Abstract
An essential feature of Alzheimer's disease (AD) is the accumulation of amyloid-β (Aβ) peptides in the brain, many years to decades before the onset of overt cognitive symptoms. We suggest that during this very extended early phase of the disease, soluble Aβ oligomers and amyloid plaques alter the function of local neuronal circuits and large-scale networks by disrupting the balance of synaptic excitation and inhibition (E/I balance) in the brain. The analysis of mouse models of AD revealed that an Aβ-induced change of the E/I balance caused hyperactivity in cortical and hippocampal neurons, a breakdown of slow-wave oscillations, as well as network hypersynchrony. Remarkably, hyperactivity of hippocampal neurons precedes amyloid plaque formation, suggesting that hyperactivity is one of the earliest dysfunctions in the pathophysiological cascade initiated by abnormal Aβ accumulation. Therapeutics that correct the E/I balance in early AD may prevent neuronal dysfunction, widespread cell loss and cognitive impairments associated with later stages of the disease.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: Alzheimer's disease, amyloid-β, in vivo calcium imaging, mouse models
1. Introduction
Alzheimer's disease (AD) is the most common cause of intellectual decline in the elderly population worldwide [1]. AD is characterized by slowly progressive memory deficits, cognitive impairments and dementia. The diagnosis is established by these clinical features combined with biomarker evidence for amyloid-β (Aβ) accumulation (as measured by cerebrospinal fluid (CSF) levels of Aβ1–42 or positron emission tomography (PET)-amyloid imaging) and/or neuronal degeneration (as measured by CSF levels of tau and phosphorylated tau as well as fluorodeoxyglucose (FDG)-PET or structural magnetic resonance imaging (MRI)) in the brain [2]. Current treatments are unsatisfactory as they provide only symptomatic relief and are effective in only a subset of affected individuals [3].
It is becoming increasingly clear that the pathogenic cascade that causes AD begins decades before first clinical symptoms become evident [4,5]. For instance, in people at risk of AD abnormal Aβ accumulation and amyloid deposition, as measured by CSFAβ and amyloid-PET, was detected 25 years before symptom onset [6]. There is growing evidence from functional MRI (fMRI) that this ‘preclinical’ stage of AD is associated with profound functional alterations of brain networks that seem to be structurally largely intact. For example, hippocampal hyperactivation and impaired deactivation of the default-mode network during memory-encoding have been demonstrated in people at genetic risk for AD [7–9], cognitively normal individuals with evidence for Aβ accumulation [10–12] and people with early AD [13–15].
Major unresolved issues include the questions of why neuronal circuits become dysfunctional in response to high Aβ levels and how circuit abnormalities can be repaired. As these problems cannot be studied easily in humans with existing techniques, transgenic mouse models overproducing human mutant Aβ are in many cases the method of choice for such investigations. Indeed, recent experimental evidence obtained in mouse model studies suggest that a disruption in the balance of excitation and inhibition (E/I balance) underlies both the functional impairment of local neuronal circuits as well as that of large-scale networks in the amyloid-depositing brain. Remarkably, restoration of the E/I balance can rescue neuronal circuit dysfunctions and ameliorate behavioural impairments in mouse models, providing a paradigm for targeting the E/I balance in humans with AD.
2. Impairment of cortical neurons by amyloid plaques
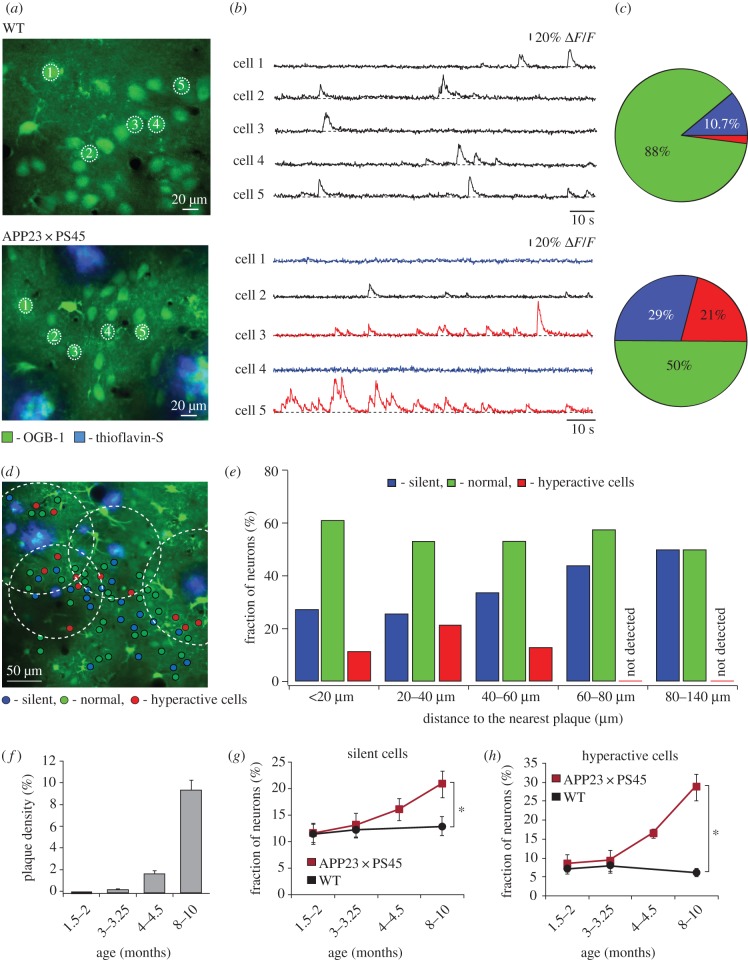
