Abstract
There is long-term (trophic) purinergic signalling involving cell proliferation, differentiation, motility and death in the development and regeneration of most systems of the body, in addition to fast purinergic signalling in neurotransmission, neuromodulation and secretion. It is not always easy to distinguish between short- and long-term signalling. For example, adenosine triphosphate (ATP) can sometimes act as a short-term trigger for long-term trophic events that become evident days or even weeks after the original challenge. Examples of short-term purinergic signalling during sympathetic, parasympathetic and enteric neuromuscular transmission and in synaptic transmission in ganglia and in the central nervous system are described, as well as in neuromodulation and secretion. Long-term trophic signalling is described in the immune/defence system, stratified epithelia in visceral organs and skin, embryological development, bone formation and resorption and in cancer. It is likely that the increase in intracellular Ca2+ in response to both P2X and P2Y purinoceptor activation participates in many short- and long-term physiological effects.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: neurotransmission, neuromodulation, secretion, vessels, bone, cancer
1. Introduction
The proposal that purine nucleotides are extracellular signalling molecules, as well as an intracellular energy source, was first reported by Drury & Szent-Györgyi [1]. Then in 1970, adenosine 5′-triphosphate (ATP) was shown to be a transmitter in autonomic neuromuscular transmission [2] and in a later review the term ‘purinergic’ signalling was introduced [3]. This concept was not accepted by many for the next 20 years. Separate purinergic receptor families, P1 (adenosine) and P2 (ATP/adenosine 5′-diphosphate (ADP)) were described in 1978 [4], but the turning point in acceptance of purinergic signalling came after the receptors for purines and pyrimidines were cloned and characterized in the early 1990s [5]. Four P1 receptor subtypes (A1, A2A, A2B, A3), seven P2X ion channel receptors (P2X1–7) and eight G-protein-coupled receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) are currently recognized [6]. Activation of P2 receptors leads to increase in intracellular Ca2+: from extracellular sources for P2X receptors and from intracellular sites for P2Y receptors. Perhaps because of their ancient origin, the array of purinoceptor subtypes has a unique property of being extraordinarily widely distributed throughout living cells and tissues [7]. In contrast to all other chemical transmitters, which are, as a rule, segregated to certain cell types and certain functions, the receptors for purines and pyrimidines are found everywhere, and it is almost impossible to find a cell without sensitivity to ATP and its analogues. There has been a rapid expansion of the field since 1995 [8,9].
2. Short-term purinergic signalling
ATP was shown to be a transmitter released from non-adrenergic, non-cholinergic nerves to produce short-term purinergic signalling from inhibitory enteric nerves in the guinea pig taenia coli [2] and from excitatory parasympathetic nerves in the urinary bladder [10]. Short-term purinergic signalling was demonstrated when ATP was identified as a cotransmitter with noradrenalin in sympathetic nerves in the taenia coli [11], cat nictitating membrane [12], vas deferens [13,14] and in blood vessels [15,16]. ATP is also a cotransmitter with acetylcholine in motor nerves supplying developing skeletal muscle [17], bladder [18] and carotid body [19] and in sensory-motor nerves with substance P and calcitonin gene-related peptide [20]. Later ATP was shown to be a cotransmitter, mediating short-term purinergic signalling, in neurons in the central nervous system (CNS) [8,21,22]. The involvement of short-term purinergic signalling in the control of vascular tone is illustrated in figure 1. Purinergic synaptic transmission between nerves was shown in the coeliac ganglion [24,25] and the medial habenula in the brain [26]. ATP released during synaptic transmission can activate astrocyte receptors, which in turn initiate Ca2+ signals and propagate Ca2+ waves in the astroglial networks via the activation of P2Y receptors and the diffusion of inositol trisphosphate (IP3) through the gap junctions [27]. Ionotropic P2X receptors are responsible for rapid astrocytic signalling, whereas metabotropic P2Y receptors mediate long-term effects [28].
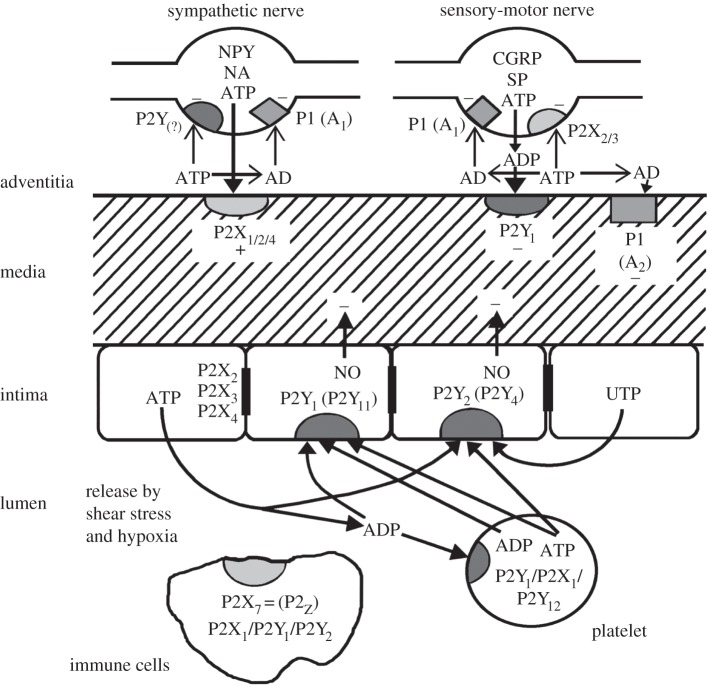
Figure 1.
Short-term (acute) purinergic signalling controlling vascular tone. Schematic illustrating the main receptor subtypes for purine and pyrimidines present in most blood vessels. Perivascular nerves in the adventitia release ATP as a cotransmitter: ATP is released with noradrenalin (NA) and neuropeptide Y (NPY) from sympathetic nerves to act on smooth muscle P2X1 and, in some vessels, P2X2, P2X4 and P2Y2 purinoceptors, resulting in vasoconstriction. ATP is also released together with calcitonin gene-related peptide (CGRP) and substance P (SP) from sensory nerves during ‘axon reflex’ activity and broken down to adenosine diphosphate (ADP) to act on smooth muscle P2Y1 purinoceptors in some regions of some vessels resulting in vasodilatation. P1(A1) purinoceptors on nerve terminals of sympathetic and sensory nerves mediate adenosine (AD) (arising from enzymatic breakdown of ATP) modulation of transmitter release. P2X2/3 purinoceptors are present on a subpopulation of sensory nerve terminals. P1(A2) purinoceptors on vascular smooth muscle mediate vasodilatation. Endothelial cells release ATP and uridine 5′-triphosphate (UTP) during shear stress and hypoxia to act on P2Y1, P2Y2 and sometimes P2Y4 purinoceptors leading to the production of nitric oxide (NO) and subsequent vasodilatation. ATP, following its release from aggregating platelets, also acts on these endothelial receptors. Blood-borne platelets possess P2Y1 and P2Y12 ADP-selective purinoceptors as well as P2X1 receptors, while immune cells of various kinds possess P2X7, as well as P2X1, P2Y1 and P2X2 purinoceptors. P2X2, P2X3 and P2X4 receptors have also been identified on endothelial cell membranes. (Modified from [23], with permission from Lippincott Williams and Wilkins.)
Short-term signalling involved in prejunctional neuromodulation via both P1 and P2 receptors was also recognized in both peripheral [20,29,30] and CNSs [31,32]. Purinoceptors are extensively present in the CNS, where they mediate neuronal excitability and they are important for signalling in neuronal–glial circuitry, being an important gliotransmitter [8,33,34].
