Abstract
In the central nervous system (CNS), during both brain and spinal cord development, purinergic and pyrimidinergic signalling molecules (ATP, UTP and adenosine) act synergistically with peptidic growth factors in regulating the synchronized proliferation and final specification of multipotent neural stem cells (NSCs) to neurons, astrocytes or oligodendrocytes, the myelin-forming cells. Some NSCs still persist throughout adulthood in both specific ‘neurogenic’ areas and in brain and spinal cord parenchyma, retaining the potentiality to generate all the three main types of adult CNS cells. Once CNS anatomical structures are defined, purinergic molecules participate in calcium-dependent neuron-to-glia communication and also control the behaviour of adult NSCs. After development, some purinergic mechanisms are silenced, but can be resumed after injury, suggesting a role for purinergic signalling in regeneration and self-repair also via the reactivation of adult NSCs. In this respect, at least three different types of adult NSCs participate in the response of the adult brain and spinal cord to insults: stem-like cells residing in classical neurogenic niches, in particular, in the ventricular–subventricular zone (V-SVZ), parenchymal oligodendrocyte precursor cells (OPCs, also known as NG2-glia) and parenchymal injury-activated astrocytes (reactive astrocytes). Here, we shall review and discuss the purinergic regulation of these three main adult NSCs, with particular focus on how and to what extent modulation of intracellular calcium levels by purinoceptors is mandatory to determine their survival, proliferation and final fate.
This article is part of the themed issue ‘Evolution brings Ca2+ and ATP together to control life and death’.
Keywords: purines, P2 receptors, calcium, neural stem cells
1. Introduction
Extracellular nucleotides and their receptors have pivotal roles from the very beginning of life. P2 receptors are expressed by oocytes, sperm and Sertoli cells, and adenosine triphosphate (ATP) is essential for sperm movement. In the majority of living organisms, ATP acts as a key sperm-to-egg signal in the process of fertilization and, immediately after zygote formation, time-specific release of ATP from cells of the developing organism together with their transient expression of P2 receptor subtypes orchestrate embryological, fetal and postnatal development. This suggests the involvement of nucleotides (and of the intracellular calcium rises generated by their receptors) in the synchronized proliferation, differentiation, migration and death of cells during these complex events (for review, see [1,2]).
In a similar way to other organs, in the central nervous system (CNS), purinergic and pyrimidinergic signalling molecules (ATP, UTP and adenosine) act synergistically with peptidic growth factors during both brain and spinal cord development in regulating the synchronized proliferation and final specification of multipotent neural stem cells (NSCs) to neurons, astrocytes or oligodendrocytes, the myelin-forming cells, which are formed by intermediate precursors known as either oligodendrocyte precursor cells (OPCs) or NG2-glia [3].
Some NSCs, however, still persist throughout adulthood in both specific ‘neurogenic’ areas and in parenchyma, retaining the potentiality to generate all the three main types of adult CNS cells (see also later sections).
Once CNS anatomical structures are defined, purinergic molecules participate in calcium-dependent neuron-to-glia communication and also control the behaviour of adult NSCs. After development, some purinergic mechanisms are silenced but can be resumed after injury, suggesting a role for purinergic signalling in regeneration and self-repair also via the reactivation of adult NSCs [4].
In this respect, recent data highlight the importance of at least three different types of adult NSCs in the response of the adult brain and spinal cord to insults: (i) stem-like cells residing in classical neurogenic niches, in particular in the ventricular–subventricular zone (V-SVZ), (ii) parenchymal NG2-glia and (iii) parenchymal injury-activated astrocytes (reactive astrocytes).
Here, we shall review and discuss the purinergic regulation of these three main adult NSCs, with particular focus on how and to what extent modulation of intracellular calcium levels by purinoceptors is mandatory to determine their survival, proliferation and final fate.
2. ‘Classical’ neurogenic areas: the ventricular–subventricular zone and the subgranular layer of the hippocampus
The more extended neurogenic area in the adult mammalian brain is the V-SVZ, located along the entire length of the walls of the lateral ventricles (figure 1a; [6]). Formerly simply known as SVZ, its current denomination as V-SVZ has integrated the concept of the contribution of the ependymal cells (ECs) contacting the ventricle with the physiology of the neurogenic niche.
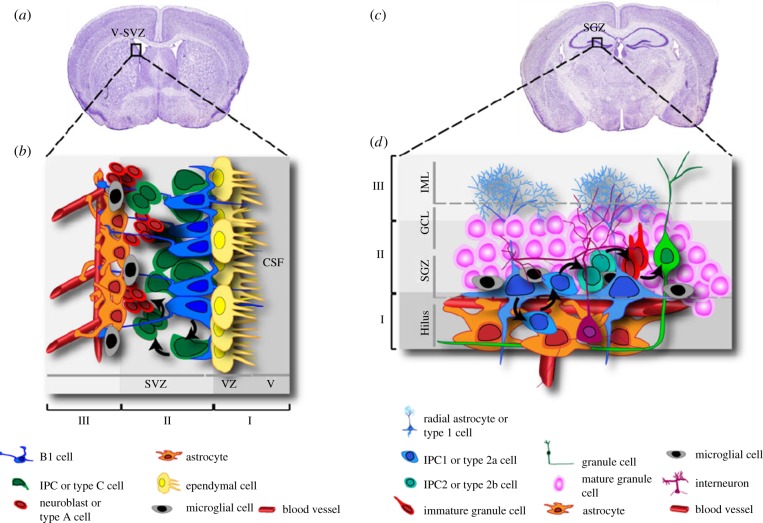
Figure 1.
Schematic of the V-SVZ and SGZ neurogenic niches in the adult brain. (a,c) Coronal sections of the adult rodent brain showing the localization of the V-SVZ and of the hippocampal SGZ, respectively. (b,d) Schematic drawing of the organization and relationships of the different cell populations residing in the V-SVZ (b) and in the SGZ (d). See text for details. IPC, intermediate progenitor cells. Reprinted from [5] under a Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0).
Within the V-SVZ, three main cell types are morphologically and functionally distinguished: astrocyte-like stem cells (type B1 cells) give rise to clusters of transit-amplifying cells (type C cells or intermediate progenitor cells, IPCs), which, in turn, generate migrating neuroblasts (type A cells; figure 1b). In rodents, newborn neurons proliferate and migrate along the rostral migratory stream (RMS) to the olfactory bulbs (OBs), where they differentiate and integrate in existing neuronal networks [5,7]. It is worth mentioning that, despite the presence, in humans, of a lateral ventricular extension connecting the SVZ to the OB [8], in the adult human brain the RMS remains elusive, possibly owing to the limited importance of the olfactory system in our species. Rather, in the human brain new interneurons are postnatally generated in the corpus striatum, thus identifying a unique pattern of adult neurogenesis [9].
By releasing a plethora of mediators, other cells residing in the close proximity of the V-SVZ, like astrocytes, microglia and cells of the vessel wall (figure 1b), contribute to the modulation of the properties of this neurogenic niche (see later sections).
Type B1 cells are the actual NSCs; they express typical astrocyte markers, like the l-glutamate/l-aspartate transporter (GLAST), glial fibrillary acidic protein (GFAP) and the intermediate filament nestin [7]. Type B1 cells inherit the apical–basal polarity from their precursors, the embryonic radial glia, with their primary cilium directly contacting the cerebrospinal fluid within the lumen of the ventricles (figure 1b). This structure is considered as essential for the reception of growth factors and morphogens and for the subsequent transduction of signals [10]. Their basal side is in close connection with blood vessels at particular sites where V-SVZ vasculature lacks coverage by both astrocytes and pericytes [11], thus being able to receive regulatory signalling molecules released from endothelial cells or circulating in the blood. Although type B1 cells mostly generate new neurons, they are endowed with pluripotency and can also give rise to young migrating oligodendrocytes through intermediate type C cells that move out of the V-SVZ into the corpus callosum, neighbouring striatum and fimbria fornix to differentiate into both non-myelinating and myelinating oligodendrocytes [12]. Moreover, it has been recently shown that type B1 cells can physiologically also generate astrocytes located in the corpus callosum and RMS, but not in the cortex or striatum. This ability decreases progressively with age and sustains a slow, but continuous turnover of astrocytes that normally undergo apoptosis in the corpus callosum [13].
Type C cells are scattered along the network of migrating neuroblasts, they are immunopositive for nestin, the transcription factor distal-less homeobox-2 (Dlx-2) and Mash1, and proliferate at high rate. They are considered as intermediate precursors for type A cells and are not found in the RMS [14].
Type A cells correspond to proliferating, migrating neural precursor cells, showing immunopositivity for typical neuronal markers, like polysialylated neural cell adhesion molecule (PSA-NCAM) and β-Tubulin class III, along with the marker of immature neurons doublecortin (DCX) [7]. Once they reach the OB, type A cells differentiate into periglomerular or granular cells following a specific regional identity [14].