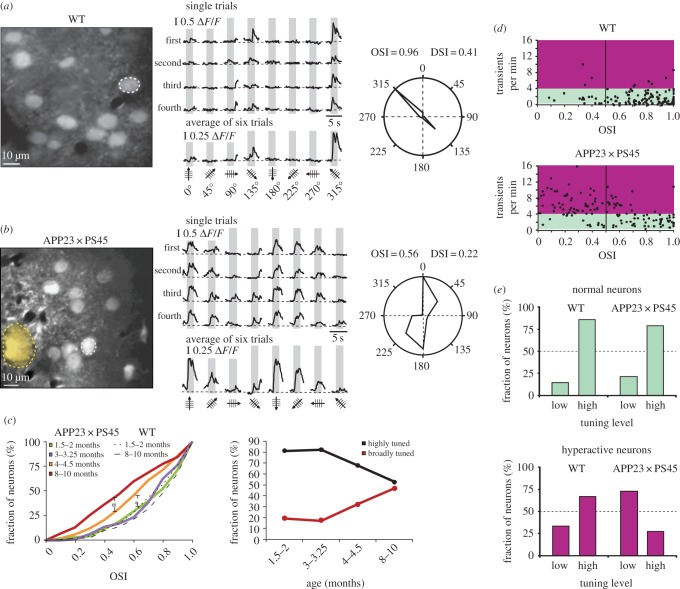
Two-photon imaging in combination with fluorescent calcium indicators allows functional analyses of neuronal activity in the intact mouse brain with single-cell and single-action potential accuracy [16]. At the same time, amyloid plaques can be directly visualized and characterized in the living brain tissue [17]. By using this optical approach in the APP23 × PS45 model of AD, we revealed that neurons in layer 2/3 of the amyloid-depositing frontal cortex are functionally impaired in vivo [18] (figure 1). In striking contrast with a dominant hypothesis in the field at that time that emphasized a progressive ‘synaptic dismantling’ in AD [20], we observed a 16-fold increase in the fraction of excessively active neurons and only a threefold increase in the fraction of functionally silent neurons in APP23 × PS45 mice when compared with wild-type littermates (figure 1a–c). Surprisingly, hyperactive neurons were found predominantly near the amyloid plaques, whereas silent neurons were more evenly distributed in the cortex (figure 1d,e). We also noted that the fractions of both hyperactive and hypoactive neurons increased with the progression of the disease (figure 1f–h).
Figure 1.
Functional impairments of cortical neurons in mouse models of AD in vivo. (a) In vivo two-photon calcium image of layer 2/3 neurons in the frontal cortex of a wild-type (WT) mouse (top panel) and an APP23 × PS45 transgenic mouse with thioflavin-S labelled amyloid plaques (bottom panel). (b) Spontaneous Ca2+-transients from neurons marked in (a): blue, silent neurons; black, normal neurons; red, hyperactive neurons. (c) Relative fractions of silent (blue), normal (green) and hyperactive (red) neurons. (d) Activity map of cortical region in an APP23 × PS45 mouse with neurons colour-coded according to the frequency of their spontaneous Ca2+ transients. Broken line circles are centred at the respective plaques and delineate the area located less than 60 µm from the plaque border. Adapted from [18]. Reproduced with permission from AAAS. (e) Bar graphs showing the abundance of silent (blue), normal (green) and hyperactive (red) neurons at different distances from the border of the nearest plaque. (f) Age-dependent increase in plaque burden in the cortex of APP23 × PS45 mice. (g,h) Relative proportions of silent (g) and hyperactive (h) neurons in WT (black) and APP23 × PS45 (red) mice at four different age groups (1.5–2, 3–3.25, 4–4.5 and 8–10 months). Error bars indicate s.e.m. Adapted from [19].
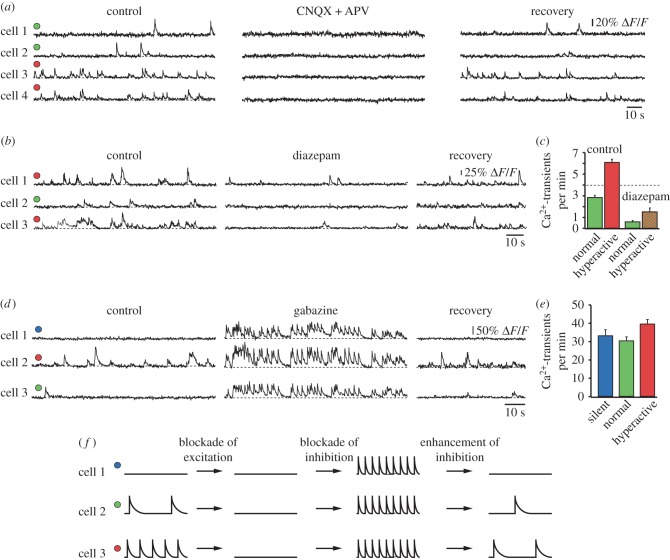
Experimental evidence indicated that hyperactivity was of synaptic origin and unrelated to spontaneous Ca2+-release from overfilled intracellular Ca2+-stores [21] or increased intrinsic neuronal excitability (figure 2a). Instead, we found that there was a redistribution of synaptic drive between hyperactive and silent neurons, with a relative reduction in GABAAergic inhibition of hyperactive neurons and an enhanced inhibition of silent neurons (figure 2b–e). Figure 2f illustrates these results schematically. Interestingly, the relative decrease in inhibition caused not only hyperactivity but, often, also an abnormal synchronization of neuronal firing, which may underlie the previously reported higher incidence of epileptiform activity in mouse models as compared to wild-type mice [22,23]. Meanwhile, neuronal hyperactivity has been observed in various transgenic mouse models of AD, including APP23 × PS45 [18,24], APP23 [25], PDAPP [26], Tg2576 [26], APPswe/PS1Δ9 [27] and ARTE 10 [28] mice. Furthermore, hyperactivity can be induced by direct application of exogenous Aβ into the brains of wild-type mice [24] and even through small elevations of endogenous Aβ [29].