Purinoceptors are present in all peripheral tissues, being involved in short-term as well as long-term regulation of different functions, including neuromuscular and synaptic transmission and secretion in gut [35], and secretion in kidneys [36], liver [37] and reproductive systems [38]. In vascular [16] and respiratory systems, ATP mediates reflex activities via activation of sensory nerves [39]. Activation of purinoceptors can mediate rapid responses in the immunological system [40], in blood cells [41], skin [42], bones and muscles [43], urinary tract [44] and heart [45]. Short-term purinergic signalling also takes place in secretion from endocrine [46] and non-endocrine cells [35]. P2X3 and P2X2/3 receptors are involved in nociception [47]. Purinergic signalling via P2Y12 receptors is well established for control of platelet aggregation.
3. Long-term (trophic) purinergic signalling
ATP and it analogues are involved in tissue remodelling in response to injury and play a key role in the regulation of subsequent repair and regeneration [48]. Stimulation of purinoceptors triggers astrogliosis, the generalized response of astrocytes to brain damage, involving cell proliferation and remodelling of the neural circuitry [49,50]. Reactive astrogliosis is instrumental for both the formation of scar and limitation of brain-damaged area (through anisomorphic astrogliosis), as well as for the post-insult remodelling and recovery of neural function (by isomorphic astrogliosis). The initial events in the responses of astroglia to purinergic signalling are instrumental for glial Ca2+ excitability or can initiate long-term effects [51]. For reactive astrogliosis, not only was increase in intracellular calcium absolutely necessary, but ATP was also shown to be one of the key factors involved in its initiation via the activation of P2Y G-protein-coupled receptors linked to phospholipase C and IP3 [52]. These trophic/astrogliotic proliferative effects of P2 agonists were found both in vitro, in glial cultures, and in vivo, in nucleus accumbens of rats [53–56]. P2X receptors mediate long-term potentiation in the hippocampus [57]. Activation of P2X receptors can have multiple effects on synaptic plasticity, either inhibiting or facilitating the long-term changes of synaptic strength depending on the physiological context [58]. Long-term purinergic signalling also occurs in chronic inflammation and neuropathic pain [59].
(a). Embryological development
P2 receptor subtypes appear transiently during both embryological and postnatal development, suggesting that ATP is involved in the sequential proliferation, differentiation, motility and death of cells during the complex events involved [8,60,61]. For example, in Xenopus embryos a novel P2Y8 receptor was cloned and shown to be transiently expressed in the neural plate and tube from stages 13 to 18 and again at stage 28, when secondary neurulation occurs in the tail bud [62]. Transient expression of P2Y1 receptors in the limb buds of chick embryos mediates rapid cell proliferation [63]. During postnatal development of cerebellum [64] and skeletal muscle [65] changes in expression of P2X receptor subtypes have been described. Purinergic signalling in development is likely to involve cross-talk between several other signalling pathways, including growth factors, cytokines and extracellular matrix components [61]. During early development of the myotube P2X5 receptors were present, followed by P2X6 receptor expression, and then P2X2 receptors were expressed during the development of the neuromuscular junction. ATP-evoked Ca2+ transients in the chicken retina were the strongest as early as E3, but were drastically reduced at E11–13.5 [66]. Similar mechanisms are involved in adult neurogenesis [67].
(b). Bone formation and resorption
Osteoclast activity and bone resorption are activated by ADP via P2Y1 receptors, whereas ATP and uridine 5′-triphosphate (UTP) signalling via P2Y2 receptors in osteoblasts inhibits bone growth and mineralization (figure 2) [43,69,70]. P2X7 receptors have trophic regulatory roles in bone formation and resorption [71,72]. Osteoblasts activated by P2X7 receptors show enhanced differentiation and bone formation [73], whereas P2X7 receptor activation of osteoclasts evokes apoptosis and bone resorption [74–76].
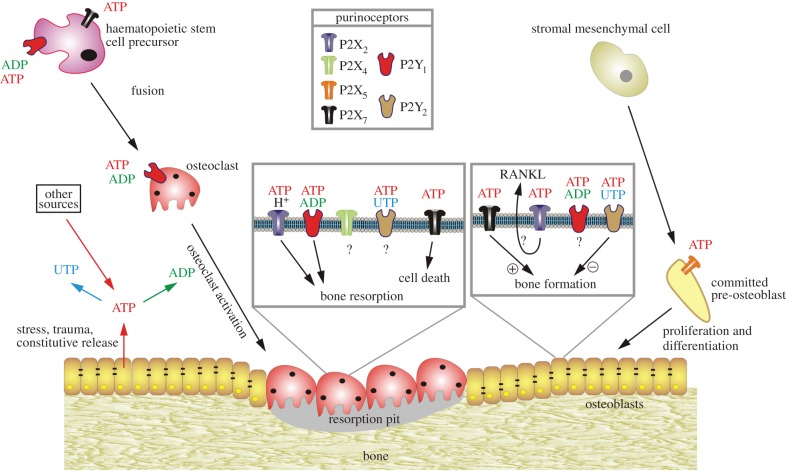
Figure 2.
Schematic diagram illustrating the potential functions of extracellular nucleotides and P2 receptors in modulating bone cell function. ATP released from osteoclasts (e.g. through shear stress or constitutively) or from other sources can be degraded to adenosine 5′-diphosphate (ADP) or converted into uridine 5′-triphosphate (UTP) through ecto-nucleotidases. All three nucleotides can function separately on specific P2 receptor subtypes, as indicated by the colour coding. ATP is a universal agonist, whereas UTP is only active at the P2Y2 receptor and ADP is only active at the P2Y1 receptor. ADP acting on P2Y1 receptors seems to stimulate both the formation (i.e. fusion) of osteoclasts from haematopoietic precursors and the resorptive activity of mature osteoclasts. For the latter, a synergistic action of ATP and protons by the P2X2 receptor has been proposed. ADP could also stimulate resorption indirectly through actions on osteoclasts, which in turn release pro-resorptive factors (e.g. receptor activator of nuclear factor κB ligand, RANKL). ATP at high concentrations might facilitate fusion of osteoclast progenitors through P2X7 receptor pore formation or induce cell death of mature osteoclasts through P2X7 receptors. In osteoblasts, ATP, through P2X5 receptors, might enhance proliferation and/or differentiation. By contrast, UTP, through P2Y2 receptors, is a strong inhibitor of bone formation by osteoblasts. For some receptors (e.g. P2X4 and P2Y2 receptors on osteoclasts or P2X2 receptors on osteoblasts), evidence for expression has been found but their role is still unclear. (Reproduced from [68], with permission.)
(c). Vascular remodelling in atherosclerosis and post-angioplasty restenosis
ATP and UTP acting via P2Y2 receptors cause proliferation of vascular smooth muscle cells. Proliferation of endothelial cells is produced by ADP acting via P2Y1 receptors. Adenosine via A2 receptors mediates inhibition of smooth muscle proliferation but stimulation of endothelial cell proliferation (figure 3) [23]. This suggests that the increase in vascular smooth muscle and endothelial cells in both atherosclerosis and hypertension may be mediated by the trophic actions of purines and pyrimidines released from nerves and endothelial cells [77–79] and in post-angioplasty restenosis [80]. P2Y4 receptors appear to be regulators of angiogenesis [81]. DNA synthesis and migration of vascular endothelial cells in vasa vasorum is increased by ATP in diseased pulmonary vessels [82]. Microvascular disease is characterized by an increased wall–lumen ratio in diabetic patients. This is probably because of an increase in vascular smooth muscle cells leading to higher rates of restenosis after angioplasty. Release of ATP, induced by high glucose, stimulates vascular smooth muscle cell growth via P2Y receptors [83]. An unusual type of long-term purinergic signalling is the evidence that at a critical concentration ATP, acting on both erythrocytes [84] and endothelial cells [85], leads to increase in ATP release into the circulating blood for several hours.
