Abstract
Life on Planet Earth, as we know it, revolves around adenosine triphosphate (ATP) as a universal energy storing molecule. The metabolism of ATP requires a low cytosolic Ca2+ concentration, and hence tethers these two molecules together. The exceedingly low cytosolic Ca2+ concentration (which in all life forms is kept around 50–100 nM) forms the basis for a universal intracellular signalling system in which Ca2+ acts as a second messenger. Maintenance of transmembrane Ca2+ gradients, in turn, requires ATP-dependent Ca2+ transport, thus further emphasizing the inseparable links between these two substances. Ca2+ signalling controls the most fundamental processes in the living organism, from heartbeat and neurotransmission to cell energetics and secretion. The versatility and plasticity of Ca2+ signalling relies on cell specific Ca2+ signalling toolkits, remodelling of which underlies adaptive cellular responses. Alterations of these Ca2+ signalling toolkits lead to aberrant Ca2+ signalling which is fundamental for the pathophysiology of numerous diseases from acute pancreatitis to neurodegeneration. This paper introduces a theme issue on this topic, which arose from a Royal Society Theo Murphy scientific meeting held in March 2016.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: calcium, adenosine triphosphate, calcium channels, evolution of calcium signalling
1. ATP and Ca2+ link cellular energetics and signalling
Calcium ions (Ca2+) and adenosine triphosphate (ATP) are those fateful molecules that defined the form of life that emerged and evolved on Planet Earth. Indeed, ATP is a universal substrate for energy storage, whereas Ca2+ is a ubiquitous intracellular signalling molecule; moreover, ATP-based energetics and Ca2+-based signalling are profoundly interdependent. By some unaccounted chance, the primordial alkaline ocean provided an environment specifically suitable for ATP metabolism, which can proceed only at a very low (sub-micromolar) concentrations of ionized Ca2+. As a result, the cytosol of the most primitive cellular ancestors contained exceedingly low levels of cytosolic free Ca2+. When the Ca2+ concentration in the ocean started to increase (due to acidification and washout of Ca2+ from the rocks) the development of a system controlling Ca2+ movements across the cellular membrane became of vital importance, and provided evolutionary pressure for emergence of cellular Ca2+ homeostatic and Ca2+-based signalling systems [1,2]. This rapid increase in the environmental [Ca2+] instigated an explosive evolution of eukaryotes and then of multicellular life forms [3]. The Ca2+ signalling system, which uses the immense transmembrane concentration gradient for Ca2+, utterly depends on ATP, as maintenance of a low cytosolic free [Ca2+] ultimately requires energy-dependent Ca2+ transportation across the plasmalemma and the endomembranes [4,5]. The ATP energy, however, is not wasted because Ca2+ signals govern the widest range of processes in virtually all cell types. The smallest change in membrane Ca2+ permeability, which occurs under physiological stimulations, induce rapid and spatio-temporally controlled changes in the cytosolic Ca2+ concentration which, in turn, regulate hundreds if not thousands of enzymes (generally denoted as Ca2+ sensors) controlling cellular responses. The Ca2+ signals are necessary attributes of life from its very beginning to its very end. A spermatozoid penetrating an oocyte instigates a specific form of Ca2+ signal that seals the oocyte membrane thus allowing conception, whereas uncontrolled Ca2+ entry, which occurs in stress and trauma, signals cell death. In between lies the realm of Ca2+ regulated cell functions. In muscle, Ca2+ signals trigger contraction, in secretory cells cytosolic [Ca2+] rises evoke secretion, in the nervous system intracellular Ca2+ fluctuations underlie neurotransmission, plasticity and integration, while long-lasting Ca2+ signals regulate expression of genes in all types of cells. The universality of these functions reflects the versatile ensemble of Ca2+ regulating molecular cascades, which, by being assembled into specific and highly plastic toolkits [6], ensure maximal adaptability of the functional outputs. Aberrant remodelling of these toolkits, however, leads to pathology, and abnormal Ca2+ signalling contributes to numerous diseases, from heart failure and pancreatitis to neurodegeneration [7–9].
