Abstract
Evolution of the nervous system progressed through cellular diversification and specialization of functions. Conceptually, the nervous system is composed from electrically excitable neuronal networks connected with chemical synapses and non-excitable glial cells that provide for homeostasis and defence. Astrocytes are integrated into neural networks through multipartite synapses; astroglial perisynaptic processes closely enwrap synaptic contacts and control homeostasis of the synaptic cleft, supply neurons with glutamate and GABA obligatory precursor glutamine and contribute to synaptic plasticity, learning and memory. In neuropathology, astrocytes may undergo reactive remodelling or degeneration; to a large extent, astroglial reactions define progression of the pathology and neurological outcome.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: astroglia, evolution, astroglial cradle, multipartite synapse, memory, neuropathology
1. Evolution of the nervous system: cellular distribution of functions
The human brain, which crowns 3.5 billion years of biological evolution, is arguably the most complex structure known to the natural sciences. The brain tissue is composed out of approximately 200 billion neurons and neuroglial cells that are connected by more than 15 trillion electrical and chemical synapses into the networks with extraordinary computing and memory storage capacity—the latter being estimated to reach a petabite mark [1]. High density of electrically excited cells, which rely on constant movement of ions across their membranes, the process requiring ATP in quantity (a single human cortical neuron is claimed to use approximately 4.7 billion ATP molecules per second [2]), makes the brain the major energy consumer in the organism [3]. The high disbursement of ATP stipulates high mitochondrial activity, whereas the oxidative phosphorylation produces reactive oxygen species that have to be scavenged to avoid profound cellular damage. Ion redistribution associated with brain activity has to be controlled, as ion accumulation in the extracellular space seriously affects neuronal excitability. Similarly, the neurotransmitters released in the course of synaptic transmission have to be properly handled to exclude associated neurotoxicity (and glutamate, the main excitatory neurotransmitter, is the most effective endogenous neurotoxin). Finally, these cellular networks and the cells making them are constantly changing their structure, which underlies neural plasticity and learning. These are only a few of the major logistical problems associated with brain function, which are managed surprisingly well in the course of about 100 years of human life. Even more remarkably, the human brain is highly resilient to the passing years and the brain appears as one of the most age-resilient systems of the human body. Indeed, the 40-year-old athlete cannot compete in the sprint with youngsters, whereas a 60-year-old academic has, as a rule, much higher intellectual output than many of his 20-year-old students. This reflects a remarkable plasticity of the human brain, which is optimized for learning thus recompensing the age-dependent alterations.
The maintenance of the brain tissue throughout life is the function of specialized cells classified as neuroglia. The neuroglial cells are represented by astroglia, oligodendroglia and NG2 glia all of neuroepithelial (i.e. ectodermal) origin, and microglial cells that derive from foetal microphages (of mesodermal descent) that enter the nervous system very early in embryogenesis [4–6]. In the human brain, the total number of glial cells is more or less similar to that of neurons, although with prominent regional differences [7,8]. Evolutionarily, emergence of neuroglia coincided with the appearance of the centralized nervous system; there is little sign of supportive cells in the diffuse nervous system [9]. The proto-astrocytes are well-defined in round worms, where they contribute to the development and maintenance of the nervous system (especially its the sensory arm; the glial cells form sensory organs of the worm, known as sensillas), although artificial ablation of this primeval glia does not exterminate the animal [10,11]. In more advanced invertebrates, glia diversifies; ganglia in leeches and ‘brains’ of insects contain many types of ‘homeostatic’ glia analogous to astrocytes, whereas axons in some types of molluscs and arthropods are covered by multiple lamellae of covering glia analogous to Schwann cells and oligodendrocytes [12–15]. The nervous tissue macrophages or primeval microglial cells are known to populate the ganglia of leeches and bivalves [16]. Conceptually, therefore, from the very beginning of evolutionary development of central nervous system, the cellular division of functions emerged: the neurons become mostly responsible for rapid propagation of signals associated with action potentials and chemical synapses, whereas neuroglia assumed the responsibility for homeostasis and defence.
This specialization occurred in evolution at least twice, because the appearance of chordates and vertebrates coincided with fundamental change in neuroglia. This fundamental change is manifested by an emergence of a new type of glial cell, the radial glia [17], which signalled the new organization of the CNS in layers in contrast to fused ganglia of invertebrates. The radial glia emerged in the Echinodermata (e.g. sea urchin, star fishes, sea cucumber), the early relatives of Chordata. Neuroglia in these species are represented, almost entirely, by the radial glia with elongated shape, long processes, perpendicular orientation to the surface of the neuroepithelium and high level of expression of intermediate filaments glial fibrillary acidic protein (GFAP) and vimentin [18]. Similarly, the radial glia are the main type of parenchymal glia in early vertebrates including elasmobranchii (sharks and rays) and teleosts (for example, zebra fish). The radial glia dominate the so-called laminar-type brains, with thin parenchyma and neurons mainly concentrated in the periventricular zone. In the larger and thicker brains with clear layered organization, the true parenchymal (i.e. protoplasmic) astrocytes are identified [19,20]. In the zebra fish (which also possesses a relatively thin brain), the radial glia is the only type of astroglia-like cells; processes of these cells extend through the entire width of the brain from the ependymal coating of the ventricles to the pial surface; zebra fish radial glial cells express GFAP, glutamine synthetase and aquaporin-4 and they seemingly perform all homeostatic functions congenital to astroglia. The reaction of zebra fish glia to injury is however idiosyncratic: instead of mounting a reactive response, the radial glia increase proliferation and neurogenesis; as a result, zebra fish never produce scars in their brain, but counteract the lesion by substituting damaged cells [21]. An increase in the thickness of the brain is associated with an appearance of specialized parenchymal astrocytes; the very same scenario [22] operates in developing mammalian brains when radial glia populating the neural tube are eventually substituted by various types of parenchymal astrocytes (although some neuroglia with radial properties remain in certain areas; for example, Bergmann glial cells in cerebellum, Müller glia in the retina or tanycytes in the hypothalamus [17]).
2. Astroglial integration into neural networks: the multipartite synapse
The neural tissue of the CNS is organized in a form of highly complex cellular networks composed of neural cells (neurons, astrocytes, NG2 cells and oligodendrocytes), microglia of mesodermal origin and blood vessels, formed by muscle and endothelial cells and containing various types of blood cells. All these elements form multiple connections that allow a high degree of coordination. In these settings, astroglia assume control over extracellular homeostasis of neurotransmitters, ions, reactive oxygen species; astrocytes are responsible for local metabolic support, for regulation of extracellular volume, for regulation and maintenance of synaptic connectivity, for secretion of numerous trophic and humoral factors and many more [7,23–29]. Astrocytes are important elements of brain cytoarchitecture; they provide for tiling in the grey matter hence creating astroglio-neurovascular units that integrate neuronal and vascular elements residing within the territorial domain of an individual protoplasmic astrocyte [30,31]. Astrocytes display a remarkable heterogeneity between brain regions; astroglia differ in morphological appearance, in expression of ion channels, receptors and transporters and in their functional specialization [7,32–37]. Differential expression of neurotransmitter receptors in astrocytes from different regions of the brain exemplifies their functional heterogeneity. Astrocytes, potentially, can express all neurotransmitter receptors existing in the brain, which was demonstrated in early experiments performed in vitro in primary cell cultures [38]. In the nervous tissue in situ, however, glial expression of neurotransmitter receptors differs substantially between brain regions, the specific repertoire of receptors being, most likely, controlled by the local neurochemical environment. Indeed, the complement of receptors borne by astrocytes in a given brain area is, as a rule, congruent to the main neurotransmitters released in this particular part of the CNS [39]. For example, glycine receptors are expressed in astrocytes in the spinal cord (where glycine acts as a main inhibitory neurotransmitter), dopamine receptors are present in astroglial cells in substantia nigra (where dopaminergic transmission dominates), whereas Bergmann glia in cerebellum express adrenoceptors, purinoceptors, histamine, GABA and glutamate receptors, all reflecting neurotransmitters released in their vicinity [40–45]. Physiological properties of astrocytes can be further sculpted by signalling influences from neighbouring neurons; these influences in particular may be mediated by Sonic Hedgehog morphogenes [46].