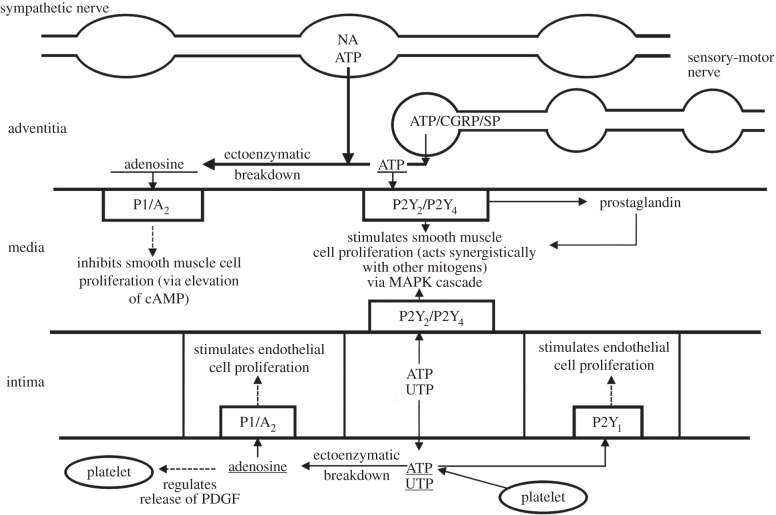
Figure 3.
Schematic diagram of long-term (trophic) actions of purines released from nerves, platelets and endothelial cells (which also release UTP) acting on P2 receptors to stimulate or inhibit cell proliferation. ATP released as a cotransmitter from sympathetic nerves and sensory-motor nerves (during axon reflex activity) stimulates smooth muscle cell proliferation via P2Y2 and/or P2Y4 receptors via a mitogen-activated protein kinase (MAPK) cascade, whereas adenosine resulting from enzymatic breakdown of ATP acts on P1 (A2) receptors to inhibit cell proliferation (via elevation of cAMP). ATP and UTP released from endothelial cells stimulate endothelial and smooth muscle cell proliferation via P2Y1, P2Y2 and P2Y4 receptors. Adenosine resulting from ATP breakdown acts on P1 (A2) receptors to stimulate endothelial cell proliferation and regulate the release of platelet-derived growth factor (PDGF) from platelets. NA, noradrenalin; CGRP, calcitonin gene-related peptide; SP, substance P. (Reproduced from [23], with permission from Lippincott, Williams and Wilkins.)
(d). Skin
Stratified squamous epithelia in rat skin as well as cornea, oesophagus, soft palate, vagina and tongue showed heavy immunostaining of the P2X5 receptor associated with cell differentiation in the spinous and granular cell layers, but not in basal cuboidal outer layers. There was heavy immunostaining of P2X7 receptors in the outer layer, associated with apoptotic cell death [86]. There is rapid turnover of the epithelium of the small intestine. P2X5 receptors are expressed on the narrow ‘stem’ of villus goblet cells, while P2X7 receptor immunoreactivity is seen only on the membranes of the enterocytes and goblet cells at the tip of the villus, where cells are undergoing apoptosis [87].
P2X5, P2X7, P2Y1 and P2Y2 receptor subtype expression was studied in healthy human epidermal keratinocytes in relation to markers for proliferation (PCNA and Ki-67), differentiation (cytokeratin KIO and involucrin) and apoptosis (TUNEL and anticaspase-3) [88]. P2Y1 and P2Y2 receptors were immunoreactive in basal and parabasal keratinocytes. Expression of P2X5 receptors within the stratum spinosum and P2X7 receptors in the stratum corneum was associated with cell differentiation (and subsequent anti-proliferation) and apoptotic cell death, respectively (figure 4). Functional experiments on cultured keratinocytes showed an increase in cell numbers in response to the P2Y1 receptor agonist 2-methylthio ADP and the P2Y2 receptor agonist UTP. By contrast, there was a significant decrease in cell numbers with the P2X5 receptor agonist ATPγS and the P2X7 receptor agonist 2′(3′)-O-(4-benzoylbenzoyl) ATP. It was also shown that P2Y1 receptors in the basal layer of the developing human fetal epidermis were associated with proliferation [89]. P2X5 receptors, predominantly in the basal and intermediate layers, were associated with differentiation, while P2X7 receptors in the periderm were associated with apoptotic cell death.
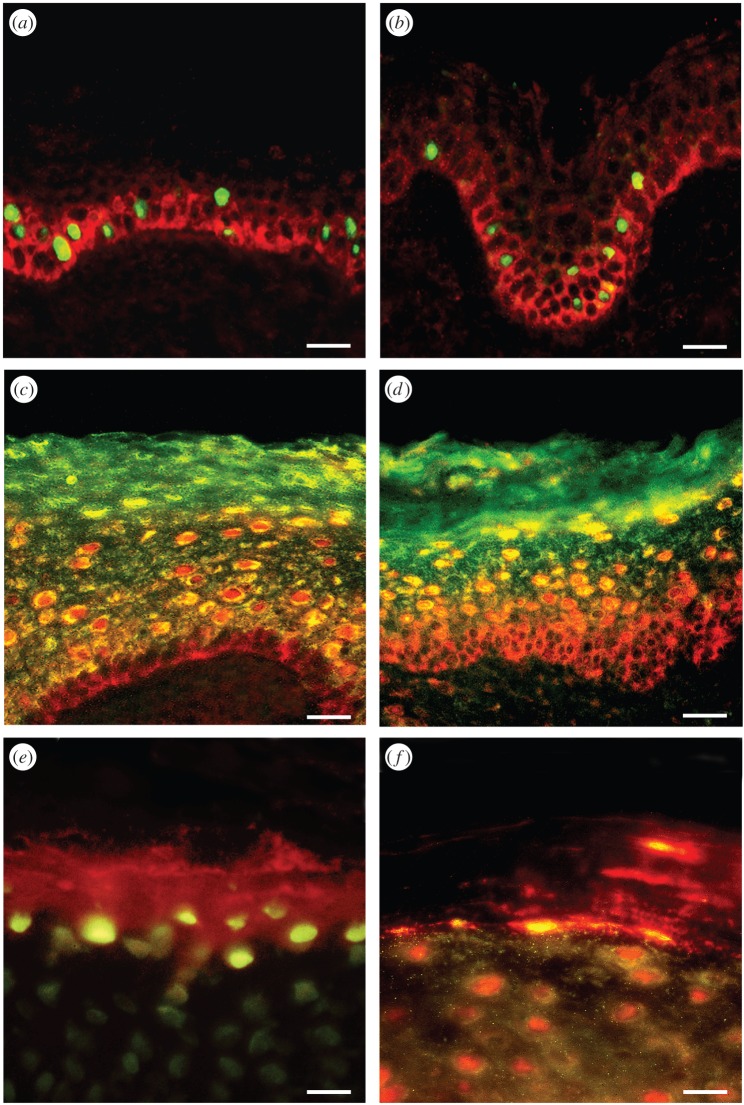
Figure 4.
