Abstract
From the very dawn of biological evolution, ATP was selected as a multipurpose energy-storing molecule. Metabolism of ATP required intracellular free Ca2+ to be set at exceedingly low concentrations, which in turn provided the background for the role of Ca2+ as a universal signalling molecule. The early-eukaryote life forms also evolved functional compartmentalization and vesicle trafficking, which used Ca2+ as a universal signalling ion; similarly, Ca2+ is needed for regulation of ciliary and flagellar beat, amoeboid movement, intracellular transport, as well as of numerous metabolic processes. Thus, during evolution, exploitation of atmospheric oxygen and increasingly efficient ATP production via oxidative phosphorylation by bacterial endosymbionts were a first step for the emergence of complex eukaryotic cells. Simultaneously, Ca2+ started to be exploited for short-range signalling, despite restrictions by the preset phosphate-based energy metabolism, when both phosphates and Ca2+ interfere with each other because of the low solubility of calcium phosphates. The need to keep cytosolic Ca2+ low forced cells to restrict Ca2+ signals in space and time and to develop energetically favourable Ca2+ signalling and Ca2+ microdomains. These steps in tandem dominated further evolution. The ATP molecule (often released by Ca2+-regulated exocytosis) rapidly grew to be the universal chemical messenger for intercellular communication; ATP effects are mediated by an extended family of purinoceptors often linked to Ca2+ signalling. Similar to atmospheric oxygen, Ca2+ must have been reverted from a deleterious agent to a most useful (intra- and extracellular) signalling molecule. Invention of intracellular trafficking further increased the role for Ca2+ homeostasis that became critical for regulation of cell survival and cell death. Several mutually interdependent effects of Ca2+ and ATP have been exploited in evolution, thus turning an originally unholy alliance into a fascinating success story.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: ATP, Ca2+, calcium, evolution, intracellular organelles, ATP receptors
1. Introduction: the early pairing of ATP and Ca2+
The course of biological evolution was preordained from the very beginning, when nucleosides become elementary units of genetic code and ATP emerged as a universal energy substrate. These events defined the molecular canvass of living cell and shaped major signalling cascades; however diverse are the living forms, the basic property of life lies in tight control over its internal milieu that has to be compatible with phosphate-based energetics. Of course, our contemplation of the habitat at the dawn of biological evolution is purely speculative, and yet the survival and diversification of all life forms including the most early ones (be these common ancestral progenotes, bacteria or else unknown forms) resulted directly from their interaction with the immediate environment. In this interaction, cells used what was readily available to them; and by whatever chance, purines and inorganic phosphates were readily available, and so ATP become central for energy metabolism.
The pyrophosphate bonds, phosphorylated nucleosides and ATP most likely occurred during the prebiotic period, being fundamental for subsequent organic evolution and RNA/DNA formation [1,2]. The exact origin of organic phosphates remains enigmatic–the most exotic under consideration invokes an extraterrestrial source: phosphates have been discovered in meteorites [3]. Of course, even accepting the extraterrestriality of ATP, we still face the question of its genesis, however distal from our planet. Conceptually, nucleotide triphosphates were available for biological evolution from the very early stage because of their requirement for genetic code, first in a RNA world [4] and then for DNA and for translationally active molecules (tRNA, mRNA, etc.). Historically, the pairing of ATP and Ca2+ could reflect an idiosyncrasy of the primordial alkaline ocean that contained only a minute concentration of Ca2+; therefore, low Ca2+ concentration was natural for the cytosol of early organisms. Rapid decrease in ocean alkalinity coincided with a similarly rapid rise in seawater Ca2+ concentration ([Ca2+]), which arguably could represent the environmental switch that triggered the emergence of complex ion homeostasis, evolutionary diversity and even multicellularity [5]. It should be noted that this hypothesis is by no means accepted by all palaeobiologists, and many believe in a slightly acid primeval ocean containing high Ca2+ [6], so the truth is yet to be established.
The natural question is: why ATP, rather than other nucleotide triphosphates? Possible driving factors in eukaryote evolution may reflect the fact that ATP had been preset for energy conservation already in bacteria before Ca2+ has been established as a signalling molecule; that Ca2+ stimulates ATP synthesis by activating dehydrogenases of the tricarbonic acid (Krebs) cycle [7]; and that ATP prevails several fold over GTP, which is formed and used in only one step by transphosphorylation from ATP to GDP [8]. Acquisition of mitochondrial precursors is considered an early and critical step of eukaryote evolution [9,10]. Formation of an endomembrane system is attributed to the first eukaryotic common ancestor, which emerged in combination with formation of multiple paralogues of molecules dedicated to signalling and trafficking [11–13]. Production of ATP by mitochondria may have been a prerequisite because it greatly increased energy available for vesicle trafficking, be it for fuelling motor proteins for vesicle transport, for activation of the triple-A ATPase N-ethylmaleimide sensitive factor [14], a SNARE chaperone, or for increasingly sophisticated Ca2+ regulation. It is of note, however, that binding of ATP and GTP to F1-ATPase is similar [15]. All in all, there are only weak arguments for the selection of ATP, rather than other nucleotide triphosphates, for energy metabolism [16]. In contrast, the high free energy of GTP hydrolysis is proposed to be advantageous in allowing efficient transition from the active to the inactive state, as it occurs with GTPases [17] when they mediate specific vesicle interactions [18,19].
Be this all as it may, ATP remains quite a unique molecule, which arguably participates in more chemical reactions than any other compound on the Earth's surface, except water. At the same time, ATP metabolism is highly demanding, being operational only at very low levels of free Ca2+. This in turn stipulates that free Ca2+ inside the cells using ATP for energetics must be exceedingly low; and very low it is set indeed, being, as a rule, around 100 nM [20–22]. This low Ca2+ requirement shaped the biological evolution and linked ATP with ion composition of the intracellular milieu, which has minute amounts of Ca2+ and relatively low concentration of Na+; maintaining the ion composition of the cytosol, in turn, became the main energy expenditure of all living cells. Thus, ATP needs control over Ca2+ and control over Ca2+ needs ATP, hence making the two an inseparable tandem.
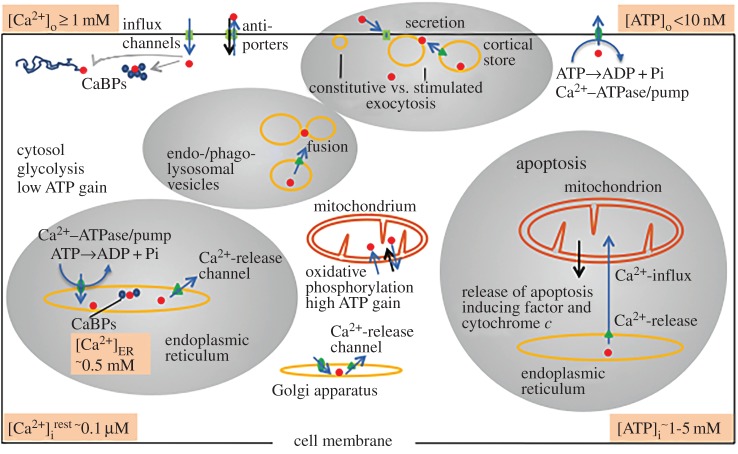
Evolution perfected ion-controlling mechanisms and used ion gradients for many purposes, most notably for cellular signalling. Calcium as a signalling ion is employed by the most primitive bacteria [23,24] and by protozoa [25,26] before the system of Ca2+ signalling became highly elaborated in higher eukaryotes. Some bacteria already contain a Ca2+–ATPase/pump that is important for Ca2+ export and bacterial [27–29] and protozoan [30,31] survival. Most importantly, all Ca2+ signalling and homeostatic molecules are controlled by Ca2+ ions themselves, which imposes multiple feedbacks thus making the system highly versatile and adaptable [32]. Eukaryotic evolution proceeded along with a significant increase in the number of Ca2+-binding proteins [33,34] as well as in the number of signalling and metabolic processes that are regulated by Ca2+ [20,35]. Exocytotic SNARE proteins, Ca2+ signalling and directionality of organelle-specific GTPases must have been early evolutionary achievements maintained throughout eukaryotic kingdoms [11,36,37]. Figure 1 gives an overview of the widely branched regulation mechanisms and effects of Ca2+ that are exerted in cooperation with ATP as an energy source, from normal cell life to programmed cell death (apoptosis).
Figure 1.
Regulatory principles in Ca2+-based signalling and ATP-based energetics. As [Ca2+]o is much higher than [Ca2+]i, Ca2+ steadily leaks into the cell (not drawn), but this is counterbalanced by the Ca2+–ATPase/pump and by antiporters. Upon stimulation, Ca2+ can flow into the cell by influx channels of different types. Rapid downregulation is executed by Ca2+-binding proteins (CaBP) and by sequestration mainly into the endoplasmic reticulum (endowed with an organellar Ca2+-pump), but also into other organelles, such as cortical stores, the Golgi apparatus, different trafficking organelles and mitochondria. In their inner membrane, mitochondria have available a uniporter for Ca2+ uptake and a antiporter system for its rapid release. Ca2+-release channels, mainly of the type InsP3R and RyR, are available in the different organelles. In the endophago/lysosomal trafficking system, such channels can mediate short-range Ca2+ signalling. Requirement of Ca2+ is also ascertained for stimulated exocytosis, whereby Ca2+ may originate from influx and/or release from cortical stores. Regulation of apoptosis (regulated cell death), among a plethora of molecules, involves release of Ca2+ from the endoplasmic reticulum and release of an ‘apoptosis-inducing factor’ as well as of cytochrome c from mitochondria. In summary, in the eukaryotic cell, there are many local signalling pathways, with many interactions, for a variety of subcellular processes.
2. Some evolutionary preconditions and basic considerations of having Ca2+ and ATP as crucial cell regulators
Signalling mediated by Ca2+ has developed early in evolution because of sheer omnipresence and abundance of this ion in the environment, whereas the ATP-centric metabolism made free Ca2+ toxic inside cells. Nonetheless, Ca2+ permeation through biomembranes, though variable and tightly controlled, is unavoidable. Like with oxygen which, when emerging in the atmosphere instigated the extinction of almost all living forms, evolution turned a disadvantage into an advantage by taming toxicity through spatial and temporal restriction [25,38,39]. Ionized Ca2+ has to be kept low in the cytosol to avoid interference with phosphate groups in free and bound form. On the other side, low Ca2+ concentration offers the advantage of economic handling. Assuming that Ca2+ was required to govern emerging intracellular trafficking, which may have developed only after full development of aerobic energy production with the consequence of high energy conservation by ATP, these two aspects represented a revolution in eukaryote evolution. Furthermore, considering the requirement of ATP for intracellular movement and of Ca2+ for membrane–vesicle interactions, the availability of both molecules in adequate concentrations was mutually interdependent from the very beginning of eukaryote evolution about 2.5 × 109 years ago. As a rule, cytosolic concentrations are kept at millimolar range for ATP and at submicromolar range for Ca2+, the [Ca2+]i being about 1000 times less than ambient concentration in the extracellular space [20]. From protists to humans, concentrations of total (free and bound) and free Ca2+ in the cytosol are very similar, being approximately 1 mM and approximately 0.1 µM, respectively. After stimulation, [Ca2+]i usually rises by up to, or even above, 10 µM. In Ca2+-storing organelles, before release upon stimulation, both total [Ca] and ionized [Ca2+] are substantially higher compared with the cytosol, from protozoa [40] to mammals [41,42]. Sequestration into stores for trapping by binding to luminal proteins inactivates Ca2+, but also makes it available for rapid release upon stimulation.
3. Availability of Ca2+ for early evolution, and its properties predestining it for intracellular functions
The prevalence of divalent cations in the earth crust and in the ocean is: Ca2+ > Mg2+ > Ba2+ > Sr2+. While Ca2+ is mainly used for signalling, Mg2+ binds to many proteins and serves as a cofactor in biochemical reactions. For instance, together with GTP, Mg2+ activates the protein initiation factor or, as MgATP, frequently serves as a substrate. The preference of MgATP may reside in the highest binding constant for Mg2+ over other divalent cations [43,44]. In contrast to Mg2+, Ba2+ and Sr2+ drive several subcellular functions, similarly (albeit not always) to Ca2+ (e.g. exo–endocytosis in fish neurons [45] or ciliary movements in ciliates [46]), however their effects are, as a rule, weaker. Sometimes, however, Ba2+ can surpass the effects of Ca2+, for instance in chromaffin cells and in ciliated protozoa (the ‘barium dance’ [47]). It is plausible that Ba2+ and Sr2+ may have been overlooked by evolution because of their minor availability in nature. An alternative view considers different coordinated binding of Mg2+, Ca2+ and Ba2+ to peptide fragments, depending on polar aliphatic residues and ion radius [48], thus reflecting some gross dependency from the primary protein structure. Binding is much more restricted to Ca2+ when proteins possess specific Ca2+-binding motifs. Here specificity can be quite selective. For example, different paralogues and isoforms of the Ca2+-sensor synaptotagmin bind Ca2+ considerably better than Ba2+, Mg2+ or Sr2+ [49]. By these different aspects, during evolution, Ca2+ may have been selected as the main signalling molecule, while the other abundant divalent metal, Mg2+, was chosen for other functions.