Figure 2.
Impaired excitation–inhibition (E/I) balance in the amyloid-bearing mouse cortex. (a) Spontaneous Ca2+-transients in normal (green circles) and hyperactive (red circles) cortical neurons before, during and after local application of the glutamate receptor antagonists CNQX and APV. (b) Ca2+-transients in normal and hyperactive neurons before, during and after local application of the GABAA-receptor agonist diazepam. (c) Summary graph illustrating the effect of diazepam on the frequency of Ca2+-transients. (d) Ca2+-transients in silent (blue), normal and hyperactive neurons before, during and after local application of the GABAA-receptor antagonist gabazine. (e) Summary graph illustrating the effect of gabazine on the frequency of Ca2+-transients. Adapted from [18]. Reprinted with permission from AAAS. (f) Schematic model summarizing the results shown in (a–e).
Two-photon calcium imaging has also been used to study glia cells in mouse models of AD, both astrocytes [30,31] and microglia [32]. Remarkably, similarly to what is observed in neurons, these cell types can become hyperactive (that is, they exhibit an increased number of Ca2+-transients) in response to Aβ-induced pathology. Hyperactive astrocytes are mostly found near amyloid plaques [31]. In view of the fact that astrocytes can secrete gliotransmitters (e.g. adenosine triphosphate, glutamate, d-serine) in a calcium-dependent manner [33], hyperactive astrocytes may directly enhance neuronal activity. Furthermore, they may release pro-inflammatory factors and induce microglial activation, which may contribute further to neurotoxicity through excessive cytokine release [34]. Finally, other functions of astrocytes, including buffering of extracellular potassium during neuronal activity and the uptake of neurotransmitters (e.g. glutamate, GABA) from the extracellular space may also be disturbed. Overall, these observations and considerations suggest that glia cells contribute significantly to various aspects of brain dysfunction in AD [35–37].
3. Hippocampal hyperactivity precedes amyloid plaque formation and neuronal silencing
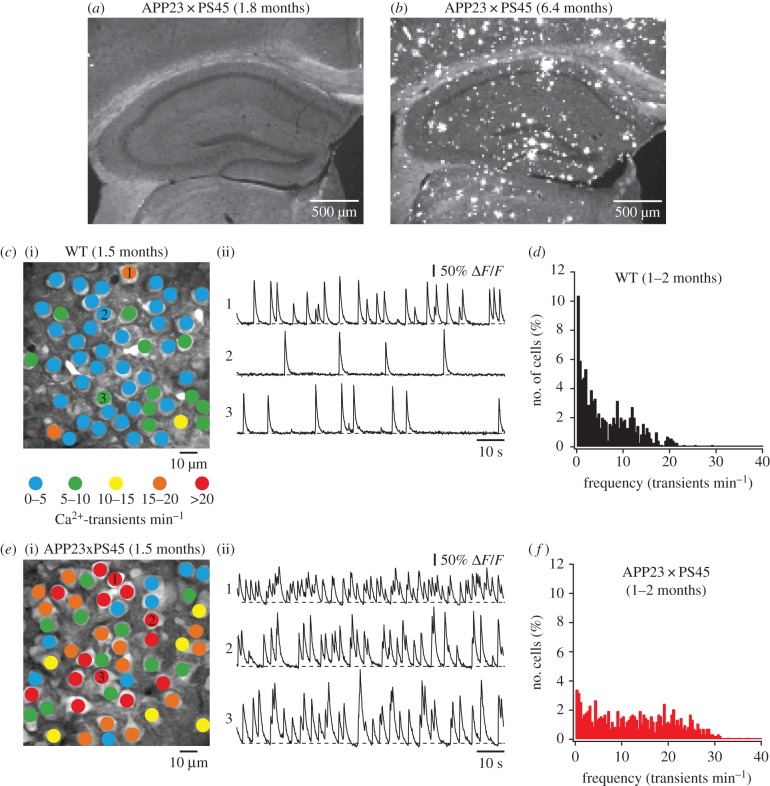
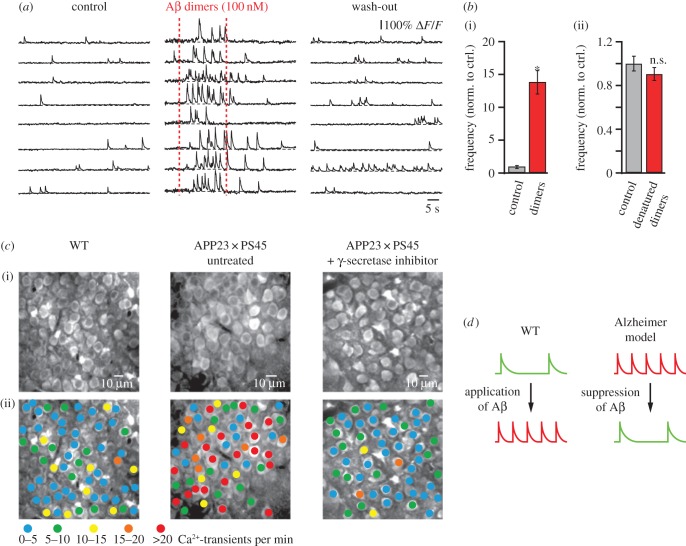
While the use of two-photon microscopy was initially restricted to superficial brain areas such as the neocortex, the development of surgical techniques to implant a hippocampal window [24,38] has enabled the in vivo investigation of hippocampal neurons. By employing this approach in the APP23 × PS45 model (figure 3), we found that in the CA1 region of the hippocampus many neurons were hyperactive already before plaque formation in 1–2 months-old mice [24]. We demonstrated that hippocampal hyperactivity can be induced by direct application of soluble Aβ dimers in wild-type mice (figure 4a,b) and that it can be rescued in APP23 × PS45 mice by acute treatment with a γ-secretase inhibitor, which reduced the levels of soluble Aβ in the brain (figure 4c). Figure 4d summarizes these experimental results. The observations suggested that soluble Aβ oligomers, rather than amyloid plaques, are the underlying cause for neuronal dysfunction. Furthermore, the experimental finding that neuronal hyperactivity precedes the formation of amyloid plaques and neuronal silencing indicates that hyperactivity represents the initial step in the pathophysiological cascade of AD. We hypothesize that the predominant occurrence of hyperactive neurons in the vicinity of amyloid plaques in later stages of the disease may be related to the enrichment of oligomers in the microenvironment of plaques [39], or, alternatively, owing to the activity-dependent release of Aβ [40,41] plaques may develop preferentially near the hyperactive neurons. Remaining open questions concern the cellular mechanisms by which soluble Aβ species induce neuronal hyperactivity and