Activation of glial receptors conveys chemical transmission from neurons to astrocytes, and initiates a specific form of astroglial excitability mediated by spatio-temporal cytosolic changes in the concentration of several ions, most notably Ca2+, Na+ and K+. Astroglial calcium signalling [45,47,48] can be global (mainly owing to Ca2+ release from the endoplasmic reticulum Ca2+ store) or local (mainly mediated by Ca2+ entry through plasmalemmal channels and Na+/Ca2+ exchanger); these Ca2+ signals regulate cellular metabolism and gene expression, as well as trafficking and release of secretory vesicles. Dynamic changes in cytosolic Na+ concentration, which occur in response to neuronal activity, specifically regulate numerous homeostatic molecular cascades expressed in astrocytes [49–51]. Increases in intracellular Na+ in astrocytes originate from Na+ influx through ionotropic receptors (such as AMPA/NMDA glutamate receptors or P2X purinoceptors [52,53], which all conduct Na+ currents), by Na+/Ca2+ exchanger and most notably by Na+-dependent glutamate transporter. The transient receptor potential ‘canonical’ (TRPC) channels expressed in astroglia provide a link between Ca2+ release from the endoplasmic reticulum and Na+ influx: TRPC channels are activated by store-operated mechanism and generate both Na+ and Ca2+ currents [54]. The transmembrane Na+ gradient in its turn controls many solute carrier transporters expressed in astroglial membrane that are responsible for removal of neurotransmitters (aforementioned glutamate transporters, GABA transporters, glycine transporters, concentrating adenosine transporters), for release of glutamine (which is the obligatory precursor for neuronal glutamate and GABA), for accumulation of ascorbic acid, for transport of protons and bicarbonate, and many more. Besides, astroglial Na+ regulates sodium–potassium pump and inward rectifying K+ channels (which are the main components of the K+ buffering system), glutamine synthetase and mitochondrial Ca2+ transport. All in all, [Na+]i transients are an important part of glial ion excitability that coordinates neuronal activity with glial homeostatic responses.
As has been briefly alluded to before, the evolution of the nervous system progressed through division of function and cellular specialization. The chemical synapse, which is the central element of neural connectivity, similarly evolved through functional specialization of its cellular compartments. In the CNS, most of the synapses are composed from several elements and hence are known as multipartite synapses [55–57]. The main components of multipartite synapses of the CNS are (i) the presynaptic terminal, (ii) the postsynaptic part that is often represented by the dendritic spine, (iii) the perisynaptic process of the astrocyte, (iv) the process of a neighbouring microglial cell that periodically contacts the synaptic structure, and (v) the extracellular matrix, which is present in the synaptic cleft and also extends extrasynaptically. The neuronal part of a synapse is fully specialized for chemical transmission: the presynaptic terminal contains a rather substantial pool of synaptic vesicles and proteins responsible for multiple stages of exocytotic release. The postsynaptic membrane contains neurotransmitter receptors; in addition, the postsynaptic neuronal compartment is densely packed with numerous proteins responsible for synaptic plasticity [58]. The microglial process (which is now believed to be a fully legitimate part of a central synapse) regularly surveys the synapse and when necessary prunes it [59,60]; the extracellular matrix regulates various aspects of receptor trafficking in pre- and postsynaptic parts, regulates synaptogenesis, modulates membrane excitability and provides a platform for integrin signalling [61]. The perisynaptic process of an astrocyte, which intimately covers both pre- and postsynaptic parts (the degree of coverage varies across the CNS; on average, 60–70% of central synapses are enwrapped by astroglial membranes), contains most of the molecules responsible for homeostatic control of the synaptic cleft and of the synapse at large. The generic functions of the astroglial compartment are many; they include regulation and promotion of synaptogenesis [62], synaptic maturation [24], synaptic maintenance and synaptic extinction [63], and thus the perisynaptic astroglial comportment can be considered as a synaptic cradle [57,64] that fosters, sustains and eliminates synapses in the course of life, thus shaping the neural connectome.
The perisynaptic astroglial processes originate from peripheral astroglial processes of parenchymal astrocytes (which could be protoplasmic astrocytes in the cerebrum or Bergmann glial cells in the cerebellum [65,66]). The perisynaptic process is quite a peculiar structure, with an extremely high surface-to-volume ratio; the perisynaptic process is exceedingly thin (approx. 100 nm or even less) and is almost completely devoid of organelles [67,68], although it may contain very small (0.2–0.4 µm) spherical mitochondria [69]. The perisynaptic processes specifically express ezrin and radixin (which can be used a immunocytochemical markers [70]); these two are known to associate with actin and hence contribute to filopodial movements and may therefore participate in morphological plasticity of astroglial processes [70,71]. Rapid filopodial movements of astroglial processes have indeed been detected in vitro and in situ [70,72], and it is conceivable that morphological remodelling of perisynaptic astroglial processes can be an important component of synaptic plasticity [73]. Astroglial perisynaptic processes control homeostasis of the synaptic cleft [57]; for this purpose, they express numerous transporters, channels and enzymatic cascades, some functions of which are discussed below.
3. Singular human astrocytes
Evolution of the brain proceeded with steady increase in the organ's size, quite obviously reflecting an increase in the number of neural cells. The total number of neural cells in low vertebrates is around hundreds, in insects the brain contains approximately 100 000 cells, whereas in humans approximately 200 billion. The relative number of glial cells generally increases when progressing through the phylogenetic tree. A single ganglia in the leech Hirudo medicinalis is composed of approximately 400 neurons and only 10–12 neuroglial cells (giving a glia-to-neuron ratio of 0.025), whereas in mammals, this parameter varies between 0.3 in rodents and approximately four to eight in elephants and whales (figure 1). There are, as usual, exceptions: for instance, the buccal ganglia of the great ramshorn snail Planorbis corneus has 391 glial cells and 298 neurons, giving a glial-to-neuron ratio of approximately 1.3 [75]. Precise numbers of cells in the brain of humans and counts of different types of neuroglia are yet to be obtained and verified. All in all, it seems that the grand totals for neurons and non-neuronal cells are quite similar: likely the human brain contains more than 100 billion neurons and in excess of 100 billion glial cells (for counts and techniques used, see for example [7,76–80]). What are the numerical fractions for different types of glia similarly remains unknown; probably, it is safe to suggest that human brain contains approximately 10% of microglia, 10% of NG2 cells and the remaining 80% are shared between oligodendrocytes and astrocytes in yet undefined proportions. Numerical distribution of neurons and neuroglia varies between brain regions, and varies rather substantially: the cerebellum for example contains the largest number of neurons (ca 70 billion [81]) and comparatively few neuroglia (about 4–10 billion, giving glia-to-neuron ratio of approx. 0.1). The relatively low numbers of cerebellar astrocytes are compensated by their specific morphology—the velate astrocytes of cerebellum extend lamellar-like processes that surround most of granule neurons and possibly even partition glomeruli into independent units [82,83]. In the cerebral cortex, the ratio between astrocytes and neurons is approximately 1.65 [84], whereas in the brainstem, glial cells may outnumber neurons by a factor of 10 [8]. In the white matter (that comprises more than 50% of the total brain volume), neuronal somata are absent and hence the glia-to-neuron ratio is (formally) infinite.
Figure 1.
Phylogenetical advance of neuroglia. (a) Glia-to-neuron ratio in the nervous system of invertebrates and in the cortex of vertebrates. Glia-to-neuron ratio is generally increased in phylogenies; more or less, this ratio linearly follows an increase in the size of the brain. (b) Relative increase in glial dimensions and complexity during evolution. Linear dimensions of human astrocytes when compared with mice are approximately 2.75 times larger and their volume is 27 times larger; human astrocytes have approximately 10 times more processes and every astrocyte in human cortex enwraps approximately 20 times more synapses. (c) Comparison of morphological appearance (at the same magnification) of mouse, monkey and human protoplasmic astrocytes. Scale bar, 10 µm. (a,b) Reproduced with permission from [7] and (c) from [74]. (Online version in colour.)