Binding to proteins is widely different for Ca2+ and Mg2+: relatively loose and reversible coordinative binding of Ca2+ to specific domains of dedicated Ca2+-binding proteins at low concentrations is an important factor. This enables low-capacity/high-affinity Ca2+-binding proteins for rapid and efficient signal transfer by conformational change. The archetypal and most widely distributed, multifunctional example is calmodulin [50], whose four EF-hand loops are hierarchically occupied, the lowest-affinity loop IV also binding Mg2+. Gradual conformational changes can thus serve for the transmission of a chemical signal into a mechanical one, be it at a subunit of plasmalemmal ion channels in protozoa [51] or intracellular Ca2+-release channels in mammals [52]. The Ca2+-sensor for membrane fusion, synaptotagmin, possesses distinct C2 domains for cooperative Ca2+-binding [53]. These C2 domains stick out from β-barrel structures and increase lipophilic properties upon Ca2+ binding. This mediates membrane binding and perturbation of adjacent membranes for Ca2+-dependent fusion [54].
An unusually high number of acidic amino acid residues in cytosolic Ca2+-binding proteins allows Ca2+ binding even at relatively high concentration. These high-capacity/low-affinity Ca2+-binding proteins, such as parvalbumin, serve as immobile Ca2+ buffers for rapid downregulation of cytosolic Ca2+ [55,56]. Centrin can operate not only by high-affinity binding sites belonging to the EF-hand motif, but also by its negative charges [57]. Centrin is the dominant cytosolic Ca2+-buffer in the cortex of ciliated protozoa [58]. In Ca2+ stores, high-capacity/low-affinity Ca2+-binding proteins are defined by their excess of acidic amino acids residues for Ca2+ storage and rapid release upon stimulation [59]. Calreticulin and calnexin in the endoplasmic reticulum are examples; they also exert a Ca2+-dependent chaperone function for folding newly synthesized proteins [60].
4. The specific case of magnesium
Magnesium is the fourth most abundant ion in eukaryotic cells, and, incidentally, it is the most abundant free divalent cation (total Mg2+ content of the body is approx. 24 g or approx. 1100 mmol, whereas ionized cytosolic [Mg2+] fluctuates between 0.25 and 1 mM [61,62]). This high cytosolic concentration could be another explanation for why evolution favoured Ca2+ over Mg2+ for signalling purposes. Well-defined domain structures evolved for Ca2+ binding, whereas Mg2+ buffers remain generally unknown; identifying Mg2+-binding domains is a tedious task and they generally are considered elusive. A similar conclusion arose from the bioinformatic analysis of Mg2+-binding proteins, the so-called magnesome, which includes rather diffuse Mg2+-binding motifs [63]. This reflects essential differences between the Mg2+ and the Ca2+ ion (and some other earth alkali metals, with the exception of Ba2+ and Sr2+ that closely resemble Ca2+). These differences [62,64] are in ionic radii (smaller for Mg2+), charge density, water shell binding (higher hydration for Mg2+), binding by inner and outer sphere coordination, etc. Coordination geometry is particularly important for binding to DNA, RNA and to various proteins and their substrates [65]. Several hundred enzymes are known to interact with Mg2+, mainly through using soluble MgATP, rather than ATP per se as a substrate. A large part of the cellular effects of Mg2+ are because of its abundance in ionized form, absence of toxicity (as opposed to Ca2+), less strict and less specific binding.
Data mining and molecular dynamics analyses have been applied to identify possible coordination structures based on crystal data for actin, myosin, DNA polymerase, RNA polymerase, DNA helicase and mitochondrial F1-ATPase [66]. These analyses identified a broad range of molecules being affected by Mg2+ and revealed a diversity of both transitory and stable coordination arrangements between Mg2+ and ATP. In eubacteria, Mg2+ stabilizes the structure of 16S rRNA in a conformation favourable for binding to the ribosomal 30S subunit [67]. Similarly, the binding of transcriptional activator protein c of bacteriophage Mu to bacterial DNA is enhanced by Mg2+-induced change from a more β-sheath-like to a more α-helical conformation, as evidenced by circular dichroism analysis [68]. All these processes are not related to cell signalling.
However, indirectly, Mg2+ may contribute to intercellular signalling, because Mg2+ (and not Ca2+) controls interaction between the subunits of trimeric GTP-binding proteins (G-proteins) with GTP and GDP, respectively, thus determining their signal transduction activity [69]. As alluded to above, in the calmodulin molecule, which acts as an activator of many enzymes and ion channels, four Ca2+-binding EF-hand loops show hierarchical Ca2+ binding with different binding constants, which depend on [Mg2+]i [70] and is different for each loop [71]. In addition, Ca2+ and Mg2+ often exert different, in part even antagonistic, effects on enzymes, such as protein kinases and protein phosphatases. For example, Ca2+ and Mg2+ differentially regulate cAMP-dependent protein kinase A [72]. Another example is associated with protein phosphatase type 2C, which is activated by physiological [Mg2+]i, in contrast to Ca2+, which is inhibitory at micromolar concentrations [73].
5. Why ATP has been selected as an energy-storing nucleotide?
Interactions of Ca2+ and Mg2+ with ATP are different, with CaATP being much less soluble. As mentioned, Mg2+ binds to ATP to form a complex that serves as a substrate for many enzymes, such as ATPases including Ca2+–ATPases/pumps for extrusion or sequestration of Ca2+. Why among nucleotide triphosphates did ATP prevail over GTP, CTP, TTP, UTP, ITP? There may be evolutionary (‘historical’) reasons, e.g. because ATP had already been established in energy conservation. If so—why? Any other nucleotide triphosphate, if available in similar concentration, might do equally well because of similar free energy [74]. However, functions of the different nucleotide triphosphates may have been diversified already at the early evolutionary stages. As we see now, ATP has been selected preferably for energy conservation, whereas GTP is mainly used for signalling, e.g. for membrane interactions. This mainly concerns monomeric GTP-binding proteins (Rab-type GTPases) in the context of vesicle/membrane interaction [18,19,75], in addition to nucleo-cytoplasmic transport and microtubule dynamics. Only rarely is GTP used in bioenergetics, e.g. in only one step of the tricarbonic acid cycle. The reduction of nicotinamide dinucleotide (NAD) to NADH for subsequent exploitation by oxidative phosphorylation in the mitochondria greatly increases energy gain. Other nucleotide triphosphates are used for specific biosynthetic processes, such as N-glycosylation of proteins in the endoplasmic reticulum. Thus, ATP prevalence in energy metabolism may result from rather stochastic early evolutionary specialization, although the early pairing of ATP and Ca2+ could also play a role. Using different nucleotide triphosphates beyond ATP and GTP, including UTP and CTP, for different purposes may be advantageous for specific regulation of a range of processes.
In summary, it may be due to the evolutionary priority to have energetics and metabolism based on ATP, rather than on other nucleotide triphosphates. During evolution, ATP and Ca2+ became intimately connected in functional terms for many activation and deactivation processes (protein phosphorylation and dephosphorylation), whereas GTP has been assigned other functions.
6. Intracellular Ca2+ fluxes and Ca2+ regulation
Cellular Ca2+ fluxes are mediated by uniporters (influx- and release channels), primary active (Ca2+–ATPase/pump) and secondary active transport systems (e.g. H+/Ca2+ exchangers or related antiporters), all to be found in the plasmalemma and in the endomembranes [20,22,76,77]; intracellular Ca2+ dynamics is further regulated by Ca2+ buffers [56]. All these mechanisms were invented early in evolution, be it in ancestors of unikonts, such as choanoflagellates [78], or be it in bikonts, such as ciliates [38,40]. Cytosol contains both mobile and immobile Ca2+ buffers of different capacity and affinity. Mobile high-affinity Ca2+ buffers hinder Ca2+ diffusion, thus favouring localization of Ca2+ signalling events that often occur in a form of microdomains. In the endoplasmic reticulum, in contrast, low-affinity Ca2+ buffers allow diffusion and rapid equilibration of Ca2+ within the lumen through Ca2+ tunnels [79]. From immobile buffers, including high-capacity/low-affinity binding proteins, Ca2+ can slowly dissociate to be fed into active transport systems. For primary active transport, the ATP → ADP system is energetically roughly the same as it would be for pyrophosphatase activity The preference for the ATP → ADP system can be explained by the requirement of only one energy limiting step for ATP re-synthesis, rather than two for the pyrophosphatase system, whereas the same amount of free energy is available for both [80]. Thus, pyrophosphatase-driven Ca2+-sequestration is used only exceptionally, for example in acidocalcisomes of trypanosome parasites [81].
Furthermore, to keep energy expenditure low, Ca2+ influx and/or release from internal stores is frequently restricted to selective sites. The textbook example is the nerve terminal, where voltage-dependent Ca2+-influx channels are restricted to the active zone where transmitter is released by exocytosis [82,83]. In ciliated protozoa, this type of channels is restricted to cilia [84]. In the central nervous system of mammals and in ciliates, these Ca2+ channels are inactivated by formation of a Ca2+/calmodulin complex [46,85,86] using the very same Ca2+ they had conducted. This is another example of a principle conserved from ciliates to the human brain: it is generally appropriate to limit [Ca2+]i increase to a small volume and short time, with values just supporting activation and, thus, greatly reducing ATP consumption for re-establishing [Ca2+]i.
The principle of local restriction is also often applied to intracellular signalling. For intracellular Ca2+ release, the cell possesses different types of Ca2+-release channels activated by distinct compounds represented by (i) inositol 1,4,5-trisphosphate (InsP3) formed from phosphatidyl inositol 4,5-bisphosphate (PInsP2, a component of lipid membranes) and binding to InsP3-receptor-type Ca2+-release channels (InsP3R, [87]); (ii) by Ca2+ ions themselves, which activate ryanodine-receptors (RyR) evolutionarily related to the InsP3-receptor at the protozoan level where intermediates of the two channel types are also found [26,38,39,88,89]; and (iii) by nicotinic acid adenosine dinucleotide phosphate (NAADP), which activates two-pore channels (TPCs) of acidic Ca2+ stores [90]. In metazoans, RyRs are activated by Ca2+ [91] and by cyclic adenosine diphosphoribose (cADPR) formed from the glycolytic H+ acceptor NAD [92]. Both NAD and nicotinamide adenine dinucleotide phosphate (NADP), but probably also PInsP2, have been available in early eukaryote evolution—at least the key components [93] and enzymes [94] are found in protozoa. Both channel types, InsP3R and RyR, are also predicted by database mining for choanoflagellates [78,95]. An intermediate form between InsP3R and RyR was suggested from the nucleotide sequence in Purkinje cells [96]. Similar to Paramecium, the two types or Ca2+-release channels coexist in Ascidian egg cells [97]. In conclusion, there are diverse ways to release Ca2+ locally by different activators, only some being considered here. Considerable similarities between InsP3Rs and RyRs from ciliated protozoa [88] to mammals [98] indicate that both may possibly have evolved from a common ancestral molecule, assembled from six transmembrane domains [38,89].
Another set of intracellular channels is represented by the TPCs that are members of the voltage-gated ion channel superfamily; the TPCs contain 12 transmembrane domains, being in essence a half of a voltage-gated channel [99–101]. These channels reside in acidic compartments (which form acidic Ca2+ stores), notably in the endo/lysosomal compartments and in the plant vacuoles [90,102], and are activated by NAADP, derived from the metabolite, NADP [90,99,103,104].
In evolution, TPCs are present in monokonts, from choanoflagellates and the social amoeba Dictyostelium [105]) to mammals [100], and in bikonts, from protozoa to green plants [101,106]. In plants, TPCs can be activated by osmotic stress [107] as well as by Ca2+ ions (this Ca2+-dependent activation contributes to regulation of seed germination and stomatal movement [108]). Paramecium, which is another bikont, also seems to possesses TPCs [109]. In the parasitic monokont, Trypanosoma, NAADP does not trigger Ca2+ response in contrast to the bikont apicomplexan parasite Toxoplasma gondii, a relative of ciliates [110]. The T. gondii also possesses a plant-like vacuole and acidocalcisomes, which may bear TPC channels encoded by orthologous genes that are absent in Apicomplexa [106].