which of their various forms are most toxic. For instance, Aβ dimers were shown to reduce the re-uptake of synaptic glutamate [42] and Aβ1–40 monomers and dimers were found to enhance the presynaptic release of glutamate [43]. A combination of these effects could increase extracellular levels of residual glutamate and, thereby, promote neuronal hyperactivity. We and others have provided evidence for a potential contribution of reduced GABAAergic inhibition, mainly via a functional impairment of inhibitory interneurons [44], a decline of GABAAcurrents [45] and a redistribution of inhibitory and excitatory drive within neuronal circuits [18]. Intriguingly, the enhancement of GABAAergic inhibition can rescue hyperactivity [18] as well as memory impairments in mouse models of AD [46,47]. These findings are in line with the observation that the apolipoprotein E4 (APOE4) genotype, which is associated with a loss of GABAergic interneurons in the hippocampus, is the main genetic risk factor for AD. Also in this case, the enhancement of GABAAergic inhibition can rescue behavioural deficits [46].
Figure 3.
Hyperactivity precedes amyloid plaque formation in the hippocampus. (a,b) Confocal fluorescence images of sagittal hippocampal sections from a young APP23 × PS45 mouse without (a) and an aged APP3 × PS45 mouse with several (b) amyloid plaques. Plaques were labelled with thioflavin-S. (c) (i) Activity map of hippocampal region in a WT mouse with neurons colour-coded according to the frequency of their spontaneous activity. (ii) Ca2+-transients of the corresponding neurons marked in (i). (d) Histogram showing the frequency distribution of Ca2+-transients in WT mice (n = 693 cells in six mice). (e) Activity map of the hippocampus and example traces from individual neurons in a young APP23 × PS45 mouse without plaques. (f) Histogram of frequency distribution of Ca2+-transients in APP23 × PS45 mice before plaque formation (n = 818 cells in seven mice). Adapted from [24].
Figure 4.
Hippocampal hyperactivity is determined by soluble Aβ. (a) Ca2+-transient activity in CA1 hippocampal neurons in a WT mouse before, during and after local application of Aβ dimer solution (100 nM). (b) (i) Summary graph illustrating the effect of amyloid dimers on the frequency of Ca2+-transients. (ii) Summary graph showing that heat-denatured dimers have no significant effect on neuronal activity. Error bars indicate s.e.m. (c) (i) In vivo two-photon image of CA1 hippocampal neurons in a WT, an untreated APP23 × PS45, and a γ-secretase inhibitor (LY-411575)-treated APP23 × PS45 mouse. (ii) Activity maps of the hippocampal region shown in top panel with neurons colour-coded according to the frequency of their spontaneous Ca2+-transients. Adapted from [24]. (d) Schematic model summarizing the results shown in (a–c).
There is increasing experimental support that the neuronal dysfunctions found in mouse models are, at least in part, observed also in human patients. Thus, the observation that hyperactivity is followed by a functional silencing of neurons in advanced disease stages is consistent with results from longitudinal human fMRI imaging showing hyperactivation of the hippocampal region before the appearance of severe clinical AD symptoms and a massive loss of hippocampal activity over time [48]. In addition, evidence from human FDG-PET imaging indicates that increased glucose metabolism in brain regions with high amyloid plaque burden can precede cognitive and metabolic decline in later disease stages [49–51]. Interestingly, FDG-PET in combination with 3D-microscopic autoradiography in an AD mouse model (the APPswe/PS1M146 L model) revealed that the glucose hypermetabolism is most pronounced in the direct vicinity of amyloid plaques [52], which could be related to the increased fractions of hyperactive neurons at these locations.