Astroglial evolution led to substantial changes in their appearances, which is particularly evident in the brains of higher primates and humans. First, classical protoplasmic and fibrous astrocytes in the human brain are substantially larger and exceedingly more complex when compared with rodents or even monkeys (figure 1b,c; [37,74,85]). The average size of the territorial domain of a human protoplasmic astrocyte is approximately 2.5 times larger that formed by a rat astrocyte (142 versus 56 µm), whereas the volume of the human protoplasmic astrocyte domain is approximately 16.5 times larger than the volume of the domain associated with rat astrocyte. Human protoplasmic astrocytes have approximately 10 times more primary processes emanating from their somata, and correspondingly much more complex arborization of terminal processes. As a result, a single human protoplasmic astrocyte envelops approximately two million synapses residing in its territorial domain, whereas rodent astrocytes cover approximately 20 000–100 000 synaptic contacts [30,85]. Similarly, human fibrous astrocytes are approximately 2.2 times larger than their rat namesakes [85].
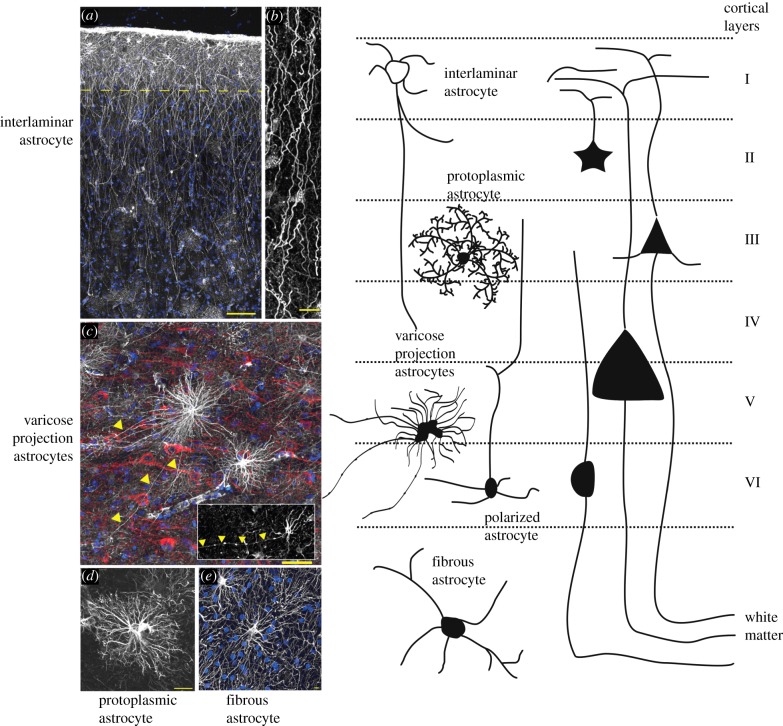
In the higher primates and in humans, several specific types of astroglial cells, which are absent in all other species, have been detected. First, these are interlaminar astrocytes; small (approx. 10 µm) somata of these cells are located in the cortical layer I, whereas their exceptionally long (approx. 1 mm) processes extend to cortical layers III–V; these processes run parallel to each other, forming the so-called palisade (figure 2b; [86–88]). The second type of human astrocytes is represented by polarized astrocytes; these are uni- or bipolar cells whose somata are placed close to the white matter in layers V and VI. These cells send one or two very long processes that end in the neuropil [85]. Finally, the brain of humans (only) contains varicose projection astrocytes that have up to five long (up to 1 mm) unbranched processes that extend in all directions from somata located in the deep cortical layers. The processes of these cells have evenly spaced varicosities (figure 2; [85]). The role of all these human-specific astroglia remains unknown.
Figure 2.
Morphological heterogeneity and subtypes of astrocytes in human cortex. (a) Pial surface and layers 1–2 of human cortex. GFAP staining in white; 4′,6-diamidino-2-phenylindole (DAPI), in blue. Scale bar, 100 µm. Yellow dashed line indicates border between layers 1 and 2. (b) Interlaminar astrocyte processes. Scale bar, 10 µm. (c) Varicose projection astrocytes reside in layers 5–6 and extend long processes characterized by evenly spaced varicosities. Inset: varicose projection astrocyte from chimpanzee cortex. Yellow arrowheads indicate varicose projections. Scale bar, 50 µm. (d) Typical human protoplasmic astrocyte. Scale bar, 20 µm. (e) Human fibrous astrocytes in white matter. Scale bar, 10 µm. (Reproduced with permission from [37]).
Do these larger and extraordinarily more complex human astrocytes contribute to the computing power of human brain? This question was directly addressed when human glial progenitors were injected into the ventricles of newborn mice [89]. These progenitors differentiated into mature protoplasmic and fibrous astrocytes as well as into oligodendrocytes. The human astrocytes maintained their complex structure in the mouse brain; they also were substantially larger then host astrocytes [89]. Quite unexpectedly, the human glia bestow a growth advantage to the mouse brain, whereas human astrocytes populate large parts of both grey and white matter [90]. When ‘humanized’ (i.e. carrying human glia) chimeric mice reach adulthood they became smarter than their littermates that did not receive human glia progenitor grafts. The humanized mice outwitted their naive relatives on multiple cognition tests, including novel object recognition and fear conditioning [89]. At a cellular level, the presence of human astrocytes leads to a reduction in long-term potentiation threshold measured in hippocampal slices. Overall, this analysis provides direct evidence that human astrocytes boost the cognitive abilities of mice, possibly by increasing neural plasticity.
4. Astroglial homeostatic cascades
(a). Neurotransmitter homeostasis
Neuronal networks communicate using chemical transmitters, which have to be constantly replenished. Turnover and homeostasis of the major neurotransmitters in the CNS (glutamate, GABA and adenosine) constitute one of the most fundamental functions of astroglia. Glutamatergic and GABAergic transmission require astroglia for maintenance through continuous supply of glutamine (glutamate–glutamine–GABA shuttle). Glutamine is synthesized in astrocytes either through the tricarboxylic acid cycle [91,92] or directly from glutamate by glutamine synthetase. Glutamine synthetase is a specific astroglial enzyme discovered by Michael Norenberg and Antonio Martinez-Hernandez [93,94]. Glutamate is accumulated into astrocytes by two types of astroglia-specific transporters, the excitatory amino acid transporters 1 and 2 (EAAT1, EAAT2). Importantly, both EAAT1/2 and glutamine synthetase are specifically concentrated in the perisynaptic astroglial processes surrounding identified glutamatergic synapses [68,95]. Glutamine produced in astrocytes is transported back to neurons by using a coordinated system of neutral amino acid transporters: astrocytes express Na+-coupled neutral amino acid transporters SN1/SNAT3/SLC38A3 and SN2/SNAT5/SLC38A5 that provide for glutamine efflux (so-called system N); neurons have distinct complement (the system A) of ATA1/SNAT1/SLC38A1 and ATA2/SNAT2/SLC38A2 amino acid transporters, which are specialized for glutamine influx [96]. Astroglial transporters are electroneutral (Na+ influx is balanced by equivalent efflux of H+), whereas the neuronal system is electrogenic (glutamine molecule is co-transported with Na+). As a result, glutamine influx is accompanied with depolarization and may, in principle, trigger neuronal excitation [97]. Astroglial secretion of glutamine is directly coupled with neuronal activity: increase in the latter (and hence increase in glutamine transport into astrocyte) stimulates release of glutamine by astrocytes [98,99]. Inhibition of the glutamate–glutamine–GABA shuttle suppresses glutamatergic as well as GABAergic transmission in the CNS [100,101].
Astrocytes also contribute to GABA transmission through expression of Na+-dependent GABA transporters GAT1/SLC6A1 and GAT3/GAT3. These transporters are involved in the removal of GABA from the synaptic cleft. The reversal potential of astroglial GABA transporters is quite close to the resting potential (approx. −70 or even −80 mV); therefore, rather moderate increases in cytosolic Na+ concentration reverse the transporter, thus leading to GABA release from astroglial compartment [102]. Astrocytes also express Na+-dependent glycine transporters of GluT1/SLC6A9 type, which also can be reversed upon an increase in cytosolic Na+ concentration [103]. Finally, astrocytes are critical elements of the purinergic transmission because they catabolize adenosine that occurs in the CNS following ATP hydrolysis. Astrocytes accumulate adenosine by equilibrating (ENT1/SLC29A1) or Na+-dependent concentrative (CNT2/SLC28A2) transporters [104]. Accumulated adenosine is subsequently catabolized by adenosine kinase, which, in the CNS, is preferentially expressed in astrocytes [26].