Different types of Ca2+-release channels frequently coexist within the membrane of Ca2+ storage organelles [111,112]. The RyRs and InsP3Rs localized in the ER membrane may be functionally linked to TPCs in neighbouring acidic organelles [90,113–116]; Ca2+ release from TPCs can trigger Ca2+-induced Ca2+ release (CICR) [115]. From the fact that InsP3Rs and RyRs can be activated by TPC activity, it has been speculatively concluded that TPCs are evolutionarily older than other Ca2+-release channels [90]. According to this hypothesis, channels that allegedly emerged later in evolution (i.e. InsP3Rs and RyRs) may serve for signal amplification through consecutive channel activation [90,107,113–115]. In addition, NAADP can also activate Ca2+-influx through TPCs localized in the plasmalemma [115], which was confirmed in patch-clamp studies [90]. The TPCs were also proposed to be responsible for topologically restricted Ca2+ signals, which may locally ignite phosphoinositol turnover, store-operated Ca2+ entry (SOCE) activity and possibly also cAMP signalling [117]. From a more conventional perspective, Ca2+ signals arising from TPCs have been considered relevant for membrane fusions along the endolysosomal pathway [118]. For the time being, however, all these effects remain hypothetical. In summary, TPCs are universally distributed in eukaryotes, with the exception of some unicellular parasites. Otherwise, however, our knowledge about TPCs remains rather incomplete.
Transient receptor potential (TRP) channels owe their name to a phenotype of a Drosophila mutant whose electrical photoreceptor response is abnormally short (i.e. transient). Mammals possess 28 TRP channels classified into six subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin) and TRPP (polycystin) [119,120]. The TRP channels are widely distributed between different cell types and may be present in plasmalemma as well as in endomembranes (with only nucleus and mitochondrion being devoid of them). The TRP channels are strictly cationic with highly variable Ca2+ permeability; some TRP channels can conduct Mg2+ [119,120]. The intracellular TRP channels may mediate Ca2+ release from the stores; plasmalemmal TRP channels can be linked to intracellular Ca2+ release through a store-operated mechanism [120]. The TRP channels are broadly distributed throughout many species along the phylogenetic ladder. In protozoa, systematic analysis is scant and assignment to eukaryotic, specifically mammalian types is difficult. For the bikont Paramecium, originally no TRP channel genes had been found [106], but then three genes were retrieved [121]. Parasitic relatives of Paramecium, Apicomplexa, Toxoplasma gondii and probably also Plasmodium spp. all possess genes encoding TRP channels [106]. Parasitic flagellates Leishmania and Trypanosoma also possess TRP channel, but for all unicellular parasites, molecular characterization is yet to be done [110]. The green flagellate Chlamydomonas reinhardtii contains 19 genes encoding TRP channels [122], one of which shows considerable similarity to a human form and is localized probably in the flagellar membrane, contributing to sensory transduction [121]. Data mining revealed TRP channels encoding genes in Dictyostelium [121], where one of the members, PKD2, is localized in the cell surface and governs rheotaxis [123]. Choanoflagellates are endowed with TRP channels genes [124]; expanded molecular data mining revealed TRPA, TRPC, TRPM, TRPML and TRPV types [78]. Higher plants (viriplantae) apparently do not express TRP channels [122,125].
There is a crosstalk between intracellular Ca2+ stores, exemplified, for example, by variable coupling of endoplasmic reticulum and mitochondria [126,127], as well as between stores and plasmalemmal Ca2+ influx. In cardiomyocytes, Ca2+ entering the cytosol through voltage-gated channels directly activates RyRs, thus initiating CICR [128]. In skeletal muscle, activation of L-type plasmalemmal Ca2+ channels directly activates RyR type II, causing depolarization-induced Ca2+ release. Emptying the ER Ca2+ stores triggers plasmalemmal store-operated Ca2+-entry, SOCE [128], which ubiquitously occurs in non-excitable cells [129,130]. Activation of SOCE involves intraluminal Ca2+-sensor (Stim) and an influx channel (Orai and associated proteins or TRPC channels) in the plasmalemma [131,132]. Although operational SOCE is well characterized in the ciliate, Paramecium [40], Orai and Stim have not been detected (as yet), in contrast to informatics data obtained from choanoflagellates [78] that are placed at the basis of metazoans. However, several alternative modes of cell membrane–cortical store coupling are discussed, for instance by extended synaptotagmins with super-numerary C2 domains [133] which also occur in ciliates [38]. Altogether, SOCE appears to be an evolutionarily old mechanism.
The intracellular Ca2+ dynamics in the nerve terminal has to be exceptionally fast, in contrast to stimulus–secretion coupling in most other cells where no specific intimate structural and functional coupling exists and where the Ca2+ response, therefore, is less precise (an obvious example being the difference in vesicular secretion between neurons and neuroglia [134]). An exception is the ciliate, Paramecium, where a rapidly activated SOCE underlies the fastest known exocytosis of dense core-secretory vesicles important for predator defence [40,135]. The final target of Ca2+ during any type of secretion is the C2-domain-bearing protein synaptotagmin, and closely related Ca2+-sensors, such as extended synaptotagmin isoforms of which several (probably with different kinetics) are known [53].
Elaborated Ca2+ signalling is also required for intracellular trafficking from organelle to organelle, which can be concluded from the distribution of synaptotagmins [136,137]. The signal may come from the very same trafficking organelles, as they contain Ca2+-release channels of different types, including InsP3Rs and RyRs. This is true from protozoa [38,88] to mammals [98], even though other types of Ca2+-release channels can also be involved (not considered here). Organelles of the endophagocytotic/lysosomal pathway contain Ca2+ at different concentrations [138]; release of Ca2+ drives local vesicle–vesicle interactions and fusion. Requirement for local availability of Ca2+ has been shown for instance for endosomes [139], endoplasmic reticulum and Golgi vesicles [140,141], as well as for lysosome–phagosome fusion [142]. Besides influx channels, endocrine cells also possess InsP3Rs in their secretory vesicles [143]. Again, limiting the signal to the organelles involved is a prerequisite for avoiding toxicity and to keep energy cost low.
Which Ca2+-release channel is the oldest? Different types of Ca2+-release channels, i.e. InsP3R, RyR, TPCs and TRPCs are all present at the lowest evolutionary stage when monokonts separated from bikonts, which happened approximately 1.6 × 109 years ago [144]. This makes it difficult to decide upon the nature of ancestral Ca2+-release channel, although low selectivity of TRPCs [120] may argue in their favour. The mechanosensor TRPY1 of yeast may be such a prototype [145] (of note however, yeasts emerged by secondary reduction). Channels with high selectivity for Ca2+, such as InsP3Rs and RyRs, require Ca2+ stores endowed with uptake mechanisms for specific sequestration of Ca2+; therefore, high selectivity of Ca2+-release channels reflects availability of Ca2+ in the store lumen (for TPCs, see above). Whichever is the oldest, Ca2+-release channels appear as inevitable prerequisite for ‘constructing’ large eukaryotic cells with abundant vesicle trafficking.
7. Molecular targets of Ca2+ in cells
Salient features of targets for Ca2+ signals are disparate in different cell types. For membrane–vesicle interaction, various SNARE proteins and synaptotagmin-type Ca2+-sensors are present in spatially separated cellular compartments [146,147], in contrast to non-stimulated (constitutive) exocytosis that may not depend on Ca2+ signals, if one makes painstaking scrutiny [148]. Endocytosis via clathrin-coated pits or uncoated vesicles is synchronized with exocytosis by the pulse of Ca2+ required for both processes in mammalian [149,150] as well as in protozoan cells [151]. Endocytosis requires dephosphorylation of the large GTPase dynamin, by the Ca2+/calmodulin-activated phosphatase 2B/calcineurin [152]—an enzyme maintained from protozoa [153] to human, where it is essential for many signalling processes, including long-term potentiation and immune defence [154–156]. Calcineurin is a true multipurpose enzyme [157]. Nevertheless, in evolution, only one of the two calcineurin subunits is maintained in plants, where it serves for coping with ionic stress [125]. Unfortunately, no data are available for characaean algae placed at the roots of higher plant evolution. The situation with trafficking vesicles deep inside a cell is different because it requires locally restricted and finely tuned Ca2+ signals. The availability of synaptotagmin at the surface of vesicles of the endophagolysosomal vesicles [136,137] and of Ca2+ in their lumen allows for local signalling and fusion. Conceptually, Ca2+ ions have to be provided ‘at the place’, because diffusion from the far remote influx channels at the cell surface would be too inefficient, as Ca2+ would be trapped on its way through the cell by Ca2+-binding proteins.
The Ca2+/CaM-activated protein kinase (‘CaM kinase’) is important for neurotransmission, because it regulates, by phosphorylation of synapsin, the release of vesicles from the actin web, thus facilitating their access to the cell membrane [158]. During evolution, CaM kinase has been maintained only in monokonts from myxamoebae Dictyostelium to man [159]. This is different for bikonts, from ciliates up to viridiplantae, where CaM kinase proper does not exist. Here, Ca2+-dependent protein kinases are kinases with integrated CaM-like motifs [125,160]. The situation is again different for cyclases for cyclic nucleotide formation. These are Ca2+-dependent enzymes from ciliates [161,162] to humans [163]. Thus, via cyclases, cyclic nucleotide-activated protein kinases at the end of a signalling cascade also depend (indirectly) on Ca2+.
The multiple ways in which Ca2+-dependent cell motility is regulated are well characterized. The Ca2+-sensitive molecules involve severing proteins (gelsolin), actin bundling proteins (caldesmon), troponin, etc. In muscle cells, Ca2+ binds to troponin C; the resulting conformational change of the troponin–tropomyosin complex allows the interaction of the myosin head with the actin filaments, which causes cell contraction by tilting of the myosin [164]. During stimulus–contraction coupling, this movement from molecular to macroscopic scale is fuelled by ATP hydrolysis. Ca2+ exerts pleiotropic effects on ciliary activity, especially in ciliated protozoa. The availability of a minimum of cytosolic ionized Ca2+ is required for ciliary/flagellar activity [165], which can be further modulated by Ca2+ [166]. In ciliates, normal ciliary beat depends on Ca2+ [165], as does any change in beat activity. For instance, ciliary beat is reversed when intraciliary [Ca2+] increases by activation of voltage-gated Ca2+ channels, as described for ciliated protozoa [166], and in ctenophores (comb jellies). In both systems, these channels are restricted to the cilia [84,167]. In ciliates, Ca2+ mediates dynein subunit phosphorylation by Ca2+ followed by calmodulin [168]; this type of regulation appears to be absent in cilia at a higher evolutionary level. In ciliates, the Ca2+-regulatory mechanism involves activation of a cGMP-activated protein kinase [169] formed by a Ca2+-dependent cyclase [170]. It also should be mentioned that many basic metabolic processes, such as glycogen turnover, are regulated by Ca2+. In metazoans, the same is true of intercellular communication through gap junctions (which close when [Ca2+] rises in the cytosol [171]). This may contribute to epigenetic effects achieved during evolution by non-structural RNA species, which can be transferred from one cell to another, thus harmonizing tissues.
Intimate links between ATP and Ca2+ are observed in mitochondria [172,173]. Mitochondrial Ca2+ is connected with ATP production through stimulation of some mitochondrial dehydrogenases [7], of oxidative phosphorylation [174] and of ATP synthase [175]. This requires rapid crosstalk between the cytosol and the organelle. The key player is the mitochondrial calcium uniporter (MCU) for rapid Ca2+ influx [176,177]; the MCU and its regulators are present at the protozoan level. An MCU-encoding gene is found in Trypanosoma [31]. Paramecium also possesses MCU and associated MIUC protein, as well as the MCU regulator, EMRE [176,178]. Accordingly, in Paramecium, during synchronous exocytosis, Ca2+ rapidly (on a subsecond time scale) flushes into mitochondria; most of the Ca2+, however, is released back into the cytosol within seconds, with only a minor fraction remaining in the organelle [109,179]. Mitochondrial exchangers, H+/Ca2+ and Na+/Ca2+ (NCLX) are known in higher eukaryotes [172,173], but they have not been identified in protozoa.