2. Calcium signalling and purinergic transmission in the nervous system
The unparalleled computing power of the human brain is defined by in excess of 15 trillion connections that integrate approximately 300 billion neural cells into a cellular network optimized for parallel information processing. Operation of these connections, which are defined as chemical and electrical synapses, is ultimately controlled by ATP and Ca2+. Conceptually, neuronal cells are represented by electrically excitable (i.e. capable of generating propagating action potentials) neurons and electrically non-excitable neuroglial cells. Both types of neural cells communicate through chemical messengers, which are either secreted into the extracellular space or directly propagated via gap junctions in cellular syncytia. Neuronal connectivity is mainly mediated through chemical synapses, in which neurotransmitters released from axonal terminals diffuse to the target cell and activate receptors, which control excitability of postsynaptic cells. Neurotransmitters are localized in the synaptic vesicles concentrated in axonal terminals; concentration of neurotransmitters in these vesicles occurs through specific vesicular transporters (for glutamate, GABA or ATP) that use H+ gradients established by vesicular ATPases. Exocytosis of synaptic vesicles is governed by local microdomains of high ionized [Ca2+] that rapidly evolve following opening of voltage-dependent Ca2+ channels [10]; this being the fundamental mechanism of excitation–secretion coupling [11]. At the postsynaptic site, activation of neurotransmitter receptors triggers depolarization and produces complex cytosolic Ca2+ signals which in turn regulate synaptic plasticity and postsynaptic integration. Pathological remodelling of postsynaptic Ca2+ signalling is a leading mechanism in synaptic dysfunction which translates into cognitive deficiency, for example, in neurodegenerative diseases [12]. Preservation of physiological Ca2+ signalling, and hence of a healthy Ca2+ signalling toolkit, seems to be imperative for lifelong adaptive plasticity. Several lines of evidence point to a particular role for vitamin D in long-term regulation of Ca2+ homeostasis (together with redox homeostasis, which is again inseparable from ATP metabolism); incidentally, vitamin D deficiency leads to an increased prevalence of age-dependent neurodegeneration and senile dementia, which may, at least in part, be associated with impaired Ca2+ signalling [13]. Several Ca2+-dependent signalling cascades are fundamental for the integration of neuronal networks with their supportive neuroglial environment. Evolution of the nervous system went through a deep specialization and separation of function between different classes of neural cells: neurons perfected their electrical excitability for fast synaptic transmission, whereas all homeostatic and defensive tasks were shifted to glia. Astroglia, in particular, became principal homeostatic cells of the CNS, being responsible for maintaining the controlled environment that permits proper operation of neuronal ensembles [14]. Astrocytes are integrated into multicellular syncytia through gap junctions; these permit intercellular diffusion of various molecules including second messengers. Diffusion of inositol 1,4,5-trisphosphate (IP3) underlies long-range propagating Ca2+ waves that serve as a substrate for astroglial excitability. Astroglial Ca2+ signals control multiple vital functions, from secretion to mounting defensive reactive responses [8,15].
The roles of ATP in synaptic transmissions are many; apart from excitation–secretion coupling, ATP controls multiple stages in vesicular formation and maturation, and it is indispensable for restoring ionic gradients. In the context of synaptic physiology, however, ATP plays another fundamental role, being a major neurotransmitter. ATP as a signalling molecule for intercellular communications is unique, being almost ubiquitous: purinergic transmission is present in all living forms, from protists and fungi to plants and animals [16]. In the nervous system, purinergic transmission is similarly ubiquitous without any apparent anatomical segregation that is characteristic for other neurotransmitters (for example, dopamine being present mainly in the midbrain and nigro-striatum and acetylcholine (ACh) in the brainstem, hippocampus and parts of the cortex as well as being the main peripheral neurotransmitter). ATP released from axonal terminals as well as from astroglia acts as a co-transmitter in many central synapses and as a transmitter in the periphery and contributes to both electrical excitability and numerous trophic effects, being involved in regulation of development, metabolism, structural plasticity and ageing [17].
Purinergic signalling is mediated through an extended family of purinoceptors, classified into four major groups of P1/A1 adenosine receptors, P2X ionotropic ATP receptors, P2Y metabotropic purinoceptors and P0 adenine receptors [18]. Metabotropic P2Y receptors, which are activated by ATP, ADP and UTP, mainly exert trophic effects; in particular, these receptors control neurogenesis in development and adulthood [19]. The P2X ionotropic receptors are trimeric cationic (i.e. Na+/K+/Ca2+) ligand-gated channels assembled (in homo- or heteromeric fashion) from seven subunits known as P2X1 to P2X7 according to the historic order of cloning [20]. These P2X receptors are widely distributed through neurons and neuroglia and also contribute to many aspects of synaptic plasticity. The P2X7 receptor, in particular, is widespread in microglial cells and its activation controls microglial defensive and immune capabilities. All P2X receptors are permeable to Ca2+ and their activation triggers complex Ca2+ signals, which regulate synaptic transmission. Ionotropic purinoceptors share their topology with the acid-sensitive ion channel (ASIC), which is also widely distributed through neural cells. Proton ATPases are invariably present in synaptic vesicles and their lumen is therefore rich in protons and hence intensely acidic (pH approximately 5). As a result, vesicular release of neurotransmitters is invariably accompanied by a rapid localized pH decrease; these H+ microdomains activate ASICs, populating presynaptic membranes, which may represent a new (and yet not fully understood) mechanism of synaptic plasticity [21].
