Abstract
Impaired epithelial barrier function is a hallmark of inflammatory bowel diseases, such as colitis, contributing to diarrhoea and perpetuating inflammation. We show that the zinc sensing receptor, ZnR/GPR39, triggers intracellular Ca2+ signalling in colonocytes thereby inducing occludin expression. Moreover, ZnR/GPR39 is essential for epithelial barrier recovery in the dextran sodium sulfate (DSS) ulcerative colitis model. Loss of ZnR/GPR39 results in increased susceptibility to DSS-induced inflammation, owing to low expression of the tight junction protein occludin and impaired epithelial barrier. Recovery of wild-type (WT) mice from the DSS insult was faster than that of ZnR/GPR39 knockout (KO) mice. Enhanced recovery of the epithelial layer and increased crypt regeneration were observed in WT mice compared with ZnR/GPR39 KO, suggesting that ZnR/GPR39 is promoting epithelial barrier integrity following DSS insult. Indeed, cell proliferation and apical expression of occludin, following the DSS-induced epithelial erosion, were increased in WT tissue but not in ZnR/GPR39 KO tissue. Importantly, survival following DSS treatment was higher in WT mice compared with ZnR/GPR39 KO mice. Our results support a direct role for ZnR/GPR39 in promoting epithelial renewal and barrier function following DSS treatment, thereby affecting the severity of the disease. We suggest ZnR/GPR39 as a novel therapeutic target that can improve epithelial barrier function in colitis.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: zinc, ZnR/GPR39, Ca2+ signalling, tight junction, intestinal epithelium, colitis
1. Background
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), are characterized by chronic dysregulation of the mucosal layer of the gastrointestinal system [1]. They share a relapsing and remitting chronic condition and involve poorly characterized genetic component, immune dysregulation and environmental factors. Ulcerative colitis is associated with increased infiltration of inflammatory cells and oedema, distortion of crypt structure and loss of the epithelial barrier that is followed by erosion and ulcerations. Of major importance in the etiology of the disease are alterations in tight junctions [2]. Tight junctions connect the epithelial cells, and induce a continuous boundary between the lumen and the mucosal layer thereby protecting from paracellular permeation and subsequent inflammation. Occludin is a critical component of the tight junction complexes but its localization to the apical cell surface may be disrupted by pathological stimuli [3,4]. Although mechanisms underlying IBD are not fully understood, breakdown of the physical epithelial tight junction barrier often precedes the onset of inflammation. Furthermore, animal models of experimental colitis, suggest that dysregulation of the tight junctions enhances epithelial apoptosis and induces colitis [5].
Zinc enhances functional integrity of the digestive system [6–8]. Zinc deficiency is frequently found in IBD patients and linked to attenuated renewal of the epithelium and the onset severe diarrhoea [9–11]. Consistently, zinc supplementation to Crohn's disease patients during clinical remission or in animal models of experimental colitis reduced intestinal permeability and mucosal damage [12–15]. A specific Zn2+ sensing receptor (ZnR) was functionally described in this tissue [16,17] and was later associated with GPR39 as the molecular moiety mediating its activity [18,19]. The ZnR/GPR39 triggers cellular Ca2+ signalling pathways and enhances cell proliferation, differentiation and survival [20]. The ZnR/GPR39 is prominently expressed in the gastrointestinal tract [21], and the analysis of ZnR/GPR39 knockout (KO) mice suggested a mild gastric phenotype of accelerated gastric emptying and higher volume of gastric fluid secretion [22]. Importantly, these mice have normal life expectancy. The role for ZnR/GPR39 in intestinal diseases linked to barrier function, however, had remained elusive. We show, here, that ZnR/GPR39 activates intracellular Ca2+ signalling and promotes occludin expression and recovery in the dextran sodium sulfate (DSS) ulcerative colitis model.
2. Material and methods
(a). Caco-2 imaging
Caco-2 colonocytic cells were grown in DMEM medium [20] containing 100 U ml–1 penicillin, 0.1 mg ml–1 streptomycin, 2 mM glutamine, 10% foetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), 1% (v/v) nonessential amino acids (Biological Industries) and 2 mM sodium pyruvate (Sigma-Aldrich, Rehovot, Israel) in a 5% CO2 humidified atmosphere at 37°C. Fluorescent imaging measurements were acquired on cells loaded with Fura-2 acetoxymethyl ester (AM; TEF-Labs, Austin, TX, USA) in Ringer's solution (30 min 2.5 µM). Fura-2 was excited at 340 and 380 nm, and imaged with a 510 nm long-pass filter, the ratio of the signal is shown in the traces. Bar graphs show the rate of the initial response and are the means of at least three independent experiments, with averaged responses of 7–10 cells from each of the n slides, as marked.
(b). Occludin recovery in Caco-2 cells
Caco-2 cells were differentiated on coverslips for 14 days. Subsequently, cells were treated with 0.05% Ca2+ for 16 h to deplete occludin [23] and then activation of ZnR/GPR39 was triggered by Zn2+ (200 µM, 2 min) in the presence or the absence of Ca2+ chelators. Cells were harvested into lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 10 μM MgCl2, 25 mM NaF), in the presence of Protease Inhibitor Cocktail (1 : 50, Sigma-Aldrich). Protein concentrations were determined and whole-cell lysates (12 μg) were separated on 10% SDS-PAGE and blotted onto nitrocellulose membranes [20]. Antibodies raised against occludin (Invitrogen, Life Technologies) were used, and blots were quantified using ImageJ Gels Plugin.
(c). Induction of colitis and clinical evaluation
Experimental procedures were performed in accordance with a protocol approved by the committee for the Ethical Care and Use of Animal in Experiments at the Faculty of Health Science at Ben-Gurion University of the Negev. ZnR/GPR39 KO mice and wild-type (WT) littermates, of C57BL background, 8–10 weeks old were used in this study [22]. Mice were given DSS (MP Biomedicals) freshly prepared daily, at a concentration of 2.5% in drinking water for 6 days (disease phase), followed by 4 days of water (without DSS, recovery phase) administration. The control group received water without DSS during the whole experiment. The experimental protocol used in the current project was calibrated for this strain of mice [24]. Since high mortality rates in both genotypes were monitored using the standard protocol [25], we used 2.5% DSS for 6 days only. Mice exhibited severe disease activity clinically and histologically, after 6 days of DSS: extensive area of complete destruction of the mucosa with ulcers and active inflammation, along with small regions of architectural distortion of crypt, parallel to severe disease activity in UC patients [26].
Clinical evaluation was done daily for each mouse, by a person blind to the genotype—the disease activity index was determined by scoring changes in weight, haemoccult positivity or gross bleeding and stool consistency [25]. The score for each parameter was between 0 and 4; when 4 represents the most severe condition (table 1). The score for weight changes were obtained as percentage of initial weight. Stool blood kit (Cenogenics) was used in order to determine severity of bleeding.
Table 1.