8. Ca2+ as a regulator of programmed cell death
In mammalian cells, apoptosis is triggered by release of Ca2+ from the endoplasmic reticulum, influx of Ca2+ into mitochondria [180] and release of cytochrome c, parallelled by a rise in [Ca2+]i, and further Ca2+ entry into mitochondria. A protease (caspase) cascade is activated and DNA is cut to nucleosome-size fragments (or multiples, called DNA ladder). Breakdown of subcellular structures, autophagy and phagocytosis of cell fragments [181] are additional events. Altogether, there are multiple effects of Ca2+ during apoptosis in mammalian cells [182], and mitochondria, before degeneration, play a key role in this process [183]. Toxic levels of [Ca2+] and failure of energy supply in conjunction with cytochrome c release [183], functional and structural disturbance of mitochondria and phagocytosis are final events.
Some molecules pertinent to apoptosis can be encountered already in unikont protists, such as choanoflagellates [184]. In bikont protists, such as ciliates, the macronucleus (equivalent to the soma, as opposed to the generative micronucleus), rather than the whole cell, is degraded in a process called ‘nuclear death’ [185]. This includes DNA fragmentation and autophagy of mitochondria, although the role for ATP and Ca2+ remain unsettled. The apoptotic stratagem is only slightly more complex in lower metazoans [186], including secondary reduction of the process in the nematode C. elegans [184]. Altogether, many important details of apoptosis at the roots of unikonts and bikonts are not yet well understood, as summarized recently [187]. It appears that these crucial aspects of apoptosis, including mitochondrial function and involvement of Ca2+, have only successively evolved in the course of metazoan evolution.
9. Intercellular signalling: purinergic transmission links to Ca2+ signals
The concentration gradient for ATP (aimed extracellularly) is likely the highest existing in living cells. Indeed, intracellular concentration of ATP is set at approximately 1–5–10 mM, whereas the extracellular concentration does not exceed 1–10 nM creating thus a difference of approximately 1 million times. An extremely low concentration of extracellular ATP results from constant operation of ectonucleotidases [188], which degrade ATP to ADP, AMP and adenosine. This ATP degrading system has ancient evolutionary roots, being present in bacteria, in early eukaryotes and in protists [188–190]. The steep concentration gradient favours ATP exit upon opening of transmembrane pores and following plasmalemmal damage; it should not be surprising therefore, that evolution rapidly began to use ATP as an intercellular signalling molecule. Already some prokaryotes are known to release ATP, which could be used for intercellular communications [191], whereas the plasmalemmal channels-mediated ATP release is described in polyphenic fungi, type Candida albicans [192].
Biological effects of extracellular ATP are many, and they are widespread throughout virtually all life forms [190]. In prokaryotes, exposure to ATP affects bacterial growth and development, sporulation and germination, changes ion fluxes and alters gene expression [193–196]. In protozoans, extracellular ATP controls multiple responses; in social amoeba, Dictyostelium discoideum, ATP inhibits amoeboid movement, and triggers depolarization and Ca2+ influx; in the ciliates, Paramecium and Tetrahymena, ATP induces avoidance reactions through altering the rate of cilia beating and swimming [47]; in Trypanosoma cruzi, ATP induces parasitosis [193]. Purinergic signalling operates in all kingdoms of life, in plants, in fungi and in animals [190,197,198]. Chemical signalling by ATP, adenine nucleotides and adenosine is present in various forms virtually in all organisms and contributes to a wide variety of functions, from food detection (purinoceptors are an important part of food search behaviour in crustaceans such as lobsters and in insects such as mosquitoes [199,200]) to neurotransmission [201]. ATP-mediated transmission is operative in most (if not in all) organs and systems in vertebrates [197].
At the molecular level, purinergic chemical transmission is mediated by several systems responsible for transmitter release, and by specific plasmalemmal purinoceptors. Purinergic receptors are represented by: (i) ionotropic P2X receptors that are ATP-gated cationic plasmalemmal channels [202,203] and (ii) by metabotropic, G-protein-coupled seven-transmembrane receptors classified as P1 adenosine receptors, P2Y nucleotide (ATP, ADP, UTP, UDP and UDP-sugar) receptors and P0 adenine receptors [204,205]. The ATP-gated ion channels are similar in their architecture to P2X receptors, and had appeared already in protozoa; hitherto, P2X receptors have been identified and characterized in D. discoideum, in the marine green alga Ostreococcus tauri, in the choanoflagellate Monosiga brevicollis, in trematode Schistosoma mansoni and in a tardigrade Hypsibius dujardini [206–210]. The P2X receptors retain their structure throughout evolution, being composed of three subunits with each subunit containing two transmembrane domains.
Purinergic metabotropic receptors generally emerged at later evolutionary stages. Although the rather unique cAMP metabotropic receptors (defined as CAR1–4) are present in D. discoideum [211], the proper metabotropic purinoceptors appeared later. The most evolutionarily ancient adenosine receptors were identified in the sea anemone Nematostella vectensis, and they are abundant in insects, in bivalves (mussels) and in echinoderms [212]. The P2Y metabotropic receptors seem to emerge in vertebrates: the earliest homologues have been found in sharks and skate, Raja erinacea [213]. The evolutionary roots of P0 adenine receptors are unknown; these have been cloned only from mouse and hamster [205].
Release of ATP seems to be a common feature of life forms, and it has been identified in the earliest life forms: ATP is secreted by bacteria, by protozoa, by yeasts and plants, and by many types of cells in all multicellular animals [193]. The pathways for ATP release are represented by (i) transmembrane diffusion via ATP-permeable channels; (ii) active transport; and (iii) exocytosis [214,215]. Arguably, diffusional ATP release that uses plasmalemmal ion (usually anion) channels or unpaired hemichannels of connexin and innexin family, or pannexins (which though related to connexins/innexins do not form gap junctional channels and hence cannot be properly called hemichannels; there are indications that pannexins act as anion channels [216]) is the most ancient and general mechanism; indeed, diffusional ATP release is found throughout phylogeny and is present in many types of cells in mammals, from kidney and osteoclasts to neurons and neuroglia [134,217–220]. Active plasmalemmal transport of ATP is associated with ABC transporters that are expressed already in protists [221]. Finally, exocytotic ATP release that uses vesicles bearing specific nucleotide transports (VNUT/SLC17A9) allows spatially restricted and tightly regulated ATP release characteristic for nervous system, the VNUT-bearing vesicles are found in invertebrates and characterized in the nematode C. elegans, in sea anemone, in sea urchin and in some insects [222,223]; they are present in vertebrates, where they are expressed in various tissues including the brain [215].
In its physiological action, ATP transmission is tightly connected with Ca2+ signalling. Indeed, generation of Ca2+ signals seems to be the general consequence of activation of purinoceptors. All P2X receptors are permeable to Ca2+: the PCa/Pmonovalent is approximately 5 for P2X1 receptors, approximately 2.5 for P2X2 receptors, approximately 1.5 for P2X3 receptors, approximately 4.2 for P2X4 receptors and approximately 1.5 for P2X5 receptors; the heteromeric receptors similarly had an appreciable Ca2+ permeability. The P2X7 receptors' permeability for Ca2+ may vary depending on the state of the pore: dilation of the latter increases PCa/Pmonovalent [193,224–226]. Fractional Ca2+ currents through P2X receptors measured directly varied between 3 and 15 depending on channel composition (with rat P2X1 and human P2X4 receptors having maximal fractional Ca2+ currents at 12.4% and 15%, respectively [227]). This is remarkable, because Ca2+ fluxes through P2X receptors are fully comparable to Ca2+ current through NMDA receptors (fractional Ca2+ permeability approx. 14% [227]) and hence P2X-mediated Ca2+ signals may seriously contribute to postsynaptic plasticity. Activation of ionotropic purinergic receptors triggers Ca2+ signals in many types of cells [228–232]. Incidentally, P2X receptors may also serve as intracellular Ca2+-release channels, as has been shown in D. discoideum [233], further corroborating an intimate relation between ATP and Ca2+ from the very early evolution of cellular signalling. The P2X receptors seem to operate in the membranes of other organelles, including mitochondria, lysosomes and nuclei [234], although whether activation of these intracellular receptors is linked to Ca2+ movements remains to be found.
Metabotropic purinoceptors are distributed even more abundantly than P2X channels; the P2Y receptors and A1 adenosine receptors are expressed virtually in every cell type in mammals [197,235]. In many cells, these receptors are coupled, via several G proteins, to phospholipase C that produces InsP3 and initiates Ca2+ release from the endoplasmic reticulum. The ATP-induced Ca2+ signalling mediated through the metabotropic route seems to be almost universal in non-excitable cells, being present in immune cells [236], in neuroglia [237], in kidney [238], in cells of urinary tract [239], in skin, bone and cartilages [240], in endocrine glands [241], etc. The P2Y receptors and their downstream Ca2+ signalling can be activated not only by ATP, but also by other nucleotides including ADP, UTP, UDP and UDP-glucose [242,243]. The adenosine-mediated signalling and A1 receptors are similarly widely distributed, being abundant in the nervous system, in the heart, in the lungs, in the blood vessels and so on [244–246] and in many cases, these receptors are coupled with cellular Ca2+ signalling. Adenosine is the product of ATP catabolism and is also released (through equilibrating transports) when cells experience high ATP breakdown (for example under stress or in pathology), thus directly coupling ATP biochemistry with ATP/Ca2+ intercellular signalling.
10. Conclusion
During evolution, inevitable leakage of Ca2+ into the eukaryotic cell was not only a problem—because of toxicity even in moderate concentrations—but also a chance. A compromise has been developed between ATP-based energetics and very low solubility of CaATP. However, considerable amounts of ATP have to be invested to keep [Ca2+] low in the cell, thus enabling Ca2+ to serve as a low cost second messenger. As a result, ATP is used by the cell preferably as an energy-storing molecule, whereas Ca2+ mainly serves for intracellular signalling. Intracellular Ca2+ signals can be generated by Ca2+ influx, by Ca2+ release from stores or by a combination of both. The termination of signal is achieved by binding to cytosolic buffers, sequestration and extrusion. There are many intracellular targets for Ca2+, the number of Ca2+-binding proteins increasing considerably during evolution. Another ancient strategy of the eukaryotic cell is to exploit the unavoidable leakage of nucleotide triphosphates from the cell as an extracellular messenger by purinergic receptors. Incidentally, this mirrors the stratagem of how the cell exploits leakage of Ca2+, but in opposite direction. Again, Ca2+ signals triggered by activation of purinoceptors provide for intracellular signalling. Considering the legion of cellular activities that depend on, or are modulated by Ca2+, this is an essential signal for life and death of a cell.
Acknowledgements
The work of H.P. cited herein was supported by the German Research Council. A.V. was supported by the Wellcome Trust, by Alzheimer's research foundation (UK) and by the Federal Target Program 'Research and development in priority areas of the development of the scientific and technological complex of Russia for 2014--2020' of the Ministry of Education and Science of Russia, contract 14.581.21.0016 (Project ID RFMEFI58115X0016).