The second region in the adult mammalian brain retaining substantial neurogenesis is the dentate gyrus (DG) of the hippocampus (figure 1c), where neurogenesis has been extensively studied over the years, mainly owing to its major role in learning and memory processes. In particular, neural progenitors in this area are located in the subgranular zone (SGZ), a very thin (i.e. only one to three nuclei wide) layer of cells [15]. Three types of neural progenitors have been identified, based on their morphology and on the expression of specific molecular markers (figure 1d). Type I (or radial astrocytes) represents the real NSCs, which rarely enter into the cell cycle and have a radial process spanning the entire granular cell layer and ramifying in the inner molecular layer (IML; figure 1d). These cells express nestin, GFAP, Sox2 and the brain lipid-binding protein, but do not express S100β, a marker of mature astrocytes [15]. Type I cells generate type II IPCs, which divide faster and are irregularly shaped, and in turn differentiate in postmitotic, immature granule cell neurons positive for DCX and the neuronal marker NeuroD1 (neurogenic differentiation 1) [15]. Immature granule cells are generated in excess and most of them die within the first two weeks whereas the few surviving neurons migrate into the IML, where they elongate their dendrites and axons, and integrate into the DG circuitry [16]. Interestingly, the generation of new DCX positive immature neurons in hippocampal DG has been recently shown to be controlled by the P2Y-like GPR17 receptor [17], whose activation can be counteracted by both purinergic antagonists like cangrelor and by montelukast, a classical antagonist of cysteinyl-leukotriene receptors [18] that is already marketed as an anti-asthmatic agent. In detail, montelukast was shown to be able to revert the cognitive decline observed in old rodents via an implementation of the proliferation of DCX immature neurons in the DG. This effect was specifically related to blockade of GPR17 on these cells, as demonstrated in neurospheres from both GPR17 KO mice and FoxO1 null mice, where this transcription factor controlling GPR17 expression had been deleted.
Birth of new neurons that integrate locally in functional circuits underlying hormone production and release has been observed in the hypothalamus in response to insulin-like growth factor 1 (IGF-1) administration, leading to the identification of a specific ‘neurogenic niche’ in the walls of the third ventricle [19], which will not be analysed in this paper. As mentioned, precursors in neurogenic areas derive from embryonic radial glial cells, whose proliferation and differentiation are tightly controlled by intercellular calcium waves. ATP release and [Ca2+]i transients are connected by autocrine loops, where secretion of ATP-containing vesicles depends on [Ca2+]i transients and activation of exocytosis through the v-SNARE system [20]. Following its hydrolysis to ADP, ATP activates metabotropic P2Y1 receptors, linked to intracellular calcium mobilization; calcium waves are then propagated into neighbouring cells by intercellular signalling through connexin hemichannels [4], resulting in cell cycle synchronization of migrating neural progenitors and radial glia cells in the V-SVZ for cortex development [21]. This provides an additional link between calcium and the purinergic system in progenitor cells. In fact, spontaneous calcium oscillations in neural progenitors have been shown to depend on the activation of P2R, probably owing to the release of ATP. Reduced calcium transients evoked by P2Y1R were observed in precursor cells isolated from connexin43 (Cx43)-null mice [22]. Blockade of P2Y1R in precursors from wild-type (WT) mice did not alter their differentiation, as measured by the ratio of nestin : GFAP expression levels, but reduced their proliferation rate and their migration distance to distances similar to those observed in Cx43-null cells. Conversely, forced overexpression of P2Y1R in Cx43-null neurospheres led to the generation of spontaneous calcium oscillation and restored the migration pattern observed in WT neural progenitors [22]. Overall, these data provided some of the first evidence linking the purinergic system with calcium mobilization in precursor cells, which, in turn, profoundly affect their functions.
To confirm the existence of a basal fine-tuning of NSC functions and fate by purines and pyrimidines, receptors and metabolizing enzymes for nucleotides and nucleosides are widely expressed in the SVZ and SGZ (reviewed in [23]). Specifically, high levels of the ectoenzyme nucleoside triphosphate diphosphohydrolase 2 (NTPDase2), which hydrolyses extracellular nucleoside diphosphates and triphosphates, have been detected in both neurogenic areas in the adult CNS [24–26]. Studies in NTPDase2-null mice, where increased ATP levels are foreseen, revealed increased progenitor cell proliferation in both V-SVZ and SGZ, with an expansion of intermediate progenitors but no changes in the number of newly generated neurons that died from apoptosis [26]. This suggests the possible recruitment of the P2X7R (see below).
Generation of calcium waves was observed in NSCs isolated from neurogenic areas and grown in vitro as floating neurospheres through activation of the ADP-sensitive G protein-coupled P2Y1R (with a contribution of P2Y13R) and UTP-responding P2Y2R, while no functional P2Y4Rs or P2Y6R have been identified [27]. Modulation of calcium transients by P2Y1Rs exerted opposite functional effects, with reduction of the proliferation rate and of the number of generated primary neurospheres, in contrast with an overall increased proliferation of secondary neurospheres (see also below). This is possibly owing to the enrichment in NSCs that is observed over successive passages in culture and to the presence of growth factors [27,28]. Confirmation of a most prominent role in neurogenesis played by the P2Y1R came from the demonstration that its activation promoted NSC differentiation [28] and migration [29] in vitro, whereas a dual effect on proliferation was observed depending on the presence of growth factors. In fact, stimulation of proliferation was observed in their absence or at low concentrations [30,31], while an anti-proliferative effect was achieved at standard concentrations (around 20 ng ml−1) [27]. Caution should be taken when trying to translate results obtained in vitro to an in vivo setting, because cultured stem cells can express all purinergic receptors and may consequently reveal a broader spectrum of responses than corresponding cells in vivo. Nevertheless, it has been clearly confirmed that activation of P2Y1R controls V-SVZ cell functions in vivo. In fact, upon ATP administration, the proliferation of type C cells increased, with no changes in proliferation of either type B stem cells or type A neuroblasts. To further strengthen the role of P2Y1R, an opposite effect was observed upon administration of the selective P2Y1R antagonist MRS2179 per se and a reduced number of type C cells was detected in the V-SVZ of P2Y1R-KO mice [32]. The interpretation of in vivo results is further complicated by: (i) difficulties in identifying the exact localization of purinoceptors on a specific cell population within the neurogenic niches (that do not allow us to discriminate whether agonists and antagonists exert direct effects on stem cells or whether the contribution of surrounding cells like astrocytes and microglia is needed) and (ii) by the possible localization of purinoceptors either on the somata or on the processes of cells contacting precursors in the neurogenic niches (see figure 3d).
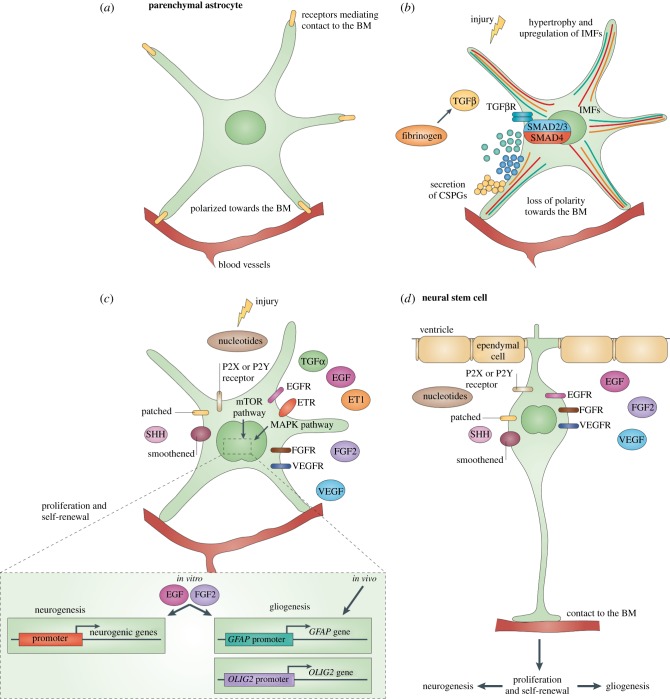
Figure 3.
Signals shared after brain injury and in the NSC niche. (a) Mature astrocytes in the healthy brain parenchyma are polarized towards (and make contact with) the basement membrane (BM) surrounding blood vessels and do not divide. (b) Loss of signalling from the BM as seen after brain injury leads to reactive astrogliosis, including loss of the polarized expression of endfeet proteins, hypertrophy and upregulation of intermediate filaments (IMFs), and secretion of chondroitin sulfate proteoglycans (CSPGs). Secretion of CSPGs is triggered by fibrinogen, which activates transforming growth factor beta (TGFβ) signalling. This pathway also promotes upregulation of glial fibrillary acidic protein (GFAP), which may inhibit axonal regeneration. (c) Signals triggering astrocyte proliferation after injury are fibroblast growth factor (FGF), epidermal growth factor (EGF) and transforming growth factor alpha (TGFα), which act via the mitogen-activated protein kinase (MAPK) or the mammalian target of rapamycin (mTOR) pathway. The latter is also activated by purinergic signalling following the release of ATP after brain injury. Proliferating reactive astrocytes do not generate other glia or neurons in vivo. However, exposure to further EGF and FGF2 signalling in vitro confers the capacity to generate neurons, astrocytes and oligodendrocytes in vitro. (d) These factors, as well as vascular endothelial growth factor (VEGF) and sonic hedgehog (SHH), are also active in the adult neurogenic niches, like the SVZ, in vivo, where they also promote proliferation of neural stem and progenitor cells. EGFR, EGF receptor; ET1, endothelin 1; ETR, ET receptor; FGFR, FGF receptor; OLIG2, oligodendrocyte transcription factor 2; TGFβR, TGFβ receptor; VEGFR, VEGF receptor. Reprinted by permission from Robel et al. [114]. (Copyright 2011 Macmillan Publishers Ltd).