Disease activity index, adapted from [25].
| score | weight loss (%) | stool consistency | occult/gross bleeding |
|---|---|---|---|
| 0 | none | normal | normal |
| 1 | 1–5 | ||
| 2 | 6–10 | loose stools | haemoccult + |
| 3 | 11–20 | ||
| 4 | >20 | diarrhoea | gross bleeding |
(d). Genotyping of mice
Polymerase chain reaction (PCR) was used to screen the mice, using tail biopsy samples that were incubated overnight at 55° in lysis buffer containing: 50 Mm KCl, 10 Mm Tris–HCl PH-8.3, 25 Mm MgCl2, 0.45% NP-40, 0.45% Tween-20 and fresh proteinase K (Sigma-Aldrich). PCR was done with Red Mix (LAROVA GmbH) solution according to the manufacturer's protocol using the following primers: 5′-ACCCTCATCTTGGTGTACCT-‘3 and 5′-ATGTAGCGCTCAAAGCTGAG-‘3 (Sigma-Aldrich) that amplified a 311 bp band from the WT allele. ZnR/GPR39 KO primers were 5′-GGAACTCTCACTCGACCTGGG-‘3 and 5′-GCAGCGCATCGCCTTCTATC-‘3 (Sigma-Aldrich) amplified a 262 bp band. After amplification, samples were mixed with Syber Green (Life Technologies) and loaded in electrophoresis agarose gel.
(e). Histological analysis
Mice were sacrificed at 0, 3, 6, 8, 10 days following the addition of DSS and distal colons were dissected. Tissues were washed in phosphate-buffered saline (PBS) solution and immediately moved to 4% paraformaldehyde (Sigma-Aldrich) for 24 h of fixation. Next, tissues were washed in ethanol at increasing concentrations (70–100%), and then moved to xylene (Biolab) and finally paraffinized. Paraffin blocks containing longitudinally positioned full-length colons were then cut into 4 µm slices and stained with haematoxylin and eosin (HE), using a standard protocol. The HE-stained tissue sections were analysed [27] by a person blind to the genotype and treatment stage. Tissues were graded by the following features: level of inflammation, severity of the oedema, extent of inflamed tissue within the wall (table 2). To directly determine the damage to the epithelial layer, we also determined level of crypt damage within the epithelium and the general epithelial damage was determined, during the recovery phase only, as the extent of injured non-regenerated tissue along the surface. Each of the indices was blindly determined and given a grade as described in table 2. For each feature, we also scored per cent of involvement along the distal colon: 1: 1–25%, 2: 26–50%, 3: 51–75%, 4: 76–100% of total colon length. We then multiplied the grades of each of the features by the involvement score, to receive a histological index in each of the features (ranging from 0–16), the histological indices (exact values) achieved using this analysis are included in the text. For simplicity of presentation in the bar graphs, all features were normalized to the relevant score of the WT.
Table 2.
Quantitative histological grading of colitis; adapted from [27].
| feature graded | grade | description |
|---|---|---|
| inflammation | 0 | none |
| 1 | slight | |
| 2 | moderate | |
| 3 | severe | |
| severity of oedema | 0 | none |
| 1 | mucosa | |
| 2 | mucosa and submucosa | |
| 3 | transmural | |
| extent of inflamed tissue within the wall (extent of damage) | 0 | none |
| 1 | mucosa | |
| 2 | mucosa and submucosa | |
| 3 | transmural | |
| crypt damage | 0 | none |
| 1 | basal 1/3 damage | |
| 2 | basal 2/3 damage | |
| 3 | only surface epithelium intact | |
| 4 | entire crypt and epithelium lost | |
| epithelial damage (used only for the recovery phase) | 0 | complete regeneration or normal surface epithelium |
| 1 | almost complete regeneration of surface epithelium | |
| 2 | regeneration of surface epithelium with crypt depletion | |
| 3 | surface epithelium not intact | |
| 4 | no epithelial repair |
For occludin staining, slices were prepared as described above and were permeabilized by incubating the slices with proteinase at concentration 1 mg ml–1 in 37°C for 10 min (for occludin staining). Blocking was then done using normal goat serum for 30 min in room temperature. Tissues were incubated for 1 h with anti-mouse polyclonal antibodies against occludin (1 : 50) (Invitrogen) and then 1 h with a secondary anti-rabbit IgG (1 : 150) fluorescently labelled with Cy3 (Jackson ImmunoResearch). At least three images were taken from each slide using florescence microscopy at 20× magnification and a single determined set of parameters for the image acquisition. Quantitative analysis was performed by counting pixels that were stained above a predetermined threshold in a constant sized region along the mucosa in each image.
(f). Cell proliferation assay
Mice were intraperitoneally injected with 50 mg kg–1 of the thymidine analogue, 5-bromo-2-deoxyuridine (BrdU) 2 h before sacrifice. Paraffin sections of distal colons were prepared as described above and were incubated with HCl 2 M at 37°C for 10 min for permeabilization. Blocking was then made using normal goat serum (Biological Industries) for 1 h in room temperature. Tissues were incubated at 4°C overnight with rat polyclonal antibodies against BrdU (1 : 400) (Sigma-Aldrich) and then 1 h at room temperature with anti- rat IgG (1 : 100) fluorescently labelled with Cy3 (Jackson ImmunoResearch). On some of the tissues, we used DAPI-containing immunomount solution (DAPI Fluoromount-G, Southern Biotech). Images were taken using a florescence microscope at 20× magnification. Number of BrdU-stained cells were counted and normalized to the number of crypts (as seen using bright-field microscopy) in the same area.
(g). Statistical analysis
Data are expressed as mean ± s.e.m. Statistical significance between groups were analysed by ANOVA multiple variables test, followed by Tukey–Kramers post-hoc test using the Statistic program, or Student's T-test as appropriate.