Double-labelling of P2Y1 and P2Y2 receptors with markers of proliferation shows colocalization within a subpopulation of basal and parabasal keratinocytes. Double-labelling of P2X5 receptors with markers of differentiated keratinocytes shows colocalization within the stratum spinosum, and double-labelling of P2X7 receptors with markers of apoptosis in human leg skin shows colocalization within the stratum corneum. (a) Ki-67 immunolabelling (a marker for proliferation) stained the nuclei (green) of a subpopulation of keratinocytes in the basal and parabasal layers of the epidermis. P2Y1 receptor immunostaining (red) was found in the basal layer on cells also staining for Ki-67. (b) PCNA immunolabelling (a marker for proliferation) stained the nuclei (green) of a subpopulation of keratinocytes. These nuclei were often distributed in clusters and found in the basal and parabasal layers of the epidermis. P2Y2 receptor immunostaining (red) was also expressed in basal and parabasal epidermal cells. (c) P2X5 receptor immunostaining (red) showed overlap (yellow) with cytokeratin K10 (green), an early marker of keratinocyte differentiation. P2X5 receptors were present in the basal layer of the epidermis up to the midgranular layer. Cytokeratin K10 was distributed in most suprabasal keratinocytes. The stratum basale stained only for P2X5 receptors, indicating that no differentiation was taking place in these cells. The colocalization of P2X5 receptors and cytokeratin K10 appeared mainly in the cytoplasm of differentiating cells within the stratum spinosum and partly in the stratum granulosum. Note that the stratum corneum also stained for cytokeratin K10, which labelled differentiated keratinocytes, even in dying cells. (d) P2X5 receptor immunostaining (red) showed overlap (yellow) with involucrin (green). P2X5 receptors were present in the basal layer of the epidermis up to the midgranular layer. Note that the pattern of staining with involucrin was similar to that seen with cytokeratin K10, except that cells from the stratum basale up to the midstratum spinosum were not labelled with involucrin, which is a late marker of keratinocyte differentiation. (e) TUNEL (green) labelled the nuclei of cells at the uppermost level of the stratum granulosum and P2X7 antibody (red) mainly stained cell fragments within the stratum corneum. (f) Anti-caspase-3 (green) colocalized with areas of P2X7 receptor immunostaining (red) both at the junction of the stratum granulosum and within the stratum corneum. Areas of colocalization were yellow. Note that the differentiating keratinocytes in the upper stratum granulosum were also positive for anti-caspase-3. Scale bars (a–d) 30 µm and (e,f) 15 µm. (Reproduced from [88], with permission.)
Purinergic signalling is involved in wound healing. In regenerating epidermis of denervated wounds, P2Y1 receptor expression was increased in keratinocytes, while P2Y2 receptor expression was decreased [90]. Nerve growth factor (NGF) treatment of denervated wounds reduced expression of P2Y1 receptors and increased expression of P2Y2 receptors. NGF treatment enhanced both P2X5 and P2Y1 receptors in keratinocytes in innervated wounds. In all experimental wound healing processes, P2X7 receptors were absent.
Human anagen hair follicles express P2Y1, P2Y2 and P2X5 receptors [91]. P2Y1 receptors were present in proliferating cells in the outer root sheath and bulb, while P2X5 receptors were associated with differentiation of the inner and outer root sheaths and medulla. P2Y2 receptors were found in cells at the edge of the cortex/medulla, while P2X7 receptors were not present.
(e). Cancer
Analysis of the purinergic receptor subtypes involved in the development of tumours in the prostate [92], bladder [93], melanoma [94,95], breast [96–98] and other organs has been described [99,100]. P2Y1 and P2Y2 receptors were expressed and involved in cell proliferation; P2X5 receptors were involved in differentiation (and were therefore antiproliferative), while P2X7 receptors were involved in cell death in many tumours (figure 5). However, P2X7 receptors have been shown to mediate both proliferation of cancer cells and apoptotic cell death [101]. It may be that low concentrations of released ATP promote proliferation, while high concentrations lead to cell death. In human melanomas, functional P2X7 receptors are expressed that mediate apoptosis [94], while P2Y1 and P2Y2 receptor agonists cause a decrease and increase in cell numbers, respectively [95]. In human squamous cell carcinoma, P2Y2, P2X5 and P2X7 receptors appear to be associated with proliferation, differentiation and cell death, respectively [102].
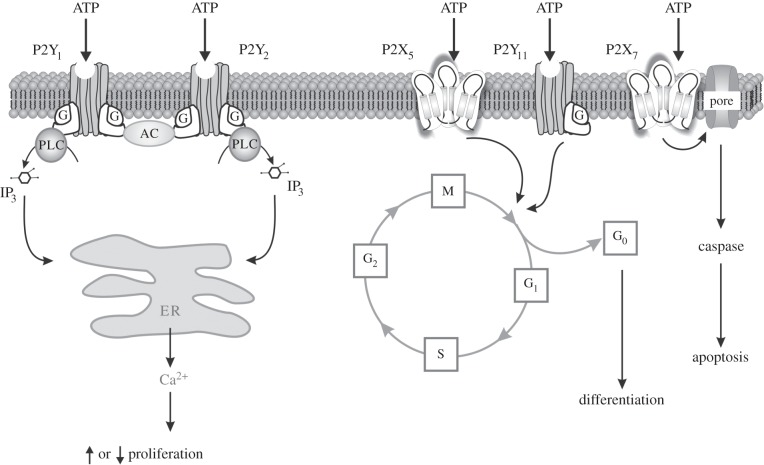
Figure 5.
Schematic diagram illustrating the different mechanisms by which P2 receptor subtypes might alter cancer cell function. P2Y1 and P2Y2 receptors could affect the rate of cell proliferation through altering the intracellular levels of cAMP by modulating adenylyl cyclase (AC) or by increasing intracellular calcium levels through the phospholipase C (PLC) pathway. P2X5 and P2Y11 receptor activation might switch the cell cycle from proliferation into a state of differentiation. The P2X7 receptor activates the apoptotic caspase enzyme system. IP3, inositol trisphosphate. (Redrawn from [99], and reproduced from [68] with permission.)
Using the HT-1376 high grade bladder cancer cell line, P2X5 and P2Y11 receptors mediated the anti-neoplastic effects of ATP, while P2X7 receptors mediated apoptotic cell death [93]. Cell lines of hormone-refractory prostate cancer showed similar results [103]. ATP reduced the in vivo growth of advanced hormone-refractory prostate cancer implanted into mice [104]. Clinical trials have demonstrated that systemic administration of ATP may have beneficial effects (prolongation of survival and reduced cachexia) in lung cancer patients [100].
4. Second messenger mechanisms and transcription factors involved in short- and long-term purinergic signalling
The second messenger mechanisms involved in short-term purinergic signalling have been analysed in a number of studies for P2X ion channel receptors [105–109]. Occupation of both P2X and P2Y receptors leads to an increase in intracellular Ca2+, P2X receptors from extracellular sources and P2Y receptors from intracellular sources [5,110]. It was shown that extracellular ATP activates the P2X channel trimeric structure by binding the three intersubunit-binding sites, which leads to conformational rearrangements that are transferred to transmembrane helices linked to ATP-binding domains by β strands [111]. Coupling of the P2Y receptor subtypes to specific G proteins was initially inferred from indirect evidence from movement of intracellular levels of IP3, calcium, cyclic AMP (cAMP) and determination of pertussis toxin sensitivity. Direct evidence followed by measuring the effect of ADP and GTP hydrolysis in vesicles reconstituted with P2Y1 and either Gαqβ1γ2 or Gα11β1γ2 [112]. G-protein-coupled P2Y receptors also modulate the activity of voltage-gated ion channels in the cell membrane through the activity of activated G proteins (see [113] for a detailed analysis).