4. Hyperactivity impairs local circuit function
How are the cellular abnormalities (hyper- and hypoactivity) linked to impaired local circuit function? To address this important issue, we analysed the primary visual cortex in the APP23 × PS45 mouse model of AD and found that a progressive deterioration of neuronal tuning for the orientation and direction of visual stimuli occurred in parallel with the age-dependent increase of amyloid plaque burden [19] (figure 5). This impairment was specific to the fraction of hyperactive neurons, which were located predominantly near amyloid plaques, as in the frontal cortex; no defects were observed in functionally normal neurons. Interestingly, in APP23 × PS45 mice, silent neurons were characterized by a total absence of visually evoked responses, whereas in wild-type mice, 20% of the silent neurons had normal responses to visual stimuli. Furthermore, in the APPswe/PS1Δ9 mouse model there was a strong decrease in the fraction of neurons that were activated by structured visual stimulation near amyloid plaques as measured by Arc expression [27]. In line with these observations in cortical neurons, it was shown that hippocampal neuronal hyperactivity is associated with a profound impairment of place cell function [53,54]. Interestingly, fMRI imaging revealed that in humans at risk of AD (APOE4 allele carriers), hyperactivity of the hippocampus was associated with diminished grid-cell-like representations in the entorhinal cortex during a virtual reality spatial memory task [55]. Such dysfunctions of cortical and hippocampal local circuits may contribute to the failure to recruit task-associated networks across the brain and thereby underlie the deficits in brain function and cognition in AD.
Figure 5.
Impaired signal processing in the visual cortex of the AD mouse model. (a) Left panel, in vivo two-photon image of layer 2/3 neurons in the visual cortex of a WT mouse. Middle panel, stimulus-evoked Ca2+-transients recorded from the orientation selective neuron indicated in the left panel by a white dotted circle. Grey regions indicate periods of visual stimulation with drifting gratings schematized by oriented arrows on the bottom of each panel. Four single trials are represented on top and the average of six trials is shown below. Right panel, polar plot showing the neuron's response function to oriented drifting gratings. The responses to each of the eight directions tested were normalized with respect to the maximal response. Then, the function was constructed by connecting the eight values. (b) Left panel, in vivo two-photon image of layer 2/3 neurons in the visual cortex of an APP23 × PS45 mouse. The broken yellow line delineates a thioflavin-S-positive plaque observed in the imaged focal plane. Middle panel, stimulus-evoked Ca2+-transients recorded from the neuron marked in the left panel. Right panel, polar plot showing the neuron's response function to oriented drifting gratings. (c) Left panel, cumulative distributions of the orientation selectivity indices (OSIs) determined for all responsive neurons recorded in APP23 × PS45 as well as WT mice at different age groups. Right panel, proportion of highly (OSI > 0.5) and broadly (OSI < 0.5) tuned neurons in the visual cortex of APP23 × PS45 mice (same neurons as used for analysis in left panel). (d) Scatter plots showing the relationship between the OSI and the frequency of spontaneous Ca2+-transients in WT (top panel) and APP23 × PS45 (bottom panel) mice. Coloured areas indicate the frequency domains of ‘normal’ (green) and hyperactive (purple) neurons. (e) Comparison of the fractions of normal (top panel) and hyperactive (bottom panel) neurons with low (OSI < 0.5) and high orientation tuning (OSI > 0.5) levels in WT and APP23 × PS45 mice (same neurons as used for analysis in d). Adapted from [19].
5. Aβ-induced breakdown of long-range brain circuits
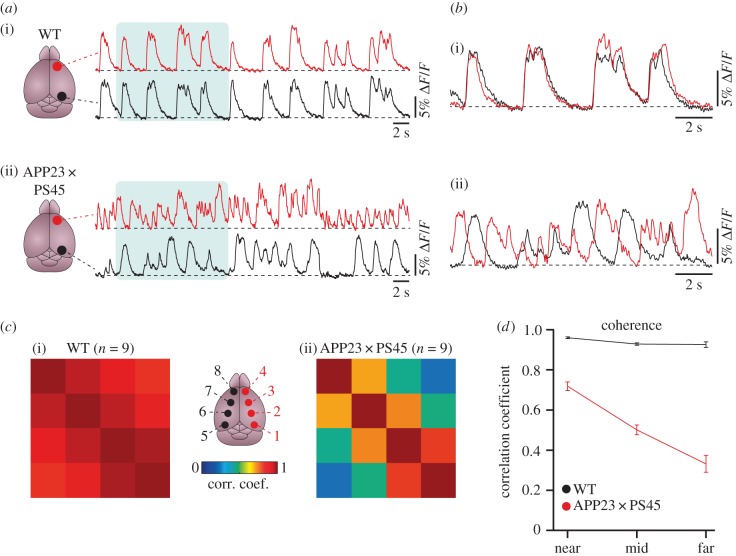
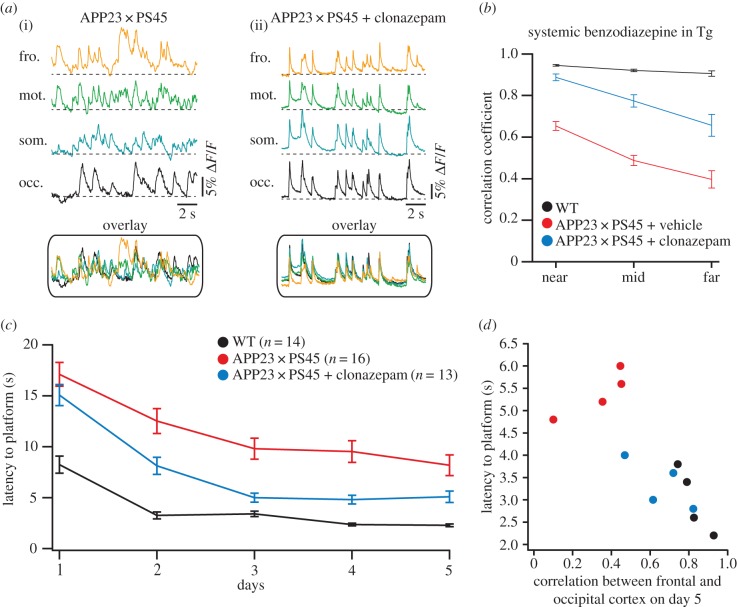
Higher brain functions such as learning and memory emerge from the interaction of myriads of neurons, organized across multiple hierarchical levels from local neuronal (micro)-circuits to communicating large-scale networks. We, therefore, tested the hypothesis that the dysfunction of individual neurons observed in AD mouse models can impair the long-range communication between distant brain regions. Indeed, by employing large-scale calcium fluorescence imaging of the mouse cortical surface, we revealed that the long-range coherence of neuronal activity across neocortical areas was massively impaired in amyloid plaques-bearing APP23 and APP23 × PS45 mouse models when compared with wild-type littermates [56] (figure 6). In these investigations we focused on the slow-wave oscillations, known to be present during non-REM sleep, quiet wakefulness and anaesthesia [57]. Increasing evidence indicates that slow oscillations play a key role in the consolidation of recently acquired memories through mechanisms that include the coordination of neuronal activity between