3. Results
(a). Znr/GPR39 triggers metabotropic Ca2+ signalling and upregulates occludin expression in Caco-2 colonocytes
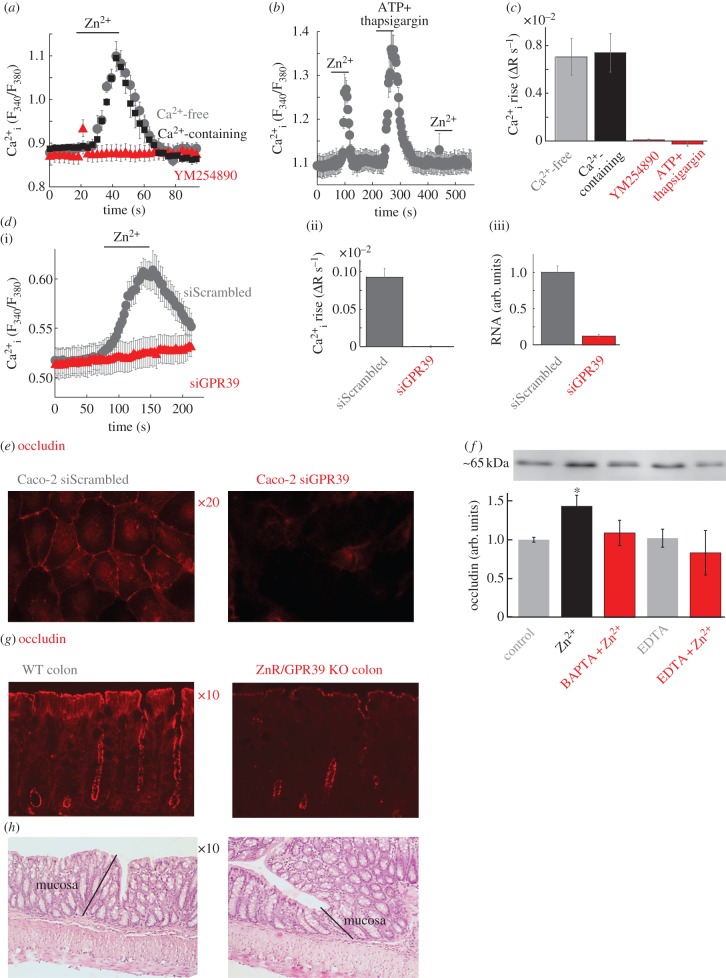
We first asked if ZnR/GPR39 triggers metabotropic Ca2+ signalling in colonocytes. Application of Zn2+ (200 µM) to Caco-2 cells loaded with the Ca2+ indicator Fura-2 triggered a robust response in the absence or the presence of extracellular Ca2+ (figure 1a,c). This response was completely eliminated by the selective Gαq inhibitor, YM254890 [28,29], suggesting that it is mediated by the IP3 pathway. We then depleted intracellular Ca2+ stores by applying ATP (100 µM), which activates the purinergic receptor to release Ca2+ from the stores, together with thapsigargin (200 nM) that inhibits the SERCA and blocks replenishment of the Ca2+ stores [30]. The Zn2+-dependent Ca2+ response was abolished following depletion of intracellular Ca2+ stores (figure 1b,c), indicating that Zn2+ activates metabotropic Ca2+ signalling in Caco-2 colonocytes. Importantly, the intracellular Ca2+ response was also abolished when ZnR/GPR39 was silenced using an siRNA construct (figure 1d), indicating that it is directly mediated by ZnR/GPR39. To determine a physiological role for ZnR/GPR39-dependent Ca2+ signalling, we asked whether ZnR/GPR39 regulates the expression of occludin, an important factor of the tight junction barrier in the colon [3]. Indeed, silencing of ZnR/GPR39 in Caco-2 cells largely downregulated occludin expression (figure 1e). Next we asked if ZnR/GPR39-dependent Ca2+ signalling is essential for regulating occludin expression. Caco-2 cells were differentiated thus forming tight junctions, and Ca2+ was then removed from the growth medium for 16 h to lower occludin expression [23]. Activation of ZnR/GPR39 by Zn2+ (200 µM) largely enhanced the recovery of occludin expression compared with control cells not treated with Zn2+ (figure 1f). The Zn2+-dependent occludin expression was abolished when Ca2+ signalling was inhibited by the intracellular Ca2+ chelator BAPTA, or when extracellular Zn2+ was chelated by EDTA (100 µM). Thus, our results indicate that ZnR/GPR39, via activation of intracellular Ca2+ signals, enhances recovery of occludin expression.
Figure 1.
ZnR/GPR39 triggers Ca2+ signalling in Caco-2 colonocytes and regulates occludin expression. (a) Caco-2 colonocytes were loaded with Fura-2 and responses were monitored in Ringer's solution with (grey) and without (black) Ca2+-free following the addition of Zn2+ (200 µM) to control cell or cells treated with the Gq inhibitor YM254890 (1 µM). Averaged response from seven cells in one slide is shown. (b) The response to Zn2+ (200 µM, as in a) was monitored before or after Ca2+ store depletion (ATP 100 µM+thapsigargin 200 nM). (c) The average initial rate of the Ca2+ response as monitored in (a,b) (n = 10). (d) The response to Zn2+ (200 µM, as in a) was monitored in Caco-2 cells treated with an siRNA construct aimed to silence ZnR/GPR39 (siGPR39) or a scrambled control (siScrambled). Panel (ii) shows the average rate of initial Ca2+ response (n = 20), panel (iii) shows mRNA level in control or siGPR39 silenced cells (n = 3). (e) Representative immunofluorescence analysis of the expression of the tight junction protein occludin in siScrambled or siGPR39 Caco-2 cells. (f) Analysis of occludin expression level after Ca2+ depletion (see Material and methods) in differentiated Caco-2 cells treated with Zn2+ (200 µM) or without it (control) in the presence of the intracellular Ca2+ chelator BAPTA-AM (5 µM) or the non-permeable Ca2+ chelator EDTA (100 µM) (n = 2, *p < 0.05). (g) Representative immunofluorescence analysis of the expression of the tight junction protein occludin in colon tissue from WT or ZnR/GPR39 KO mice. (h) Histochemical (H&E) analysis of colon tissue sections from WT and ZnR/GPR39 KO mice in sections of colon. The mucosa layer is marked, showing similar crypt formation and low extent of inflammation in both genotypes.
We therefore asked whether ZnR/GPR39 may also affect the expression of occludin in vivo. Expression of the tight junction protein occludin was fourfold lower in mice lacking ZnR/GPR39 (ZnR/GPR39 KO) compared with WT mice (figure 1g). Histological analysis of HE-stained colon tissues indicated that mucosal/submucosal organization was similar in both genotypes, crypts were intact and no oedema or excessive inflammatory cells were seen in both genotypes (figure 1h).