Another purinergic receptor involved in neural differentiation is the P2Y2R subtype, which activates PLC-β, leading to increased [Ca2+]i and generation of intercellular Ca2+ waves in NSCs in vitro [30]. Moreover, neural progenitor proliferation is modulated by an autocrine loop, with cells releasing ATP, activating P2Y receptors for proliferation maintenance. Blockade of proliferation and induction to neural differentiation occurred only when purinergic receptor activity had been antagonized and [Ca2+]i transients had diminished [33].
The effects of P2Y1R activation are counterbalanced by the ionotropic P2X7R subtype, which is highly expressed by NSCs in the SVZ, and modulates intracellular calcium spikes [34]. Its prolonged activation by ATP led to caspase-independent lysis/necrosis of NSCs, as demonstrated by cell membrane disruption accompanied by loss of mitochondrial membrane potential. Surprisingly, activation of P2X7R in NPCs causes cell death in the absence of pore formation [35]. These observations might have opposite outcomes following pathological events: in fact, high levels of extracellular ATP in inflammatory CNS lesions may delay the successful graft of newborn NPCs and therefore impair tentative repair of the damaged tissue. On the other hand, recruitment of P2X7R can inhibit excessive neuro- and gliogenesis, thus reducing the risks for development of tumours.
Apart from the physiological control of neurogenesis, it can be foreseen that the above-mentioned mechanisms involving calcium and extracellular nucleotides become increasingly prevalent upon traumatic and/or ischaemic events, when extracellular purine and neurotransmitter concentrations increase several fold, leading to an amplification of their signalling pathways under emergency conditions [36]. As elevated extracellular nucleotide concentrations are known to be responsible for astrocyte and microglia activation (see also below), it is conceivable that these reactive cell populations residing within the neurogenic niche (or in its close proximity) can participate to drive NSC final destiny and neurogenic potential. In line with that, ATP secretion by astrocytes even at basal level can promote NSC proliferation in the adult hippocampus through P2Y1R activation [36]. Moreover, in vivo intracerebroventricular infusion of high concentrations of a hydrolysis-resistant ADP analogue, thus resembling high pathological nucleotide concentrations, promotes the proliferation of type B progenitors and sustains their progression toward the generation of rapidly dividing progenitors, both directly and indirectly, through the contribution of surrounding reactive astrocytes [31].
Interestingly, traumatic and ischaemic events not only dramatically modify NSC neurogenic environment, but can also recruit them outside their ‘natural’ migratory pathway towards the site of injury, in an often unsuccessful attempt of regeneration [37], thus profoundly affecting the fate of newborn cells. It cannot be excluded that the increased concentrations of extracellular nucleotides are also involved in this effect.
(a). Ependymal cells lining spinal cord central canal
ECs lining the spinal cord central canal are considered the real stem-like cells in this part of the CNS. They stain positive for markers of immature neural cells, such as nestin, vimentin and the transcription factor Sox2, and of ciliated cells such as Fox1 and Crocc [38]. Moreover, a very recent paper showed for the first time that spinal cord ECs express ionotropic P2X4Rs and P2X7Rs, and metabotropic P2Y1Rs and P2Y4Rs, all functionally coupled to [Ca2+]i transients [39]. They also stain positive for the dualistic receptor GPR17 [40], which responds to both extracellular nucleotides and cysteinyl-leukotrienes and plays a fundamental role not only in the differentiation of NG2-glia to mature myelinating oligodendrocytes (see also below), but also in their alternative switch towards a neurogenic fate [41]. In the intact tissue, spinal cord ECs are virtually quiescent, self-renew very slowly and give rise to a very small number of neurospheres in vitro. However, after spinal cord injury (SCI) or hypoxia, ECs proliferate, migrate towards the injury site and start expressing GFAP, a marker of multipotency [38,40]. Interestingly, ECs reveal downregulation of P2Y1Rs in parallel with upregulation of P2Y4Rs one week after SCI, suggesting that the panel of expression of P2YRs may play a critical role in the modulation of neural progenitor cell expansion [39]. Activated ECs generate a high number of neurospheres and give rise to astrocytes, oligodendrocytes and, under some conditions, motoneurons when exposed to differentiating agents in vitro [42]. However, fate-mapping analysis of ECs following SCI in vivo revealed generation of astrocytes and oligodendrocytes only [43] (see also §3). Although no generation of new neurons has been observed, acute transplantation of undifferentiated ECs fully reverted the pathological increase in P2X4Rs and P2X7Rs observed after SCI and led to a partial recovery of locomotor activity [39], thus suggesting a possible bystander role played by ECs.
3. Oligodendroglial precursor cells (NG2-glia)
In the CNS, mature myelinating oligodendrocytes are formed from OPCs, also termed as NG2-glia for their expression of the membrane-spanning proteoglycan NG2. These cells derive from less differentiated NPCs and constitute a ubiquitous population of glial progenitors in the mammalian CNS, able to generate mature oligodendrocytes during early postnatal development [44]. OPCs remain abundant in grey and white matter regions of the adult CNS parenchyma [44,45] and retain the ability to proliferate and differentiate into oligodendrocytes to sustain basal myelin turnover [46,47]. More importantly, these cells promptly react to injury and demyelination by increasing their proliferation, migration and differentiation rate to repopulate the lesioned area with new myelinating oligodendrocytes [48–50]. Recently discovered properties of OPCs also point to additional neuromodulatory and neuroprotective actions of this cell population [51]. OPCs have indeed the capability to receive and respond to electrical activity [52]; moreover, it has been proposed that the expression of growth factors, cytokines, chemokines and receptors for various signalling molecules may confer to these cells the same reparative bystander actions that germinal neural progenitors exert in the lesioned CNS [51,53–55].
In early studies, changes in intercellular calcium waves have been detected in OPCs [56,57], suggesting that intracellular calcium transients can fine-tune their development. Several studies have later addressed the importance of Ca2+ signalling in process extension and OPC migration [58–62], in their differentiation, myelination and re-myelination capacity [63–66], and in retraction of membrane sheaths and cell death in mature mouse oligodendrocytes [67].
(a). Extracellular nucleotides and calcium signalling in oligodendrocyte precursor cells
Several neurotransmitters contribute to the homeostasis of OPCs and it is now well accepted that purinergic signalling is actively involved in the regulation of their physiological behaviour and reaction to injury [68]. Extracellular ATP is one of the main activity-dependent axonal signalling molecules that activate P2 receptors on neighbouring OPCs [69]. Both ATP and uracil nucleotides interact with other extracellular signals impinging on common intracellular pathways in regulating OPC proliferation and migration, various steps of OPC maturation and myelination and axonal activity [70–73]. Key roles in various OPC processes are also carried out by adenosine deriving from ATP metabolism through activation of A1 or A2 receptors [74–76]. A1 receptors are downregulated during oligodendrocyte differentiation, whereas P2Y1 and P2X7Rs are expressed at all differentiating stages and by mature myelin-forming oligodendrocytes [77].
Of interest, nucleotide-dependent mechanisms are implicated in OPC calcium oscillations, which in turn regulate neuronal integrity and stimulate myelin production by oligodendrocytes [74]. Elevation of intracellular basal calcium in these cells occurs via a number of routes by favouring calcium entry across the plasma membrane through: (i) ligand-operated channels (such as the P2X, and glutamate receptors) [78–80], (ii) voltage-operated Ca2+ channels (VOCs) activated in response to membrane depolarization [60,65], (iii) other routes, such as the opening of store-operated membrane Ca2+ channels following depletion of Ca2+ stores in the endoplasmic reticulum [81–83]. Release of calcium from internal stores of the endoplasmic reticulum can occur following activation of a variety of membrane G protein-coupled receptors, including P2YRs coupled to Gq via the inositol-1,4,5-triphosphate (IP3) pathway (figure 2a).