3. Roles of intracellular Ca2+ and ATP in the control of exocytosis
The classical work of Baker & Knight [22,23] established the critical importance of intracellular Ca2+ and ATP for the control of exocytotic secretion from permeabilized adrenal chromaffin cells (figure 1a). It is now clear that a rise in the cytosolic [Ca2+] ([Ca2+]i) is the trigger for initiating exocytosis in a wide range of cell types, but that this only happens in the presence of millimolar levels of intracellular ATP. The molecular mechanism by which a local rise in [Ca2+]i triggers release of neurotransmitters from nerve endings has been elucidated in considerable detail by the work of Sudhof and his collaborators [25]. It is interesting to compare the Ca2+-sensitivity of exocytosis to that of another secretory function, namely fluid secretion in exocrine glands, which depends on Ca2+ activation of high-conductance K+ channels (see later section). As seen in figure 1b, an increase in [Ca2+]i from 0.1 to 1 µM at the physiological membrane potential of –40 mV causes a very substantial increase in the open-state probability of the channel and, indeed, such a change in [Ca2+]i causes a doubling of exocytotic secretion, as seen in figure 1a.
Figure 1.
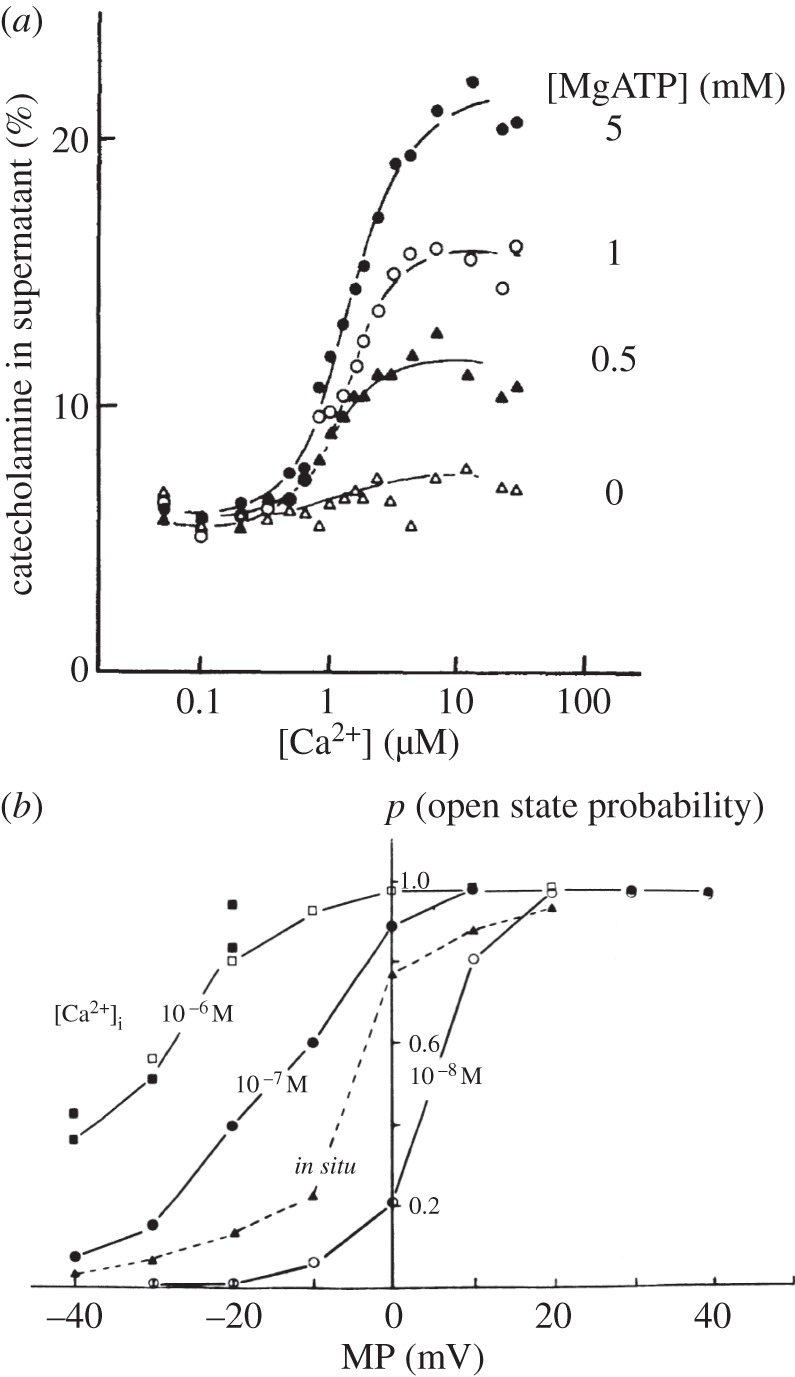
The precise relationship between the cytosolic Ca2+ concentration ([Ca2+]i) and the intensity of exocytotic secretion as well as opening of Ca2+-activated K+ channels. (a) Catecholamine release from permeabilized chromaffin cells at various levels of [Ca2+] and MgATP in the external medium, which in such experiments determines the cytosolic concentration. (b) Plots of the open-state probability of Ca2+- and voltage-activated K+ channels from a single inside-out membrane patch isolated from a pancreatic acinar cell. Comparison is made with the situation in the same membrane patch before excision (in situ). The normal membrane potential (MP) of these cells is about −40 mV. (a) Adapted from Baker & Knight [23] and (b) adapted from Maruyama et al. [24].