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Galimov EM. 2009. Concept of sustained ordering and an ATP-related mechanism of life's origin. Int. J. Mol. Sci. 10, 2019–2030. ( 10.3390/ijms10052019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponnamperuma C, Sagan C, Mariner R. 1963. Synthesis of adenosine triphosphate under possible primitive earth conditions. Nature 199, 222–226. ( 10.1038/199222a0) [DOI] [PubMed] [Google Scholar]
- 3.Bryant DE, et al. 2013. Hydrothermal modification of the Sikhote–Alin iron meteorite under low pH geothermal environments. A plausibly prebiotic route to activated phosphorus on the early Earth. Geochim. Cosmochim. Acta 109, 90–112. ( 10.1016/j.gca.2012.12.043) [DOI] [Google Scholar]
- 4.Eigen M. 1971. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58, 465–523. ( 10.1007/BF00623322) [DOI] [PubMed] [Google Scholar]
- 5.Kazmierczak J, Kempe S, Kremer B. 2013. Calcium in the early evolution of living systems: a biohistorical approach. Curr. Org. Chem. 17, 1738–1750. ( 10.2174/13852728113179990081) [DOI] [Google Scholar]
- 6.Fralick P, Riding R. 2015. Steep Rock Lake: sedimentology and geochemistry of an archean carbonate platform. Earth-Sci. Rev. 151, 132–175. ( 10.1016/j.earscirev.2015.10.006) [DOI] [Google Scholar]
- 7.Denton RM. 2009. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 1787, 1309–1316. ( 10.1016/j.bbabio.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 8.Bazil JN, Buzzard GT, Rundell AE. 2010. Modeling mitochondrial bioenergetics with integrated volume dynamics. PLoS Comput. Biol. 6, e1000632 ( 10.1371/journal.pcbi.1000632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speijer D. 2015. Birth of the eukaryotes by a set of reactive innovations: new insights force us to relinquish gradual models. Bioessays 37, 1268–1276. ( 10.1002/bies.201500107) [DOI] [PubMed] [Google Scholar]
- 10.Blackstone NW. 2015. The impact of mitochondrial endosymbiosis on the evolution of calcium signaling. Cell Calcium 57, 133–139. ( 10.1016/j.ceca.2014.11.006) [DOI] [PubMed] [Google Scholar]
- 11.Dacks JB, Field MC. 2007. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 120, 2977–2985. ( 10.1242/jcs.013250) [DOI] [PubMed] [Google Scholar]
- 12.Field MC, Dacks JB. 2009. First and last ancestors: reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins, and nuclear pore complexes. Curr. Opin. Cell Biol. 21, 4–13. ( 10.1016/j.ceb.2008.12.004) [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Cai X. 2015. Evolution of acidic Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium 57, 222–230. ( 10.1016/j.ceca.2014.12.005) [DOI] [PubMed] [Google Scholar]
- 14.Whiteheart SW, Schraw T, Matveeva EA. 2001. N-ethylmaleimide sensitive factor (NSF) structure and function. Int. Rev. Cytol. 207, 71–112. ( 10.1016/S0074-7696(01)07003-6) [DOI] [PubMed] [Google Scholar]
- 15.Volkan-Kacso S, Marcus RA. 2015. Theory for rates, equilibrium constants, and Bronsted slopes in F1-ATPase single molecule imaging experiments. Proc. Natl Acad. Sci. USA 112, 14 230–14 235. ( 10.1073/pnas.1518489112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milo R, Philips R. 2016. Cell biology by the numbers, 400 p. New York and Abingdon: Garland Science. [Google Scholar]
- 17.Goody RS. 2003. The significance of the free energy of hydrolysis of GTP for signal-transducing and regulatory GTPases. Biophys. Chem. 100, 535–544. ( 10.1016/S0301-4622(02)00304-6) [DOI] [PubMed] [Google Scholar]
- 18.Novick P, Zerial M. 1997. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496–504. ( 10.1016/S0955-0674(97)80025-7) [DOI] [PubMed] [Google Scholar]
- 19.Zhen Y, Stenmark H. 2015. Cellular functions of Rab GTPases at a glance. J. Cell Sci. 128, 3171–3176. ( 10.1242/jcs.166074) [DOI] [PubMed] [Google Scholar]
- 20.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529. ( 10.1038/nrm1155) [DOI] [PubMed] [Google Scholar]
- 21.Berridge MJ. 2006. Calcium microdomains: organization and function. Cell Calcium 40, 405–412. ( 10.1016/j.ceca.2006.09.002) [DOI] [PubMed] [Google Scholar]
- 22.Clapham DE. 2007. Calcium signaling. Cell 131, 1047–1058. ( 10.1016/j.cell.2007.11.028) [DOI] [PubMed] [Google Scholar]
- 23.Shemarova IV, Nesterov VP. 2005. Evolution of mechanisms of calcium signaling: the role of calcium ions in signal transduction in prokaryotes. Zh. Evol. Biokhim. Fiziol. 41, 12–17. [DOI] [PubMed] [Google Scholar]
- 24.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. 2007. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium 42, 345–350. ( 10.1016/j.ceca.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 25.Plattner H, Verkhratsky A. 2015. Evolution of calcium signalling. Cell Calcium 57, 121–122. ( 10.1016/j.ceca.2015.02.007) [DOI] [PubMed] [Google Scholar]
- 26.Plattner H, Verkhratsky A. 2015. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 57, 123–132. ( 10.1016/j.ceca.2014.12.004) [DOI] [PubMed] [Google Scholar]
- 27.Ambudkar SV, Lynn AR, Maloney PC, Rosen BP. 1986. Reconstitution of ATP-dependent calcium transport from streptococci. J. Biol. Chem. 261, 15 596–15 600. [PubMed] [Google Scholar]
- 28.Raeymaekers L, Wuytack E, Willems I, Michiels CW, Wuytack F. 2002. Expression of a P-type Ca2+-transport ATPase in Bacillus subtilis during sporulation. Cell Calcium 32, 93–103. ( 10.1016/S0143-4160(02)00125-2) [DOI] [PubMed] [Google Scholar]
- 29.Rosch JW, Sublett J, Gao G, Wang YD, Tuomanen EI. 2008. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol. Microbiol. 70, 435–444. ( 10.1111/j.1365-2958.2008.06425.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lourido S, Moreno SN. 2015. The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp. Cell Calcium 57, 186–193. ( 10.1016/j.ceca.2014.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Docampo R, Huang G. 2015. Calcium signaling in trypanosomatid parasites. Cell Calcium 57, 194–202. ( 10.1016/j.ceca.2014.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdakov D, Petersen OH, Verkhratsky A. 2005. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38, 303–310. ( 10.1016/j.ceca.2005.06.010) [DOI] [PubMed] [Google Scholar]
- 33.Williams DB. 2006. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 119, 615–623. ( 10.1242/jcs.02856) [DOI] [PubMed] [Google Scholar]
- 34.Morgan RO, Martin-Almedina S, Iglesias JM, Gonzalez-Florez MI, Fernandez MP. 2004. Evolutionary perspective on annexin calcium-binding domains. Biochim. Biophys. Acta 1742, 133–140. ( 10.1016/j.bbamcr.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 35.Berridge MJ, Bootman MD, Lipp P. 1998. Calcium: a life and death signal. Nature 395, 645–648. ( 10.1038/27094) [DOI] [PubMed] [Google Scholar]
- 36.Stenmark H. 2012. The Rabs: a family at the root of metazoan evolution. BMC Biol. 10, 68 ( 10.1186/1741-7007-10-68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloepper TH, Kienle CN, Fasshauer D. 2008. SNAREing the basis of multicellularity: consequences of protein family expansion during evolution. Mol. Biol. Evol. 25, 2055–2068. ( 10.1093/molbev/msn151) [DOI] [PubMed] [Google Scholar]
- 38.Plattner H. 2015. Molecular aspects of calcium signalling at the crossroads of unikont and bikont eukaryote evolution: the ciliated protozoan Paramecium in focus. Cell Calcium 57, 174–185. ( 10.1016/j.ceca.2014.12.002) [DOI] [PubMed] [Google Scholar]
- 39.Plattner H. 2015. Calcium signalling in the ciliated protozoan model, Paramecium: strict signal localisation by epigenetically controlled positioning of different Ca2+-channels. Cell Calcium 57, 203–213. ( 10.1016/j.ceca.2014.09.003) [DOI] [PubMed] [Google Scholar]
- 40.Plattner H. 2014. Calcium regulation in the protozoan model, Paramecium tetraurelia. J. Eukaryot Microbiol. 61, 95–114. ( 10.1111/jeu.12070) [DOI] [PubMed] [Google Scholar]
- 41.Verkhratsky A. 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 85, 201–279. ( 10.1152/physrev.00004.2004) [DOI] [PubMed] [Google Scholar]
- 42.Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. 2002. Ca2+ dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca2+-induced Ca2+ release triggered by physiological Ca2+ entry. EMBO J. 21, 622–630. ( 10.1093/emboj/21.4.622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storer AC, Cornish-Bowden A. 1976. Concentration of MgATP2− and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem. J. 159, 1–5. ( 10.1042/bj1590001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garfinkel L, Altschuld RA, Garfinkel D. 1986. Magnesium in cardiac energy metabolism. J. Mol. Cell Cardiol. 18, 1003–1013. ( 10.1016/S0022-2828(86)80289-9) [DOI] [PubMed] [Google Scholar]
- 45.Neves G, Neef A, Lagnado L. 2001. The actions of barium and strontium on exocytosis and endocytosis in the synaptic terminal of goldfish bipolar cells. J. Physiol. 535, 809–824. ( 10.1111/j.1469-7793.2001.t01-1-00809.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brehm P, Eckert R. 1978. Calcium entry leads to inactivation of calcium channel in Paramecium. Science 202, 1203–1206. ( 10.1126/science.103199) [DOI] [PubMed] [Google Scholar]
- 47.Hennessey TM, Kuruvilla HG. 2000. Electrophysiology of Tetrahymena. Methods Cell Biol. 62, 363–377. ( 10.1016/S0091-679X(08)61543-5) [DOI] [PubMed] [Google Scholar]
- 48.Dilger JM, Valentine SJ, Glover MS, Clemmer DE. 2013. A database of alkaline-earth-coordinated peptide cross sections: insight into general aspects of structure. J. Am. Soc. Mass Spectrom. 24, 768–779. ( 10.1007/s13361-013-0579-z) [DOI] [PubMed] [Google Scholar]
- 49.Kee Y, Scheller RH. 1996. Localization of synaptotagmin-binding domains on syntaxin. J. Neurosci. 16, 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park HY, Kim SA, Korlach J, Rhoades E, Kwok LW, Zipfel WR, Waxham MN, Webb WW, Pollack L. 2008. Conformational changes of calmodulin upon Ca2+ binding studied with a microfluidic mixer. Proc. Natl Acad. Sci. USA 105, 542–547. ( 10.1073/pnas.0710810105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saimi Y, Kung C. 2002. Calmodulin as an ion channel subunit. Annu. Rev. Physiol. 64, 289–311. ( 10.1146/annurev.physiol.64.100301.111649) [DOI] [PubMed] [Google Scholar]
- 52.Taylor CW, da Fonseca PC, Morris EP. 2004. IP3 receptors: the search for structure. Trends Biochem. Sci. 29, 210–219. ( 10.1016/j.tibs.2004.02.010) [DOI] [PubMed] [Google Scholar]
- 53.Pang ZP, Sudhof TC. 2010. Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 22, 496–505. ( 10.1016/j.ceb.2010.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rizo J, Chen X, Arac D. 2006. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 16, 339–350. ( 10.1016/j.tcb.2006.04.006) [DOI] [PubMed] [Google Scholar]
- 55.Neher E. 1998. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium 24, 345–357. ( 10.1016/S0143-4160(98)90058-6) [DOI] [PubMed] [Google Scholar]
- 56.Schwaller B. 2010. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2, a004051 ( 10.1101/cshperspect.a004051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang B, Mengersen A, Lee VD. 1988. Molecular cloning of cDNA for caltractin, a basal body-associated Ca2+-binding protein: homology in its protein sequence with calmodulin and the yeast CDC31 gene product. J. Cell Biol. 107, 133–140. ( 10.1083/jcb.107.1.133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sehring IM, Klotz C, Beisson J, Plattner H. 2009. Rapid downregulation of the Ca2+-signal after exocytosis stimulation in Paramecium cells: essential role of a centrin-rich filamentous cortical network, the infraciliary lattice. Cell Calcium 45, 89–97. ( 10.1016/j.ceca.2008.06.004) [DOI] [PubMed] [Google Scholar]
- 59.Cho JH, et al. 2000. Calsequestrin, a calcium sequestering protein localized at the sarcoplasmic reticulum, is not essential for body-wall muscle function in Caenorhabditis elegans. J. Cell Sci. 113, 3947–3958. [DOI] [PubMed] [Google Scholar]
- 60.Williams RJ. 2006. The evolution of calcium biochemistry. Biochim. Biophys. Acta 1763, 1139–1146. ( 10.1016/j.bbamcr.2006.08.042) [DOI] [PubMed] [Google Scholar]
- 61.Grubbs RD. 2002. Intracellular magnesium and magnesium buffering. Biometals 15, 251–259. ( 10.1023/A:1016026831789) [DOI] [PubMed] [Google Scholar]