(b). Acute colitis phase: DSS treatment
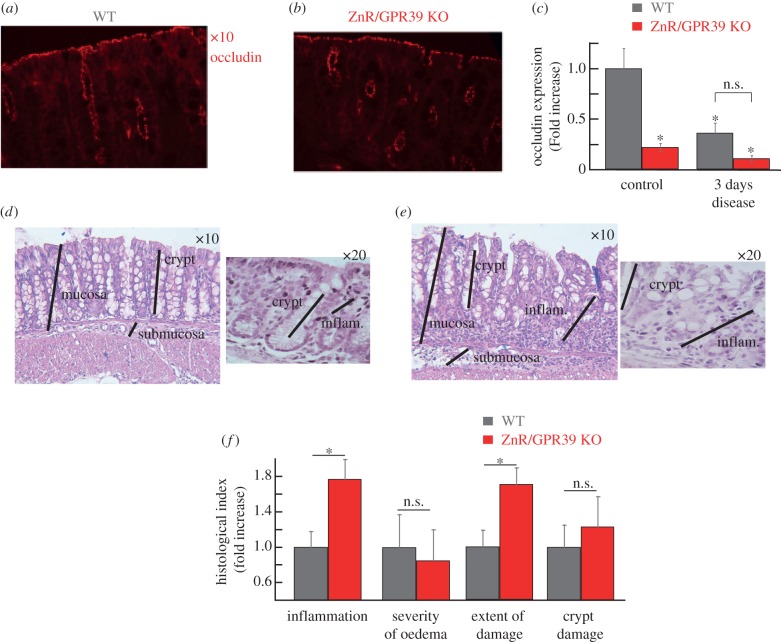
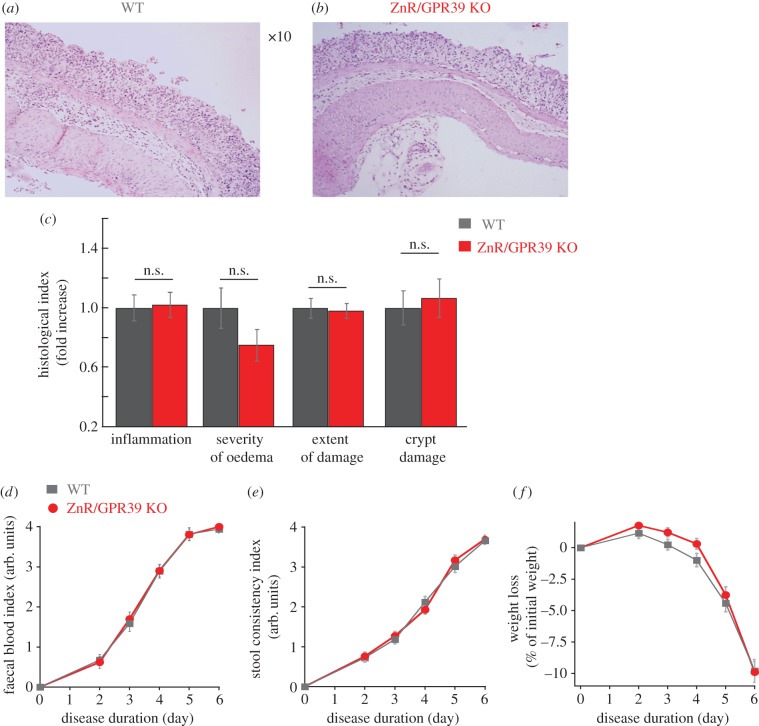
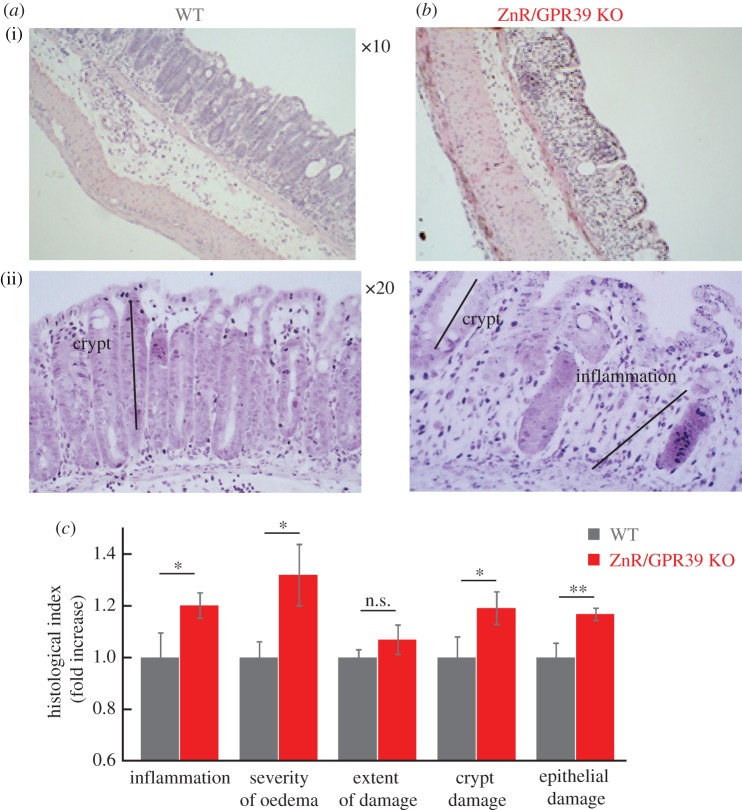
To determine whether the impaired Ca2+ signalling and epithelial barrier, in the absence of ZnR/GPR39, results in enhanced susceptibility to inflammatory disease we employed the DSS-induced acute colitis model [31]. Substantial decreases in occludin levels were observed in WT tissues after 3 days of DSS treatment compared with the untreated controls (figure 2a). No change was observed in occludin expression level in ZnR/GPR39 KO mice (figure 2b), consistent with the very low basal occludin expression levels. Importantly, 3 days of DSS treatment attenuate occludin expression in WT mice, reducing it to the level monitored in ZnR/GPR39 KO mice already under basal conditions (figure 2c). Histological analysis of colon tissue sections on the thirrd day of DSS treatment indicated that tissue organization was still preserved in WT tissues but much less preserved in ZnR/GPR39 KO (figure 2d–e). Moreover, inflammation was almost absent in the WT tissue (figure 2d) while regions containing infiltrating inflammatory cells within the mucosa of ZnR/GPR39 KO mice were observed (figure 2e). For quantifying mucosal integrity, we analysed four features along the colon: level of inflammation, severity of oedema, extent of tissue damage within the wall and extent of crypt damage (Material and methods). Following 3 days of DSS treatment, the level of inflammation and the extent of tissue damage indices were significantly higher in ZnR/GPR39 KO (inflammation: 3.6 ± 0.4; extent: 2.8 ± 0.3) compared with WT (inflammation: 2.0 ± 0.4; extent: 1.6 ± 0.3; p < 0.05) mice (figure 2f, note that for simplicity of presentation indices are normalized to WT in the bar graphs). These findings indicate that in ZnR/GPR39 KO tissue the destruction of the epithelial layer was almost complete, while this layer was partially preserved in WT mice, as reflected by the larger extent of tissue damage (figure 2f). Moreover, crypt shortening was observed in both genotypes but loss of crypts was more severe in the ZnR/GPR39 KO mice in some regions along the colon length. In agreement with the major effect on the epithelial layer, WT mice were more resistant to the inflammatory response in the mucosal layer. On day 6 of DSS treatment, histological scores showed complete loss of crypts, focal ulcerations, severe infiltration of inflammatory cells to the mucosa and severe oedema in the submucosa (figure 3a–c). Importantly, there were no differences between the WT and the ZnR/GPR39 KO tissues following 6 days of DSS treatment. The tissues exhibit nearly complete erosion of the epithelial layer throughout the length of the colon. Note that ZnR/GPR39 activity was monitored in colonocytes (figure 1), which were similarly absent in both genotypes on the 6th day of DSS treatment. Clinical parameters were also determined throughout the 6 days of DSS treatment (Material and methods). Changes in haemoccult positivity, gross bleeding, stool consistency and weight were monitored daily. We observed no significant differences between WT and ZnR/GPR39 KO mice in all parameters during all 6 days of DSS treatment, disease phase (figure 3d–f). Previous studies indicated that clinical manifestations during the acute disease phase do not necessarily reflect the histological severity of the disease in the colon tissue [32], in agreement with our results. Because the clinical and histopathological scores were similar between the genotypes on the 6th day of DSS treatment, we continued to study whether ZnR/GPR39 affects a recovery phase, following removal of DSS.
Figure 2.