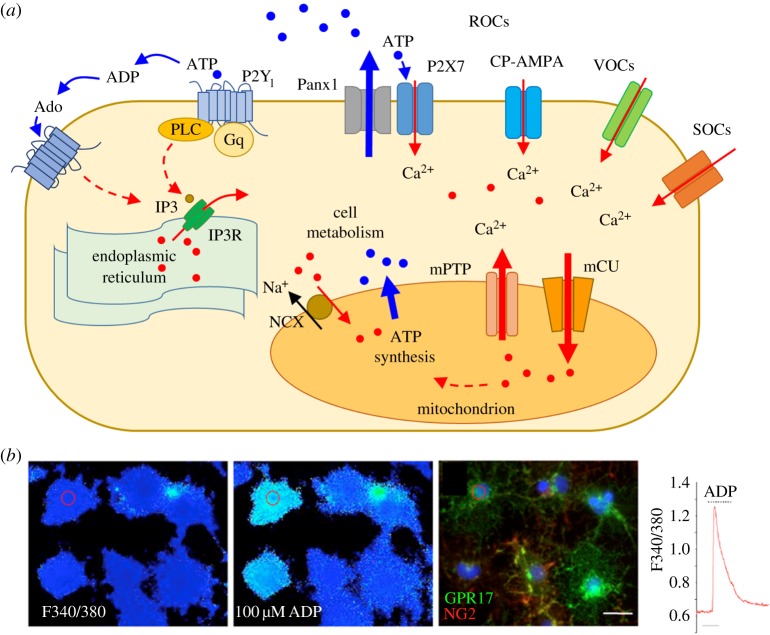
Figure 2.
Calcium signalling in OPCs. (a) Schematic of the main routes activating Ca2+ signalling in OPCs. Calcium enters OPCs through ROCs (receptor-operated channels, such as P2X7 and CP-AMPA), VOCs (voltage-operated channels) and SOCs (store-operated channels). IP3 receptors (IP3Rs) on the endoplasmic reticulum promote the release of Ca2+ from the intracellular stores via Gq-coupled receptors, mainly P2Y1 (activated by ATP and ADP) and in part A1 and A2A receptors (primarily Gi- and Gs-coupled, respectively, but secondarily coupled to Gq) activated by Ado (adenosine). In disease, activation of P2X7Rs promotes the opening of a non-selective pore, namely pannexin1 (Panx1), which contributes to massive release of ATP in the extracellular milieu. Mitochondria take up intracellular Ca2+ through the mCU (mitochondrial calcium uniporter) and maintain the Ca2+ homeostasis by both the mPTP (mitochondrial permeability transition pore) and NCX (Na+/Ca2+ exchanger) localized on the inner membrane. ATP synthesized by the mitochondrial respiratory chain is then available for cell metabolism. (b) Series of pseudocolour images of the same Fura-2 loaded cells. First micrograph from the left shows a field of cells at F340/380; the second image shows response of the same group of cells after the application of 100 µM ADP (at peak of Ca2+ response); changes of fluorescence from dark to light blue indicates increases of intracellular calcium concentrations [Ca2+]i. Post-fixation staining of the same field of cells with NG2 and GPR17 indicates that several NG2+ and GPR17+ cells responded to ADP (see cell highlighted with circle as an example). On the right, representative trace of Ca2+ increases recorded from pre-oligodendrocytes after application of ADP. Adapted from [84].
The first in vivo evidence that, upon release during axonal action potential, ATP evokes rapid and transient Ca2+ rises in single OPCs was attested by confocal calcium imaging on optic nerves from a transgenic reporter mouse line in which adult NG2-glia were identified by the expression of the red fluorescent protein DsRed [85]. The predominant mechanism was the activation of metabotropic P2Y1R, because response was abolished by the pre-incubation with the P2Y1R antagonist MRS2179, but also the P2X7R induced significant calcium increases [85]. The Ca2+ signals increased in both amplitude and duration with increasing stimulus strength, indicating that responses in these cells matched with axonal activity; however, it was clearly demonstrated that ATP is not only released by axons during action potentials, but also comes from astrocytes, in response to axonal activity through vesicles, reverse transport, hemichannels and the P2X7R [86]. The close connections between OPCs and astrocytes was demonstrated by direct enwrapment of short segments of astroglial processes, thus enabling the local passage of Ca2+ signals from astrocytes to OPCs and vice versa [85]. These results strongly favour the view of a close interdependence among OPCs, astrocytes and neurons in synaptic transmission [87]. It has been proposed that P2Y1Rs may have a major role in the physiological signalling of OPCs, as it is activated by nanomolar concentrations of ATP in optic nerve glia, whereas P2X7R becomes activated when extracellular ATP is in the micromolar range [73], i.e. under pathological conditions. Thus, low levels of ATP would activate a transient self-limiting P2Y1R-mediated release of Ca2+, whereas at higher levels of signalling, Ca2+ influx through P2X7 would prolong the ATP-evoked Ca2+ signals for hundreds of seconds [85]. However, this fascinating hypothesis has not been demonstrated yet.
During neuron-to-glia signalling, P2Y1Rs are also responsible for reduced expression of the calcium-permeable AMPA receptors (CP-AMPARs), which play important roles in OPC proliferation, migration, differentiation and neuron–glia signalling [88,89]. This has led to the hypothesis that, under physiological conditions, ATP primes OPCs for differentiation into myelinating cells by both reducing the expression of CP-AMPARs and promoting differentiation via activation of adenosine receptors [90]. On the other hand, CP-AMPARs also make these cells susceptible to ischaemic damage, with important implications for cell survival [91]. While P2Y1R activation and consequent release of Ca2+ from intracellular stores induced rectification of the glutamate I-V receptors, suggesting that P2Y1R may promote OPC survival under stress or ischaemia/hypoxia conditions, this has not yet been proved unequivocally.
Intracellular calcium transients elicited by several purinergic agonists have been also recorded in OPC cultures demonstrating the in vitro functionality of P2Y1, P2Y2, and P2Y6Rs ([84]; figure 2b). The P2Y-like receptor GPR17, activated by UDP, UDP-sugars and cysteinyl-leukotrienes [18,92], is expressed at an intermediate stage of OPC differentiation. While GPR17's molecular mechanism of action in oligodendrocyte physiology is still unclear and probably involves both modulation of cAMP levels and of K+ conductance [93], its stimulation promotes the transition from early OPCs to intermediate oligodendrocytes [84,93–95]. Conversely, at later differentiation stages, GPR17 plays an inhibitory role and has to be downregulated to allow cells' terminal differentiation [95,96]. Although GPR17 is primarily coupled to a Gi protein, in transfected systems Ca2+ fluxes via Gq were also reported using the endogenous ligands UDP-glucose and LTD4 [18] and the synthetic non-specific agonist, MDL29,951 [97]. However, whether and how Ca2+ signalling contributes to the pathophysiological roles of GPR17 in native systems still remain to be established. Single cell calcium imaging showed that adenosine receptors also contribute to the robust Ca2+ responses induced by electrical stimulations of axons in both OPCs and immature oligodendrocytes, suggesting that intracellular calcium dynamics may underlie the positive effects of adenosine on OPC maturation and myelination [74]. These data reveal that not only ATP, but also the whole purinergic system is involved in calcium signalling, with different and synergic contributions to OPC proliferation, differentiation and migration.
(b). Adenosine triphosphate and calcium in oligodendrocyte death
As mentioned above, ATP-gated P2XR channels equal NMDA receptors in their calcium permeability [98]. In OPCs, P2X7R seems to be the only functionally active P2X receptor subtype [73], but its physiological role is still elusive [99]. Instead, the role of P2X7Rs in pathological conditions has been well described. P2X7R overactivation opens the non-selective channel pannexin1 (Panx1), which becomes permeable to ATP, allowing massive calcium entry into cells, thus contributing to cell death [100]. OPC cultures exhibit high sensitivity to oxygen–glucose deprivation (OGD) toxicity, which can be attenuated by Brilliant Blue G (BBG), a P2X7R-preferring antagonist [101]. In SCI, high levels of extracellular ATP in spinal cord resulted in massive cell death, whereas the P2X7R antagonist oxidized ATP (oATP) significantly diminished death of OPCs in both grey and white matter [102].
Of relevance, during neuroinflammatory diseases such as multiple sclerosis, activated immune system cells, astrocytes, dead oligodendrocytes and neurons release high amounts of ATP and other nucleotides, which may evoke excitotoxic degeneration of cells [77]. Panx1-mediated ATP release contributes to development of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis; Panx1 knockout (KO) mice, in which ATP release was found to be decreased and P2X7R upregulated in spinal cord of chronic EAE phase (probably owing to a compensatory mechanism), displayed a delayed onset of EAE clinical signs and decreased mortality compared to WT animals [103].
All these data, obtained in several models of disease, suggest that P2X7R contributes to damage exacerbation; however, it has also been reported that the expression of P2X7R was downregulated in OPC cultures and in ischaemic cerebral cortex, subcortical white matter and hippocampus in a paradigm of perinatal hypoxia-ischaemia, probably in an attempt to prevent the excessive receptor stimulation in the early stages of damage [99].
As previously mentioned, during injury, OPCs can also migrate to the lesion site following an ATP gradient to promote repair; although it has not been demonstrated in vivo, P2X7R stimulation in vitro showed that this receptor can indeed mediate chemotaxis of OPCs via receptor coupling to Fyn kinase [104].