Although both Ca2+ and ATP are needed for secretion, the relationship between the control of intracellular Ca2+ and ATP levels varies markedly between cell types. As an example of this, it is instructive to compare the sequence of events in two different cell types present in the same organ, namely the pancreas (figure 2). The quantitatively dominant exocrine acinar cells secrete digestive (pro)enzymes, which are required for the digestion of food products, whereas the endocrine beta-cells secrete insulin, required for the control of blood sugar levels. In both cases, there is a need for Ca2+ signals to stimulate exocytosis (stimulus–secretion coupling) and a requirement for adjusting metabolism to the increased energy demand of the secretion process (stimulus–metabolism coupling).
Figure 2.
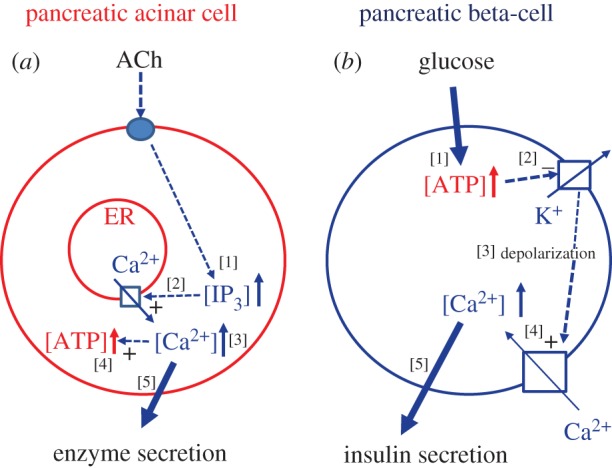
Schematic and simplified diagrams illustrating the different relationships between stimulant-evoked changes in the cytosolic Ca2+ and ATP concentrations in two different cell types in the same organ, namely (a) pancreatic acinar cells and (b) pancreatic beta-cells. For further explanation, see text.
In the pancreatic acinar cells, ACh—released from peri-acinar nerve endings—activates receptors on the baso-lateral membrane generating IP3 inside the cells which, in turn, releases Ca2+ from the endoplasmic reticulum (ER) [26,27]. The subsequent rise in [Ca2+]i in the apical (secretory) region, where the IP3 receptors are localized, causes immediate uptake of Ca2+ into the peri-granular mitochondria via the mitochondrial Ca2+ uniporter in the inner mitochondrial membrane. Recent work suggests that the gating of this pathway is controlled by a Ca2+ sensor in the mitochondrial matrix [28]. The rise in the intra-mitochondrial [Ca2+]i, in turn, activates the three Ca2+-sensitive dehydrogenases in the Krebs cycle, stimulating ATP production [26]. In spite of the enhanced ATP utilization, due to the energy requirement for exocytosis, the overall result of this regulation is that the intracellular ATP level increases. As shown in figure 2a, the sequence of events in the pancreatic acinar cells is such that the initial rise in [Ca2+]i triggers mitochondrial ATP generation. The rise in [Ca2+]i also opens Ca2+-sensitive ion channels in the plasma membrane which is important for fluid secretion (not shown in figure 2, but see next section). The initial release of Ca2+ from intracellular stores, particularly from the ER, also triggers opening of store-operated Ca2+ channels (not included in figure 2, but see later section), which enable store refilling [29,30].
In the neighbouring insulin-secreting beta-cells, a very different relationship exists between regulation of Ca2+ and ATP (figure 2b). The increase in the plasma glucose concentration after a meal results in glucose uptake into the beta-cells and its metabolism then leads to an increased mitochondrial ATP production. The resulting rise in the ratio of ATP/ADP closes ATP/ADP-sensitive K+ channels in the beta-cell membrane [31,32]. The consequence is membrane depolarization, which activates voltage-sensitive Ca2+ channels causing Ca2+ influx. The resulting rise in [Ca2+]i triggers exocytotic insulin secretion [31]. Thus, in the beta-cells, the rise in the intracellular ATP concentration precedes the rise in [Ca2+]i, whereas in the acinar cells the Ca2+ signal occurs first and then drives the rise in ATP concentration (figure 2). The common feature of the stimulus–secretion/stimulus–metabolism coupling events in the pancreatic acinar and beta-cells is the increase in both the intracellular ATP and Ca2+ levels.
4. Roles of intracellular Ca2+ and ATP in controlling fluid and electrolyte movements
Cytosolic Ca2+ signals, in addition to stimulating exocytotic secretion of enzymes, hormones or neurotransmitters, also activate fluid secretion in exocrine glands, such as the salivary glands, the lacrimal gland, the sweat glands and the pancreas [27,33,34]. In the resting (unstimulated) condition, the salivary glands, for example, secrete little or no fluid, but nerve stimulation—via release of ACh or noradrenalin from parasympathetic or sympathetic nerve endings, respectively, in the gland tissue—will induce an immediate and substantial fluid flow. For the salivary glands, as well as the lacrimal and sweat glands, fluid secretion is the principal organ function, whereas in the pancreas it is the necessary vehicle for the washout of secreted (pro)enzymes into the gut [34].