The transcription factors involved in long-term trophic signalling are more complex, as indicated in figure 6. A role for calcium influx in cell proliferation has been proposed [114]. External calcium concentration is important for calcium channel function and it also regulates calcium sensing receptor activity. Activation of the P2Y11 receptor by ATP, for example, leads to a rise in cAMP and in IP3 and cytosolic calcium, whereas activation by UTP was shown to produce calcium mobilization without IP3 or cAMP increase [115].
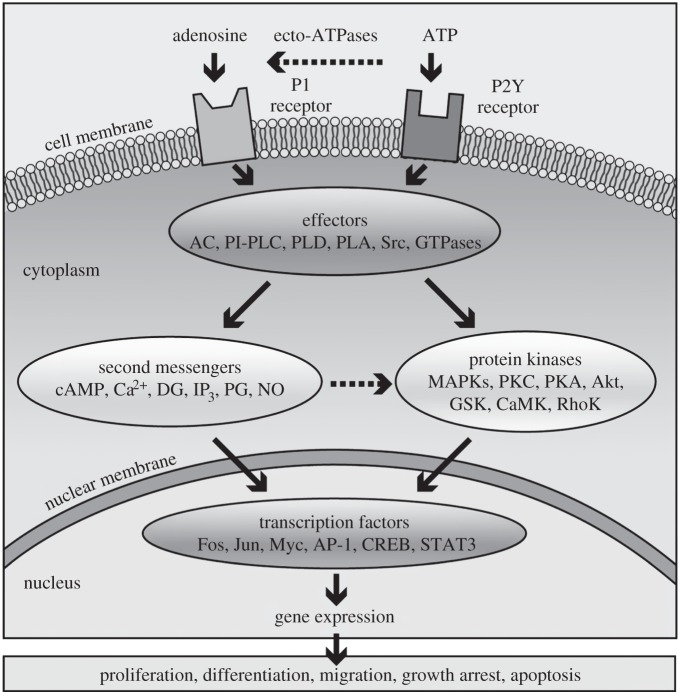
Figure 6.
Schematic overview of purinergic signalling mechanisms that regulate long-term, trophic effects. Extracellular nucleotides and nucleosides bind to purinergic receptors coupled to signal transducing effector molecules. Activation of the effectors leads to generation of second messengers and/or stimulation of protein kinases that regulate expression of genes needed for long-term, trophic actions. In some cases, P2X receptors such as P2X7 are also coupled to protein kinase cascades and can mediate proliferation and apoptosis. Cell-specific and/or receptor subtype-specific differences are likely to account for variations in signalling pathways and functional outcomes. It should be noted that the list of elements is not meant to be all-inclusive. Other protein kinases, e.g. MEK, PI3 K, are upstream of the listed kinases involved in purinergic signalling while others are downstream, e.g. p70S6 K. In addition, dashed arrows indicate that not all listed elements are activated by the upstream component, e.g. not all P1 receptors are coupled to all listed effectors. AC, adenylyl cyclase; AP-1, activator protein-1; CaMK, calcium–calmodulin protein kinase; CREB, cyclic AMP response element binding protein; DG, diacylglycerol; GSK, glycogen synthase kinase; IP3, inositol trisphosphate; MAPKs, mitogen-activated protein kinases (including extracellular signal regulated protein kinase (ERK), p38 MAPK and stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK)); MEK, MAPK/ERK kinase; NO, nitrous oxide; PG, prostaglandin; PI3 K, phosphoinositide 3-kinase; PI-PLC, phosphatidylinositol-specific phospholipase C; PKA, protein kinase A; PKC, protein kinase C; PLD, phospholipase D; PLA, phospholipase A; STAT3, signal transducer and activator of transcription-3. (Reproduced from [8], with permission.)
5. Conclusion
Trimeric P2X ion channel receptors largely mediate short-term purinergic signalling, although there are examples of P2X receptor-mediated long-term signalling. P1 and P2Y G-protein-coupled receptors are predominantly involved in long-term (trophic) purinergic signalling, but there are also examples of mediation of short-term events. Examples of both types of purinergic signalling are explored and the intracellular translational mechanisms involved discussed. Knowledge of the underlying mechanisms involved in both short- and long-term purinoceptor-mediated signalling will help in the development of purinergic drugs for therapeutic purposes.
Acknowledgements
The author thanks Dr Gillian E. Knight for her excellent editorial assistance.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Drury AN, Szent-Györgyi A. 1929. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J. Physiol. 68, 213–237. ( 10.1113/jphysiol.1929.sp002608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G, Campbell G, Satchell D, Smythe A. 1970. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 40, 668–688. ( 10.1111/j.1476-5381.1970.tb10646.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. 1972. Purinergic nerves. Pharmacol. Rev. 24, 509–581. [PubMed] [Google Scholar]
- 4.Burnstock G. 1978. A basis for distinguishing two types of purinergic receptor. In Cell membrane receptors for drugs and hormones: a multidisciplinary approach (eds Straub RW, Bolis L), pp. 107–118. New York, NY: Raven Press. [Google Scholar]
- 5.Ralevic V, Burnstock G. 1998. Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492. [PubMed] [Google Scholar]
- 6.Burnstock G. 2007. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64, 1471–1483. ( 10.1007/s00018-007-6497-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G, Knight GE. 2004. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 240, 31–304. ( 10.1016/S0074-7696(04)40002-3) [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. 2007. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797. ( 10.1152/physrev.00043.2006) [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. 2014. The Paton Lecture: purinergic signalling: from discovery to current developments. Exp. Physiol. 99, 16–34. ( 10.1113/expphysiol.2013.071951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G, Dumsday B, Smythe A. 1972. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br. J. Pharmacol. 44, 451–461. ( 10.1111/j.1476-5381.1972.tb07283.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su C, Bevan JA, Burnstock G. 1971. [3H]adenosine triphosphate: release during stimulation of enteric nerves. Science 173, 337–339. ( 10.1126/science.173.3994.336) [DOI] [PubMed] [Google Scholar]
- 12.Langer SZ, Pinto JEB. 1976. Possible involvement of a transmitter different from norepinephrine in residual responses to nerve stimulation of cat nictitating membrane after pretreatment with reserpine. J. Pharmacol. Exp. Ther. 196, 697–713. [PubMed] [Google Scholar]
- 13.Fedan JS, Hogaboom GK, O'Donnell JP, Colby J, Westfall DP. 1981. Contributions by purines to the neurogenic response of the vas deferens of the guinea-pig. Eur. J. Pharmacol. 69, 41–53. ( 10.1016/0014-2999(81)90600-2) [DOI] [PubMed] [Google Scholar]
- 14.Sneddon P, Burnstock G. 1984. Inhibition of excitatory junction potentials in guinea-pig vas deferens by α,β-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur. J. Pharmacol. 100, 85–90. ( 10.1016/0014-2999(84)90318-2) [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G. 1988. Sympathetic purinergic transmission in small blood vessels. Trends Pharmacol. Sci. 9, 116–117. ( 10.1016/0165-6147(88)90185-X) [DOI] [PubMed] [Google Scholar]
- 16.Burnstock G, Ralevic V. 2014. Purinergic signalling and blood vessels in health and disease. Pharmacol. Rev. 66, 102–192. ( 10.1124/pr.113.008029) [DOI] [PubMed] [Google Scholar]
- 17.Henning RH. 1997. Purinoceptors in neuromuscular transmission. Pharmacol. Ther. 74, 115–128. ( 10.1016/S0163-7258(97)00015-6) [DOI] [PubMed] [Google Scholar]
- 18.Lawrence GW, Aoki KR, Dolly JO. 2010. Excitatory cholinergic and purinergic signaling in bladder are equally susceptible to botulinum neurotoxin A consistent with co-release of transmitters from efferent fibers. J. Pharmacol. Exp. Ther. 334, 1080–1086. ( 10.1124/jpet.110.169342) [DOI] [PubMed] [Google Scholar]