Clinical and histological analysis of colon tissue at the acute phase of DSS treatment. (a,b) Representative immunofluorescence analysis of the expression of the tight junction protein occludin in colon tissue from WT or ZnR/GPR39 KO mice following 3 days DSS treatment. (c) Quantification of occludin expression levels during basal (figure 1f) or disease condition (figure 2a) using a threshold analysis (see Material and methods). Results were normalized to the average count in the WT group at baseline/control conditions (n = 15–35 slices from six to eight mice for each treatment, *p < 0.05 compared to control WT). (d,e) H&E analysis of colon tissue sections from WT and ZnR/GPR39 KO mice in sections of colon following 3 days DSS treatment. The black lines mark the mucosal layer, intact crypts in the mucosa or the inflamed mucosal layer (inflam). (f) Histological evaluation along the colon was done to obtain indices of: the inflammation level, severity of oedema, extent of inflamed tissue within the wall (extent of damage) and crypt damage (see Material and methods). Each of the indices is normalized to the level in WT tissues. (n = 22–23 per genotype, *p < 0.05 compared to WT).
Figure 3.
Clinical and histological analysis of colon tissue at the acute phase of DSS treatment. (a,b) Representative HE-stained colon tissue sections obtained following 6 days of DSS treatment from WT (a) and ZnR/GPR39 KO (b). (c) Histological evaluation along the colon, performed as described in figure 1, shows no significant differences in the severity of the injury to WT and ZnR/GPR39 KO mice. (d) Faecal blood and (e) faecal consistency indices were monitored during the 6 days of DSS treatment (n = 22–23, per genotype). The scores for blood index and stool consistency were between 0 and 4, when 0 indicates the healthy stage (see Material and methods). (f) Weight loss during DSS treatment is shown. During the first 2 days of DSS treatment, the weight of treated mice increased by 0.6 ± 0.4% in WT mice and 1.5 ± 0.4% in ZnR/GPR39 KO mice (n = 22–23, per genotype).
(c). Recovery phase: following DSS removal
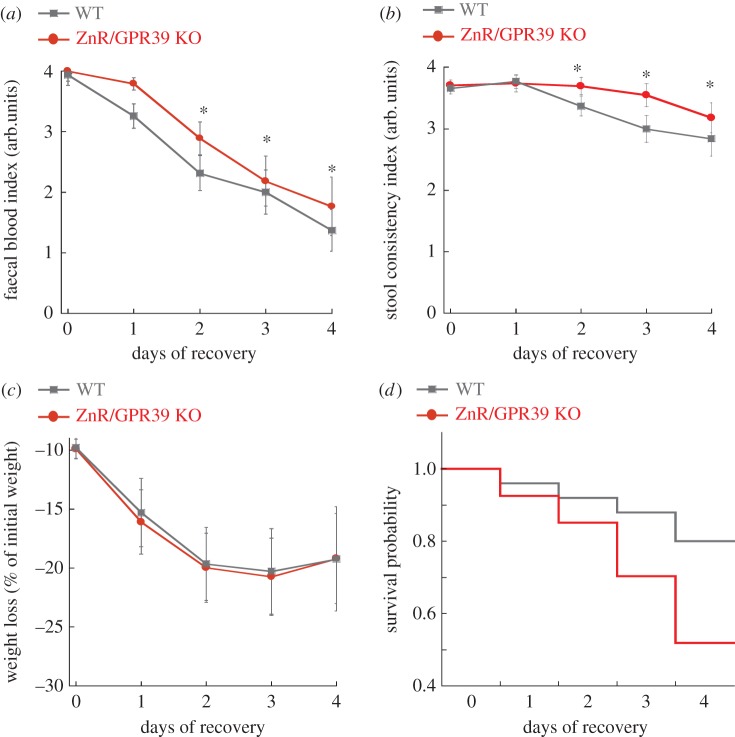
The mice were allowed to recover by removing DSS from their drinking water, yielding a period that bears resemblance to the remission period in IBD patients [33]. Analysis of clinical symptoms revealed that WT mice showed significantly faster decrease in faecal blood indices (figure 4a) and watery stool consistency (figure 4b) compared with ZnR/GPR39 KO mice, although both genotypes continued to lose weight even following the removal of DSS (figure 4c). These differences were seen on the second day of the recovery period and remained statistically significant even after 4 days, indicating a more rapid recovery of WT compared to ZnR/GPR39 KO mice. In addition, ZnR/GPR39 KO mice had lower overall survival probability compared with WT mice (figure 4d, Kaplan–Meier probability p < 0.05), averaged daily mortality rate during the recovery period was 14 ± 1% in ZnR/GPR39 KO compared to 7.5 ± 0.5% in WT (p < 0.05). Thus, during the recovery from DSS-induced colitis, clinical symptoms were ameliorated in WT mice compared to ZnR/GPR39 KO mice.
Figure 4.
ZnR/GPR39 ameliorates clinical symptoms of the disease and enhances survival during recovery from DSS treatment. Clinical scores were monitored daily during the recovery period, following removal of DSS treatment, while on a regular drinking regime. Day 0 is the 6th day of DSS and shows the data as figure 3. (a) Faecal blood and (b) stool consistency indices as well as (c) weight loss were determined in WT versus ZnR/GPR39 KO mice (as in figure 3 and Material and methods). (d) Survival probability during the recovery period is presented using the Kaplan–Maier plot, p < 0.05 between WT and ZnR/GPR39 KO mice.
Histological analysis of distal colon tissue sections on day 2 of the recovery (figure 5a–b) showed no significant differences between the genotypes in the extent of tissue damage within the colon wall (4.3 ± 0.1 in WT versus 4.5 ± 0.2 in ZnR/GPR39 KO). This finding is consistent with the severe acute response triggered following destruction of the epithelial layer during 6 days of toxin treatment in both genotypes. By contrast, the indices for immune cells infiltration (inflammation index of 9.0 ± 0.9 in WT versus 10.8 ± 0.4 in ZnR/GPR39 KO; p < 0.05) and the severity of oedema (4.5 ± 0.3 in WT versus 6.0 ± 0.5 in ZnR/GPR39 KO; p < 0.05) were significantly higher in tissues from ZnR/GPR39 KO mice compared with WT, suggesting partial recovery of the inflammatory response in WT but not in the ZnR/GPR39 KO mice. Importantly, the crypt damage index, reflecting the direct loss of crypts along the colon, was significantly higher in the ZnR/GPR39 KO (13.9 ± 0.7 in ZnR/GPR39 KO versus 11.7 ± 0.9 in WT; p < 0.05). In addition, the index of epithelial damage was significantly higher in ZnR/GPR39 KO mice compared with WT (15.5 ± 0.3 in ZnR/GPR39 KO versus 13.3 ± 0.7 in WT; p < 0.01). As such, in tissue from ZnR/GPR39 KO mice we observed fewer and relatively short crypts with scarce regions covered by surface epithelium, indicating mild regeneration from the destruction by DSS. But, in tissue from WT mice we observed more regions along the colon where the surface epithelial layer recovered from the damage and substantial numbers of crypts were regenerated already 2 days following removal of DSS (figure 5a–c, note that indices in c are normalized to WT).
Figure 5.