(b). Astroglia control ion homeostasis
Tight control over extracellular ion concentrations is of critical importance for CNS functions, as even minor changes may have far-reaching consequences. At the same time, fluctuations in extracellular ion concentration may represent a physiologically relevant mechanism for regulation of overall excitability of neurons and regulate functional status of the brain. Astrocytes actively control extracellular cations (K+, Na+ and Ca2+) as well as extracellular protons. Astroglial K+ buffering system (discovered by Leif Herz in 1965 [105]) reflects concerted operation of Na+/K+ ATPase, inward rectifying Kir4.1 channels, and possibly some other transporters such as the K+/Cl− co-transporter KCC1/SLC12A4 [106,107]. All these systems mediate local transmembrane K+ transport; local increases in K+ inside astrocytes are rapidly equilibrated through astroglial syncytia by diffusion through connexins/gap junctional channels (the spatial K+ buffering [108]). Astrocytes are important regulators of pH in the brain interstitial space, being endowed with Na+/H+ exchanger NHE-1/SLC9A1, 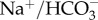 co-transporter NBC/SLC4A4, the lactate transporter MCT-1/SLC16A1, which expels 1H+ together with each lactate molecule, and glutamate transporters EAAT1/2, which remove a single proton from the extracellular space with each molecule of glutamate [109]. Astrocytes may also supply extracellular milieu with Ca2+ ions (which can be depleted upon strong neuronal activity)—decrease in extracellular Ca2+ triggers Ca2+ release from astroglial internal stores and this Ca2+ can subsequently be extruded through the Na+/Ca2+ exchanger [110]. Astrocytes also participate in regulation of Cl− (providing Cl− efflux through anion channels) and Zn2+ [111].
co-transporter NBC/SLC4A4, the lactate transporter MCT-1/SLC16A1, which expels 1H+ together with each lactate molecule, and glutamate transporters EAAT1/2, which remove a single proton from the extracellular space with each molecule of glutamate [109]. Astrocytes may also supply extracellular milieu with Ca2+ ions (which can be depleted upon strong neuronal activity)—decrease in extracellular Ca2+ triggers Ca2+ release from astroglial internal stores and this Ca2+ can subsequently be extruded through the Na+/Ca2+ exchanger [110]. Astrocytes also participate in regulation of Cl− (providing Cl− efflux through anion channels) and Zn2+ [111].
Consequences of astroglial ion regulation can be significant, although the role of physiological fluctuations in ion composition of interstitial fluid (ISF) remains somewhat underappreciated. Recent findings of rapid and significant fluctuations of extracellular K+ Ca2+, Mg2+ and H+ associated with the sleep/wake cycle highlight the importance of ion regulation in the CNS [112]. These fluctuations in ion concentrations are likely to represent a robust physiological mechanism that controls global excitability of the CNS and thus defines the functional status of the brain.
5. Astrocytes and global homeostasis of the brain: the glymphatic system
Astrocytes are key cellular contributors to the brain-wide perivascular pathway that supports the exchange of cerebrospinal fluid and ISF through the recently discovered glymphatic system [113,114]. The perivascular drainage system is composed from the vascular muscle cells, endothelial cells, pericytes and astroglial perivascular endfeet. These latter plaster the vascular wall with approximately 98% coverage [115]; the endfeet are separated by narrow (approx. 50 nm) clefts that allow certain exchange between perivascular space and brain parenchyma. The interchange of ISF and CSF between perivascular space and brain tissue is driven by pressure gradients arising from arterial pulsatility or vasomotion or respiration; importantly, the CSF transport into brain parenchyma critically depends on aquaporin water channels densely expressed in astroglial endfeet (see [116] for detailed overview). This continuous interchange of fluids underlies the clearance of numerous solute molecules providing for brain cleansing. The glymphatic system is possibly connected to the sinus-associated lymphatic vessels, thus creating a global brain lymphatic drainage complex [117]. The status of the glymphatic system is very much affected by sleep/wake cycle; the activity of glymphatic clearance markedly increases during the sleep period [118]. The glymphatic clearance severely decreases with ageing [119], which may be responsible for increased vulnerability of the aged brain to neurodegeneration, this latter being associated with accumulation of various pathological proteins. Very recently, the glymphatic system has been demonstrated in the human brain [120,121].
6. Astroglial plasticity and memory
Current neuroscience philosophy regards learning and memory through the prism of synaptic plasticity, when structural changes, occurring at the synaptic level, outlast memory stabilization. This concept, proposed by Santiago Ramon y Cajal more than a century ago [122], implies numerous molecular changes that occur in all parts of the synapse, leading to a remodelling of the neural networks that impact onto behaviour [123]. Astrocytes, being in intimate contact with synaptic structures, are likely to contribute to various aspects of memory formation, storage and retention [124].
Astroglial processes that surround synapses not only provide for tight homeostatic control over synaptic cleft and supply neurons with various substances, but they also seem to stabilize the synapse and prevent it remodelling. In the process of learning, astroglial processes undergo rapid and profound changes; for example, in the lateral amygdala, the implicit memory consolidation of Pavlovian threat conditioning is accompanied with retraction of astroglial processes from the existing synapses. This retraction apparently allows the enlargement of synaptic contacts [125]. From the temporal perspective, astroglial processes undergo morphological metamorphosis in a matter of minutes, hence allowing rapid synaptic remodelling with subsequent stabilization. Simultaneously, retraction or extension of astroglial processes affects neurochemical environment by allowing or denying neurotransmitter spillover [126,127], which might be another mechanism contributing to memory formation. Astroglia may also be important for energy support of synaptic remodelling during memory formation, which has been shown to depend rather critically on glycogenolysis that occurs specifically in astrocytes [128,129]. Similarly, there is evidence linking long-term memory formation with astroglia-dependent supply of neurons with the energy substrate, lactate [130]. It is of importance that astroglial energy metabolism is regulated mainly through adrenergic input; and noradrenalin, in the adult brain, is the main neurotransmitter triggering Ca2+ excitability of astrocytes [131]. It is therefore conceivable that astrocytic Ca2+ signalling regulates their energy metabolism, including ATP production and glycogen degradation. At the same time, noradrenalin controls many aspects of astroglial morphological plasticity. It is well recognized, from early in vitro studies, that stimulation of adrenergic receptors or increase in their downstream second messenger cAMP induces rapid stellation of cultured astrocytes [132–135]. Hence, noradrenergic innervation can integrate various aspects of astroglial plasticity in the memory-related plasticity.
7. Neuropathology as a homeostatic failure: central role for astroglia
From the broad perspective, neuroglia are homeostatic and defensive cells in the CNS; any type of insult to the nervous tissue initiates active glial response, whereas neurons are left to be stressed, to die or to recover. The neurological diseases can, conceptually, be regarded as homeostatic failure, and hence neuroglial performances, reactions and deficiencies are central elements that shape neuropathological evolution and define neurological outcome. This glia-centric view on neuropathology started to develop very recently, and yet it seems now obvious that a glial component can be distinguished in every form of neurological disease [136–142], although understanding the precise role of glia and potential of glia as a target for cell-specific therapies requires tenacious research.
Astroglial contribution to neuropathology is multifaceted, and astrocytes are known to undergo heterogeneous pathological remodelling, which is directly linked to the disease context. In many pathological conditions, astroglial cells undergo degeneration, atrophy and loss of function; these changes are particularly characteristic for psychiatric diseases such as major depression and schizophrenia, in which decrease in astroglial densities is the leading histopathological manifestation [143–146]. Astrodegenerative morphological changes in neuropsychiatric diseases coincide with functional abnormalities such as decreased glutamate and GABA uptake [147,148], or increased production of kinurenic acid incidentally associated with higher risk of schizophrenia [149,150]. Moreover, ablation of astrocytes in the prefrontal cortex (by injection of specific toxin l-α-aminoadipic acid) was sufficient to induce depression-like symptoms in adult rats [143]. The leading mechanism of Wernicke encephalopathy (clinically expressed as Korsakoff syndrome) is directly associated with profound downregulation of astroglial glutamate transporters with ensuing excitotoxic neuronal death [151,152]. Astrocytes therefore are increasingly considered as a legitimate target for cell-specific therapy in various neuropsychiatric conditions [153,154].