In recent years, adult OPCs have also been implicated in glioma formation. According to the recent proposed ‘cancer stem cell’ hypothesis, mutated OPCs have been found to be responsible for the growth of the tumour and its resistance to therapies [105–107]. Of relevance in this context, recent data pointed to intracellular Ca2+ as a key positive regulator of tumourigenesis in glioma, through influence on processes involved in the quiescence, maintenance, proliferation or migration of adult OPCs [106]. Moreover, several data obtained in the C6 glioma cell line showed that purinergic signalling participates in the development and progression of glioma through the release of ATP. P2X7R, P2Y1R, P2Y12R and A2B activation by ATP, ADP and adenosine result in increased production of chemokines, augmented cell proliferation and tumour invasiveness (for review, see [108]). In line, purinergic-dependent calcium signalling has been highlighted to be crucial for the behaviour of transformed glioma cells [109]. Understanding whether and how the manipulation of purinergic-dependent Ca2+ events in mutant OPCs could affect the transformation of these cells into gliomas would greatly help the set-up of novel antitumoural strategies.
Importantly, mitochondria are also involved in OPC Ca2+ homeostasis (figure 2a). In basal conditions, mitochondrial calcium is generally maintained at low concentrations, but these organelles can take up and store high Ca2+ concentrations. Of note, a proper function of mitochondria has been reported to be required for correct OPC maturation and myelination [110,111]. In vitro evidence has shown that cytokine-induced OPC injury involves mitochondrial dysfunction [112,113], while inhibition of OPC differentiation by tumour necrosis factor alpha (TNF-α) is accompanied by alterations in mitochondrial calcium uptake, mitochondrial membrane potential and respiratory complex I activity [113]. However, an evaluation of the purinergic contribution to mitochondrial calcium transport in OPCs has not been defined yet.
4. Parenchymal astrocytes
Astrocytes residing in both brain and spinal cord parenchyma have long been recognized to play key roles in the regulation of neural network activity through modulation of extracellular transmitter levels and ion homeostasis and by directly participating in synaptic activity in the now called ‘tripartite synapsis’ [114]. Notably, these astrocytes do express a wide panel of both P2X and P2YRs, most of which are linked to mobilization of intracellular calcium levels [115]. It has also been known for a quite long time that as a result of injury parenchymal astrocytes become activated, a phenomenon characterized by increased expression of GFAP and marked enlargement of astrocyte cell body and fibres (reactive astrogliosis) [116,117].
For many years, this phenomenon has been viewed as both a positive and a detrimental event for CNS recovery. On one side, reactive astrocytes were recognized to be responsible for the formation of the glial scar, which physically separates the damaged tissue from the surviving one, thus impeding axonal regeneration; on the other side, reactive astrogliosis was soon shown to be important for functional recovery owing to release of beneficial cytokines and growth factors and other trophic actions favouring tissue remodelling and repair in the lesioned area (reviewed in [118]).
However, a seminal breakthrough in reactive astrogliosis was made when evidence was provided that, after injury, reactive astrocytes re-acquire properties similar to those typical of the astrocyte-like cells found in the adult V-SVZ (see §1; [114] and references therein) and in spinal cord central canal [40]. In these niches, astrocyte-like cells indeed behave as progenitor/stem-like cells that, under some circumstances, are able to differentiate to all the three main types of CNS cells, i.e. adult quiescent astrocytes, neurons and oligodendrocytes (see §1). This behaviour is, in turn, reminiscent of that of radial glia, the ubiquitous glial cell type during development, also acting as stem and progenitor cells, which are the source of many, if not most, neurons in vertebrate brains (see §1).
The concept that, among other features, reactive astrocytes re-acquire ‘stemcellness’ was strongly suggested by work performed in mice subjected to either brain trauma or ischaemia owing to middle cerebral artery occlusion [119]. Specifically, it was demonstrated that, when placed in vitro, only reactive astrocytes from the ischaemic side of the brain could generate neurospheres characterized by both self-maintenance and multipotency, as shown by their ability to proliferate and generate astrocytes, neurons and oligodendrocytes under specific differentiation protocols. Astrocytes obtained from the contralateral healthy hemisphere of the same animals were not able to generate neurospheres, to confirm that it is only after injury that astrocytes dedifferentiate to multipotent precursor cells re-expressing stem cell properties that are normally repressed in the adult brain under healthy conditions. In a similar way, upon injury, ECs lining spinal cord central canal were found to be activated, started proliferating and expressing GFAP, suggesting a shift to astrocyte-like progenitor cells [40]. Unfortunately, as already pointed out in §1, generation of new neurons from injury-reactivated adult stem-like cells is strongly hampered by the local unfavourable environment typical of the inflamed CNS, which prevents these cells from fully expressing their regenerative properties. However, very recent evidence suggests that parenchymal reactive astrocytes may be more prone to express these properties compared with V-SVZ precursors, because, following ischaemic brain injury, some striatal astrocytes were indeed shown to transdifferentiate into functional mature neurons [120]. Obviously, elucidation of the molecular pathways involved in the transition from quiescent to reactive astrocytes would greatly help the set-up of novel neuroreparative strategies.
In this respect, comparison between signals orchestrating the shift of normal parenchymal astrocytes to reactive cells after injury and signals that are active in the endogenous NSC niches have revealed a striking degree of overlap, as shown by involvement of the same molecules (summarized in figure 3). Normally, adult astrocytes do not divide and are polarized towards, and in contact with, the basement membrane (BM) surrounding blood vessels (figure 3a). Leakage of the blood–brain barrier as a result of injury disrupts signalling from the BM to astrocytes, leading to loss of polarization, upregulation of intermediate filaments (IMFs) and hypertrophy. Blood-borne fibrinogen induces secretion of chondroitin sulfate proteoglycans (CSPGs), which activates transforming growth factor beta (TGF-β) signalling (figure 3b). In addition to this, owing to damage, a number of peptide growth factors, such as fibroblast growth factor (FGF), epidermal growth factor (EGF) and transforming growth factor alpha (TGF-α) are released in the extracellular milieu to induce astrocytic proliferation via ERK1/2 and the mitogen-activated protein kinase (MAPK) or the mammalian target of rapamycin (mTOR) pathway. Purinergic signals are one of the first and most potently activated after injury, as ATP is immediately released by damaged cells (for a review, see [121]) and may also interact with growth factor signalling in this context [116,117] to phosphorylate the immediate early gene products c-FOS and c-JUN and increase astrocyte proliferation and GFAP expression (figure 3c). These same factors, as well as vascular endothelial growth factor (VEGF) and sonic hedgehog (SHH), are also active in the adult neurogenic niches in vivo, where they promote proliferation of neural stem and progenitor cells (figure 3d). However, while in neurogenic niches the proliferating effects of ATP have been univocally related to P2 receptor-mediated elevation of cytosolic Ca2+ concentrations in a synergistic manner with mitogenic growth factors ([30]; see also §1), the involvement of intracellular calcium in the effects mediated by ATP in adult reactive astrocytes is far less defined.
In early studies, exposure of cultured rat cortical astrocytes to ATP and the ATP derivative α,β-methyleneATP resulted in significant elongation of GFAP-positive astrocytic processes, suggesting induction of reactive astrogliosis [122]. In line with the concepts discussed above, this was preceded (and probably mediated) by a very early ERK1/2 activation in the absence of any apparent mobilization of intracellular calcium levels, suggesting this effect to be independent of calcium signalling. Data with a number of P2 receptor antagonists and with pertussis toxin, a G-protein inhibitor, suggested this effect to be mediated by a P2YR subtype that is at variance with the majority of known P2YRs [123] did not seem to be linked to increases of intracellular calcium levels. To investigate in more detail the role of calcium in α,β-methyleneATP-mediated reactive astrogliosis, the intracellular calcium chelator BAPTA-AM was used. BAPTA-AM induced a marked reduction of basal ERK1/2 phosphorylation compared to cultures maintained in standard medium, indicating that basal ERK1/2 phosphorylation in control unstimulated astrocytes is owing to both calcium-dependent and -independent mechanisms. Nevertheless, challenge of cultures with α,β-methyleneATP in the presence of BAPTA-AM resulted in a 7.9-fold stimulation of ERK1/2 phosphorylation that was very similar to that obtained in standard calcium-containing medium. On this basis, authors concluded that the purine analogue could activate intracellular pathways leading to reactive astrogliosis even in the absence of calcium [122]. However, it has to be said that, since then, detection of calcium signalling has undergone significant technical improvements and that lack of sufficiently sensitive methods at those times has prevented the measurement of small, but biologically significant, early astrocytic calcium increases contributing to induction of reactive astrogliosis. In this respect, using transgenic mice that express an ultrasensitive genetically encoded Ca2+ indicator, YC-Nano50, in an astrocyte-specific manner, Kanemaru and co-workers [124] have recently reported the in vivo visualization of spontaneous subtle and localized astrocytic Ca2+ signals (Ca2+ twinkles), which are preferentially displayed in fine astrocytic processes in living mice brain.