- 19.Zapata P. 2007. Is ATP a suitable co-transmitter in carotid body arterial chemoreceptors? Respir. Physiol. Neurobiol. 157, 106–115. ( 10.1016/j.resp.2007.01.002) [DOI] [PubMed] [Google Scholar]
- 20.Burnstock G. 2009. Purines and sensory nerves. Handbk Exp. Pharmacol. 194, 333–392. ( 10.1007/978-3-540-79090-7_10) [DOI] [PubMed] [Google Scholar]
- 21.Pankratov Y, Lalo U, Castro E, Miras-Portugal MT, Krishtal O. 1999. ATP receptor-mediated component of the excitatory synaptic transmission in the hippocampus. Prog. Brain Res. 120, 237–249. ( 10.1016/S0079-6123(08)63559-1) [DOI] [PubMed] [Google Scholar]
- 22.Jo YH, Role LW. 2002. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J. Neurosci. 22, 4794–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnstock G. 2002. Purinergic signalling and vascular cell proliferation and death. Arterioscler. Thromb. Vasc. Biol. 22, 364–373. ( 10.1161/hq0302.105360) [DOI] [PubMed] [Google Scholar]
- 24.Evans RJ, Derkach V, Surprenant A. 1992. ATP mediates fast synaptic transmission in mammalian neurons. Nature 357, 503–505. ( 10.1038/357503a0) [DOI] [PubMed] [Google Scholar]
- 25.Silinsky EM, Gerzanich V. 1993. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J. Physiol. 464, 197–212. ( 10.1113/jphysiol.1993.sp019630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards FA, Gibb AJ, Colquhoun D. 1992. ATP receptor-mediated synaptic currents in the central nervous system. Nature 359, 144–147. ( 10.1038/359144a0) [DOI] [PubMed] [Google Scholar]
- 27.Hamilton NB, Attwell D. 2010. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci 11, 227–238. ( 10.1038/nrn2803) [DOI] [PubMed] [Google Scholar]
- 28.Brini M, Calì T, Ottolini D, Carafoli E. 2014. Neuronal calcium signaling: function and dysfunction. Cell. Mol. Life Sci. 71, 2787–2814. ( 10.1007/s00018-013-1550-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gourine AV, Wood J, Burnstock G. 2009. Purinergic signalling in autonomic control. Trends Neurosci. 32, 241–248. ( 10.1016/j.tins.2009.03.002) [DOI] [PubMed] [Google Scholar]
- 30.Chandaka GK, Salzer I, Drobny H, Boehm S, Schicker KW. 2011. Facilitation of transmitter release from rat sympathetic neurons via presynaptic P2Y1 receptors. Br. J. Pharmacol. 164, 1522–1533. ( 10.1111/j.1476-5381.2011.01466.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro JA, Sebastião AM. 2010. Modulation and metamodulation of synapses by adenosine. Acta Physiol. 199, 161–169. ( 10.1111/j.1748-1716.2010.02115.x) [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Khakh BS. 2014. Slow neuromodulation mediated by ATP P2X receptors. Neuron 83, 257–259. ( 10.1016/j.neuron.2014.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. 2009. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32, 19–29. ( 10.1016/j.tins.2008.10.001) [DOI] [PubMed] [Google Scholar]
- 34.Verkhratsky A, Krishtal OA, Burnstock G. 2009. Purinoceptors in neuroglia. Mol. Neurobiol. 39, 190–208. ( 10.1007/s12035-009-8070-3) [DOI] [PubMed] [Google Scholar]
- 35.Burnstock G. 2014. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 10, 3–50. ( 10.1007/s11302-013-9397-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnstock G, Evans L, Bailey M. 2014. Purinergic signalling in the kidney in health and disease. Purinergic Signal. 10, 71–101. ( 10.1007/s11302-013-9400-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnstock G, Vaughn B, Robson S. 2014. Purinergic signalling in the liver in health and disease. Purinergic Signal. 10, 51–70. ( 10.1007/s11302-013-9398-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burnstock G. 2014. Purinergic signalling in the reproductive system in health and disease. Purinergic Signal. 10, 157–187. ( 10.1007/s11302-013-9399-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnstock G, Brouns I, Adriaensen D, Timmermans JP. 2012. Purinergic signalling in the airways. Pharmacol. Rev. 64, 834–868. ( 10.1124/pr.111.005389) [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G, Boeynaems J-M. 2014. Purinergic signalling and immune cells. Purinergic Signal. 10, 529–564. ( 10.1007/s11302-014-9427-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnstock G. 2015. Blood cells: an historical account of the roles of purinergic signalling. Purinergic Signal. 11, 411–434. ( 10.1007/s11302-015-9462-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnstock G, Knight GE, Greig AVH. 2012. Purinergic signalling in healthy and diseased skin. J. Invest. Dermatol. 132, 526–546. ( 10.1038/jid.2011.344) [DOI] [PubMed] [Google Scholar]
- 43.Burnstock G, Arnett TR, Orriss IR. 2013. Purinergic signalling in the musculoskeletal system. Purinergic Signal. 9, 541–572. ( 10.1007/s11302-013-9381-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnstock G. 2014. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 10, 103–155. ( 10.1007/s11302-013-9395-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnstock G, Pelleg A. 2015. Cardiac purinergic signalling in health and disease. Purinergic Signal. 11, 1–46. ( 10.1007/s11302-014-9436-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burnstock G. 2014. Purinergic signalling in endocrine organs. Purinergic Signal. 10, 189–231. ( 10.1007/s11302-013-9396-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnstock G. 2009. Purinergic receptors and pain. Curr. Pharm. Des. 15, 1717–1735. ( 10.2174/138161209788186335) [DOI] [PubMed] [Google Scholar]
- 48.Burnstock G. 2016. An introduction to the roles of purinergic signalling in neurodegeneration and neuroregeneration. Neuropharmacology 104, 4–17. ( 10.1016/j.neuropharm.2015.05.031) [DOI] [PubMed] [Google Scholar]
- 49.Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. 2007. Glia: the fulcrum of brain diseases. Cell Death Differ. 14, 1324–1335. ( 10.1038/sj.cdd.4402144) [DOI] [PubMed] [Google Scholar]
- 50.Li L, et al. 2008. Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab. 28, 468–481. ( 10.1038/sj.jcbfm.9600546) [DOI] [PubMed] [Google Scholar]
- 51.Abbracchio MP, Verderio C. 2006. Pathophysiological roles of P2 receptors in glial cells. In Novartis Foundation Symposium No. 276, Purinergic Signalling in Neuron–Glia Interactions, London, 7–9 June 2005 (eds DJ Chadwick, J Goode), pp. 91–103. Chichester, UK: John Wiley & Sons, Ltd. [PubMed]
- 52.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M. 2013. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc. Natl Acad. Sci. USA 110, 11 612–11 617. ( 10.1073/pnas.1300378110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neary JT. 1996. Trophic actions of extracellular ATP on astrocytes, synergistic interactions with fibroblast growth factors and underlying signal transduction mechanisms. In P2 purinoceptors: localization, function and transduction mechanisms (eds Chadwick DJ, Goode JA), pp. 130–141. Chichester, UK: John Wiley and Sons. [DOI] [PubMed] [Google Scholar]