Another important element of astrogliopathology is represented by pathological remodelling when astrocytes acquire abnormal phenotype that contributes to the development of neurological disease. Examples of such pathological remodelling could be found in Alexander disease, where expression of sporadically mutant GFAP affects astroglial biochemistry that impacts on the developing brain and results in massive leukomalacia [155]; in hepatic encephalopathy, where astrocytes undergo profound remodelling of their homeostatic functions [156]; and in epilepsy, where astrocytes change their characteristic appearance, lose gap-junctional coupling and ability to buffer extracellular K+, which together precipitate development of seizures [157].
In neurodegenerative pathologies, both astrodegeneration and astroglial reactivity appear at different stages of the disease or may coexist [142]. In amyotrophic lateral sclerosis, for example, early degeneration and death of astroglia result in increased excitotoxicity. Astroglia-specific expression of amyotrophic lateral sclerosis (ALS)-associated hSOD1 mutant gene, or grafting of hSOD1 mutant-expressing astrocyte precursors into the spinal cord of rodents mimicked symptoms of the disease [158,159]. Inversely, silencing of astroglial hSOD-1 delayed and lessened the symptomatology [160]. In Alzheimer's disease, both astrodegeneration and astroglial reactivity are observed. Reactive astrocytes are generally associated with senile plaques, whereas atrophic astrocytes may occur at early stages and contribute (owing to reduced synaptic coverage and overall homeostatic capabilities) to early cognitive deficits [139].
8. Conclusion
Astrocytes are principal homeostatic cells of the central nervous system. They evolved through specialization and diversification of function and assumed the responsibility for all homeostatic needs of the brain. Astroglial cells are integrated into neural networks through multipartite synapses, and their remarkable morphological and functional plasticity contribute to learning and memory. In neuropathology, astrocytes may undergo reactivity and degeneration, which are specific to the disease context and may, to a large extent, define pathological progression.
Competing interests
We declare we have no competing interests.
Funding
The work of A.V. was supported by the Wellcome Trust, by Alzheimer's research foundation (UK) and by the Federal Target Program ‘Research and development in the priority areas of the development of the scientific and technological complex of Russia for 2014–2020’ of the Ministry of Education and Science of Russia, contract 14.581.21.00.16 (Project ID RFMEFI158115X0016). M.N. is supported by Joint Programme-Neurodegenerative Disease Research (JPND) project funded by the European Union's Horizon 2020 research and innovation programme under grant agreement No. 643417 (DACAPO-AD).
References
- 1.Bartol TM, Bromer C, Kinney J, Chirillo MA, Bourne JN, Harris KM, Sejnowski TJ. 2015. Nanoconnectomic upper bound on the variability of synaptic plasticity. Elife 4, e10778 ( 10.7554/eLife.10778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, Chen W. 2012. Quantitative imaging of energy expenditure in human brain. Neuroimage 60, 2107–2117. ( 10.1016/j.neuroimage.2012.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magistretti PJ. 2009. Neuroscience. Low-cost travel in neurons. Science 325, 1349–1351. ( 10.1126/science.1180102) [DOI] [PubMed] [Google Scholar]
- 4.Molofsky AV, Deneen B. 2015. Astrocyte development: a guide for the perplexed. Glia 63, 1320–1329. ( 10.1002/glia.22836) [DOI] [PubMed] [Google Scholar]
- 5.Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. 2014. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276, 29–47. ( 10.1016/j.neuroscience.2013.11.029) [DOI] [PubMed] [Google Scholar]
- 6.Ginhoux F, Prinz M. 2015. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 7, a020537 ( 10.1101/cshperspect.a020537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verkhratsky A, Butt AM. 2013. Glial physiology and pathophysiology, 560 p. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 8.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. 2009. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541. ( 10.1002/cne.21974) [DOI] [PubMed] [Google Scholar]
- 9.Hartline DK. 2011. The evolutionary origins of glia. Glia 59, 1215–1236. ( 10.1002/glia.21149) [DOI] [PubMed] [Google Scholar]
- 10.Oikonomou G, Shaham S. 2011. The glia of Caenorhabditis elegans. Glia 59, 1253–1263. ( 10.1002/glia.21084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacaj T, Tevlin M, Lu Y, Shaham S. 2008. Glia are essential for sensory organ function in C. elegans. Science 322, 744–747. ( 10.1126/science.1163074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu K, Terakawa S. 1999. Fenestration nodes and the wide submyelinic space form the basis for the unusually fast impulse conduction of shrimp myelinated axons. J. Exp. Biol. 202, 1979–1989. [DOI] [PubMed] [Google Scholar]
- 13.Edwards TN, Meinertzhagen IA. 2010. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 90, 471–497. ( 10.1016/j.pneurobio.2010.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartenstein V. 2011. Morphological diversity and development of glia in Drosophila. Glia 59, 1237–1252. ( 10.1002/glia.21162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker RJ, Auld VJ. 2006. Roles of glia in the Drosophila nervous system. Semin. Cell Dev. Biol. 17, 66–77. ( 10.1016/j.semcdb.2005.11.012) [DOI] [PubMed] [Google Scholar]
- 16.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. 2011. Physiology of microglia. Physiol. Rev. 91, 461–553. ( 10.1152/physrev.00011.2010) [DOI] [PubMed] [Google Scholar]
- 17.Sild M, Ruthazer ES. 2011. Radial glia: progenitor, pathway, and partner. Neuroscientist 17, 288–302. ( 10.1177/1073858410385870) [DOI] [PubMed] [Google Scholar]
- 18.Mashanov VS, Zueva OR, Heinzeller T, Aschauer B, Naumann WW, Grondona JM, Cifuentes M, Garcia-Arraras JE. 2009. The central nervous system of sea cucumbers (Echinodermata: Holothuroidea) shows positive immunostaining for a chordate glial secretion. Front. Zool. 6, 11 ( 10.1186/1742-9994-6-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ari C, Kalman M. 2008. Glial architecture of the ghost shark (Callorhinchus milii, Holocephali, Chondrichthyes) as revealed by different immunohistochemical markers. J. Exp. Zool. B Mol. Dev. Evol. 310, 504–519. ( 10.1002/jez.b.21223) [DOI] [PubMed] [Google Scholar]
- 20.Ari C, Kalman M. 2008. Evolutionary changes of astroglia in Elasmobranchii comparing to amniotes: a study based on three immunohistochemical markers (GFAP, S-100, and glutamine synthetase). Brain Behav. Evol. 71, 305–324. ( 10.1159/000129654) [DOI] [PubMed] [Google Scholar]
- 21.Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J. 2010. Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60, 343–357. ( 10.1002/glia.22269) [DOI] [PubMed] [Google Scholar]
- 22.Reichenbach A, Neumann M, Bruckner G. 1987. Cell length to diameter relation of rat fetal radial glia—does impaired K+ transport capacity of long thin cells cause their perinatal transformation into multipolar astrocytes? Neurosci. Lett. 73, 95–100. ( 10.1016/0304-3940(87)90038-3) [DOI] [PubMed] [Google Scholar]
- 23.Kimelberg HK. 2010. Functions of mature mammalian astrocytes: a current view. Neuroscientist 16, 79–106. ( 10.1177/1073858409342593) [DOI] [PubMed] [Google Scholar]
- 24.Eroglu C, Barres BA. 2010. Regulation of synaptic connectivity by glia. Nature 468, 223–231. ( 10.1038/nature09612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danbolt NC. 2001. Glutamate uptake. Prog. Neurobiol. 65, 1–105. ( 10.1016/S0301-0082(00)00067-8) [DOI] [PubMed] [Google Scholar]
- 26.Boison D, Chen JF, Fredholm BB. 2010. Adenosine signaling and function in glial cells. Cell Death Differ. 17, 1071–1082. ( 10.1038/cdd.2009.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deitmer JW, Rose CR. 1996. pH regulation and proton signalling by glial cells. Prog. Neurobiol. 48, 73–103. ( 10.1016/0301-0082(95)00039-9) [DOI] [PubMed] [Google Scholar]