In addition to this, the same authors have shown that astrocytic calcium signalling and the downstream function of N-cadherin, a calcium-dependent cell–cell adhesion glycoprotein, play indispensable roles in the reactive response to a neocortical stab wound injury (SWI) and, highly relevant to this review, they have linked these mechanisms to ATP signalling [124]. Specifically, they first demonstrated that brain injury induces inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ signalling in astrocytes and that this is required for SWI-associated astrogliosis. Moreover, type2 IP3 receptor knockout (IP3R2KO) mice, deficient in astrocytic Ca2+ signalling, showed impaired reactive astrogliosis and increased injury-associated neuronal death. Next, they explored the mechanisms of Ca2+-dependent reactive astrogliosis by comparing the gene expression profiles of Ca2+ signal-silent and -active astrocytes in culture and found that Ca2+ signal-active astrocytes have very low levels of the translational repressor pumilio 2 (Pum2). They then reasoned that proteins whose translation is regulated by Pum2 are expected to be Ca2+-dependently upregulated during astrogliosis and found that N-cadherin was indeed markedly increased in reactive astrocytes. In previous studies, N-cadherin and its family proteins were initially characterized by their function in cell–cell adhesion, but were subsequently found also to regulate other cellular functions, including differentiation, proliferation, cell polarization and migration, which are likely to be at the basis of the detected reactive astrogliosis [124]. Interestingly, these authors also found that the Ca2+-mobilizing agonists ATP and ET1 induced downregulation of Pum2 and concomitantly upregulated N-cadherin in cultured astrocytes, thus linking astrocytic calcium mobilization by purinergic signalling to reactive astrogliosis. These findings are consistent with previous studies demonstrating that N-cadherin expression was increased when astrocytes were subjected to rapid and reversible mechanical strain and that this effect was reproduced in a time- and concentration-dependent manner by treatment of cultures with ATP [125]. Globally, these data support ATP as an initiation signal in reactive astrogliosis and suggest that mobilization of intracellular calcium by classical IP3-linked P2Y receptors plays a major role in mediating the transition of quiescent to activated astrocytes.
Acknowledgement
Authors thank Dr Marta Boccazzi for useful discussion.
Authors' contributions
All authors equally contributed to this manuscript.
Competing interests
The authors have no competing interests.
Funding
The work has been supported by Fondazione Italiana Sclerosi Multipla grant no. 2013/R/1 and ERA-NET ‘Network of European Funding for Neuroscience Research’ (NEURON) project ‘RENEW-IT’, NEURON to M.P.A.
References
- 1.Burnstock G, Verkhratsky A. 2010. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 1, e9 ( 10.1038/cddis.2009.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. 2016. Short- and long-term (trophic) purinergic signalling. Phil. Trans. R. Soc. B 371, 20150422 ( 10.1098/rstb.2015.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neary JT, Zimmermann H. 2009. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 32, 189–198. ( 10.1016/j.tins.2009.01.002) [DOI] [PubMed] [Google Scholar]
- 4.Ulrich H, Abbracchio MP, Burnstock G. 2012. Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev. 8, 755–767. ( 10.1007/s12015-012-9372-9) [DOI] [PubMed] [Google Scholar]
- 5.Donegà M, Giusto E, Cossetti C, Pluchino S. 2013. Systemic neural stem cell-based therapeutic interventions for inflammatory CNS disorders. In Neural stem cells—new perspectives (ed. Bonfanti L.). InTech; Available from http://www.intechopen.com/books/neural-stem-cells-new-perspectives/systemic-neural-stem-cell-based-therapeutic-interventions-for-inflammatory-cns-disorders . (http://www.intechopen.com/books/neural-stem-cells-new-perspectives/systemic-neural-stem-cell-based-therapeutic-interventions-for-inflammatory-cns-disorders 10.5772/55426. ( doi:10.5772/55426 ) [DOI] [Google Scholar]
- 6.Bond AM, Ming GL, Song H. 2015. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 17, 385–395. ( 10.1016/j.stem.2015.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716. ( 10.1016/S0092-8674(00)80783-7) [DOI] [PubMed] [Google Scholar]
- 8.Curtis MA, et al. 2007. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315, 1243–1249. ( 10.1126/science.1136281) [DOI] [PubMed] [Google Scholar]
- 9.Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. 2014. Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083. ( 10.1016/j.cell.2014.01.044) [DOI] [PubMed] [Google Scholar]
- 10.Falcao AM, Marques F, Novais A, Sousa N, Palha JA, Sousa JC. 2012. The path from the choroid plexus to the subventricular zone: go with the flow! Front. Cell. Neurosci. 6, 34 ( 10.3389/fncel.2012.00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. 2008. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288. ( 10.1016/j.stem.2008.07.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. 2006. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26, 7907–7918. ( 10.1523/JNEUROSCI.1299-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohn J, Orosco L, Guo F, Chung SH, Bannerman P, Mills Ko E, Zarbalis K, Deng W, Pleasure D. 2015. The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J. Neurosci. 35, 3756–3763. ( 10.1523/JNEUROSCI.3454-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorelli R, Azim K, Fischer B, Raineteau O. 2015. Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis. Development 142, 2109–2120. ( 10.1242/dev.119966) [DOI] [PubMed] [Google Scholar]
- 15.Nicola Z, Fabel K, Kempermann G. 2015. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 9, 53 ( 10.3389/fnana.2015.00053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J. 2005. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J. Neurosci. 25, 10 366–10 368. ( 10.1523/JNEUROSCI.3452-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marschallinger J, et al. 2015. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 6, 8466 ( 10.1038/ncomms9466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciana P, et al. 2006. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25, 4615–4627. ( 10.1038/sj.emboj.7601341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. 2010. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur. J. Neurosci. 31, 1533–1548. ( 10.1111/j.1460-9568.2010.07220.x) [DOI] [PubMed] [Google Scholar]
- 20.Striedinger K, Meda P, Scemes E. 2007. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia 55, 652–662. ( 10.1002/glia.20494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser T, Resende RR, Ulrich H. 2013. Implications of purinergic receptor-mediated intracellular calcium transients in neural differentiation. Cell Commun. Signal. 11, 12 ( 10.1186/1478-811X-11-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scemes E, Duval N, Meda P. 2003. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J. Neurosci. 23, 11 444–11 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavaliere F, Donno C, D'Ambrosi N. 2015. Purinergic signaling: a common pathway for neural and mesenchymal stem cell maintenance and differentiation. Front. Cell. Neurosci. 9, 211 ( 10.3389/fncel.2015.00211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. 2003. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur. J. Neurosci. 17, 1355–1364. ( 10.1046/j.1460-9568.2003.02567.x) [DOI] [PubMed] [Google Scholar]
- 25.Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sevigny J, Robson SC, Braun N. 2005. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J. Neurosci. Res. 80, 600–610. ( 10.1002/jnr.20508) [DOI] [PubMed] [Google Scholar]
- 26.Gampe K, et al. 2015. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells 33, 253–264. ( 10.1002/stem.1846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stafford MR, Bartlett PF, Adams DJ. 2007. Purinergic receptor activation inhibits mitogen-stimulated proliferation in primary neurospheres from the adult mouse subventricular zone. Mol. Cell. Neurosci. 35, 535–548. ( 10.1016/j.mcn.2007.04.013) [DOI] [PubMed] [Google Scholar]
- 28.Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H. 2009. Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J. Cell Sci. 122, 2524–2533. ( 10.1242/jcs.044891) [DOI] [PubMed] [Google Scholar]
- 29.Grimm I, Ullsperger SN, Zimmermann H. 2010. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol. 199, 181–189. ( 10.1111/j.1748-1716.2010.02092.x) [DOI] [PubMed] [Google Scholar]
- 30.Mishra SK, et al. 2006. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development 133, 675–684. ( 10.1242/dev.02233) [DOI] [PubMed] [Google Scholar]
- 31.Boccazzi M, Rolando C, Abbracchio MP, Buffo A, Ceruti S. 2014. Purines regulate adult brain subventricular zone cell functions: contribution of reactive astrocytes. Glia 62, 428–439. ( 10.1002/glia.22614) [DOI] [PubMed] [Google Scholar]
- 32.Suyama S, Sunabori T, Kanki H, Sawamoto K, Gachet C, Koizumi S, Okano H. 2012. Purinergic signaling promotes proliferation of adult mouse subventricular zone cells. J. Neurosci. 32, 9238–9247. ( 10.1523/JNEUROSCI.4001-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. 2007. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev. Biol. 302, 356–366. ( 10.1016/j.ydbio.2006.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messemer N, et al. 2013. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology 73, 122–137. ( 10.1016/j.neuropharm.2013.05.017) [DOI] [PubMed] [Google Scholar]