- 54.Abbracchio MP, Saffrey MJ, Höpker V, Burnstock G. 1994. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience 59, 67–76. ( 10.1016/0306-4522(94)90099-X) [DOI] [PubMed] [Google Scholar]
- 55.Franke H, Krügel U, Schmidt R, Grosche J, Reichenbach A, Illes P. 2001. P2 receptor-types involved in astrogliosis in vivo. Br. J. Pharmacol. 134, 1180–1189. ( 10.1038/sj.bjp.0704353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbracchio MP, Ceruti S. 2006. Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal. 2, 595–604. ( 10.1007/s11302-006-9016-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pankratov YV, Lalo UV, Krishtal OA. 2002. Role for P2X receptors in long-term potentiation. J. Neurosci. 22, 8363–8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burnstock G. 2013. Purinergic mechanisms and pain—an update. Eur. J. Pharmacol. 716, 24–40. ( 10.1016/j.ejphar.2013.01.078) [DOI] [PubMed] [Google Scholar]
- 59.Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. 2009. P2X receptors and synaptic plasticity. Neuroscience 158, 137–148. ( 10.1016/j.neuroscience.2008.03.076) [DOI] [PubMed] [Google Scholar]
- 60.Burnstock G. 2001. Purinergic signalling in development. In Handbook of experimental pharmacology, volume 151/I. Purinergic and pyrimidinergic signalling I - Molecular, nervous and urinogenitary system function (eds Abbracchio MP, Williams M), pp. 89–127. Berlin, Germany: Springer. [Google Scholar]
- 61.Zimmermann H. 2006. Nucleotide signaling in nervous system development. Pflugers Arch. 452, 573–588. ( 10.1007/s00424-006-0067-4) [DOI] [PubMed] [Google Scholar]
- 62.Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. 1997. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J. Biol. Chem. 272, 12 583–12 590. ( 10.1074/jbc.272.19.12583) [DOI] [PubMed] [Google Scholar]
- 63.Meyer MP, Gröschel-Stewart U, Robson T, Burnstock G. 1999. Expression of two ATP-gated ion channels, P2X5 and P2X6, in developing chick skeletal muscle. Dev. Dyn. 216, 442–449. ( 10.1002/(SICI)1097-0177(199912)216:4/5%3C442::AID-DVDY12%3E3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 64.Xiang Z, Burnstock G. 2005. Changes in expression of P2X purinoceptors in rat cerebellum during postnatal development. Dev. Brain Res. 156, 147–157. ( 10.1016/j.devbrainres.2005.02.015) [DOI] [PubMed] [Google Scholar]
- 65.Ryten M, Hoebertz A, Burnstock G. 2001. Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev. Dyn. 221, 331–341. ( 10.1002/dvdy.1147) [DOI] [PubMed] [Google Scholar]
- 66.Sugioka M, Fukuda Y, Yamashita M. 1996. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J. Physiol. 493, 855–863. ( 10.1113/jphysiol.1996.sp021428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burnstock G. 2015. Purinergic signalling in neuroregeneration. Neural Regen. Res. 10, 1919 ( 10.4103/1673-5374.165300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burnstock G, Verkhratsky A. 2010. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 1, e9 ( 10.1038/cddis.2009.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoebertz A, Arnett TR, Burnstock G. 2003. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol. Sci. 24, 290–297. ( 10.1016/S0165-6147(03)00123-8) [DOI] [PubMed] [Google Scholar]
- 70.Orriss IR, Key ML, Brandao-Burch A, Burnstock G, Arnett TR. 2012. The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: the role of P2X receptors. Bone 51, 389–400. ( 10.1016/j.bone.2012.06.013) [DOI] [PubMed] [Google Scholar]
- 71.Grol MW, Panupinthu N, Korcok J, Sims SM, Dixon SJ. 2009. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signal. 5, 205–221. ( 10.1007/s11302-009-9139-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Meyer R, Duncan RL, Turner CH. 2009. P2X7 nucleotide receptor plays an important role in callus remodeling during fracture repair. Calcif. Tissue Int. 84, 405–412. ( 10.1007/s00223-009-9237-7) [DOI] [PubMed] [Google Scholar]
- 73.Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ. 2008. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J. Cell Biol. 181, 859–871. ( 10.1083/jcb.200708037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gartland A, Buckley KA, Bowler WB, Gallagher JA. 2003. Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif. Tissue Int. 73, 361–369. ( 10.1007/s00223-002-2098-y) [DOI] [PubMed] [Google Scholar]
- 75.Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ. 2004. Extracellular nucleotides act through P2X7 receptors to activate NF-κΒ in osteoclasts. J. Bone Miner. Res. 19, 642–651. ( 10.1359/JBMR.040108) [DOI] [PubMed] [Google Scholar]
- 76.Ohlendorff SD, et al. 2007. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet. Genomics 17, 555–567. ( 10.1097/FPC.0b013e3280951625) [DOI] [PubMed] [Google Scholar]
- 77.Di Virgilio F, Solini A. 2002. P2 receptors: new potential players in atherosclerosis. Br. J. Pharmacol. 135, 831–842. ( 10.1038/sj.bjp.0704524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erlinge D, Burnstock G. 2008. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 4, 1–20. ( 10.1007/s11302-007-9078-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ralevic V, Burnstock G. 2003. Involvement of purinergic signalling in cardiovascular diseases. Drug News Persp. 16, 133–140. ( 10.1358/dnp.2003.16.3.876886) [DOI] [PubMed] [Google Scholar]
- 80.Seye CI, Kong Q, Yu N, Gonzalez FA, Erb L, Weisman GA. 2006. P2 receptors in atherosclerosis and postangioplasty restenosis. Purinergic Signal. 2, 471–480. ( 10.1007/s11302-006-9015-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horckmans M, Nicolas L, Dol-Gleizes F, Savi P, Gachet C, Boeynaems JM, Robaye B, Communi D. 2008. Role of P2Y4 nucleotide receptor in angiogenesis and inflammation. Purinergic Signal. 4, S118 ( 10.1007/s11302-008-9116-0) [DOI] [Google Scholar]
- 82.Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. 2008. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 11, 169–182. ( 10.1007/s10456-007-9087-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nilsson J, Nilsson LM, Chen YW, Molkentin JD, Erlinge D, Gomez MF. 2006. High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arterioscler. Thromb. Vasc. Biol. 26, 794–800. ( 10.1161/01.ATV.0000209513.00765.13) [DOI] [PubMed] [Google Scholar]
- 84.Trams EJ, Kauffman H, Burnstock G. 1980. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J. Theor. Biol. 87, 609–621. ( 10.1016/0022-5193(80)90239-8) [DOI] [PubMed] [Google Scholar]
- 85.Bodin P, Burnstock G. 1996. ATP-stimulated release of ATP by human endothelial cells. J. Cardiovasc. Pharmacol. 27, 872–875. ( 10.1097/00005344-199606000-00015) [DOI] [PubMed] [Google Scholar]