cortical areas, thalamus and the hippocampus [58]. In the AD mouse models, we found that the activity correlations between these widely distributed brain regions were substantially impaired. Notably, such impairment of slow-wave oscillations can also be induced by direct application of Aβ in wild-type mice, suggesting a direct ‘functional disturbance’ through soluble Aβ oligomers rather than an involvement of amyloid plaques-mediated structural damages. This view is supported by the observations that the normalization of the E/I balance, through the application of a low dose of a benzodiazepine that enhances GABAAergic inhibition, can rescue the impairment of slow-wave oscillations (figure 7a,b). By contrast, application of gabazine, which acts by blocking GABAAergic receptors, in wild-type mice resulted in a profound disturbance in the long-range coherence of the slow-wave oscillations, which was similar to that in mouse models of AD. Remarkably, the restoration of slow-wave oscillations by normalization of the E/I-balance resulted in a profound improvement of memory deficits in the APP23 × PS45 mice (figure 7c,d).
Figure 6.
Impaired slow-wave oscillations in mouse models of AD in vivo. (a) Example traces of slow-wave oscillations from the frontal (red) and occipital (black) cortex in a WT (i) and an APP23 × PS45 mouse (ii). (b) Superimposed traces from the shaded areas in (a). (c) Cross-correlation matrix from the cortical regions indicated in the scheme at centre of WT (i) and APP23 × PS45 (ii) mice. (d) Summary graph displaying the average cross-correlation coefficients and standard errors plotted against the cortical distance (categorized as ‘near’ for two neighbouring cortical regions, ‘mid’ for domain pairs separated by one region and ‘far’ for domain pairs separated by two regions) in WT (black) and APP23 × PS45 mice (red). Adapted from [56].
Figure 7.
Pharmacological rescue of slow-wave activity and memory deficits in AD mouse models. (a) Slow-wave oscillations in an untreated APP23 × PS45 (i) and a benzodiazepine-treated (ii) APP23 × PS45 mouse (occ., occipital; som., somatosensory; mot., motor; fro., frontal cortex). (b) Summary graph showing the average cross-correlation coefficients and standard errors plotted against the cortical distance in WT (black), untreated APP23 × PS45 (red) and benzodiazepine-treated (blue) APP23 × PS45 mice. (c) Results from a discriminatory water maze showing the mean time required to reach the platform (latency) for WT (black), untreated APP23 × PS45 (red) and benzodiazepine-treated APP23 × PS45 mice (blue). (d) The latency to find the platform on day 5 is plotted against the correlation between frontal and occipital cortex in WT (black), untreated APP23 × PS45 (red) and benzodiazepine-treated APP23 × PS45 (blue) mice. Each circle represents an individual animal. All error bars indicate s.e.m. Adapted from [56].
Our observations in mouse models of AD are of clinical interest because sleep is often considerably disrupted among people with AD; in many cases long before the onset of cognitive symptoms [59]. Based on our experimental results, we hypothesized that the Aβ-induced impairment of slow-wave oscillations and resulting functional decoupling of cortical–hippocampal–thalamic networks during non-REM sleep could contribute to memory decline in AD. In line with this hypothesis, a recent cross-sectional study in 26 older humans showed that a high amyloid burden in the medial prefrontal cortex, as measured by amyloid-PET, correlated with decreased slow-wave activity during non-REM sleep in that region [60]. Importantly, the impaired slow-wave activity was associated with an impaired overnight hippocampus-dependent memory consolidation.
6. Rescue of circuit dysfunction by correcting the synaptic excitation and inhibition balance
The experimental results reviewed above suggest that therapies that directly modulate the impaired E/I balance in the AD brain may be beneficial, perhaps even at advanced stages of AD. This hypothesis is supported by results obtained in mouse models of AD, showing that the enhancement of GABAAergic inhibition can normalize the activity status of hyperactive neurons, restore slow-wave oscillations and improve memory deficits [18,56]. Furthermore, the antiepileptic drug levetiracetam was shown to reduce neuronal hypersynchrony and behavioural deficits in the hAPPJ20 mouse model of AD [61]. Moreover, treatment with low doses of levetiracetam in people with early AD reduced hippocampal hyperactivation and improved performance in a hippocampus-dependant memory task [15,62].
Finally, a recent study employing a chemogenetic approach with designer receptors exclusively activated by designer drugs (DREADDs) in AD mouse models (the 5XFAD and PSZAPP models) indicated that reduction of neuronal hyperactivity can prevent the further build-up of amyloid plaques and synapse loss [63], suggesting that hyperactivity is not only a consequence of Aβ accumulation but a cellular mechanism that directly promotes the pathogenesis of AD.