- 35.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, Kanellopoulos JM. 2009. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J. Neurochem. 109, 846–857. ( 10.1111/j.1471-4159.2009.06008.x) [DOI] [PubMed] [Google Scholar]
- 36.Cao X, et al. 2013. Astrocytic adenosine 5′-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 31, 1633–1643. ( 10.1002/stem.1408) [DOI] [PubMed] [Google Scholar]
- 37.Addington CP, Roussas A, Dutta D, Stabenfeldt SE. 2015. Endogenous repair signaling after brain injury and complementary bioengineering approaches to enhance neural regeneration. Biomark. Insights 10, 43–60. ( 10.4137/BMI.S20062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. 2008. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6, e182 ( 10.1371/journal.pbio.0060182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Villafuertes R, Rodriguez-Jimenez FJ, Alastrue-Agudo A, Stojkovic M, Miras-Portugal MT, Moreno-Manzano V. 2015. Purinergic receptors in spinal cord-derived ependymal stem/progenitor cells and their potential role in cell-based therapy for spinal cord injury. Cell Transplant. 24, 1493–1509. ( 10.3727/096368914X682828) [DOI] [PubMed] [Google Scholar]
- 40.Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, Bramanti P, Cuzzocrea S, Abbracchio MP. 2009. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain 132, 2206–2218. ( 10.1093/brain/awp147) [DOI] [PubMed] [Google Scholar]
- 41.Boccazzi M, Lecca D, Marangon D, Guagnini F, Abbracchio MP, Ceruti S. A new role for the P2Y-like GPR17 receptor in the generation of neurons from oligodendrocyte precursor cells in vitro. Purinergic Signalling. Submitted. [DOI] [PMC free article] [PubMed]
- 42.Moreno-Manzano V, et al. 2009. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 27, 733–743. ( 10.1002/stem.24) [DOI] [PubMed] [Google Scholar]
- 43.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. 2010. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482. ( 10.1016/j.stem.2010.07.014) [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama A, Komitova M, Suzuki R, Zhu X. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10, 9–22. ( 10.1038/nrn2495) [DOI] [PubMed] [Google Scholar]
- 45.Dawson MR, Polito A, Levine JM, Reynolds R. 2003. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24, 476–488. ( 10.1016/S1044-7431(03)00210-0) [DOI] [PubMed] [Google Scholar]
- 46.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. 2010. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681. ( 10.1016/j.neuron.2010.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. 2013. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. ( 10.1016/j.neuron.2013.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zawadzka M, et al. 2010. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6, 578–590. ( 10.1016/j.stem.2010.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Chopp M, Zhang ZG. 2013. Oligodendrogenesis after cerebral ischemia. Front. Cell. Neurosci. 7, 201 ( 10.3389/fncel.2013.00201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McTigue DM, Wei P, Stokes BT. 2001. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 21, 3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boda E, Buffo A. 2014. Beyond cell replacement: unresolved roles of NG2-expressing progenitors. Front. Neurosci. 8, 122 ( 10.3389/fnins.2014.00122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Biase LM, Nishiyama A, Bergles DE. 2010. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 30, 3600–3611. ( 10.1523/JNEUROSCI.6000-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martino G, Pluchino S. 2006. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 7, 395–406. ( 10.1038/nrn1908) [DOI] [PubMed] [Google Scholar]
- 54.Butti E, et al. 2012. Subventricular zone neural progenitors protect striatal neurons from glutamatergic excitotoxicity. Brain 135, 3320–3335. ( 10.1093/brain/aws194) [DOI] [PubMed] [Google Scholar]
- 55.Pluchino S, et al. 2005. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436, 266–271. ( 10.1038/nature03889) [DOI] [PubMed] [Google Scholar]
- 56.Borges K, Ohlemeyer C, Trotter J, Kettenmann H. 1994. AMPA/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience 63, 135–149. ( 10.1016/0306-4522(94)90012-4) [DOI] [PubMed] [Google Scholar]
- 57.Takeda M, Nelson DJ, Soliven B. 1995. Calcium signaling in cultured rat oligodendrocytes. Glia 14, 225–236. ( 10.1002/glia.440140308) [DOI] [PubMed] [Google Scholar]
- 58.Simpson PB, Armstrong RC. 1999. Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia 26, 22–35. ( 10.1002/(SICI)1098-1136(199903)26:1%3C22::AID-GLIA3%3E3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 59.Yoo AS, Krieger C, Kim SU. 1999. Process extension and intracellular Ca2+ in cultured murine oligodendrocytes. Brain Res. 827, 19–27. ( 10.1016/S0006-8993(99)01282-2) [DOI] [PubMed] [Google Scholar]
- 60.Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW, Macklin WB, Colwell C, Campagnoni AT. 2009. Golli myelin basic proteins regulate oligodendroglial progenitor cell migration through voltage-gated Ca2+ influx. J. Neurosci. 29, 6663–6676. ( 10.1523/JNEUROSCI.5806-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao L, et al. 2013. NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration. Glia 61, 2078–2099. ( 10.1002/glia.22578) [DOI] [PubMed] [Google Scholar]
- 62.Harlow DE, Saul KE, Komuro H, Macklin WB. 2015. Myelin proteolipid protein complexes with αv integrin and AMPA receptors in vivo and regulates AMPA-dependent oligodendrocyte progenitor cell migration through the modulation of cell-surface GluR2 expression. J. Neurosci. 35, 12 018–12 032. ( 10.1523/JNEUROSCI.5151-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soliven B. 2001. Calcium signalling in cells of oligodendroglial lineage. Microsc. Res. Tech. 52, 672–679. ( 10.1002/jemt.1051) [DOI] [PubMed] [Google Scholar]
- 64.Paez PM, Cheli VT, Ghiani CA, Spreuer V, Handley VW, Campagnoni AT. 2012. Golli myelin basic proteins stimulate oligodendrocyte progenitor cell proliferation and differentiation in remyelinating adult mouse brain. Glia 60, 1078–1093. ( 10.1002/glia.22336) [DOI] [PubMed] [Google Scholar]
- 65.Cheli VT, Santiago Gonzalez DA, Spreuer V, Paez PM. 2015. Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp. Neurol. 265, 69–83. ( 10.1016/j.expneurol.2014.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boscia F, et al. 2012. Silencing or knocking out the Na+/Ca2+ exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 19, 562–572. ( 10.1038/cdd.2011.125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamins JA, Nedelkoska L. 1996. Release of intracellular calcium stores leads to retraction of membrane sheets and cell death in mature mouse oligodendrocytes. Neurochem. Res. 21, 471–479. ( 10.1007/BF02527712) [DOI] [PubMed] [Google Scholar]
- 68.Fumagalli M, Lecca D, Abbracchio MP. 2015. CNS remyelination as a novel reparative approach to neurodegenerative diseases: the roles of purinergic signaling and the P2Y-like receptor GPR17. Neuropharmacology 104, 82–93. ( 10.1016/j.neuropharm.2015.10.005) [DOI] [PubMed] [Google Scholar]
- 69.Fields RD, Stevens B. 2000. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 23, 625–633. ( 10.1016/S0166-2236(00)01674-X) [DOI] [PubMed] [Google Scholar]
- 70.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. 2006. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832. ( 10.1016/j.neuron.2006.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fumagalli M, Lecca D, Abbracchio MP. 2011. Role of purinergic signalling in neuro-immune cells and adult neural progenitors. Front. Biosci. 16, 2326–2341. ( 10.2741/3856) [DOI] [PubMed] [Google Scholar]
- 72.Lecca D, Ceruti S, Fumagalli M, Abbracchio MP. 2012. Purinergic trophic signalling in glial cells: functional effects and modulation of cell proliferation, differentiation, and death. Purinergic Signal. 8, 539–557. ( 10.1007/s11302-012-9310-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonte C, Aloisi F, Visentin S. 2005. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia 50, 132–144. ( 10.1002/glia.20160) [DOI] [PubMed] [Google Scholar]
- 74.Stevens B, Porta S, Haak LL, Gallo V, Fields RD. 2002. Adenosine: a neuron–glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868. ( 10.1016/S0896-6273(02)01067-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coppi E, Cellai L, Maraula G, Pugliese AM, Pedata F. 2013. Adenosine A(2)A receptors inhibit delayed rectifier potassium currents and cell differentiation in primary purified oligodendrocyte cultures. Neuropharmacology 73, 301–310. ( 10.1016/j.neuropharm.2013.05.035) [DOI] [PubMed] [Google Scholar]
- 76.Coppi E, Cellai L, Maraula G, Dettori I, Melani A, Pugliese AM, Pedata F. 2015. Role of adenosine in oligodendrocyte precursor maturation. Front. Cell. Neurosci. 9, 155 ( 10.3389/fncel.2015.00155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matute C, et al. 2007. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 27, 9525–9533. ( 10.1523/JNEUROSCI.0579-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kastritsis CH, McCarthy KD. 1993. Oligodendroglial lineage cells express neuroligand receptors. Glia 8, 106–113. ( 10.1002/glia.440080206) [DOI] [PubMed] [Google Scholar]
- 79.Kirchhoff F, Kettenmann H. 1992. GABA triggers a [Ca2+]i increase in murine precursor cells of the oligodendrocyte lineage. Eur. J. Neurosci. 4, 1049–1058. ( 10.1111/j.1460-9568.1992.tb00131.x) [DOI] [PubMed] [Google Scholar]
