects of ageing continues to focus on articular chondrocytes, further studies are needed to determine whether similar mechanisms are at play in cartilage as well as other joint tissues affected by OA, in order to find common targets for intervention.
A primary goal of ageing research is to better understand common mechanisms that could be targeted with the intent of delaying loss of function in more than a single system, which would result in improved ‘healthspan’ rather than simply extending lifespan. Heterochronic parabiosis experiments, in which the circulatory systems of young and old animals are joined, have revealed systemic factors that alter the ageing phenotype, including growth and differentiation factor 11 (GDF11), oxytocin and IL-15, which might be able to reduce ageing in multiple organ systems108. An important aim of future studies will be to determine whether these ageing-associated factors influence the development of OA.
Another emerging approach is the development of senolytic agents that would target and remove senescent cells62,64. These and other therapies that target ageing processes could be developed into novel treatments for OA (BOX 3). Given the prevalence of conditions such as OA in older adults and the importance of the musculoskeletal system in physical function, achieving improvements in healthspan will certainly require interventions that benefit the musculoskeletal system.
Box 3. Therapeutic targeting of ageing-related processes in osteoarthritis.
Interventions designed to extend ‘healthspan’ by targeting systemic ageing-related processes could potentially delay or prevent various chronic diseases, including osteoarthritis111,112.
Senolytics, such as dasatanib and quercetin, are being designed to specifically kill senescent cells; removal of these cells might alleviate their harmful effects on neighbouring cells, including the release of proinflammatory factors that characterize the senescence-associated secretory phenotype (SASP)62.
Agents that activate 5′-AMP-activated protein kinase (AMPK), such as metformin, could restore physiological levels of autophagy and promote proteostasis112.
Sirtuin activators could restore normal nutrient sensing and energy metabolism112.
Antioxidants targeted to the mitochondria or designed to restore homeostatic redox signalling could counteract mitochondrial dysfunction and altered intracellular signalling113.
Key points.
Ageing-associated changes promote the development of osteoarthritis (OA), but ageing and OA are independent processes
Several hallmarks of ageing could contribute to OA: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication
The increase in fat mass and related metabolic changes that occur with ageing can result in ageing-related inflammation (referred to as ‘inflammaging’), a chronic low-grade systemic proinflammatory state
Elevated levels of reactive oxygen species can contribute to OA by causing oxidative damage and disrupting normal cell signalling, leading to imbalanced anabolic and catabolic activity and ultimately cell death
Chondrocytes can undergo cellular senescence with age and OA in response to growth signals released as a result of underlying cellular damage
The non-enzymatic crosslinking of collagen that occurs with ageing alters the mechanical properties of cartilage, and the resulting changes to mechanotransduction pathways reduce extracellular matrix synthesis by chondrocytes
Acknowledgments
The authors’ research work is funded by grants from the National Institute on Aging (RO1 AG044034 and F32 AG050399).
Glossary
- Telomere attrition
The shortening and deterioration of the protective caps on the ends of chromosomes associated with ageing.
Biographies
Richard Loeser is the Herman and Lousie Smith Distiguished Professor in the Division of Rheumatology, Allergy and Immunology at the University of North Carolina School of Medicine and the Director of Basic and Translational Research in the Thurston Arthritis Research Center, Chapel Hill, North Carolina, USA. He trained in both rheumatology and geriatrics at the Wake Forest School of Medicine in Winston-Salem, North Carolina, and has spent more than 25 years carrying out research in the field of cartilage biology and osteoarthritis (OA) with a major interest in how ageing contributes to OA.
John Collins is a postdoctoral research fellow at the University of North Carolina Thurston Arthritis Research Center, Chapel Hill, North Carolina, USA. He earned his PhD in 2014 from the Institute of Ageing and Chronic Disease, University of Liverpool, UK, for his research in the field of musculoskeletal biology. Under the mentorship of Dr Richard Loeser, he is currently investigating the role of oxidative stress in ageing and osteoarthritic joint tissues, with a focus on how age-related oxidative stress disrupts cell signalling.
Brian Diekman is currently investigating ageing and cellular senescence as a postdoctoral research associate in the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, North Carolina, USA, under the direction of Dr Norman Sharpless. After earning a BSE in Biomedical Engineering from Duke University, Durham, North Carolina, in 2005, he received a Fulbright Student Grant to perform stem cell research at the Regenerative Medicine Institute in Galway, Ireland. Dr Diekman earned his PhD in Biomedical Engineering from Duke University in 2012 after performing a series of projects in the field of cartilage tissue engineering in the Orthopaedic Bioengineering Laboratory directed by Dr Farshid Guilak.