7. Conclusion
In AD research, we are witnessing a fundamental paradigm shift. The classic view that the structural damage by amyloid plaques and the loss of neurons underlies cognitive impairment in AD through a reduced cortical activity is challenged by recent findings from animal and human studies showing that, in fact, excess neuronal activity, hypersynchrony and altered brain oscillations are key features of the disease. Growing experimental evidence suggests that these functional impairments are predominantly driven by the abnormal accumulation of soluble Aβ in the brain. This process may start in patients decades before the occurrence of the first clinical symptoms. In view of a potential key role of neuronal hyperactivity for AD pathogenesis, we suggest that the therapeutic correction of neuronal circuit impairments, as early as possible, could prevent or slow down the onset of cognitive impairment in patients with AD.
Competing interests
We have no competing interests.
Funding
This work was funded by an Advanced European Research Council grant to A.K., the European Union FP7 program (Project Corticonic) and the Deutsche Forschungsgemeinschaft (RTG 1373 and SFB870). M.A.B. was supported by the Alzheimer Forschung Initiative.
References
- 1.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. 2015. Alzheimer's disease. Nat. Rev. Dis. Primers 1, 15056 ( 10.1038/nrdp.2015.56) [DOI] [PubMed] [Google Scholar]
- 2.McKhann GM, et al. 2011. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 263–269. ( 10.1016/j.jalz.2011.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golde TE, Schneider LS, Koo EH. 2011. Anti-Aβ therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron 69, 203–213. ( 10.1016/j.neuron.2011.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen WJ, et al. 2015. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. J. Am. Med. Assoc. 313, 1924–1938. ( 10.1001/jama.2015.4668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ossenkoppele R, et al. 2015. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. J. Am. Med. Assoc. 313, 1939–1949. ( 10.1001/jama.2015.4669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ, et al. 2012. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 367, 795–804. ( 10.1056/NEJMoa1202753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. 2000. Patterns of brain activation in people at risk for Alzheimer's disease. N. Engl. J. Med. 343, 450–456. ( 10.1056/NEJM200008173430701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillon G, Lopera F, Stern CE. 2010. Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann. Neurol. 68, 865–875. ( 10.1002/ana.22105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machulda MM, et al. 2011. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol. 68, 1131–1136. ( 10.1001/archneurol.2011.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling RA, et al. 2009. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188. ( 10.1016/j.neuron.2009.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. 2009. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 29, 12 686–12 694. ( 10.1523/JNEUROSCI.3189-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. 2010. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 67, 584–587. ( 10.1016/j.biopsych.2009.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickerson BC, et al. 2005. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. ( 10.1212/01.wnl.0000171450.97464.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorg C, et al. 2007. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 104, 18 760–18 765. ( 10.1073/pnas.0708803104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakker A, et al. 2012. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. ( 10.1016/j.neuron.2012.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. 2003. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl Acad. Sci. USA 100, 7319–7324. ( 10.1073/pnas.1232232100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichhoff G, Busche MA, Garaschuk O. 2008. In vivo calcium imaging of the aging and diseased brain. Eur. J. Nucl. Med. Mol. Imaging 35(Suppl 1), S99–S106. ( 10.1007/s00259-007-0709-6) [DOI] [PubMed] [Google Scholar]
- 18.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. 2008. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science 321, 1686–1689. ( 10.1126/science.1162844) [DOI] [PubMed] [Google Scholar]
- 19.Grienberger C, Rochefort NL, Adelsberger H, Henning HA, Hill DN, Reichwald J, Staufenbiel M, Konnerth A. 2012. Staged decline of neuronal function in vivo in an animal model of Alzheimer's disease. Nat. Commun. 3, 774 ( 10.1038/ncomms1783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selkoe DJ. 2002. Alzheimer's disease is a synaptic failure. Science 298, 789–791. ( 10.1126/science.1074069) [DOI] [PubMed] [Google Scholar]
- 21.Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. 2007. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Inv. 117, 1230–1239. ( 10.1172/JCI30447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palop JJ, et al. 2007. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55, 697–711. ( 10.1016/j.neuron.2007.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minkeviciene R, et al. 2009. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 29, 3453–3462. ( 10.1523/JNEUROSCI.5215-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. 2012. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 109, 8740–8745. ( 10.1073/pnas.1206171109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier FC, et al. 2014. Longitudinal PET-MRI reveals β -amyloid deposition and rCBF dynamics and connects vascular amyloidosis to quantitative loss of perfusion. Nat. Med. 20, 1485–1492. ( 10.1038/nm.3734) [DOI] [PubMed] [Google Scholar]
- 26.Busche MA, Grienberger C, Keskin AD, Song B, Neumann U, Staufenbiel M, Forstl H, Konnerth A. 2015. Decreased amyloid-β and increased neuronal hyperactivity by immunotherapy in Alzheimer's models. Nat. Neurosci. 18, 1725–1727. ( 10.1038/nn.4163) [DOI] [PubMed] [Google Scholar]
- 27.Rudinskiy N, Hawkes JM, Betensky RA, Eguchi M, Yamaguchi S, Spires-Jones TL, Hyman BT. 2012. Orchestrated experience-driven Arc responses are disrupted in a mouse model of Alzheimer's disease. Nat. Neurosci. 15, 1422–1429. ( 10.1038/nn.3199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siskova Z, et al. 2014. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer's disease. Neuron 84, 1023–1033. ( 10.1016/j.neuron.2014.10.024) [DOI] [PubMed] [Google Scholar]
- 29.Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. 2009. Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat. Neurosci. 12, 1567–1576. ( 10.1038/nn.2433) [DOI] [PubMed] [Google Scholar]