- 86.Gröschel-Stewart U, Bardini M, Robson T, Burnstock G. 1999. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tiss. Res. 296, 599–605. ( 10.1007/s004410051321) [DOI] [PubMed] [Google Scholar]
- 87.Gröschel-Stewart U, Bardini M, Robson T, Burnstock G. 1999. P2X receptors in the rat duodenal villus. Cell Tiss. Res. 297, 111–117. ( 10.1007/s004410051338) [DOI] [PubMed] [Google Scholar]
- 88.Greig AVH, Linge C, Terenghi G, McGrouther DA, Burnstock G. 2003. Purinergic receptors are part of a functional signalling system for proliferation and differentiation of human epidermal keratinocytes. J. Invest. Dermatol. 120, 1007–1015. ( 10.1046/j.1523-1747.2003.12261.x) [DOI] [PubMed] [Google Scholar]
- 89.Greig AVH, Linge C, Cambrey A, Burnstock G. 2003. Purinergic receptors are part of a signalling system for keratinocyte proliferation, differentiation and apoptosis in human fetal epidermis. J. Invest. Dermatol. 121, 1145–1149. ( 10.1046/j.1523-1747.2003.12567.x) [DOI] [PubMed] [Google Scholar]
- 90.Greig AVH, James SE, McGrouther DA, Terenghi G, Burnstock G. 2003. Purinergic receptor expression in the regenerating epidermis in a rat model of normal and delayed wound healing. Exp. Dermatol. 12, 860–871. ( 10.1111/j.0906-6705.2003.00110.x) [DOI] [PubMed] [Google Scholar]
- 91.Greig AVH, Linge C, Burnstock G. 2008. Purinergic receptors are part of a signalling system for proliferation and differentiation in distinct cell lineages in human anagen hair follicles. Purinergic Signal. 4, 331–338. ( 10.1007/s11302-008-9108-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Janssens R, Boeynaems JM. 2001. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br. J. Pharmacol. 132, 536–546. ( 10.1038/sj.bjp.0703833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shabbir M, Ryten M, Thompson CS, Mikhailidis DP, Burnstock G. 2007. Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int. 101, 106–112. ( 10.1111/j.1464-410X.2007.07286.x) [DOI] [PubMed] [Google Scholar]
- 94.White N, Butler PEM, Burnstock G. 2005. Human melanomas express functional P2X7 receptors. Cell Tiss. Res. 321, 411–418. ( 10.1007/s00441-005-1149-x) [DOI] [PubMed] [Google Scholar]
- 95.White N, Ryten M, Clayton E, Butler P, Burnstock G. 2005. P2Y purinergic receptors regulate the growth of human melanomas. Cancer Lett. 224, 81–91. ( 10.1016/j.canlet.2004.11.027) [DOI] [PubMed] [Google Scholar]
- 96.Vandewalle B, Hornez L, Revillion F, Lefebvre J. 1994. Effect of extracellular ATP on breast tumor cell growth, implication of intracellular calcium. Cancer Lett. 85, 47–54. ( 10.1016/0304-3835(94)90237-2) [DOI] [PubMed] [Google Scholar]
- 97.Dixon CJ, Bowler WB, Fleetwood P, Ginty AF, Gallagher JA, Carron JA. 1997. Extracellular nucleotides stimulate proliferation in MCF-7 breast cancer cells via P2-purinoceptors. Br. J. Cancer 75, 34–39. ( 10.1038/bjc.1997.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gow IF, Thomson J, Davidson J, Shennan DB. 2005. The effect of a hyposmotic shock and purinergic agonists on K+(Rb+) efflux from cultured human breast cancer cells. Biochim. Biophys. Acta 1712, 52–61. ( 10.1016/j.bbamem.2005.04.002) [DOI] [PubMed] [Google Scholar]
- 99.White N, Burnstock G. 2006. P2 receptors and cancer. Trends Pharmacol. Sci. 27, 211–217. ( 10.1016/j.tips.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 100.Burnstock G, Di Virgilio F. 2013. Purinergic signalling in cancer. Purinergic Signal. 9, 491–540. ( 10.1007/s11302-013-9372-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adinolfi E, et al. 2012. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 72, 2957–2969. ( 10.1158/0008-5472.CAN-11-1947) [DOI] [PubMed] [Google Scholar]
- 102.Greig AVH, Linge C, Healy V, Lim P, Clayton E, Rustin MH, McGrouther DA, Burnstock G. 2003. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J. Invest. Dermatol. 121, 315–327. ( 10.1046/j.1523-1747.2003.12379.x) [DOI] [PubMed] [Google Scholar]
- 103.Shabbir M, Ryten M, Thompson CS, Mikhailidis DP, Burnstock G. 2008. Characterisation of calcium-independent purinergic receptor-mediated apoptosis in hormone refractory prostate cancer. BJU Int. 101, 352–359. ( 10.1111/j.1464-410X.2007.07293.x) [DOI] [PubMed] [Google Scholar]
- 104.Shabbir M, Thompson CS, Jarmulowicz M, Mikhailidis DP, Burnstock G. 2008. Effect of extracellular ATP on the growth of hormone refractory prostate cancer in vivo. BJU Int. 102, 108–112. ( 10.1111/j.1464-410X.2008.07578.x) [DOI] [PubMed] [Google Scholar]
- 105.North RA. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067. ( 10.1152/physrev.00015.2002) [DOI] [PubMed] [Google Scholar]
- 106.Erb L, Liao Z, Seye CI, Weisman GA. 2006. P2 receptors: intracellular signaling. Pflugers Arch. 452, 552–562. ( 10.1007/s00424-006-0069-2) [DOI] [PubMed] [Google Scholar]
- 107.Roberts JA, Digby HR, Kara M, El Ajouz S, Sutcliffe MJ, Evans RJ. 2008. Cysteine substitution mutagenesis and the effects of methanethiosulfonate reagents at P2X2 and P2X4 receptors support a core common mode of ATP action at P2X receptors. J. Biol. Chem. 283, 20 126–20 136. ( 10.1074/jbc.M800294200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silberberg SD, Swartz KJ. 2009. Structural biology: trimeric ion-channel design. Nature 460, 580–581. ( 10.1038/460580a) [DOI] [PubMed] [Google Scholar]
- 109.Keceli B, Kubo Y. 2014. Signal transmission within the P2X2 trimeric receptor. J. Gen. Physiol. 143, 761–782. ( 10.1085/jgp.201411166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Egan TM, Khakh BS. 2004. Contribution of calcium ions to P2X channel responses. J. Neurosci. 24, 3413–3420. ( 10.1523/JNEUROSCI.5429-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hattori M, Gouaux E. 2012. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485, 207–212. ( 10.1038/nature11010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waldo GL, Harden TK. 2004. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol. Pharmacol. 65, 426–436. ( 10.1124/mol.65.2.426) [DOI] [PubMed] [Google Scholar]
- 113.Abbracchio MP, et al. 2006. International Union of Pharmacology. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341. ( 10.1124/pr.58.3.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Borowiec AS, Bidaux G, Pigat N, Goffin V, Bernichtein S, Capiod T. 2014. Calcium channels, external calcium concentration and cell proliferation. Eur. J. Pharmacol. 739, 19–25. ( 10.1016/j.ejphar.2013.10.072) [DOI] [PubMed] [Google Scholar]
- 115.White PJ, Webb TE, Boarder MR. 2003. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol. Pharmacol. 63, 1356–1363. ( 10.1124/mol.63.6.1356) [DOI] [PubMed] [Google Scholar]