Footnotes
Author contributions
All authors researched data for the article and contributed to discussion of content, writing the article, and reviewing and editing the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Review criteria
The authors searched the PubMed database for articles using the search terms “ageing”, “cellular senescence”, “oxidative stress”, “AMPK”, “autophagy” and “sirtuin”, combined with “chondrocyte”, “cartilage” or “osteoarthritis”, and years starting at 2010. Full-length original manuscripts published in English were selected for further review. Key references from identified manuscripts that reported important earlier studies of interest were also reviewed.
References
- 1.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014;28:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Prieto-Alhambra D, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Losina E, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. (Hoboken) 2013;65:703–711. doi: 10.1002/acr.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March LM, Bachmeier CJ. Economics of osteoarthritis: a global perspective. Baillieres Clin. Rheumatol. 1997;11:817–834. doi: 10.1016/s0950-3579(97)80011-8. [DOI] [PubMed] [Google Scholar]
- 6.Cram P, et al. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308:1227–1236. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauli C, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–1141. doi: 10.1016/j.joca.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa A, et al. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012;64:696–704. doi: 10.1002/art.33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse B, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9:1065–1075. doi: 10.1111/j.1474-9726.2010.00633.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attur M, et al. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. 2015;67:2905–2915. doi: 10.1002/art.39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scanzello CR, Loeser RF. Inflammatory activity in symptomatic knee osteoarthritis: not all inflammation is local. Arthritis Rheumatol. 2015;67:2797–2800. doi: 10.1002/art.39304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschi C, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 15.Ershler WB. Interleukin-6: a cytokine for gerontologists. J. Am. Geriatr. Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 16.Morrisette-Thomas V, et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014;139:49–57. doi: 10.1016/j.mad.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spector TD, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 18.Livshits G, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford study. Arthritis Rheum. 2009;60:2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolen JS, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 20.de Hooge AS, et al. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage. 2005;13:66–73. doi: 10.1016/j.joca.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Delmonico MJ, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23:1955–1965. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine. 2013;80:568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Guilak F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Kim TN, Kim SH. Sarcopenic obesity is more closely associated with knee osteoarthritis than is nonsarcopenic obesity: a cross-sectional study. Arthritis Rheum. 2012;64:3947–3954. doi: 10.1002/art.37696. [DOI] [PubMed] [Google Scholar]
- 27.Scott D, Blizzard L, Fell J, Jones G. Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res. (Hoboken) 2012;64:30–37. doi: 10.1002/acr.20545. [DOI] [PubMed] [Google Scholar]
- 28.Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J. Orthop. Res. 2010;28:1149–1154. doi: 10.1002/jor.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann. Rheum. Dis. 2003;62:108–112. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastiaansen-Jenniskens YM, et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann. Rheum. Dis. 2012;71:288–294. doi: 10.1136/ard.2011.153858. [DOI] [PubMed] [Google Scholar]
- 31.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobasheri A, Matta C, Zakany R, Musumeci G. Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80:237–244. doi: 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 34.Aigner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 35.Kozhemyakina E, et al. Identification of a Prg4-positive articular cartilage progenitor cell population. Arthritis Rheumatol. 2015;67:1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 37.Bovee JV, Hogendoorn PC, Wunder JS, Alman BA. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat. Rev. Cancer. 2010;10:481–488. doi: 10.1038/nrc2869. [DOI] [PubMed] [Google Scholar]
- 38.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppe JP, et al. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchida AI, et al. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res. Ther. 2014;16:441. doi: 10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 43.Rose J, et al. DNA damage, discoordinated gene expression and cellular senescence in osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2012;20:1020–1028. doi: 10.1016/j.joca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Harbo M, et al. The relationship between ultra-short telomeres, aging of articular cartilage and the development of human hip osteoarthritis. Mech. Ageing Dev. 2013;134:367–372. doi: 10.1016/j.mad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Jurk D, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 2011;193:257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philipot D, et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodelling in osteoarthritis. Arthritis Res. Ther. 2014;16:R58. doi: 10.1186/ar4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 49.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc. Natl Acad. Sci. USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoshiyama Y, et al. Chondrocyte clusters adjacent to sites of cartilage degeneration have characteristics of progenitor cells. J. Orthop. Res. 2015;33:548–555. doi: 10.1002/jor.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-α in normal and osteoarthritic chondrocytes. J. Bone Joint Surg. Am. 2003;85-A(Suppl. 3):59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 52.Blaney Davidson EN, et al. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res. Ther. 2007;9:R102. doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johmura Y, et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol. Cell. 2014;55:73–84. doi: 10.1016/j.molcel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Krenning L, Feringa FM, Shaltiel IA, van den Berg J, Medema RH. Transient activation of p53 in G2 phase is sufficient to induce senescence. Mol. Cell. 2014;55:59–72. doi: 10.1016/j.molcel.