The mechanism by which cytosolic Ca2+ signals activate exocrine fluid secretion is now well established [33] and involves opening of Ca2+-activated K+ channels (figure 1b) in the baso-lateral and Ca2+-activated Cl− channels in the apical acinar membrane, allowing operation of the Na+, K+, 2Cl− co-transporter in the baso-lateral membrane. Thus Cl− is taken up across the baso-lateral membrane by a combination of three transport proteins, namely the Ca2+-activated K+ channels, the Na+, K+, 2Cl− co-transporters and the Na+/K+ pumps, and released into the glandular lumen via the apical Cl− channels. K+ recirculates across the baso-lateral membrane via Na+/K+ pumps, K+ channels and the Na+, K+, 2Cl− co-transporters. Na+ moves into the glandular lumen via para-cellular pathways, through leaky tight junctions, attracted by the lumen negativity created by the opening of the apical Ca2+-activated Cl− channels [33].
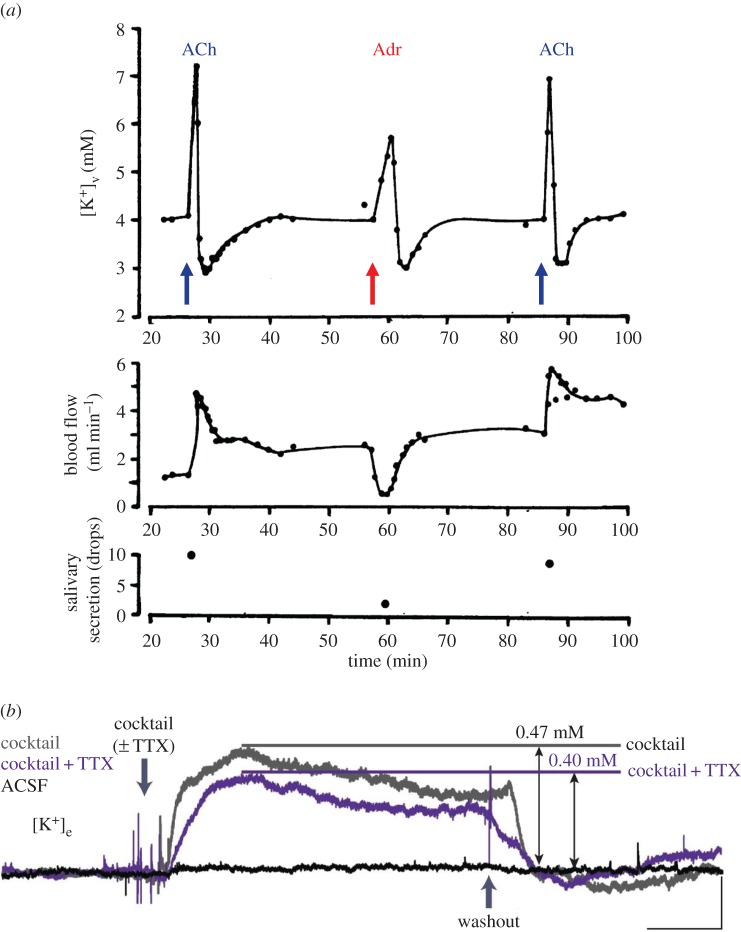
Ca2+ activation of the Cl− and K+ channels causes a very marked rise in the K+ concentration in the extracellular (interstitial) fluid of the glands, as seen, for example, by the sharp rise in [K+] in the venous outflow from the perfused submandibular gland upon intra-arterial injection of ACh or adrenalin (figure 3a). In the exocrine glands, the substantial change in the interstitial fluid [K+] associated with activation of fluid secretion is not in itself of major importance, but simply reflects necessary ionic shifts required for fluid formation. It is, however, very interesting that Nedergaard and her collaborators [36] have very recently shown that neuromodulators, including catecholamines, induce increases in the extracellular [K+] in brain cortical slices electrically silenced by tetrodotoxin (figure 3b) and that in vivo arousal is linked to AMPA receptor—independent elevations of external [K+], and concomitant decreases in the external concentrations of Ca2+, Mg2+ and H+ [36]. Importantly, local cortical activity of sleeping mice could be converted to the stereotypical EEG pattern of wakefulness by imposing a change in the extracellular ion composition [36]. Thus, the kind of ion changes seen in glandular tissues (figure 3a) not only can be observed in the brain (figure 3b), but appear to play an extremely important role in the transition between different behavioural states.
Figure 3.
Stimulant-evoked changes in the extracellular [K+] in a salivary gland and the brain. (a) Results from an experiment on an isolated perfused submandibular gland in which changes in the [K+] in the venous effluent from the gland, evoked by brief intra-arterial injections of ACh or adrenalin (Adr), are monitored. [K+] in the arterial inflow was 4 mM. (b) Traces of [K+]e (e, extracellular fluid) shifts in cortical brain slices after administration of a neuromodulator cocktail (noradrenalin, ACh, dopamine, orexin and histamine). Scale bars (lower right corner), horizontal, 5 min; vertical, 0.2 mM [K+]e. For further explanation, see text. (a) Adapted from Petersen [35] and (b) adapted from Ding et al. [36].