ZnR/GPR39 promotes crypts regeneration during the recovery period. (a,b) Representative HE-stained colon tissues from WT (a) and ZnR/GPR39 KO (b) mice obtained on the second day of the recovery phase. (c) Histological evaluation along the colon was done to obtain indices of: the inflammation level, severity of oedema, extent of inflamed tissue within the wall (extent of damage), crypt damage and extent of epithelial damage (regions of destructed tissue along the epithelial layer). For clarity of presentation in a bar graph, each of the indices is normalized to the level in WT tissues. (n = 28–30 per genotype, *p < 0.05 compared to WT).
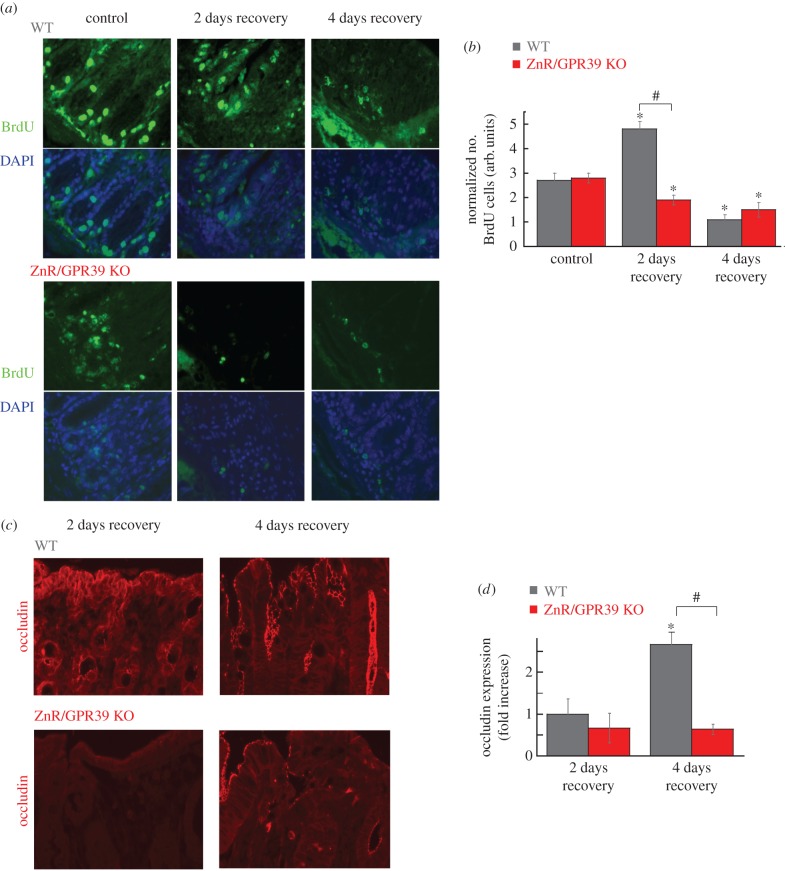
We then monitored BrdU incorporation, as an in situ proliferative marker, to study if the differences in epithelial recovery between WT and ZnR/GPR39 KO are directly linked to proliferation. Quantitative analysis was done by counting the number of BrdU-stained cells per crypt (as observed under bright-field microscopy of the same field of view). Slices from the distal colon were obtained from control mice, before DSS treatment (control) to determine whether ZnR/GPR39 KO mice have slower proliferation rates in the normal tissue. In control tissues, under basal conditions, we did not monitor differences in proliferating colonocytes between genotypes, showing 2.7 ± 0.3 cells crypt−1 in WT mice and 2.8 ± 0.2 cell crypt−1 in ZnR/GPR39 KO mice (figure 6a left panels and b). This is important because it allows us to determine whether the proliferation rates are changed under conditions of recovery from the injury when this process plays an important role in tissue repair. We then studied the differences in proliferation rates on the second and fourth days of the recovery period (figure 6a–b). On the second day of the recovery period, crypt cell proliferation among WT mice dramatically increased compared with control mice untreated with DSS (4.8 ± 0.3 cells crypt−1, p < 0.05 compared to control, figure 5a(i)), indicating that proliferation of epithelial cells is accelerated, apparently to facilitate repair of the injured epithelial layer. In striking contrast to the WT colon, in ZnR/GPR39 KO mice the proliferation within regenerated crypts during the recovery phase from DSS was not enhanced, and even decreased compared with untreated mice (1.9 ± 0.2 cells crypt−1, p < 0.05, figure 6a bottom panels). BrdU-stained cells were counted only in crypts that had well-defined morphology and structure, and therefore this parameter reflects cell proliferation rates in preserved/renewed crypts and argues against this being a result of the general larger damage of the epithelial layer seen in ZnR/GPR39 KO mice. After 4 days of recovery, proliferation rate in WT mice decreased to 1.1 ± 0.2 cells crypt−1, suggesting a shift to a differentiation phase. Yet, in tissues from ZnR/GPR39 KO mice, even on the fourth day of recovery, the number of BrdU-stained cells remained low at 1.5 ± 0.3 cells crypt−1. Thus, in WT mice we monitored a large increase in rates of proliferation during the initial recovery, thereby rapidly renewing the epithelial layer. By contrast, in ZnR/GPR39 KO mice we observed attenuated epithelial proliferation even during the recovery period. These results suggest that ZnR/GPR39 is required for promoting proliferation during the recovery phase following epithelial erosion but not during the basal state, when proliferating cell numbers were similar in the presence or the absence of the receptor.
Figure 6.
ZnR/GPR39 promotes cell proliferation. (a) Representative BrdU-stained tissues of WT or ZnR/GPR39 KO colon from control (non-treated) mice or following DSS treatment at 2 days or 4 days of the recovery period (×20). DAPI staining was added as a control. Note that DAPI staining is seen in epithelial as well as in inflammatory cells nuclei within the mucosa. (b) Analysis of epithelial proliferation, using BrdU-stained tissue, comparing the number of stained cells to the number of crypts observed in a field. (n = 37 per genotype, *p < 0.05 compared to control WT, #p < 0.05, between WT and ZnR/GPR39 KO on day 2 of recovery). (c) Representative images of occludin expression levels during the recovery period, on the second and fourth days following removal of DSS, recovery phase. (d) Quantitative analysis was performed as in figure 1 (n = 10–30 slices from 6 to 10 mice from each treatment and each genotype, *p < 0.05 compared to 2 days of recovery in WT, #p < 0.05 compared to 4 days of recovery in WT).
Differentiation and formation of tight junctions are further essential for the complete recovery of epithelial barrier [34], thus we compared the expression levels of occludin during the recovery period. In colon sections from the second day of recovery, when peak proliferation was monitored, WT and ZnR/GPR39 KO mice still exhibit low levels of occludin expression. Importantly, on the fourth day of recovery, occludin levels were increased in WT mice, by about threefold, compared to the second day (figure 6c–d). By contrast, occludin expression in ZnR/GPR39 KO mice did not change from day 2 to day 4 of the recovery period (figure 6c,d). Altogether our results suggest that ZnR/GPR39 expression is required for the formation and function of the tight epithelial barrier during DSS-induced colitis and the recovery period.