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Ashraf S, et al. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthritis Cartilage. 2016;24:196–205. doi: 10.1016/j.joca.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Gossan N, et al. The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum. 2013;65:2334–2345. doi: 10.1002/art.38035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khapre RV, Kondratova AA, Susova O, Kondratov RV. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle. 2011;10:4162–4169. doi: 10.4161/cc.10.23.18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudek M, et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Invest. 2016;126:365–376. doi: 10.1172/JCI82755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker DJ, et al. Naturally occurring p16ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Y, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takayama K, et al. Involvement of ERCC1 in the pathogenesis of osteoarthritis through the modulation of apoptosis and cellular senescence. J. Orthop. Res. 2014;32:1326–1332. doi: 10.1002/jor.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laberge RM, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones DP. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hui W, et al. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 2016;75:449–458. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finkel T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheumatol. 2015;67:2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 72.Loeser RF. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011;23:492–496. doi: 10.1097/BOR.0b013e3283494005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koike M, et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015;5:11722. doi: 10.1038/srep11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aigner T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 75.Ruiz-Romero C, et al. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteomics. 2008;8:179–189. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott JL, et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010;69:1502–1510. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK–ERK MAPK signaling pathways. J. Biol. Chem. 2009;284:31972–31981. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S. Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1. Arthritis Rheumatol. 2014;66:2201–2209. doi: 10.1002/art.38641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxid. Redox Signal. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wood ST, et al. Cysteine-mediated redox regulation of cell signaling in chondrocytes stimulated with fibronectin fragments. Arthritis Rheumatol. 2016;68:117–126. doi: 10.1002/art.39326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins JA, et al. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signalling in aging chondrocytes. J. Biol. Chem. 2016;291:6641–6654. doi: 10.1074/jbc.M115.693523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petursson F, et al. Linked decreases in liver kinase B1 and AMP-activated protein kinase activity modulate matrix catabolic responses to biomechanical injury in chondrocytes. Arthritis Res. Ther. 2013;15:R77. doi: 10.1186/ar4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terkeltaub R, Yang B, Lotz M, Liu-Bryan R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum. 2011;63:1928–1937. doi: 10.1002/art.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 2011;7:579–587. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67:1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bohensky J, Leshinsky S, Srinivas V, Shapiro IM. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatr. Nephrol. 2010;25:633–642. doi: 10.1007/s00467-009-1310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015;74:1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 92.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burnett C, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kugel S, Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends Biochem. Sci. 2014;39:72–81. doi: 10.1016/j.tibs.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mostoslavsky R, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 96.Kanfi Y, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 97.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagai K, et al. Depletion of SIRT6 causes cellular senescence, DNA damage, and telomere dysfunction in human chondrocytes. Osteoarthritis Cartilage. 2015;23:1412–1420. doi: 10.1016/j.joca.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 99.Wu Y, et al. Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci. Rep. 2015;5:17602. doi: 10.1038/srep17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr. Rheumatol. Rep. 2014;16:451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verzijl N, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 102.Vos PA, et al. Elevation of cartilage AGEs does not accelerate initiation of canine experimental osteoarthritis upon mild surgical damage. J. Orthop. Res. 2012;30:1398–1404. doi: 10.1002/jor.22092. [DOI] [PubMed] [Google Scholar]
- 103.Li Y, et al. Establishment of a rabbit model to study the influence of advanced glycation end products accumulation on osteoarthritis and the protective effect of pioglitazone. Osteoarthritis Cartilage. 2016;24:307–314. doi: 10.1016/j.joca.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Kim JH, et al. Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc. Natl Acad. Sci. USA. 2015;112:9424–9429. doi: 10.1073/pnas.1505700112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Madej W, et al. Ageing is associated with reduction of mechanically-induced activation of Smad2/3P signaling in articular cartilage. Osteoarthritis Cartilage. 2016;24:146–157. doi: 10.1016/j.joca.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 106.Blaney Davidson EN, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 107.Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol. Biol. Cell. 2012;23:3731–3742. doi: 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:1966–1971. doi: 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loeser RF. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol. 2013;25:108–113. doi: 10.1097/BOR.0b013e32835a9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Longo VD, et al. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]