- 80.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. 1994. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron 12, 357–371. ( 10.1016/0896-6273(94)90277-1) [DOI] [PubMed] [Google Scholar]
- 81.Belachew S, Malgrange B, Rigo JM, Rogister B, Leprince P, Hans G, Nguyen L, Moonen G. 2000. Glycine triggers an intracellular calcium influx in oligodendrocyte progenitor cells which is mediated by the activation of both the ionotropic glycine receptor and Na+-dependent transporters. Eur. J. Neurosci. 12, 1924–1930. ( 10.1046/j.1460-9568.2000.00085.x) [DOI] [PubMed] [Google Scholar]
- 82.Deitmer JW, Verkhratsky AJ, Lohr C. 1998. Calcium signalling in glial cells. Cell Calcium 24, 405–416. ( 10.1016/S0143-4160(98)90063-X) [DOI] [PubMed] [Google Scholar]
- 83.Simpson PB, Mehotra S, Lange GD, Russell JT. 1997. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J. Biol. Chem. 272, 22 654–22 661. ( 10.1074/jbc.272.36.22654) [DOI] [PubMed] [Google Scholar]
- 84.Fumagalli M, et al. 2011. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J. Biol. Chem. 286, 10 593–10 604. ( 10.1074/jbc.M110.162867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamilton N, Vayro S, Wigley R, Butt AM. 2010. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 58, 66–79. ( 10.1002/glia.20902) [DOI] [PubMed] [Google Scholar]
- 86.Parpura V, Scemes E, Spray DC. 2004. Mechanisms of glutamate release from astrocytes: gap junction ‘hemichannels’, purinergic receptors and exocytotic release. Neurochem. Int. 45, 259–264. ( 10.1016/j.neuint.2003.12.011) [DOI] [PubMed] [Google Scholar]
- 87.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. 2005. Synantocytes: the fifth element. J. Anat. 207, 695–706. ( 10.1111/j.1469-7580.2005.00458.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gudz TI, Komuro H, Macklin WB. 2006. Glutamate stimulates oligodendrocyte progenitor migration mediated via an αv integrin/myelin proteolipid protein complex. J. Neurosci. 26, 2458–2466. ( 10.1523/JNEUROSCI.4054-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bergles DE, Roberts JD, Somogyi P, Jahr CE. 2000. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. ( 10.1038/35012083) [DOI] [PubMed] [Google Scholar]
- 90.Zonouzi M, Renzi M, Farrant M, Cull-Candy SG. 2011. Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nat. Neurosci. 14, 1430–1438. ( 10.1038/nn.2942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fern R, Moller T. 2000. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J. Neurosci. 20, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lecca D, et al. 2008. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE 3, e3579 ( 10.1371/journal.pone.0003579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coppi E, et al. 2013. UDP-glucose enhances outward K+ currents necessary for cell differentiation and stimulates cell migration by activating the GPR17 receptor in oligodendrocyte precursors. Glia 61, 1155–1171. ( 10.1002/glia.22506) [DOI] [PubMed] [Google Scholar]
- 94.Daniele S, Trincavelli ML, Fumagalli M, Zappelli E, Lecca D, Bonfanti E, Campiglia P, Abbracchio MP, Martini C. 2014. Does GRK-beta arrestin machinery work as a ‘switch on’ for GPR17-mediated activation of intracellular signaling pathways? Cell. Signal. 26, 1310–1325. ( 10.1016/j.cellsig.2014.02.016) [DOI] [PubMed] [Google Scholar]
- 95.Fumagalli M, Bonfanti E, Daniele S, Lecca D, Martini C, Trincavelli ML, Abbracchio MP. 2015. The ubiquitin ligase Mdm2 controls oligodendrocyte maturation by intertwining mTOR with G protein-coupled receptor kinase 2 in the regulation of GPR17 receptor desensitization. Glia 12, 2327–2339. ( 10.1002/glia.22896) [DOI] [PubMed] [Google Scholar]
- 96.Chen Y, et al. 2009. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12, 1398–1406. ( 10.1038/nn.2410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hennen S, et al. 2013. Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci. Signal. 6, ra93. ( 10.1126/scisignal.2004350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.North RA. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067. ( 10.1152/physrev.00015.2002) [DOI] [PubMed] [Google Scholar]
- 99.Wang LY, Cai WQ, Chen PH, Deng QY, Zhao CM. 2009. Downregulation of P2X7 receptor expression in rat oligodendrocyte precursor cells after hypoxia ischemia. Glia 57, 307–319. ( 10.1002/glia.20758) [DOI] [PubMed] [Google Scholar]
- 100.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. 2008. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295, C752–C760. ( 10.1152/ajpcell.00228.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. 2010. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 58, 730–740. ( 10.1002/glia.20958) [DOI] [PubMed] [Google Scholar]
- 102.Wang X, et al. 2004. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 10, 821–827. ( 10.1038/nm1082) [DOI] [PubMed] [Google Scholar]
- 103.Lutz SE, et al. 2013. Contribution of pannexin1 to experimental autoimmune encephalomyelitis. PLoS ONE 8, e66657 ( 10.1371/journal.pone.0066657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng JF, Gao XF, Pu YY, Burnstock G, Xiang Z, He C. 2015. P2X7 receptors and Fyn kinase mediate ATP-induced oligodendrocyte progenitor cell migration. Purinergic Signal. 11, 361–369. ( 10.1007/s11302-015-9458-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu C, et al. 2011. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146, 209–221. ( 10.1016/j.cell.2011.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leclerc C, et al. 2016. Calcium signaling orchestrates glioblastoma development: facts and conjunctures. Biochim. Biophys. Acta 1863, 1447–1459. ( 10.1016/j.bbamcr.2016.01.018) [DOI] [PubMed] [Google Scholar]
- 107.Zong H, Verhaak RG, Canoll P. 2012. The cellular origin for malignant glioma and prospects for clinical advancements. Expert Rev. Mol. Diagn. 12, 383–394. ( 10.1586/erm.12.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Puchalowicz K, Tarnowski M, Baranowska-Bosiacka I, Chlubek D, Dziedziejko V. 2014. P2X and P2Y receptors—role in the pathophysiology of the nervous system. Int. J. Mol. Sci. 15, 23 672–23 704. ( 10.3390/ijms151223672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wypych D, Pomorski P. 2013. Calcium signaling in glioma cells—the role of nucleotide receptors. Adv. Exp. Med. Biol. 986, 61–79. ( 10.1007/978-94-007-4719-7_4) [DOI] [PubMed] [Google Scholar]
- 110.Schoenfeld R, Wong A, Silva J, Li M, Itoh A, Horiuchi M, Itoh T, Pleasure D, Cortopassi G. 2010. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 10, 143–150. ( 10.1016/j.mito.2009.12.141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. 2011. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 31, 538–548. ( 10.1523/JNEUROSCI.3516-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benarroch EE. 2009. Oligodendrocytes: susceptibility to injury and involvement in neurologic disease. Neurology 72, 1779–1785. ( 10.1212/WNL.0b013e3181a6b123) [DOI] [PubMed] [Google Scholar]
- 113.Bonora M, et al. 2014. Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 21, 1198–1208. ( 10.1038/cdd.2014.35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robel S, Berninger B, Gotz M. 2011. The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 12, 88–104. ( 10.1038/nrn2978) [DOI] [PubMed] [Google Scholar]
- 115.Fumagalli M, Brambilla R, D'Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. 2003. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43, 218 ( 10.1002/glia.10248) [DOI] [PubMed] [Google Scholar]
- 116.Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. 1994. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience 59, 67–76. ( 10.1016/0306-4522(94)90099-X) [DOI] [PubMed] [Google Scholar]
- 117.Abbracchio MP, Ceruti S, Langfelder R, Cattabeni F, Saffrey MJ, Burnstock G. 1995. Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int. J. Dev. Neurosci. 13, 685–693. ( 10.1016/0736-5748(95)00064-X) [DOI] [PubMed] [Google Scholar]
- 118.Brambilla R, Abbracchio MP. 2001. Modulation of cyclooxygenase-2 and brain reactive astrogliosis by purinergic P2 receptors. Ann. N Y Acad. Sci. 939, 54–62. ( 10.1111/j.1749-6632.2001.tb03612.x) [DOI] [PubMed] [Google Scholar]
- 119.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. 2008. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl Acad. Sci. USA 105, 3581–3586. ( 10.1073/pnas.0709002105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duan CL, Liu CW, Shen SW, Yu Z, Mo JL, Chen XH, Sun FY. 2015. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia 63, 1660–1670. ( 10.1002/glia.22837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. 2009. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 32, 79–87. ( 10.1016/j.tins.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 122.Brambilla R, Neary JT, Cattabeni F, Cottini L, D'Ippolito G, Schiller PC, Abbracchio MP. 2002. Induction of COX-2 and reactive gliosis by P2Y receptors in rat cortical astrocytes is dependent on ERK1/2 but independent of calcium signalling. J. Neurochem. 83, 1285–1296. ( 10.1046/j.1471-4159.2002.01239.x) [DOI] [PubMed] [Google Scholar]
- 123.Abbracchio MP, et al. 2006. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341. ( 10.1124/pr.58.3.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M. 2013. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc. Natl Acad. Sci. USA 110, 11 612–11 617. ( 10.1073/pnas.1300378110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tran MD, Wanner IB, Neary JT. 2008. Purinergic receptor signaling regulates N-cadherin expression in primary astrocyte cultures. J. Neurochem. 105, 272–286. ( 10.1111/j.1471-4159.2008.05214.x) [DOI] [PubMed] [Google Scholar]