4. Discussion
Reduction in the expression of occludin was seen in inflamed epithelia of both Crohn's disease and ulcerative colitis patients even at early stages of the disease [35]. A fundamental difference between WT and ZnR/GPR39 KO mice is the basal expression of occludin, in the absence of any pathological condition. Compromised epithelial barrier in the absence of ZnR/GPR39 may hence increase epithelial permeability. Indeed, during the disease phase (DSS treatment), colon tissue from ZnR/GPR39 KO mice exhibited more damage compared with WT. Moreover, enhanced faecal expulsion and accelerated gastric emptying that were described in ZnR/GPR39 KO mice [22] may also result from the low expression of occludin and compromised tight junction barrier. The low susceptibility to DSS during the disease phase may result from the severity of the chemical DSS insult which damages the epithelial layer. However, the effect of the receptor is fully manifested during the recovery when epithelial cells expressing ZnR/GPR39 enhance the buildup of the epithelial layer and barrier function. Thus, our results suggest that ZnR/GPR39 has an important role in rebuilding the epithelial barrier during remission in ulcerative disease (figure 7).
Figure 7.
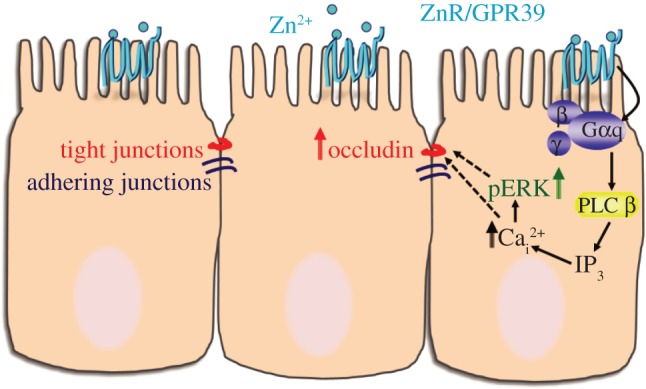
Scheme of ZnR/GPR39 signalling activated by Zn2+ to enhance tight junction formation. Our results indicate that ZnR/GPR39 signalling activates Ca2+ release from thapsigargin-dependent endoplasmic reticulum stores in colonocytes, thereby upregulating occludin expression. In vivo, ZnR/GPR39 expression is essential to enhance the recovery of occludin expression and colonocytes proliferation following barrier disruption by DSS.
Consistent with the findings in this study, extracellular Zn2+ activates ZnR/GPR39 leading to Ca2+ signalling and activating epithelial proliferation and migration [18–20,36,37]. The ZnR/GPR39 response is mediated via the IP3 pathway and induces release of intracellular Ca2+, which is essential for downstream signalling [38] and recovery of occludin expression. Remarkably, ZnR/GPR39-dependent colon epithelial cell proliferation was largely increased only during the recovery period, promoting epithelial renewal following its erosion during the disease phase. Interestingly, the Zn2+ transporter Zip14 has been recently shown to regulate occludin expression [39]. Further studies will be required to study the interaction of ZnR/GPR39 and intracellular Zn2+ transporters in regulating proliferation and differentiation.
One could argue that ZnR/GPR39 may have a direct role on the immune response, as it is well known that zinc deficiency affects immune system cells resulting in higher susceptibility to infections [40–42]. Our results, however, suggest a prominent role for ZnR/GPR39-dependent regulation of the epithelial barrier as the pathway for attenuating the DSS-induced response. Consistent with this hypothesis, the inflammatory index was lower in WT mice during the DSS-induced disease phase when ZnR/GPR39 was functional on WT epithelial cells. By contrast, when erosion of the epithelial layer was complete (sixth day of DSS) we found no differences in the inflammatory response between genotypes. Indeed, zinc supplementation to Crohn's disease patients during clinical remission reduced intestinal permeability [12]. A similar role on epithelial barrier, and thereby permeability, was described for Zn2+ in lung bacterial infection [43]. Importantly, a direct effect of ZnR/GPR39 on the epithelial barrier is also supported by the faster renewal of the epithelial surface layer along the mucosa with crypt reorganization and increased proliferation rate of epithelial cells in WT compared with ZnR/GPR39 KO mice. The enhanced recovery of the epithelial barrier in WT mice was reflected by amelioration of the clinical symptoms (faecal blood and diarrhoea) during the recovery phase in the WT mice compared to ZnR/GPR39 KO.
The essential role of zinc for function of the digestive system is documented in many studies [7,10,13,44], but as the specific molecular targets of this ion are unknown the regime of treatment is controversial. By identifying a molecular target of Zn2+ in the digestive tract, ZnR/GPR39, we provide a tool to study the effects of zinc treatment in IBD in a controllable manner. To date, there is no known medical cure for IBD that targets the epithelial layer, we show that ZnR/GPR39 directly promotes intestinal barrier formation and function and may therefore provide an effective therapeutic target in ulcerative diseases.
Acknowledgements
The GPR39 KO mice were kindly provided by D. Moechars from Johnson & Johnson Pharmaceutical Research and Development, a Division of Janssen Pharmaceutica. We thank Elena Voronov for technical help with the DSS model.
Ethics
Experimental procedures were performed in accordance with a protocol approved by the committee for the Ethical Care and Use of Animal in Experiments at the Faculty of Health Science at Ben-Gurion University of the Negev.
Authors' contributions
L.S., M.M. and L.C. performed experiments, analysed data and wrote the manuscript; I.S. conceived and wrote the manuscript; M.H. conceived and planned the experiments, analysed data and wrote the manuscript.
Competing interests
We have no competing interests to declare.
Funding
This work was supported by the Israel Science Foundation (grant no. 978/13 to M.H.).