- 28.Pellerin L, Magistretti PJ. 2012. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 32, 1152–1166. ( 10.1038/jcbfm.2011.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. 2016. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 35, 239–257. ( 10.15252/embj.201592705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bushong EA, Martone ME, Jones YZ, Ellisman MH. 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedergaard M, Ransom B, Goldman SA. 2003. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530. ( 10.1016/j.tins.2003.08.008) [DOI] [PubMed] [Google Scholar]
- 32.Emsley JG, Macklis JD. 2006. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2, 175–186. ( 10.1017/S1740925X06000202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matyash V, Kettenmann H. 2010. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 63, 2–10. ( 10.1016/j.brainresrev.2009.12.001) [DOI] [PubMed] [Google Scholar]
- 34.Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. 2003. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J. Neurosci. 23, 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. 2015. Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol. 7, a020362 ( 10.1101/cshperspect.a020362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Marques J, Lopez-Mascaraque L. 2013. Clonal identity determines astrocyte cortical heterogeneity. Cereb. Cortex 23, 1463–1472. ( 10.1093/cercor/bhs134) [DOI] [PubMed] [Google Scholar]
- 37.Oberheim NA, Goldman SA, Nedergaard M. 2012. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 814, 23–45. ( 10.1007/978-1-61779-452-0_3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verkhratsky A, Kettenmann H. 1996. Calcium signalling in glial cells. Trends Neurosci. 19, 346–352. ( 10.1016/0166-2236(96)10048-5) [DOI] [PubMed] [Google Scholar]
- 39.Verkhratsky A. 2010. Physiology of neuronal–glial networking. Neurochem. Int. 57, 332–343. ( 10.1016/j.neuint.2010.02.002) [DOI] [PubMed] [Google Scholar]
- 40.Kirchhoff F, Mulhardt C, Pastor A, Becker CM, Kettenmann H. 1996. Expression of glycine receptor subunits in glial cells of the rat spinal cord. J. Neurochem. 66, 1383–1390. ( 10.1046/j.1471-4159.1996.66041383.x) [DOI] [PubMed] [Google Scholar]
- 41.Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A. 1999. Glutamate-triggered calcium signalling in mouse Bergmann glial cells in situ: role of inositol-1,4,5-trisphosphate-mediated intracellular calcium release. Neuroscience 92, 1051–1059. ( 10.1016/S0306-4522(99)00067-6) [DOI] [PubMed] [Google Scholar]
- 42.Kirischuk S, Moller T, Voitenko N, Kettenmann H, Verkhratsky A. 1995. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J. Neurosci. 15, 7861–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. 1996. Calcium signalling in mouse Bergmann glial cells mediated by α1-adrenoreceptors and H1 histamine receptors. Eur. J. Neurosci. 8, 1198–1208. ( 10.1111/j.1460-9568.1996.tb01288.x) [DOI] [PubMed] [Google Scholar]
- 44.Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. 2004. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res 1029, 120–123. ( 10.1016/j.brainres.2004.09.014) [DOI] [PubMed] [Google Scholar]
- 45.Verkhratsky A, Orkand RK, Kettenmann H. 1998. Glial calcium: homeostasis and signaling function. Physiol. Rev. 78, 99–141. [DOI] [PubMed] [Google Scholar]
- 46.Todd Farmer WT, et al. 2016. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–854. ( 10.1126/science.aab3103) [DOI] [PubMed] [Google Scholar]
- 47.Verkhratsky A, Rodriguez JJ, Parpura V. 2012. Calcium signalling in astroglia. Mol. Cell Endocrinol. 353, 45–56. ( 10.1016/j.mce.2011.08.039) [DOI] [PubMed] [Google Scholar]
- 48.Rusakov DA. 2015. Disentangling calcium-driven astrocyte physiology. Nat. Rev. Neurosci. 16, 226–233. ( 10.1038/nrn3878) [DOI] [PubMed] [Google Scholar]
- 49.Rose CR, Verkhratsky A. 2016. Principles of sodium homeostasis and sodium signalling in astroglia. Glia, E-pub ahead of print. ( 10.1002/glia.22964) [DOI] [PubMed] [Google Scholar]
- 50.Kirischuk S, Parpura V, Verkhratsky A. 2012. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 35, 497–506. ( 10.1016/j.tins.2012.04.003) [DOI] [PubMed] [Google Scholar]
- 51.Parpura V, Verkhratsky A. 2012. Homeostatic function of astrocytes: Ca2+ and Na+ signalling. Transl. Neurosci. 3, 334–344. ( 10.2478/s13380-012-0040-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verkhratsky A, Kirchhoff F. 2007. NMDA receptors in glia. Neuroscientist 13, 28–37. ( 10.1177/1073858406294270) [DOI] [PubMed] [Google Scholar]
- 53.Verkhratsky A, Krishtal OA, Burnstock G. 2009. Purinoceptors on neuroglia. Mol. Neurobiol. 39, 190–208. ( 10.1007/s12035-009-8063-2) [DOI] [PubMed] [Google Scholar]
- 54.Verkhratsky A, Reyes RC, Parpura V. 2014. TRP channels coordinate ion signalling in astroglia. Rev. Physiol. Biochem. Pharmacol. 166, 1–22. ( 10.1007/112_2013_15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araque A, Parpura V, Sanzgiri RP, Haydon PG. 1999. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22, 208–215. ( 10.1016/S0166-2236(98)01349-6) [DOI] [PubMed] [Google Scholar]
- 56.Halassa MM, Fellin T, Haydon PG. 2007. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 13, 54–63. ( 10.1016/j.molmed.2006.12.005) [DOI] [PubMed] [Google Scholar]
- 57.Verkhratsky A, Nedergaard M. 2014. Astroglial cradle in the life of the synapse. Phil. Trans. R Soc. B 369, 20130595 ( 10.1098/rstb.2013.0595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayford M, Siegelbaum SA, Kandel ER. 2012. Synapses and memory storage. Cold Spring Harb. Perspect. Biol. 4, a005751 ( 10.1101/cshperspect.a005751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tremblay ME, Lowery RL, Majewska AK. 2010. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 ( 10.1371/journal.pbio.1000527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kettenmann H, Kirchhoff F, Verkhratsky A. 2013. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18. ( 10.1016/j.neuron.2012.12.023) [DOI] [PubMed] [Google Scholar]
- 61.Dityatev A, Rusakov DA. 2011. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 21, 353–359. ( 10.1016/j.conb.2010.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfrieger FW. 2002. Role of glia in synapse development. Curr. Opin. Neurobiol. 12, 486–490. ( 10.1016/S0959-4388(02)00358-6) [DOI] [PubMed] [Google Scholar]
- 63.Chung WS, et al. 2013. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. ( 10.1038/nature12776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nedergaard M, Verkhratsky A. 2012. Artifact versus reality—how astrocytes contribute to synaptic events. Glia 60, 1013–1023. ( 10.1002/glia.22288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. 1999. Microdomains for neuron–glia interaction: parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 2, 139–143. ( 10.1038/5692) [DOI] [PubMed] [Google Scholar]
- 66.Reichenbach A, Derouiche A, Kirchhoff F. 2010. Morphology and dynamics of perisynaptic glia. Brain Res. Rev. 63, 11–25. ( 10.1016/j.brainresrev.2010.02.003) [DOI] [PubMed] [Google Scholar]
- 67.Peters A, Palay SL, Webster HF. 1991. The fine structure of the nervous system: the neurons and supporting cells, 3rd edn New York, NY: Oxford University Press. [Google Scholar]
- 68.Derouiche A. 2003. The perisynaptic astrocyte process as a glial compartment-immunolabeling for glutamine synthetase and other glial markers. Adv. Mol. Cell Biol. 31, 147–163. ( 10.1016/S1569-2558(03)31006-9) [DOI] [Google Scholar]
- 69.Derouiche A, Haseleu J, Korf HW. 2015. Fine astrocyte processes contain very small mitochondria: glial oxidative capability may fuel transmitter metabolism. Neurochem. Res. 40, 2402–2413. ( 10.1007/s11064-015-1563-8) [DOI] [PubMed] [Google Scholar]
- 70.Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A. 2011. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl Acad. Sci. USA 108, 12915–12919. ( 10.1073/pnas.1100957108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Derouiche A, Frotscher M. 2001. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia 36, 330–341. ( 10.1002/glia.1120) [DOI] [PubMed] [Google Scholar]
- 72.Hirrlinger J, Hulsmann S, Kirchhoff F. 2004. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur. J. Neurosci. 20, 2235–2239. ( 10.1111/j.1460-9568.2004.03689.x) [DOI] [PubMed] [Google Scholar]
- 73.Heller JP, Rusakov DA. 2015. Morphological plasticity of astroglia: understanding synaptic microenvironment. Glia 63, 2133–2151. ( 10.1002/glia.22821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oberheim NA, Wang X, Goldman S, Nedergaard M. 2006. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 29, 547–553. ( 10.1016/j.tins.2006.08.004) [DOI] [PubMed] [Google Scholar]