- 62.Jahnen-Dechent W, Ketteler M. 2012. Magnesium basics. Clin. Kidney J. 5, 3–i14. ( 10.1093/ndtplus/sfr163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piovesan D, Profiti G, Martelli PL, Casadio R. 2012. The human ‘magnesome’: detecting magnesium binding sites on human proteins. BMC Bioinformatics 13(Suppl 14), S10 ( 10.118/1471-2105-13-S14-S10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowan JA. 1998. Magnesium activation of nuclease enzymes—the importance of water. Inorg. Chim. Acta 275–276, 24–27. ( 10.1016/S0020-1693(98)00073-5) [DOI] [Google Scholar]
- 65.Black CB, Huang HW, Cowan JA. 1994. Biological coordination chemistry of magnesium, sodium, and potassium ions. Protein and nucleotide binding sites. Coord. Chem. Rev. 135/136, 165–202. ( 10.1016/0010-8545(94)80068-5) [DOI] [Google Scholar]
- 66.Bojovschi A, Liu MS, Sadus RJ. 2014. Mg2+ coordinating dynamics in Mg:ATP fueled motor proteins. J. Chem. Phys. 140, 115102 ( 10.1063/1.4867898) [DOI] [PubMed] [Google Scholar]
- 67.Ivanov AV, Malygin AA, Karpova GG. 2013. Mg2+ ions affect the structure of the central domain of the 18S rRNA in the vicinity of the ribosomal protein S13 binding site. Mol. Biol. (Mosk) 47, 157–166. [DOI] [PubMed] [Google Scholar]
- 68.De A, Ramesh V, Mahadevan S, Nagaraja V. 1998. Mg2+ mediated sequence-specific binding of transcriptional activator protein C of bacteriophage Mu to DNA. Biochemistry 37, 3831–3838. ( 10.1021/bi972171v) [DOI] [PubMed] [Google Scholar]
- 69.Higashijima T, Ferguson KM, Sternweis PC, Smigel MD, Gilman AG. 1987. Effects of Mg2+ and the bg-subunit complex on the interactions of guanine nucleotides with G proteins. J. Biol. Chem. 262, 762–766. [PubMed] [Google Scholar]
- 70.Nelson MR, Chazin WJ. 1998. Calmodulin as a calcium sensor. In Calmodulin and signal transduction (eds van Eldik LJ, Watterson MD), pp. 17–64. San Diego, MA: Academic Press. [Google Scholar]
- 71.Malmendal A, Linse S, Evenas J, Forsen S, Drakenberg T. 1999. Battle for the EF-hands: magnesium-calcium interference in calmodulin. Biochemistry 38, 11 844–11 850. ( 10.1021/bi9909288) [DOI] [PubMed] [Google Scholar]
- 72.Knape MJ, Ahuja LG, Bertinetti D, Burghardt NC, Zimmermann B, Taylor SS, Herberg FW. 2015. Divalent metal ions Mg2+ and Ca2+ have distinct effects on protein kinase A activity and regulation. ACS Chem. Biol. 10, 2303–2315. ( 10.1021/acschembio.5b00271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klumpp S, Selke D, Hermesmeier J. 1998. Protein phosphatase type 2C active at physiological Mg2+: stimulation by unsaturated fatty acids. FEBS Lett. 437, 229–232. ( 10.1016/S0014-5793(98)01237-X) [DOI] [PubMed] [Google Scholar]
- 74.Brüssow H. 2007. The quest for food. A natural history of eating, 870 p. Heiedelberg, Germany: Springer. [Google Scholar]
- 75.Grosshans BL, Ortiz D, Novick P. 2006. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl Acad. Sci. USA 103, 11 821–11 827. ( 10.1073/pnas.0601617103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petersen OH, Michalak M, Verkhratsky A. 2005. Calcium signalling: past, present and future. Cell Calcium 38, 161–169. ( 10.1016/j.ceca.2005.06.023) [DOI] [PubMed] [Google Scholar]
- 77.Booth IR, Miller S, Muller A, Lehtovirta-Morley L. 2015. The evolution of bacterial mechanosensitive channels. Cell Calcium 57, 140–150. ( 10.1016/j.ceca.2014.12.011) [DOI] [PubMed] [Google Scholar]
- 78.Cai X, Wang X, Patel S, Clapham DE. 2015. Insights into the early evolution of animal calcium signaling machinery: a unicellular point of view. Cell Calcium 57, 166–173. ( 10.1016/j.ceca.2014.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petersen OH, Verkhratsky A. 2007. Endoplasmic reticulum calcium tunnels integrate signalling in polarised cells. Cell Calcium 42, 373–378. ( 10.1016/j.ceca.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 80.Cooper GM. 2000. The cell: a molecular approach, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 81.Docampo R, Ulrich P, Moreno SN. 2010. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Phil. Trans. R. Soc. B 365, 775–784. ( 10.1098/rstb.2009.0179). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robitaille R, Adler EM, Charlton MP. 1990. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron 5, 773–779. ( 10.1016/0896-6273(90)90336-E) [DOI] [PubMed] [Google Scholar]
- 83.Dunlap K, Luebke JI, Turner TJ. 1995. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 18, 89–98. ( 10.1016/0166-2236(95)80030-6) [DOI] [PubMed] [Google Scholar]
- 84.Machemer H, Ogura A. 1979. Ionic conductances of membranes in ciliated and deciliated Paramecium. J. Physiol. 296, 49–60. ( 10.1113/jphysiol.1979.sp012990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia XM, et al. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395, 503–507. ( 10.1038/26758). [DOI] [PubMed] [Google Scholar]
- 86.Levitan IB. 1999. It is calmodulin after all! Mediator of the calcium modulation of multiple ion channels. Neuron 22, 645–648. ( 10.1016/S0896-6273(00)80722-9) [DOI] [PubMed] [Google Scholar]
- 87.Mak DO, Foskett JK. 2015. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: a single-channel point of view. Cell Calcium 58, 67–78. ( 10.1016/j.ceca.2014.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ladenburger EM, Plattner H. 2011. Calcium-release channels in Paramecium. Genomic expansion, differential positioning and partial transcriptional elimination. PLoS ONE 6, e27111 ( 10.1371/journal.pone.0027111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plattner H, Verkhratsky A. 2013. Ca2+ signalling early in evolution: all but primitive. J. Cell Sci. 126, 2141–2150. ( 10.1242/jcs.127449) [DOI] [PubMed] [Google Scholar]
- 90.Galione A. 2015. A primer of NAADP-mediated Ca2+ signalling: from sea urchin eggs to mammalian cells. Cell Calcium 58, 27–47. ( 10.1016/j.ceca.2014.09.010) [DOI] [PubMed] [Google Scholar]
- 91.Verkhratsky A, Shmigol A. 1996. Calcium-induced calcium release in neurones. Cell Calcium 19, 1–14. ( 10.1016/S0143-4160(96)90009-3) [DOI] [PubMed] [Google Scholar]
- 92.Lee HC. 2012. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J. Biol. Chem. 287, 31 633–31 640. ( 10.1074/jbc.R112.349464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leondaritis G, Siokos J, Skaripa I, Galanopoulou D. 2013. Genome-wide analysis of the phosphoinositide kinome from two ciliates reveals novel evolutionary links for phosphoinositide kinases in eukaryotic cells. PLoS ONE 8, e78848 ( 10.1371/journal.pone.0078848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nusblat AD, Bright LJ, Turkewitz AP. 2012. Conservation and innovation in Tetrahymena membrane traffic: proteins, lipids, and compartments. Methods Cell Biol. 109, 141–175. ( 10.1016/B978-0-12-385967-9.00006-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mackrill JJ. 2012. Ryanodine receptor calcium release channels: an evolutionary perspective. Adv. Exp. Med. Biol. 740, 159–182. ( 10.1007/978-94-007-2888-2_7) [DOI] [PubMed] [Google Scholar]
- 96.Mignery GA, Sudhof TC, Takei K, De Camilli P. 1989. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature 342, 192–195. ( 10.1038/342192a0) [DOI] [PubMed] [Google Scholar]
- 97.Albrieux M, Sardet C, Villaz M. 1997. The two intracellular Ca2+ release channels, ryanodine receptor and inositol 1,4,5-trisphosphate receptor, play different roles during fertilization in ascidians. Dev. Biol. 189, 174–185. ( 10.1006/dbio.1997.8674) [DOI] [PubMed] [Google Scholar]
- 98.Ponting CP. 2000. Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem. Sci 25, 48–50. [DOI] [PubMed] [Google Scholar]
- 99.Calcraft PJ, et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600. ( 10.1038/nature08030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. 2014. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 7, ra109. ( 10.1126/scisignal.2005450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. 2010. Calcium signaling via two-pore channels: local or global, that is the question. Am. J. Physiol. Cell Physiol. 298, C430–C441. ( 10.1152/ajpcell.00475.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel S, Docampo R. 2010. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 20, 277–286. ( 10.1016/j.tcb.2010.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee HC. 2005. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J. Biol. Chem. 280, 33 693–33 696. ( 10.1074/jbc.R500012200) [DOI] [PubMed] [Google Scholar]
- 104.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. 2008. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 88, 841–886. ( 10.1152/physrev.00035.2007) [DOI] [PubMed] [Google Scholar]
- 105.Watanabe K. 2011. The localization and functional analysis of two-pore channel proteins. PhD Thesis. Oxford, Oxford University.
- 106.Prole DL, Taylor CW. 2011. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE 6, e26218 ( 10.1371/journal.pone.0026218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. 2013. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 126, 60–66. ( 10.1242/jcs.118836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. 2005. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434, 404–408. ( 10.1038/nature03381) [DOI] [PubMed] [Google Scholar]
- 109.Plattner H, Sehring IM, Mohamed IK, Miranda K, De Souza W, Billington R, Genazzani A, Ladenburger EM. 2012. Calcium signaling in closely related protozoan groups (Alveolata): non-parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma). Cell Calcium 51, 351–382. ( 10.1016/j.ceca.2012.01.006) [DOI] [PubMed] [Google Scholar]
- 110.Docampo R, Moreno SN, Plattner H. 2014. Intracellular calcium channels in protozoa. Eur. J. Pharmacol. 739, 4–18. ( 10.1016/j.ejphar.2013.11.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu H, Martinoia E, Szabo I. 2015. Organellar channels and transporters. Cell Calcium 58, 1–10. ( 10.1016/j.ceca.2015.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Solovyova N, Verkhratsky A. 2003. Neuronal endoplasmic reticulum acts as a single functional Ca2+ store shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurones. Pflugers Arch. 446, 447–454. ( 10.1007/s00424-003-1094-z) [DOI] [PubMed] [Google Scholar]
- 113.Guse AH. 2012. Linking NAADP to ion channel activity: a unifying hypothesis. Sci. Signal. 5, ppe18. ( 10.1126/scisignal.2002890) [DOI] [PubMed] [Google Scholar]
- 114.Guse AH, Lee HC. 2008. NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10. ( 10.1126/scisignal.144re10) [DOI] [PubMed] [Google Scholar]
- 115.Patel S, Marchant JS, Brailoiu E. 2010. Two-pore channels: regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47, 480–490. ( 10.1016/j.ceca.2010.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Penny CJ, Kilpatrick BS, Eden ER, Patel S. 2015. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium 58, 387–396. ( 10.1016/j.ceca.2015.03.006) [DOI] [PubMed] [Google Scholar]
- 117.Levine TP, Patel S. 2016. Signalling at membrane contact sites: two membranes come together to handle second messengers. Curr. Opin. Cell Biol. 39, 77–83. ( 10.1016/j.ceb.2016.02.011) [DOI] [PubMed] [Google Scholar]
- 118.Patel S. 2015. Function and dysfunction of two-pore channels. Sci. Signal. 8, re7 ( 10.1126/scisignal.aab3314) [DOI] [PubMed] [Google Scholar]
- 119.Gees M, Colsoul B, Nilius B. 2010. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2, a003962 ( 10.1101/cshperspect.a003962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nilius B, Owsianik G. 2011. The transient receptor potential family of ion channels. Genome Biol. 12, 218 ( 10.1186/gb-2011-12-3-218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arias-Darraz L, Cabezas D, Colenso CK, Alegria-Arcos M, Bravo-Moraga F, Varas-Concha I, Almonacid DE, Madrid R, Brauchi S. 2015. A transient receptor potential ion channel in Chlamydomonas shares key features with sensory transduction-associated TRP channels in mammals. Plant Cell 27, 177–188. ( 10.1105/tpc.114.131862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wheeler GL, Brownlee C. 2008. Ca2+ signalling in plants and green algae: changing channels. Trends Plant Sci. 13, 506–514. ( 10.1016/j.tplants.2008.06.004) [DOI] [PubMed] [Google Scholar]