5. Abnormal Ca2+–ATP relationships drive pathological processes
The precise pattern (spatial extension and timing) of physiological Ca2+ signals varies enormously between different cell types but, generally, such signals consist of repetitive [Ca2+]i spikes and often these are confined to a specific sub-cellular space where local Ca2+ regulation is required. Localized Ca2+ signalling is possible because of the restricted diffusion of Ca2+ in comparison with, for example, Mg2+ [37]. However, localized Ca2+ signals can only be transient; sustained [Ca2+]i elevations will always become global and this, usually, leads to pathological effects. Examples of such pathology are described by Maleth & Hegyi [34] and Peng et al. [27]. Sustained global [Ca2+]i elevations are toxic, partly because the Ca2+ signal reaches parts of the cell that under normal physiological circumstances should not have been invaded and partly because overloading of mitochondria with Ca2+ causes a decrease in ATP formation due to depolarization of the inner mitochondrial membrane caused by opening of the permeability transition pore [38]. Cytosolic Ca2+ overloading initiates a ‘circulus vitiosus’ in which the markedly reduced mitochondrial ATP generation will prevent Ca2+ pumps, both in the plasma membrane and in the ER, from clearing the cytosol of the excessive Ca2+ content, resulting in a further [Ca2+]i elevation [26].
6. Store-operated Ca2+ entry, its role in pathology and how it can be inhibited
In electrically non-excitable cells, such as epithelial and immune cells, which do not possess voltage-gated Ca2+ channels, Ca2+-release activated Ca2+ (CRAC) channels constitute the main Ca2+ entry pathway and these channels, as the name implies, are opened as a direct consequence of Ca2+ release from intracellular stores, via a now well characterized molecular machinery [30,39]. By monitoring simultaneously the emptying of the ER Ca2+ store (after inhibition of the ER Ca2+ pumps by thapsigargin) and the CRAC current, it can be seen that the rise of the current follows closely the decrease in the intra-store [Ca2+] [29]. Excessive primary Ca2+ release from intracellular stores is generally the initiating event in the actions of toxic agents or excessive concentrations of neurotransmitters and hormones [27,29], but cytosolic Ca2+ overload requires Ca2+ entry through the CRAC pathway and this is therefore increasingly seen as an attractive molecular target for drug therapy [27,29,30].
The principal argument against CRAC inhibition as a potential treatment against, for example, asthma [30] or pancreatitis [27,29,34] is that CRAC channels are widely distributed in the body, so that unintended side-effects would be likely to occur. Nevertheless, it has recently been shown in three different in vivo mouse models of experimental pancreatitis that CRAC inhibition is a remarkably effective treatment [40]. With regard to this disease, there is a ‘window of opportunity’ in the acute stage [41], where inhibition of not only pancreatic Ca2+ entry but also Ca2+ entry into immune cells would be beneficial to combat this inflammatory disease. Unfortunately, at this stage, there are no CRAC channel blockers approved for clinical use, but this could soon change [30].
Interplay of Ca2+ signalling between different, but neighbouring, cell types can amplify the effects of toxic agents but, if CRAC channels are involved in both types of cells, pharmacological inhibition of this Ca2+ entry pathway can be particularly effective. This seems to be the case in pancreatitis. In the quantitatively dominant acinar cells, toxic agents, such as fatty acid ethyl esters or bile acids, cause excessive intracellular Ca2+ release followed by excessive CRAC-mediated Ca2+ entry [27,29,34]. If sustained, this would activate trypsin inside the cells and lead to necrosis with leakage of activated proteases into the interstitial fluid. Here, one of the proteases, kallikrein, would liberate bradykinin (BK) from kininogen and BK would act on type 2 BK receptors in the neighbouring stellate cells. This, in turn, would cause intracellular Ca2+ release and subsequent Ca2+ entry into the stellate cells via CRAC channels [42,43]. Ca2+ signal generation in the stellate cells, via an unknown mechanism, exacerbates the toxic effects of fatty acid ethyl esters and bile acids on the acinar cells [42]. CRAC channel inhibition would diminish excessive Ca2+ signal generation in both acinar and stellate cells [27,34,42,43], and this may be the reason for the remarkable success of such inhibition in treating pancreatitis in the mouse in vivo [40].