- 75.Pentreath VW, Radojcic T, Seal LH, Winstanley EK. 1985. The glial cells and glia–neuron relations in the buccal ganglia of Planorbis corneus (L.): cytological, qualitative and quantitative changes during growth and ageing. Phil. Trans. R. Soc. Lond. B 307, 399–455. ( 10.1098/rstb.1985.0002) [DOI] [PubMed] [Google Scholar]
- 76.Herculano-Houzel S, Lent R. 2005. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 25, 2518–2521. ( 10.1523/JNEUROSCI.4526-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV. 2012. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur. J. Neurosci. 35, 1–9. ( 10.1111/j.1460-9568.2011.07923.x) [DOI] [PubMed] [Google Scholar]
- 78.Pakkenberg H. 1966. The number of nerve cells in the cerebral cortex of man. J. Comp. Neurol. 128, 17–20. ( 10.1002/cne.901280103) [DOI] [PubMed] [Google Scholar]
- 79.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. 2008. Neocortical glial cell numbers in human brains. Neurobiol. Aging 29, 1754–1762. ( 10.1016/j.neurobiolaging.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 80.Reichenbach A. 1989. Glia:neuron index: review and hypothesis to account for different values in various mammals. Glia 2, 71–77. ( 10.1002/glia.440020202) [DOI] [PubMed] [Google Scholar]
- 81.Andersen BB, Korbo L, Pakkenberg B. 1992. A quantitative study of the human cerebellum with unbiased stereological techniques. J. Comp. Neurol. 326, 549–560. ( 10.1002/cne.903260405) [DOI] [PubMed] [Google Scholar]
- 82.Chan-Palay V, Palay SL. 1972. The form of velate astrocytes in the cerebellar cortex of monkey and rat: high voltage electron microscopy of rapid Golgi preparations. Z. Anat. Entwicklungsgesch 138, 1–19. ( 10.1007/BF00519921) [DOI] [PubMed] [Google Scholar]
- 83.Hoogland TM, Kuhn B. 2010. Recent developments in the understanding of astrocyte function in the cerebellum in vivo. Cerebellum 9, 264–271. ( 10.1007/s12311-009-0139-z) [DOI] [PubMed] [Google Scholar]
- 84.Sherwood CC, et al. 2006. Evolution of increased glia–neuron ratios in the human frontal cortex. Proc. Natl Acad. Sci. USA 103, 13 606–13 611. ( 10.1073/pnas.0605843103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oberheim NA, et al. 2009. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287. ( 10.1523/JNEUROSCI.4707-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colombo JA, Reisin HD. 2004. Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res. 1006, 126–131. ( 10.1016/j.brainres.2004.02.003) [DOI] [PubMed] [Google Scholar]
- 87.Colombo JA, Sherwood CC, Hof PR. 2004. Interlaminar astroglial processes in the cerebral cortex of great apes. Anat. Embryol. (Berl.) 208, 215–218. ( 10.1007/s00429-004-0391-4) [DOI] [PubMed] [Google Scholar]
- 88.Colombo JA, Yanez A, Puissant V, Lipina S. 1995. Long, interlaminar astroglial cell processes in the cortex of adult monkeys. J. Neurosci. Res. 40, 551–556. ( 10.1002/jnr.490400414) [DOI] [PubMed] [Google Scholar]
- 89.Han X, et al. 2013. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353. ( 10.1016/j.stem.2012.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. 2014. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 34, 16 153–16 161. ( 10.1523/JNEUROSCI.1510-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waagepetersen HS, Qu H, Schousboe A, Sonnewald U. 2001. Elucidation of the quantitative significance of pyruvate carboxylation in cultured cerebellar neurons and astrocytes. J. Neurosci. Res. 66, 763–770. ( 10.1002/jnr.10061) [DOI] [PubMed] [Google Scholar]
- 92.Cesar M, Hamprecht B. 1995. Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J. Neurochem. 64, 2312–2318. ( 10.1046/j.1471-4159.1995.64052312.x) [DOI] [PubMed] [Google Scholar]
- 93.Martinez-Hernandez A, Bell KP, Norenberg MD. 1977. Glutamine synthetase: glial localization in brain. Science 195, 1356–1358. ( 10.1126/science.14400) [DOI] [PubMed] [Google Scholar]
- 94.Norenberg MD, Martinez-Hernandez A. 1979. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 161, 303–310. ( 10.1016/0006-8993(79)90071-4) [DOI] [PubMed] [Google Scholar]
- 95.Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. 1998. EAAC1, a high-affinity glutamate tranporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb. Cortex 8, 108–116. ( 10.1093/cercor/8.2.108) [DOI] [PubMed] [Google Scholar]
- 96.Mackenzie B, Erickson JD. 2004. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 447, 784–795. ( 10.1007/s00424-003-1117-9). [DOI] [PubMed] [Google Scholar]
- 97.Rusakov DA. 2012. Astroglial glutamate transporters trigger glutaminergic gliotransmission. J. Physiol. 590, 2187–2188. ( 10.1113/jphysiol.2012.233577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uwechue NM, Marx MC, Chevy Q, Billups B. 2012. Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. J. Physiol. 590, 2317–2331. ( 10.1113/jphysiol.2011.226605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez-Lozada Z et al. 2013. GLAST/EAAT1-induced glutamine release via SNAT3 in Bergmann glial cells: evidence of a functional and physical coupling. J. Neurochem. 125, 545–554. ( 10.1111/jnc.12211) [DOI] [PubMed] [Google Scholar]
- 100.Billups D, Marx MC, Mela I, Billups B. 2013. Inducible presynaptic glutamine transport supports glutamatergic transmission at the calyx of Held synapse. J. Neurosci. 33, 17 429–17 434. ( 10.1523/JNEUROSCI.1466-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ. 2014. A local glutamate–glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81, 888–900. ( 10.1016/j.neuron.2013.12.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Unichenko P, Myakhar O, Kirischuk S. 2012. Intracellular Na+ concentration influences short-term plasticity of glutamate transporter-mediated currents in neocortical astrocytes. Glia 60, 605–614. ( 10.1002/glia.22294) [DOI] [PubMed] [Google Scholar]
- 103.Eulenburg V, Gomeza J. 2010. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 63, 103–112. ( 10.1016/j.brainresrev.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 104.Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, Hertz L. 2005. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia 52, 25–35. ( 10.1002/glia.20216) [DOI] [PubMed] [Google Scholar]
- 105.Hertz L. 1965. Possible role of neuroglia: a potassium-mediated neuronal–neuroglial–neuronal impulse transmission system. Nature 206, 1091–1094. ( 10.1038/2061091a0) [DOI] [PubMed] [Google Scholar]
- 106.Macaulay N, Zeuthen T. 2012. Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem. Res. 37, 2299–2309. ( 10.1007/s11064-012-0731-3) [DOI] [PubMed] [Google Scholar]
- 107.Olsen ML, Sontheimer H. 2008. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J. Neurochem. 107, 589–601. ( 10.1111/j.1471-4159.2008.05615.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kofuji P, Newman EA. 2004. Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056. ( 10.1016/j.neuroscience.2004.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deitmer JW, Rose CR. 2010. Ion changes and signalling in perisynaptic glia. Brain Res. Rev. 63, 113–129. ( 10.1016/j.brainresrev.2009.10.006) [DOI] [PubMed] [Google Scholar]
- 110.Zanotti S, Charles A. 1997. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J. Neurochem. 69, 594–602. ( 10.1046/j.1471-4159.1997.69020594.x) [DOI] [PubMed] [Google Scholar]
- 111.Segawa S, Nishiura T, Furuta T, Ohsato Y, Tani M, Nishida K, Nagasawa K. 2014. Zinc is released by cultured astrocytes as a gliotransmitter under hypoosmotic stress-loaded conditions and regulates microglial activity. Life Sci. 94, 137–144. ( 10.1016/j.lfs.2013.11.007) [DOI] [PubMed] [Google Scholar]
- 112.Ding F, O'Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. 2016. Changes in the composition of brain interstitial ions control the sleep–wake cycle. Science 352, 550–555. ( 10.1126/science.aad4821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. 2013. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309. ( 10.1172/JCI67677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iliff JJ, et al. 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, p147ra111. ( 10.1126/scitranslmed.3003748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. 2010. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103. ( 10.1002/glia.20990) [DOI] [PubMed] [Google Scholar]