- 123.Lima WC, Vinet A, Pieters J, Cosson P. 2014. Role of PKD2 in rheotaxis in Dictyostelium. PLoS ONE 9, e88682 ( 10.1371/journal.pone.0088682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cai X. 2008. Unicellular Ca2+ signaling ‘toolkit’ at the origin of metazoa. Mol. Biol. Evol. 25, 1357–1361. ( 10.1093/molbev/msn077) [DOI] [PubMed] [Google Scholar]
- 125.Edel KH, Kudla J. 2015. Increasing complexity and versatility: how the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium 57, 231–246. ( 10.1016/j.ceca.2014.10.013) [DOI] [PubMed] [Google Scholar]
- 126.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. 2006. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921. ( 10.1083/jcb.200604016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766. ( 10.1126/science.280.5370.1763) [DOI] [PubMed] [Google Scholar]
- 128.Protasi F. 2002. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front Biosci 7, d650–d658. ( 10.2741/A801) [DOI] [PubMed] [Google Scholar]
- 129.Lewis RS. 1999. Store-operated calcium channels. Adv. Second Messenger Phosphoprotein Res. 33, 279–307. ( 10.1016/S1040-7952(99)80014-7) [DOI] [PubMed] [Google Scholar]
- 130.Hofer AM, Fasolato C, Pozzan T. 1998. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+]. J. Cell Biol. 140, 325–334. ( 10.1083/jcb.140.2.325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. 2006. Ca2+ entry through plasma membrane IP3 receptors. Science 313, 229–233. ( 10.1126/science.1125203) [DOI] [PubMed] [Google Scholar]
- 132.Lewis RS. 2007. The molecular choreography of a store-operated calcium channel. Nature 446, 284–287. ( 10.1038/nature05637) [DOI] [PubMed] [Google Scholar]
- 133.Giordano F, et al. 2013. PI(4,5)P2-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153, 1494–1509. ( 10.1016/j.cell.2013.05.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. 2016. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 35, 239–257. ( 10.15252/embj.201592705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Plattner H, Kissmehl R. 2003. Dense-core secretory vesicle docking and exocytotic membrane fusion in Paramecium cells. Biochim. Biophys. Acta 1641, 183–193. ( 10.1016/S0167-4889(03)00092-2) [DOI] [PubMed] [Google Scholar]
- 136.Haberman Y, Grimberg E, Fukuda M, Sagi-Eisenberg R. 2003. Synaptotagmin IX, a possible linker between the perinuclear endocytic recycling compartment and the microtubules. J. Cell Sci. 116, 4307–4318. ( 10.1242/jcs.00719) [DOI] [PubMed] [Google Scholar]
- 137.Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. 2006. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J. Cell Biol. 174, 997–1007. ( 10.1083/jcb.200605004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hay JC. 2007. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep. 8, 236–240. ( 10.1038/sj.embor.7400921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mayorga LS, Beron W, Sarrouf MN, Colombo MI, Creutz C, Stahl PD. 1994. Calcium-dependent fusion among endosomes. J. Biol. Chem. 269, 30 927–30 934. [PubMed] [Google Scholar]
- 140.Chen JL, Ahluwalia JP, Stamnes M. 2002. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J. Biol. Chem. 277, 35 682–35 687. ( 10.1074/jbc.M204157200) [DOI] [PubMed] [Google Scholar]
- 141.Porat A, Elazar Z. 2000. Regulation of intra-Golgi membrane transport by calcium. J. Biol. Chem. 275, 29 233–29 237. ( 10.1074/jbc.M005316200) [DOI] [PubMed] [Google Scholar]
- 142.Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjogren M, Stendahl O. 1990. Cytosolic free calcium elevation mediates the phagosome–lysosome fusion during phagocytosis in human neutrophils. J. Cell Biol. 110, 1555–1564. ( 10.1083/jcb.110.5.1555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoo SH. 2010. Secretory granules in inositol 1,4,5-trisphosphate-dependent Ca2+ signaling in the cytoplasm of neuroendocrine cells. FASEB J. 24, 653–664. ( 10.1096/fj.09-132456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D. 2004. A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 21, 809–818. ( 10.1093/molbev/msh075) [DOI] [PubMed] [Google Scholar]
- 145.Su Z, Zhou X, Loukin SH, Haynes WJ, Saimi Y, Kung C. 2009. The use of yeast to understand TRP-channel mechanosensitivity. Pflugers Arch. 458, 861–867. ( 10.1007/s00424-009-0680-0) [DOI] [PubMed] [Google Scholar]
- 146.Rothman JE. 2014. The principle of membrane fusion in the cell (Nobel lecture). Angew. Chem. Int. Ed. Engl. 53, 12 676–12 694. ( 10.1002/anie.201402380) [DOI] [PubMed] [Google Scholar]
- 147.Sudhof TC. 2014. The molecular machinery of neurotransmitter release (Nobel lecture). Angew. Chem. Int. Ed. Engl. 53, 12 696–12 717. ( 10.1002/anie.201406359) [DOI] [PubMed] [Google Scholar]
- 148.Jaiswal JK, Rivera VM, Simon SM. 2009. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell 137, 1308–1319. ( 10.1016/j.cell.2009.04.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thomas P, Lee AK, Wong JG, Almers W. 1994. A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J. Cell Biol. 124, 667–675. ( 10.1083/jcb.124.5.667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Artalejo CR, Henley JR, McNiven MA, Palfrey HC. 1995. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc. Natl Acad. Sci. USA 92, 8328–8332. ( 10.1073/pnas.92.18.8328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Plattner H, Braun C, Hentschel J. 1997. Facilitation of membrane fusion during exocytosis and exocytosis-coupled endocytosis and acceleration of ‘ghost’ detachment in Paramecium by extracellular calcium. A quenched-flow/freeze-fracture analysis. J. Membr. Biol. 158, 197–208. ( 10.1007/s002329900257) [DOI] [PubMed] [Google Scholar]
- 152.Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH. 1999. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J. Biol. Chem. 274, 25 963–25 966. ( 10.1074/jbc.274.37.25963) [DOI] [PubMed] [Google Scholar]
- 153.Fraga D, Sehring IM, Kissmehl R, Reiss M, Gaines R, Hinrichsen R, Plattner H. 2010. Protein phosphatase 2B (PP2B, calcineurin) in Paramecium: partial characterization reveals that two members of the unusually large catalytic subunit family have distinct roles in calcium-dependent processes. Eukaryot Cell 9, 1049–1063. ( 10.1128/EC.00322-09). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yakel JL. 1997. Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharmacol. Sci. 18, 124–134. ( 10.1016/S0165-6147(97)01046-8) [DOI] [PubMed] [Google Scholar]
- 155.Malleret G, et al. 2001. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104, 675–686. ( 10.1016/S0092-8674(01)00264-1) [DOI] [PubMed] [Google Scholar]
- 156.Aramburu J, Heitman J, Crabtree GR. 2004. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 5, 343–348. ( 10.1038/sj.embor.7400133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rusnak F, Mertz P. 2000. Calcineurin: form and function. Physiol. Rev. 80, 1483–1521. [DOI] [PubMed] [Google Scholar]
- 158.Cesca F, Baldelli P, Valtorta F, Benfenati F. 2010. The synapsins: key actors of synapse function and plasticity. Prog. Neurobiol. 91, 313–348. ( 10.1016/j.pneurobio.2010.04.006) [DOI] [PubMed] [Google Scholar]
- 159.Goldberg JM, Manning G, Liu A, Fey P, Pilcher KE, Xu Y, Smith JL. 2006. The Dictyostelium kinome: analysis of the protein kinases from a simple model organism. PLoS Genet. 2, e38 ( 10.1371/journal.pgen.0020038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim K, Messinger LA, Nelson DL. 1998. Ca2+-dependent protein kinases of Paramecium: cloning provides evidence of a multigene family. Eur. J. Biochem. 251, 605–612. ( 10.1046/j.1432-1327.1998.2510605.x) [DOI] [PubMed] [Google Scholar]
- 161.Kudo S, Muto Y, Nozawa Y. 1985. Regulation by calcium of hormone-insensitive adenylate cyclase and calmodulin-dependent guanylate cyclase in Tetrahymena plasma membrane. Comp. Biochem. Physiol. B 80, 813–816. [DOI] [PubMed] [Google Scholar]
- 162.Kudo S, Muto Y, Inagaki M, Hidaka H, Nozawa Y. 1985. Interaction of calcium-binding proteins with calmodulin-dependent guanylate cyclase in Tetrahymena plasma membrane. Comp. Biochem. Physiol. B 80, 495–498. ( 10.1016/0300-9629(85)90403-7) [DOI] [PubMed] [Google Scholar]
- 163.Imanishi Y, Li N, Sokal I, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K. 2002. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur. J. Neurosci. 15, 63–78. ( 10.1046/j.0953-816x.2001.01835.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coluccio LMEE. 2008. Myosin. A superfamily of molecular motors, 477 p. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 165.Naito Y, Kaneko H. 1972. Reactivated triton-extracted models of paramecium: modification of ciliary movement by calcium ions. Science 176, 523–524. ( 10.1126/science.176.4034.523) [DOI] [PubMed] [Google Scholar]
- 166.Eckert R, Brehm P. 1979. Ionic mechanisms of excitation in paramecium. Annu. Rev. Biophys. Bioeng. 8, 353–383. ( 10.1146/annurev.bb.08.060179.002033). [DOI] [PubMed] [Google Scholar]
- 167.Tamm SL. 2015. Ctenophores and termites - systems for motility. In Cilia and ciliates, flagella and flragellates (eds Hausmann K, Radek R), pp. 147–171. Stuttgart, Germany: Schweizerbart Science Publishers. [Google Scholar]
- 168.Blum JJ, Hayes A, Jamieson GA Jr, Vanaman TC. 1980. Calmodulin confers calcium sensitivity on ciliary dynein ATPase. J. Cell Biol. 87, 386–397. ( 10.1083/jcb.87.2.386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miglietta LA, Nelson DL. 1988. A novel cGMP-dependent protein kinase from Paramecium. J. Biol. Chem. 263, 16 096–16 105. [PubMed] [Google Scholar]
- 170.Schultz JE, Klumpp S. 1991. Calcium-regulated guanylyl cyclases from Paramecium and Tetrahymena. Methods Enzymol. 195, 466–474. ( 10.1016/0076-6879(91)95193-N) [DOI] [PubMed] [Google Scholar]
- 171.Peracchia C. 1978. Calcium effects on gap junction structure and cell coupling. Nature 271, 669–671. ( 10.1038/271669a0) [DOI] [PubMed] [Google Scholar]
- 172.Carafoli E. 2003. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem. Sci. 28, 175–181. ( 10.1016/S0968-0004(03)00053-7) [DOI] [PubMed] [Google Scholar]
- 173.Jacobson J, Duchen MR. 2004. Interplay between mitochondria and cellular calcium signalling. Mol. Cell Biochem. 256–257, 209–218. ( 10.1023/B:MCBI.0000009869.29827.df) [DOI] [PubMed] [Google Scholar]
- 174.Glancy B, Willis WT, Chess DJ, Balaban RS. 2013. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52, 2793–2809. ( 10.1021/bi3015983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Balaban RS. 2009. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta 1787, 1334–1341. ( 10.1016/j.bbabio.2009.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sancak Y, et al. 2013. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382. ( 10.1126/science.1242993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Murgia M, Rizzuto R. 2015. Molecular diversity and pleiotropic role of the mitochondrial calcium uniporter. Cell Calcium 58, 11–17. ( 10.1016/j.ceca.2014.11.001) [DOI] [PubMed] [Google Scholar]
- 178.Kamer KJ, Mootha VK. 2014. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 15, 299–307. ( 10.1002/embr.201337946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hardt M, Plattner H. 2000. Sub-second quenched-flow/X-ray microanalysis shows rapid Ca2+ mobilization from cortical stores paralleled by Ca2+ influx during synchronous exocytosis in Paramecium cells. Eur. J. Cell Biol. 79, 642–652. ( 10.1078/0171-9335-00087) [DOI] [PubMed] [Google Scholar]
- 180.Rizzuto R, et al. 2009. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta 1787, 1342–1351. ( 10.1016/j.bbabio.2009.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. 2008. Calcium and apoptosis: ER–mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27, 6407–6418. ( 10.1038/onc.2008.308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Orrenius S, Nicotera P, Zhivotovsky B. 2011. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 119, 3–19. ( 10.1093/toxsci/kfq268) [DOI] [PubMed] [Google Scholar]
- 183.Wang X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933. [PubMed] [Google Scholar]
- 184.Zmasek CM, Godzik A. 2013. Evolution of the animal apoptosis network. Cold Spring Harb. Perspect. Biol. 5, a008649 ( 10.1101/cshperspect.a008649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Endoh H, Kobayashi T. 2006. Death harmony played by nucleus and mitochondria: nuclear apoptosis during conjugation of Tetrahymena. Autophagy 2, 129–131. ( 10.4161/auto.2.2.2368) [DOI] [PubMed] [Google Scholar]