7. Conclusion
Precise regulation of cellular functions is vitally important for virtually all aspects of the life of multicellular organisms. Ca2+ has emerged through evolution as the most important regulator of a vast range of different cellular functions. Very subtle and precise mechanisms exist for controlling the handling of Ca2+, even in very specific sub-cellular compartments, through an impressive array of Ca2+ channels, transporters and pumps precisely localized and tuned to the specific needs of cells in different tissues. Enormous progress has been made in identifying these mechanisms and although many aspects still require more studies, we now have a good working knowledge of the basic control mechanisms of the major cellular functions. As most of such functions require energy in the form of ATP, it is essential that Ca2+ and ATP work together, so that when Ca2+ activates a particular function, for example, muscle contraction or secretion, ATP is made available. Although these links are relatively well understood at the single-cell level, they are less well understood at the integrated organ level and particularly in relation to overall brain function. In recent years, increasing attention has been paid to the role of Ca2+ and ATP in various disease states, where dysregulation of the normal relationship between Ca2+ signalling and mitochondrial ATP production occurs. Cytosolic Ca2+ overloading is a major element of many diseases and much work has been, and continues to be, done on how to prevent this. Some promising avenues have opened up recently. The translation of new insights gained at the single-cell level into a full understanding of integrated organ function in vivo and application of our knowledge of how to interfere with specific critical pathways to real clinical scenarios are far from trivial and still pose significant challenges.
Acknowledgements
We thank the Royal Society and the speakers and attendees at the Theo Murphy scientific meeting held in March 2016 from which this issue arose.
Competing interests
We declare we have no competing interests.
Funding
O.H.P. is a Medical Research Council Professor (G19/22/2).
References
- 1.Plattner H, Verkhratsky A. 2016. Inseparable tandem: evolution chooses ATP and Ca2+ to control life, death and cellular signalling. Phil. Trans. R. Soc. B 371, 20150419 ( 10.1098/rstb.2015.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plattner H, Verkhratsky A. 2015. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 57, 123–132. ( 10.1016/j.ceca.2014.12.004) [DOI] [PubMed] [Google Scholar]
- 3.Kazmierczak J, Kempe S, Kremer B. 2013. Calcium in the early evolution of living systems: a biohistorical approach. Curr. Organic Chem. 17, 1738–1750. ( 10.2174/13852728113179990081) [DOI] [Google Scholar]
- 4.Burdakov D, Petersen OH, Verkhratsky A. 2005. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38, 303–310. ( 10.1016/j.ceca.2005.06.010) [DOI] [PubMed] [Google Scholar]
- 5.Petersen OH, Michalak M, Verkhratsky A. 2005. Calcium signalling: past, present and future. Cell Calcium 38, 161–169. ( 10.1016/j.ceca.2005.06.023) [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529. ( 10.1038/nrm1155) [DOI] [PubMed] [Google Scholar]
- 7.Eisner DA, Venetucci LA, Trafford AW. 2006. Life, sudden death, and intracellular calcium. Circ. Res. 99, 223–224. ( 10.1161/01.RES.0000236790.08267.74) [DOI] [PubMed] [Google Scholar]
- 8.Lim D, Rodriguez-Arellano JJ, Parpura V, Zorec R, Zeidan-Chulia F, Genazzani AA, Verkhratsky A. 2016. Calcium signalling toolkits in astrocytes and spatio-temporal progression of Alzheimer's disease. Curr. Alzheimer Res. 13, 359–369. ( 10.2174/1567205013666151116130104) [DOI] [PubMed] [Google Scholar]
- 9.Petersen OH, Tepikin AV, Gerasimenko JV, Gerasimenko OV, Sutton R, Criddle DN. 2009. Fatty acids, alcohol and fatty acid ethyl esters: toxic Ca2+ signal generation and pancreatitis. Cell Calcium 45, 634–642. ( 10.1016/j.ceca.2009.02.005) [DOI] [PubMed] [Google Scholar]
- 10.Margas W, Ferron L, Nieto-Rostro M, Schwartz A, Dolphin AC. 2016. Effect of knockout of α2δ-1 on action potentials in mouse sensory neurons. Phil. Trans. R. Soc. B 371, 20150430 ( 10.1098/rstb.2015.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudhof TC. 2012. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 4, a011353 ( 10.1101/cshperspect.a011353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busche MA, Konnerth A. 2016. Impairments of neural circuit function in Alzheimer's disease. Phil. Trans. R. Soc. B 371, 20150429 ( 10.1098/rstb.2015.0429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MJ. 2016. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Phil. Trans. R. Soc. B 371, 20150434 ( 10.1098/rstb.2015.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkhratsky A, Nedergaard M. 2016. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Phil. Trans. R. Soc. B 371, 20150428 ( 10.1098/rstb.2015.0428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. 2016. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J 35, 239–257. ( 10.15252/embj.201592705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkhratsky A, Burnstock G. 2014. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays 36, 697–705. ( 10.1002/bies.201400024) [DOI] [PubMed] [Google Scholar]
- 17.Burnstock G. 2016. Short- and long-term (trophic) purinergic signalling. Phil. Trans. R. Soc. B 371, 20150422 ( 10.1098/rstb.2015.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnstock G, Verkhratsky A. 2012. Purinergic signalling and the nervous system. Heidelberg, Germany: Springer. [Google Scholar]