- 116.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. 2015. The glymphatic system: a beginner's guide. Neurochem. Res. 40, 2583–2599. ( 10.1007/s11064-015-1581-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iliff JJ, Goldman SA, Nedergaard M. 2015. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 14, 977–979. ( 10.1016/S1474-4422(15)00221-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie L, et al. 2013. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. ( 10.1126/science.1241224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kress BT, et al. 2014. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861. ( 10.1002/ana.24271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eide PK, Ringstad G. 2015. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol. Open 4, 2058460115609635 ( 10.1177/2058460115609635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiviniemi V, et al. 2015. Ultra-fast magnetic resonance encephalography of physiological brain activity—glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab. E-pub ahead of print. ( 10.1177/0271678X15622047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cajal S. 1894. The Croonian lecture: la fine structure des centres nerveux. Proc. R. Soc. Lond. 55, 444–468. ( 10.1098/rspl.1894.0063) [DOI] [Google Scholar]
- 123.Kandel ER, Dudai Y, Mayford MR. 2014. The molecular and systems biology of memory. Cell 157, 163–186. ( 10.1016/j.cell.2014.03.001) [DOI] [PubMed] [Google Scholar]
- 124.Zorec R, Horvat A, Vardjan N, Verkhratsky A. 2015. Memory formation shaped by astroglia. Front. Integr. Neurosci. 9, 56 ( 10.3389/fnint.2015.00056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ostroff LE, Manzur MK, Cain CK, Ledoux JE. 2014. Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J. Comp. Neurol. 522, 2152–2163. ( 10.1002/cne.23523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oliet SH, Piet R. 2004. Anatomical remodelling of the supraoptic nucleus: changes in synaptic and extrasynaptic transmission. J. Neuroendocrinol. 16, 303–307. ( 10.1111/j.0953-8194.2004.01159.x) [DOI] [PubMed] [Google Scholar]
- 127.Theodosis DT, Poulain DA, Oliet SH. 2008. Activity-dependent structural and functional plasticity of astrocyte–neuron interactions. Physiol. Rev. 88, 983–1008. ( 10.1152/physrev.00036.2007) [DOI] [PubMed] [Google Scholar]
- 128.Gibbs ME, Anderson DG, Hertz L. 2006. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54, 214–222. ( 10.1002/glia.20377) [DOI] [PubMed] [Google Scholar]
- 129.Hertz L, Gibbs ME. 2009. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J. Neurochem. 109(Suppl 1), 10–16. ( 10.1111/j.1471-4159.2009.05939.x) [DOI] [PubMed] [Google Scholar]
- 130.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. 2011. Astrocyte–neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. ( 10.1016/j.cell.2011.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ding F, O'Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. 2013. α1-adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394. ( 10.1016/j.ceca.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shain W, Forman DS, Madelian V, Turner JN. 1987. Morphology of astroglial cells is controlled by beta-adrenergic receptors. J. Cell Biol. 105, 2307–2314. ( 10.1083/jcb.105.5.2307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shao Y, Enkvist MO, McCarthy KD. 1994. Glutamate blocks astroglial stellation: effect of glutamate uptake and volume changes. Glia 11, 1–10. ( 10.1002/glia.440110103) [DOI] [PubMed] [Google Scholar]
- 134.Won CL, Oh YS. 2000. cAMP-induced stellation in primary astrocyte cultures with regional heterogeneity. Brain Res. 887, 250–258. ( 10.1016/S0006-8993(00)02922-X) [DOI] [PubMed] [Google Scholar]
- 135.Vardjan N, Kreft M, Zorec R. 2014. Dynamics of beta-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia 62, 566–579. ( 10.1002/glia.22626) [DOI] [PubMed] [Google Scholar]
- 136.Burda JE, Sofroniew MV. 2014. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248. ( 10.1016/j.neuron.2013.12.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nedergaard M, Rodriguez JJ, Verkhratsky A. 2010. Glial calcium and diseases of the nervous system. Cell Calcium 47, 140–149. ( 10.1016/j.ceca.2009.11.010) [DOI] [PubMed] [Google Scholar]
- 138.Rajkowska G, Stockmeier CA. 2013. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236. ( 10.2174/13894501113149990156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Verkhratsky A, Marutle A, Rodriguez-Arellano JJ, Nordberg A. 2014. Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer's disease. Neuroscientist 21, 552–568. ( 10.1177/1073858414547132) [DOI] [PubMed] [Google Scholar]
- 140.Verkhratsky A, Rodriguez JJ, Steardo L. 2014. Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist 20, 576–588. ( 10.1177/1073858413510208) [DOI] [PubMed] [Google Scholar]
- 141.Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M. 2012. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4, e0082 ( 10.1042/AN20120010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. 2016. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131, 323–345. ( 10.1007/s00401-015-1513-1) [DOI] [PubMed] [Google Scholar]
- 143.Banasr M, Duman RS. 2008. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry 64, 863–870. ( 10.1016/j.biopsych.2008.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bowley MP, Drevets WC, Ongur D, Price JL. 2002. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry 52, 404–412. ( 10.1016/S0006-3223(02)01404-X) [DOI] [PubMed] [Google Scholar]
- 145.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. 2002. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 12, 386–394. ( 10.1093/cercor/12.4.386) [DOI] [PubMed] [Google Scholar]
- 146.Falkai P, Bogerts B. 1986. Cell loss in the hippocampus of schizophrenics. Eur. Arch. Psychiatry Neurol. Sci. 236, 154–161. ( 10.1007/BF00380943) [DOI] [PubMed] [Google Scholar]
- 147.Choudary PV, et al. 2005. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl Acad. Sci. USA 102, 15 653–15 658. ( 10.1073/pnas.0507901102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanacora G, Treccani G, Popoli M. 2012. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77. ( 10.1016/j.neuropharm.2011.07.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477. ( 10.1038/nrn3257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schwarcz R, Hunter CA. 2007. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr. Bull. 33, 652–653. ( 10.1093/schbul/sbm030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hazell AS. 2009. Astrocytes are a major target in thiamine deficiency and Wernicke's encephalopathy. Neurochem. Int. 55, 129–135. ( 10.1016/j.neuint.2009.02.020) [DOI] [PubMed] [Google Scholar]
- 152.Hazell AS, Sheedy D, Oanea R, Aghourian M, Sun S, Jung JY, Wang D, Wang C. 2009. Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia 58, 148–156. ( 10.1002/glia.20908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Czeh B, Di Benedetto B. 2013. Antidepressants act directly on astrocytes: evidences and functional consequences. Eur. Neuropsychopharmacol. 23, 171–185. ( 10.1016/j.euroneuro.2012.04.017) [DOI] [PubMed] [Google Scholar]
- 154.Peng L, Verkhratsky A, Gu L, Li B. 2015. Targeting astrocytes in major depression. Expert Rev. Neurother. 15, 1299–1306. ( 10.1586/14737175.2015.1095094) [DOI] [PubMed] [Google Scholar]
- 155.Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. 2012. Alexander disease. J. Neurosci. 32, 5017–5023. ( 10.1523/JNEUROSCI.5384-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montana V, Verkhratsky A, Parpura V. 2014. Pathological role for exocytotic glutamate release from astrocytes in hepatic encephalopathy. Curr. Neuropharmacol. 12, 324–333. ( 10.2174/1570159X12666140903094700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bedner P, et al. 2015. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138, 1208–1222. ( 10.1093/brain/awv067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Papadeas ST, Kraig SE, O'Banion C, Lepore AC, Maragakis NJ. 2011. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. Proc. Natl Acad. Sci. USA 108, 17 803–17 808. ( 10.1073/pnas.1103141108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A. 2008. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 15, 1691–1700. ( 10.1038/cdd.2008.99) [DOI] [PubMed] [Google Scholar]
- 160.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. 2008. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253. ( 10.1038/nn2047) [DOI] [PMC free article] [PubMed] [Google Scholar]