- 30.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. 2009. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323, 1211–1215. ( 10.1126/science.1169096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delekate A, Fuchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC. 2014. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat. Commun. 5, 5422 ( 10.1038/ncomms6422) [DOI] [PubMed] [Google Scholar]
- 32.Brawek B, Schwendele B, Riester K, Kohsaka S, Lerdkrai C, Liang Y, Garaschuk O. 2014. Impairment of in vivo calcium signaling in amyloid plaque-associated microglia. Acta Neuropathol. 127, 495–505. ( 10.1007/s00401-013-1242-2) [DOI] [PubMed] [Google Scholar]
- 33.Allen NJ. 2014. Astrocyte regulation of synaptic behavior. Ann. Rev. Cell Dev. Biol. 30, 439–463. ( 10.1146/annurev-cellbio-100913-013053) [DOI] [PubMed] [Google Scholar]
- 34.Chung WS, Welsh CA, Barres BA, Stevens B. 2015. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 18, 1539–1545. ( 10.1038/nn.4142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heneka MT, et al. 2015. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14, 388–405. ( 10.1016/S1474-4422(15)70016-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. 2016. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131, 323–345. ( 10.1007/s00401-015-1513-1) [DOI] [PubMed] [Google Scholar]
- 37.De Strooper B, Karran E. 2016. The cellular phase of Alzheimer's disease. Cell 164, 603–615. ( 10.1016/j.cell.2015.12.056) [DOI] [PubMed] [Google Scholar]
- 38.Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. 2004. High-resolution in vivo imaging of hippocampal dendrites and spines. J. Neurosci. 24, 3147–3151. ( 10.1523/JNEUROSCI.5218-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koffie RM, et al. 2009. Oligomeric amyloid-β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 106, 4012–4017. ( 10.1073/pnas.0811698106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. 2011. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 14, 750–756. ( 10.1038/nn.2801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto K, et al. 2015. Chronic optogenetic activation augments Aβ pathology in a mouse model of Alzheimer disease. Cell Rep. 11, 859–865. ( 10.1016/j.celrep.2015.04.01) [DOI] [PubMed] [Google Scholar]
- 42.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. 2009. Soluble oligomers of amyloid-β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801. ( 10.1016/j.neuron.2009.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fogel H, et al. 2014. APP homodimers transduce an amyloid-β-mediated increase in release probability at excitatory synapses. Cell Rep. 7, 1560–1576. ( 10.1016/j.celrep.2014.04.02) [DOI] [PubMed] [Google Scholar]
- 44.Verret L, et al. 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721. ( 10.1016/j.cell.2012.02.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Limon A, Reyes-Ruiz JM, Miledi R. 2012. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc. Natl Acad. Sci. USA 109, 10 071–10 076. ( 10.1073/pnas.1204606109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews-Zwilling Y, et al. 2010. Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 30, 13 707–13 717. ( 10.1523/JNEUROSCI.4040-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, Meng X, Zhang J, Li Y, Wang L, Qin X, Sui N, Zhang Y. 2012. GABA attenuates amyloid toxicity by downregulating its endocytosis and improves cognitive impairment. J. Alz. Dis. 31, 635–649. ( 10.3233/JAD-2012-120535) [DOI] [PubMed] [Google Scholar]
- 48.O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. 2010. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74, 1969–1976. ( 10.1212/WNL.0b013e3181e3966e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen AD, et al. 2009. Basal cerebral metabolism may modulate the cognitive effects of Aβ in mild cognitive impairment: an example of brain reserve. J. Neurosci. 29, 14 770–14 778. ( 10.1523/JNEUROSCI.3669-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh H, Habeck C, Madison C, Jagust W. 2014. Covarying alterations in Aβ deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Hum. Brain Mapp. 35, 297–308. ( 10.1002/hbm.22173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson SC, et al. 2014. Amyloid burden and neural function in people at risk for Alzheimer's disease. Neurobiol. Aging 35, 576–584. ( 10.1016/j.neurobiolaging.2013.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poisnel G, et al. 2012. Increased regional cerebral glucose uptake in an APP/PS1 model of Alzheimer's disease. Neurobiol. Aging 33, 1995–2005. ( 10.1016/j.neurobiolaging.2011.09.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. 2005. Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 25, 6877–6886. ( 10.1523/JNEUROSCI.1744-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. 2010. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacol. 35, 1016–1025. ( 10.1038/npp.2009.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunz L, et al. 2015. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer's disease. Science 350, 430–433. ( 10.1126/science.aac8128) [DOI] [PubMed] [Google Scholar]
- 56.Busche MA, Kekus M, Adelsberger H, Noda T, Forstl H, Nelken I, Konnerth A. 2015. Rescue of long-range circuit dysfunction in Alzheimer's disease models. Nat. Neurosci. 18, 1623–1630. ( 10.1038/nn.4137) [DOI] [PubMed] [Google Scholar]
- 57.Crunelli V, Hughes SW. 2010. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat. Neurosci. 13, 9–17. ( 10.1038/nn.2445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diekelmann S, Born J. 2010. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. ( 10.1038/nrn2762) [DOI] [PubMed] [Google Scholar]
- 59.Musiek ES, Xiong DD, Holtzman DM. 2015. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 47, e148 ( 10.1038/emm.2014.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. 2015. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci. 18, 1051–1057. ( 10.1038/nn.4035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez PE, et al. 2012. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc. Natl Acad. Sci. USA 109, E2895–E2903. ( 10.1073/pnas.1121081109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. 2015. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 7, 688–698. ( 10.1016/j.nicl.2015.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan P, Grutzendler J. 2016. Attenuation of β-amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J. Neurosci. 36, 632–641. ( 10.1523/JNEUROSCI.2531-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]