- 19.Lecca D, Fumagalli M, Ceruti S, Abbracchio MP. 2016. Intertwining extracellular nucleotides and their receptors with Ca2+ in determining adult neural stem cell survival, proliferation and final fate. Phil. Trans. R. Soc. B 371, 20150433 ( 10.1098/rstb.2015.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.North RA. 2016. P2X receptors. Phil. Trans. R. Soc. B 371, 20150427 ( 10.1098/rstb.2015.0427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ievglevskyi O, Isaev D, Netsyk O, Romanov A, Fedoriuk M, Maximyuk O, Isaeva E, Akaike N, Krishtal O. 2016. Acid-sensing ion channels regulate spontaneous inhibitory activity in the hippocampus: possible implications for epilepsy. Phil. Trans. R. Soc. B 371, 20150431 ( 10.1098/rstb.2015.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker PF, Knight DE. 1978. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature 276, 620–622. ( 10.1038/276620a0) [DOI] [PubMed] [Google Scholar]
- 23.Baker PF, Knight DE. 1981. Calcium control of exocytosis and endocytosis in bovine adrenal medullary cells. Phil. Trans. R. Soc. B 296, 83–103. ( 10.1098/rstb.1981.0174) [DOI] [PubMed] [Google Scholar]
- 24.Maruyama Y, Petersen OH, Flanagan P, Pearson GT. 1983. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature 305, 228–232. ( 10.1038/305228a0) [DOI] [PubMed] [Google Scholar]
- 25.Sudhof TC. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547. ( 10.1146/annurev.neuro.26.041002.131412) [DOI] [PubMed] [Google Scholar]
- 26.Petersen OH, Tepikin AV. 2008. Polarized calcium signaling in exocrine gland cells. Annu. Rev. Physiol. 70, 273–299. ( 10.1146/annurev.physiol.70.113006.100618) [DOI] [PubMed] [Google Scholar]
- 27.Peng S, Gerasimenko JV, Tsugorka T, Gryshchenko O, Samarasinghe S, Petersen OH, Gerasimenko OV. 2016. Calcium and adenosine triphosphate control of cellular pathology: asparaginase-induced pancreatitis elicited via protease-activated receptor 2. Phil. Trans. R. Soc. B 371, 20150423 ( 10.1098/rstb.2015.0423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vais H, Mallilankaraman K, Mak DO, Hoff H, Payne R, Tanis JE, Foskett JK. 2016. EMRE is a matrix Ca2+ sensor that governs gatekeeping of the mitochondrial Ca2+ uniporter. Cell Rep. 14, 403–410. ( 10.1016/j.celrep.2015.12.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerasimenko JV, et al. 2013. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl Acad. Sci. USA 110, 13 186–13 191. ( 10.1073/pnas.1300910110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samanta K, Parekh AB. 2016. Store-operated Ca2+ channels in airway epithelial cell function and implications for asthma. Phil. Trans. R. Soc. B 371, 20150424 ( 10.1098/rstb.2015.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashcroft FM, Rorsman P. 2013. KATP channels and islet hormone secretion: new insights and controversies. Nat. Rev. Endocrinol. 9, 660–669. ( 10.1038/nrendo.2013.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proks P, Puljung MC, Vedovato N, Sachse G, Mulvaney R, Ashcroft FM. 2016. Running out of time: the decline of channel activity and nucleotide activation in adenosine triphosphate-sensitive K-channels. Phil. Trans. R. Soc. B 371, 20150426 ( 10.1098/rstb.2015.0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen OH. 1992. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J. Physiol. 448, 1–51. ( 10.1113/jphysiol.1992.sp019028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maléth J, Hegyi P. 2016. Ca2+ toxicity and mitochondrial damage in acute pancreatitis: translational overview. Phil. Trans. R. Soc. B 371, 20150425 ( 10.1098/rstb.2015.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen OH. 1970. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J. Physiol. 208, 431–447. ( 10.1113/jphysiol.1970.sp009129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding F, O'Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. 2016. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555. ( 10.1126/science.aad4821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker PF, Crawford AC. 1972. Mobility and transport of magnesium in squid giant axons. J. Physiol. 227, 855–874. ( 10.1113/jphysiol.1972.sp010062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardi P, Rasola A, Forte M, Lippe G. 2015. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev. 95, 1111–1155. ( 10.1152/physrev.00001.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parekh AB. 2010. Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug Discov. 9, 399–410. ( 10.1038/nrd3136) [DOI] [PubMed] [Google Scholar]
- 40.Wen L, et al. 2015. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology 149, 481–492e7. ( 10.1053/j.gastro.2015.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegyi P. 2016. Blockade of calcium entry provides a therapeutic window in acute pancreatitis. J. Physiol. 594, 257 ( 10.1113/JP271710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gryshchenko O, Gerasimenko JV, Gerasimenko OV, Petersen OH. 2016. Ca2+ signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific Ca2+ channel blockade. J. Physiol. 594, 281–293. ( 10.1113/JP271468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gryshchenko O, Gerasimenko JV, Gerasimenko OV, Petersen OH. 2016. Calcium signalling in pancreatic stellate cells: Mechanisms and potential roles. Cell Calcium 59, 140–144. ( 10.1016/j.ceca.2016.02.003) [DOI] [PubMed] [Google Scholar]