- 186.Rosenberg SH. 2011. Mammalian apoptosis in a parasitic worm. Proc. Natl Acad. Sci. USA 108, 6695–6696. ( 10.1073/pnas.1104151108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Plattner H. 2015. Signalling in ciliates: long- and short-range signals and molecular determinants for cellular dynamics. Biol. Rev. Camb. Philos. Soc. ( 10.1111/brv.12218) [DOI] [PubMed] [Google Scholar]
- 188.Zimmermann H, Zebisch M, Strater N. 2012. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 8, 437–502. ( 10.1007/s11302-012-9309-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vivian JP, et al. 2010. Crystal structure of a Legionella pneumophila ecto-triphosphate diphosphohydrolase, a structural and functional homolog of the eukaryotic NTPDases. Structure 18, 228–238. ( 10.1016/j.str.2009.11.014) [DOI] [PubMed] [Google Scholar]
- 190.Verkhratsky A, Burnstock G. 2014. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays 36, 697–705. ( 10.1002/bies.201400024) [DOI] [PubMed] [Google Scholar]
- 191.Ivanova EP, Alexeeva YV, Pham DK, Wright JP, Nicolau DV. 2006. ATP level variations in heterotrophic bacteria during attachment on hydrophilic and hydrophobic surfaces. Int. Microbiol. 9, 37–46. [PubMed] [Google Scholar]
- 192.Vylkova S, Sun JN, Edgerton M. 2007. The role of released ATP in killing Candida albicans and other extracellular microbial pathogens by cationic peptides. Purinergic Signal. 3, 91–97. ( 10.1007/s11302-006-9040-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burnstock G, Verkhratsky A. 2012. Purinergic signalling and the nervous system. Heidelberg, Germany: Springer. [Google Scholar]
- 194.Choi SB, Kim JU, Joo H, Min CK. 2010. Identification and characterization of a novel bacterial ATP-sensitive K+ channel. J. Microbiol. 48, 325–330. ( 10.1007/s12275-010-9231-9) [DOI] [PubMed] [Google Scholar]
- 195.Li M, Kim TJ, Kwon HJ, Suh JW. 2008. Effects of extracellular ATP on the physiology of Streptomyces coelicolor A32. FEMS Microbiol. Lett. 286, 24–31. ( 10.1111/j.1574-6968.2008.01248.x) [DOI] [PubMed] [Google Scholar]
- 196.Naseem R, Wann KT, Holland IB, Campbell AK. 2009. ATP regulates calcium efflux and growth in E. coli. J. Mol. Biol. 391, 42–56. ( 10.1016/j.jmb.2009.05.064) [DOI] [PubMed] [Google Scholar]
- 197.Burnstock G, Verkhratsky A. 2009. Evolutionary origins of the purinergic signalling system. Acta Physiol. (Oxf.) 195, 415–447. ( 10.1111/j.1748-1716.2009.01957.x) [DOI] [PubMed] [Google Scholar]
- 198.Roux SJ, Steinebrunner I. 2007. Extracellular ATP: an unexpected role as a signaler in plants. Trends Plant Sci. 12, 522–527. ( 10.1016/j.tplants.2007.09.003) [DOI] [PubMed] [Google Scholar]
- 199.Galun R, Kabayo JP. 1988. Gorging response of Glossina palpalis palpalis to ATP analogues. Physiol. Entomol. 13, 419–423. ( 10.1111/j.1365-3032.1988.tb01125.x) [DOI] [Google Scholar]
- 200.Zimmer-Faust RK. 1993. ATP: A potent prey attractant evoking carnivory. Luminol. Oceonogr. 38, 1271–1275. ( 10.4319/lo.1993.38.6.1271) [DOI] [Google Scholar]
- 201.Backus KH, Braum S, Lohner F, Deitmer JW. 1994. Neuronal responses to purinoceptor agonists in the leech central nervous system. J. Neurobiol. 25, 1283–1292. ( 10.1002/neu.480251009) [DOI] [PubMed] [Google Scholar]
- 202.Khakh BS, North RA. 2006. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532. ( 10.1038/nature04886) [DOI] [PubMed] [Google Scholar]
- 203.North RA. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067. ( 10.1152/physrev.00015.2002) [DOI] [PubMed] [Google Scholar]
- 204.Ralevic V, Burnstock G. 1998. Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492. [PubMed] [Google Scholar]
- 205.Thimm D, Knospe M, Abdelrahman A, Moutinho M, Alsdorf BB, von Kugelgen I, Schiedel AC, Muller CE. 2013. Characterization of new G protein-coupled adenine receptors in mouse and hamster. Purinergic Signal. 9, 415–426. ( 10.1007/s11302-013-9360-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Agboh KC, Webb TE, Evans RJ, Ennion SJ. 2004. Functional characterization of a P2X receptor from Schistosoma mansoni. J. Biol. Chem. 279, 41 650–41 657. ( 10.1074/jbc.M408203200) [DOI] [PubMed] [Google Scholar]
- 207.Baines A, Parkinson K, Sim JA, Bragg L, Thompson CR, North RA. 2013. Functional properties of five Dictyostelium discoideum P2X receptors. J. Biol. Chem. 288, 20 992–21 000. ( 10.1074/jbc.M112.445346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bavan S, Straub VA, Blaxter ML, Ennion SJ. 2009. A P2X receptor from the tardigrade species Hypsibius dujardini with fast kinetics and sensitivity to zinc and copper. BMC Evol. Biol. 9, 17 ( 10.1186/1471-2148-9-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fountain SJ. 2013. Primitive ATP-activated P2X receptors: discovery, function and pharmacology. Front. Cell Neurosci. 7, 247 ( 10.3389/fncel.2013.00247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fountain SJ, Cao L, Young MT, North RA. 2008. Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J. Biol. Chem. 283, 15 122–15 126. ( 10.1074/jbc.M801512200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Meima M, Schaap P. 1999. Dictyostelium development-socializing through cAMP. Semin. Cell Dev. Biol. 10, 567–576. ( 10.1006/scdb.1999.0340) [DOI] [PubMed] [Google Scholar]
- 212.Kukulski F, Levesque SA, Sevigny J. 2011. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv. Pharmacol. 61, 263–299. ( 10.1016/B978-0-12-385526-8.00009-6) [DOI] [PubMed] [Google Scholar]
- 213.Hoyle CH. 2011. Evolution of neuronal signalling: transmitters and receptors. Auton. Neurosci. 165, 28–53. ( 10.1016/j.autneu.2010.05.007) [DOI] [PubMed] [Google Scholar]
- 214.Pankratov Y, Lalo U, Verkhratsky A, North RA. 2007. Quantal release of ATP in mouse cortex. J. Gen. Physiol. 129, 257–265. ( 10.1085/jgp.200609693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pankratov Y, Lalo U, Verkhratsky A, North RA. 2006. Vesicular release of ATP at central synapses. Pflugers Arch. 452, 589–597. ( 10.1007/s00424-006-0061-x) [DOI] [PubMed] [Google Scholar]
- 216.Ma W, Compan V, Zheng W, Martin E, North RA, Verkhratsky A, Surprenant A. 2012. Pannexin 1 forms an anion-selective channel. Pflugers Arch. 463, 585–592. ( 10.1007/s00424-012-1077-z) [DOI] [PubMed] [Google Scholar]
- 217.Fields RD. 2011. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron–glia signaling. Semin. Cell Dev. Biol. 22, 214–219. ( 10.1016/j.semcdb.2011.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sabirov RZ, Dutta AK, Okada Y. 2001. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J. Gen. Physiol. 118, 251–266. ( 10.1085/jgp.118.3.251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Samuels SE, Lipitz JB, Dahl G, Muller KJ. 2010. Neuroglial ATP release through innexin channels controls microglial cell movement to a nerve injury. J. Gen. Physiol. 136, 425–442. ( 10.1085/jgp.201010476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Suadicani SO, Brosnan CF, Scemes E. 2006. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385. ( 10.1523/JNEUROSCI.3902-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sauvage V, Aubert D, Escotte-Binet S, Villena I. 2009. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol. Biochem. Parasitol. 167, 81–94. ( 10.1016/j.molbiopara.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 222.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. 2008. Identification of a vesicular nucleotide transporter. Proc. Natl Acad. Sci. USA 105, 5683–5686. ( 10.1073/pnas.0800141105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R. 2010. Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics 11, 17 ( 10.1186/1471-2164-11-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. 2009. P2X receptors and synaptic plasticity. Neuroscience 158, 137–148. ( 10.1016/j.neuroscience.2008.03.076) [DOI] [PubMed] [Google Scholar]
- 225.Lalo U, Pankratov Y, Parpura V, Verkhratsky A. 2011. Ionotropic receptors in neuronal–astroglial signalling: what is the role of ‘excitable’ molecules in non-excitable cells? Biochim. Biophys. Acta 1813, 992–1002. ( 10.1016/j.bbamcr.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 226.Egan TM, Samways DS, Li Z. 2006. Biophysics of P2X receptors. Pflugers Arch. 452, 501–512. ( 10.1007/s00424-006-0078-1) [DOI] [PubMed] [Google Scholar]
- 227.Egan TM, Khakh BS. 2004. Contribution of calcium ions to P2X channel responses. J. Neurosci. 24, 3413–3420. ( 10.1523/JNEUROSCI.5429-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Palygin O, Lalo U, Verkhratsky A, Pankratov Y. 2010. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 48, 225–231. ( 10.1016/j.ceca.2010.09.004) [DOI] [PubMed] [Google Scholar]
- 229.White SM, Imig JD, Kim TT, Hauschild BC, Inscho EW. 2001. Calcium signaling pathways utilized by P2X receptors in freshly isolated preglomerular MVSMC. Am. J. Physiol. Renal Physiol. 280, F1054–F1061. [DOI] [PubMed] [Google Scholar]
- 230.Pubill D, Dayanithi G, Siatka C, Andres M, Dufour MN, Guillon G, Mendre C. 2001. ATP induces intracellular calcium increases and actin cytoskeleton disaggregation via P2x receptors. Cell Calcium 29, 299–309. ( 10.1054/ceca.2000.0194) [DOI] [PubMed] [Google Scholar]
- 231.Zhao X, Falck JR, Gopal VR, Inscho EW, Imig JD. 2004. P2X receptor-stimulated calcium responses in preglomerular vascular smooth muscle cells involves 20-hydroxyeicosatetraenoic acid. J. Pharmacol. Exp. Ther. 311, 1211–1217. ( 10.1124/jpet.104.070797) [DOI] [PubMed] [Google Scholar]
- 232.Surprenant A, North RA. 2009. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359. ( 10.1146/annurev.physiol.70.113006.100630) [DOI] [PubMed] [Google Scholar]
- 233.Sivaramakrishnan V, Fountain SJ. 2013. Intracellular P2X receptors as novel calcium release channels and modulators of osmoregulation in Dictyostelium: a comparison of two common laboratory strains. Channels (Austin) 7, 43–46. ( 10.4161/chan.22737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Burnstock G. 2015. Intracellular expression of purinoceptors. Purinergic Signal. 11, 275–276. ( 10.1007/s11302-015-9455-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Burnstock G, Knight GE. 2004. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 240, 31–304. ( 10.1016/S0074-7696(04)40002-3) [DOI] [PubMed] [Google Scholar]
- 236.Burnstock G, Boeynaems JM. 2014. Purinergic signalling and immune cells. Purinergic Signal. 10, 529–564. ( 10.1007/s11302-014-9427-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Verkhratsky A, Krishtal OA, Burnstock G. 2009. Purinoceptors on neuroglia. Mol. Neurobiol. 39, 190–208. ( 10.1007/s12035-009-8063-2) [DOI] [PubMed] [Google Scholar]
- 238.Burnstock G, Evans LC, Bailey MA. 2014. Purinergic signalling in the kidney in health and disease. Purinergic Signal. 10, 71–101. ( 10.1007/s11302-013-9400-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Burnstock G. 2014. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 10, 103–155. ( 10.1007/s11302-013-9395-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Burnstock G, Verkhratsky A. 2010. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 1, e9 ( 10.1038/cddis.2009.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burnstock G. 2014. Purinergic signalling in endocrine organs. Purinergic Signal. 10, 189–231. ( 10.1007/s11302-013-9396-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Abbracchio MP, et al. 2003. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 24, 52–55. ( 10.1016/S0165-6147(02)00038-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Abbracchio MP, et al. 2006. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341. ( 10.1124/pr.58.3.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. 2001. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552. [PMC free article] [PubMed] [Google Scholar]
- 245.Burnstock G, Fredholm BB, Verkhratsky A. 2011. Adenosine and ATP receptors in the brain. Curr. Top. Med. Chem. 11, 973–1011. ( 10.2174/156802611795347627) [DOI] [PubMed] [Google Scholar]
- 246.Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. 2000. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 362, 364–374. ( 10.1007/s002100000313) [DOI] [PubMed] [Google Scholar]