References
- 1.Danese S, Fiocchi C. 2011. Ulcerative colitis. N. Engl. J. Med. 365, 1713–1725. ( 10.1056/NEJMra1102942) [DOI] [PubMed] [Google Scholar]
- 2.Hering NA, Fromm M, Schulzke JD. 2012. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J. Physiol. 590, 1035–1044. ( 10.1113/jphysiol.2011.224568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorfel MJ, Huber O. 2012. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 807356 ( 10.1155/2012/807356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertiaux-Vandaele N, et al. 2011. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 106, 2165–2173. ( 10.1038/ajg.2011.257) [DOI] [PubMed] [Google Scholar]
- 5.Su L, et al. 2013. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 145, 407–415. ( 10.1053/j.gastro.2013.04.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scrimgeour AG, Lukaski HC. 2008. Zinc and diarrheal disease: current status and future perspectives. Curr. Opin. Clin. Nutr. Metab. Care 11, 711–717. ( 10.1097/MCO.0b013e3283109092) [DOI] [PubMed] [Google Scholar]
- 7.Goh J, O'Morain CA. 2003. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol. Ther. 17, 307–320. ( 10.1046/j.1365-2036.2003.01482.x) [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni H, Mamtani M, Patel A. 2012. Roles of zinc in the pathophysiology of acute diarrhea. Curr. Infect. Dis. Rep. 14, 24–32. ( 10.1007/s11908-011-0222-8) [DOI] [PubMed] [Google Scholar]
- 9.Walker CL, Black RE. 2010. Zinc for the treatment of diarrhoea: effect on diarrhoea morbidity, mortality and incidence of future episodes. Int. J. Epidemiol. 39(Suppl. 1), i63–i69. ( 10.1093/ije/dyq023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikora SK, Spady D, Prosser C, El-Matary W. 2011. Trace elements and vitamins at diagnosis in pediatric-onset inflammatory bowel disease. Clin. Pediatr. 50, 488–492. ( 10.1177/0009922810397041) [DOI] [PubMed] [Google Scholar]
- 11.Ojuawo A, Keith L. 2002. The serum concentrations of zinc, copper and selenium in children with inflammatory bowel disease. Cent. Afr. J. Med. 48, 116–119. [PubMed] [Google Scholar]
- 12.Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Inca R. 2001. Zinc supplementation tightens ‘leaky gut’ in Crohn's disease. Inflamm. Bowel Dis. 7, 94–98. ( 10.1097/00054725-200105000-00003) [DOI] [PubMed] [Google Scholar]
- 13.Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D'Inca R. 2002. Effect of zinc supplementation on intestinal permeability in experimental colitis. J. Lab. Clin. Med. 139, 311–315. ( 10.1067/mlc.2002.123624) [DOI] [PubMed] [Google Scholar]
- 14.Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. 2008. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 138, 1664–1670. [DOI] [PubMed] [Google Scholar]
- 15.Luk HH, Ko JK, Fung HS, Cho CH. 2002. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur. J. Pharmacol. 443, 197–204. ( 10.1016/S0014-2999(02)01592-3) [DOI] [PubMed] [Google Scholar]
- 16.Hershfinkel M, Moran A, Grossman N, Sekler I. 2001. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl Acad. Sci. USA 98, 11 749–11 754. ( 10.1073/pnas.201193398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershfinkel M, Silverman WF, Sekler I. 2007. The zinc sensing receptor, a link between zinc and cell signaling. Mol. Med. 13, 331–336. ( 10.2119/2006-00038.Hershfinkel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharir H, Zinger A, Nevo A, Sekler I, Hershfinkel M. 2010. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 285, 26 097–26 106. ( 10.1074/jbc.M110.107490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen L, Azriel-Tamir H, Arotsker N, Sekler I, Hershfinkel M. 2012. Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin. PLoS ONE 7, e35482 ( 10.1371/journal.pone.0035482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen L, Sekler I, Hershfinkel M. 2014. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 5, e1307 ( 10.1038/cddis.2014.262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popovics P, Stewart AJ. 2011. GPR39: a Zn2+-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol. Life Sci 68, 85–95. ( 10.1007/s00018-010-0517-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moechars D, et al. 2006. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology 131, 1131–1141. ( 10.1053/j.gastro.2006.07.009) [DOI] [PubMed] [Google Scholar]
- 23.Stuart RO, Sun A, Panichas M, Hebert SC, Brenner BM, Nigam SK. 1994. Critical role for intracellular calcium in tight junction biogenesis. J. Cell Physiol. 159, 423–433. ( 10.1002/jcp.1041590306) [DOI] [PubMed] [Google Scholar]
- 24.Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, Sundberg JP. 1998. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. 274, G544–G551. [DOI] [PubMed] [Google Scholar]
- 25.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249. [PubMed] [Google Scholar]
- 26.Gramlich T, Petras RE. 2007. Pathology of inflammatory bowel disease. Semin. Pediatr. Surg. 16, 154–163. ( 10.1053/j.sempedsurg.2007.04.005) [DOI] [PubMed] [Google Scholar]
- 27.Kim CJ, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. 2009. L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim. Biophys. Acta 1790, 1161–1169. ( 10.1016/j.bbagen.2009.05.018) [DOI] [PubMed] [Google Scholar]
- 28.Sharir H, Hershfinkel M. 2005. The extracellular zinc-sensing receptor mediates intercellular communication by inducing ATP release. Biochem. Biophys. Res. Commun. 332, 845–852. ( 10.1016/j.bbrc.2005.05.036) [DOI] [PubMed] [Google Scholar]
- 29.Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, Kobori M. 2004. A novel Galphaq/11-selective inhibitor. J. Biol. Chem. 279, 47 438–47 445. ( 10.1074/jbc.M408846200) [DOI] [PubMed] [Google Scholar]
- 30.Cummins MM, O'Mullane LM, Barden JA, Cook DI, Poronnik P. 2000. Purinergic responses in HT29 colonic epithelial cells are mediated by G protein alpha -subunits. Cell Calcium 27, 247–255. ( 10.1054/ceca.2000.0120) [DOI] [PubMed] [Google Scholar]
- 31.Kim HS, Berstad A. 1992. Experimental colitis in animal models. Scand. J. Gastroenterol. 27, 529–537. ( 10.3109/00365529209000116) [DOI] [PubMed] [Google Scholar]
- 32.Perse M, Cerar A. 2012. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012, 718617 ( 10.1155/2012/718617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose WA 2nd, Sakamoto K, Leifer CA. 2012. Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol. 13, 41 ( 10.1186/1471-2172-13-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forster C. 2008. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 130, 55–70. ( 10.1007/s00418-008-0424-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruewer M, Samarin S, Nusrat A. 2006. Inflammatory bowel disease and the apical junctional complex. Ann. NY Acad. Sci. 1072, 242–252. ( 10.1196/annals.1326.017) [DOI] [PubMed] [Google Scholar]
- 36.Asraf H, Salomon S, Nevo A, Sekler I, Mayer D, Hershfinkel M. 2014. The ZnR/GPR39 interacts with the CaSR to enhance signaling in prostate and salivary epithelia. J. Cell Physiol. 229, 868–877. ( 10.1002/jcp.24514) [DOI] [PubMed] [Google Scholar]
- 37.Dubi N, Gheber L, Fishman D, Sekler I, Hershfinkel M. 2008. Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells. Carcinogenesis 29, 1692–1700. ( 10.1093/carcin/bgn027) [DOI] [PubMed] [Google Scholar]
- 38.Azriel-Tamir H, Sharir H, Schwartz B, Hershfinkel M. 2004. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 279, 51 804–51 816. ( 10.1074/jbc.M406581200) [DOI] [PubMed] [Google Scholar]
- 39.Guthrie GJ, Aydemir TB, Troche C, Martin AB, Chang SM, Cousins RJ. 2015. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G171–G178. ( 10.1152/ajpgi.00021.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. 2008. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 181, 6491–6502. ( 10.4049/jimmunol.181.9.6491) [DOI] [PubMed] [Google Scholar]
- 41.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. 2009. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit. Care Med. 37, 1380–1388. ( 10.1097/CCM.0b013e31819cefe4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad AS. 2008. Zinc in human health: effect of zinc on immune cells. Mol. Med. 14, 353–357. ( 10.2119/2008-00033.Prasad) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao S, Knoell DL. 2006. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L1132–L1141. ( 10.1152/ajplung.00207.2006) [DOI] [PubMed] [Google Scholar]
- 44.Liu J, et al. 2011. Demand for Zn2+ in acid-secreting gastric mucosa and its requirement for intracellular Ca2+. PLoS ONE 6, e19638 ( 10.1371/journal.pone.0019638) [DOI] [PMC free article] [PubMed] [Google Scholar]