Abstract
Infection tolerance is the ability of a host to limit the health effects of a given parasite load. A few recent studies have demonstrated genetic variation for tolerance, but little is known about how environmental factors affect tolerance. Here, we used the intestinal nematode Heligmosomoides polygyrus in laboratory mice to test for effects of protein malnutrition on tolerance. We performed an experiment where two different mouse strains (CBA and BALB/c) were fed either adequate-protein food or low-protein food, and trickle-infected with different doses of H. polygyrus larvae during four weeks. We found that protein malnutrition decreases tolerance measured as intestinal barrier function, but only in one of the strains (BALB/c); that is, there was a host genotype-by-environment interaction for tolerance. We conclude that nutritional status can affect tolerance and that sensitivity of tolerance to malnutrition may differ between host genotypes.
Keywords: helminth, intestinal permeability, resistance, tolerance
1. Introduction
Most organisms are hosts to a wide diversity of parasites, from pathogenic microorganisms such as viruses and bacteria to macroparasites like helminths. The health and fitness effects of an infection with a given type of parasite often vary considerably among host individuals. This variation can be a result of differences among hosts in resistance and/or tolerance to infection. Resistance and tolerance represent two fundamentally different aspects of host defence; resistance is defined as the ability to prevent infection and control parasite replication, while tolerance is the ability to limit the health effects of a given parasite load [1,2].
Causes of variation in resistance are well understood and include both genetic and various environmental factors like nutritional status. In particular, protein malnutrition is known to compromise resistance [3]; infection experiments with a variety of parasites, from viruses to helminths, have shown that malnourished individuals have weaker immune responses and higher parasite loads [4,5]. The causes of variation in tolerance are less well known. A few recent studies have demonstrated genetic variation for tolerance [6–8]. Environmental effects on tolerance have been demonstrated in a few invertebrate host–parasite systems [9], but there are, as far as we are aware, no explicit analyses of the effect of nutritional status or other environmental factors on tolerance to parasites in vertebrates.
The intestinal nematode Heligmosomoides polygyrus in laboratory mice is a common model for human and livestock helminth infections [10]. In laboratory mice, H. polygyrus infection may lead to impaired growth and occasionally mortality [11,12]. Strains like C3H or CBA maintain high parasite loads for more than 20 weeks, whereas loads are lower and parasites more rapidly cleared in, for example, SWR and BALB/c mice [13,14], indicating genetic variation for resistance. Protein malnutrition compromises Th2-mediated immunity to H. polygyrus, and thereby reduces resistance [5].
To test for effects of protein malnutrition on tolerance, we performed an experiment where two different mouse strains with contrasting levels of resistance (CBA and BALB/c) were fed either adequate-protein food or low-protein food, and were trickle-infected (to mimic the natural infection process) with different doses of H. polygyrus larvae during four weeks. We focused on the effect of protein malnutrition because this type of environmental stress is known to be an important factor affecting resistance to pathogens [3], including H. polygyrus [5]. The full-factorial set-up allowed us to test for effects of nutritional status and host genotype on both resistance and tolerance. Resistance is typically measured as the inverse of parasite load after a given challenge [15]. We used number of adult worms in the small intestine at the end of the experiment as an indication of host resistance, where higher load means lower resistance. Tolerance is best measured as the slope of a regression of fitness (or more commonly some proxy, e.g. a measure of health or infection-induced damage) against parasite load, where a steeper slope means lower tolerance [15]. We measured the effect of infection on host health/fitness in two ways: (i) relative weight change during the experiment, (ii) intestinal permeability to macromolecules (as an indication of tissue damage at the site of infection [16]; increased permeability impairs nutrient absorption and can lead to inflammatory disease [17,18]). Our aim was to test if nutritional status affected the slope of the relationship between each of these health measures and parasite load.
2. Material and methods
The experiment was performed with CBA/Ca and BALB/cN (Charles River Europe) mice. We used L3 larvae of H. polygyrus, obtained from University of Burgundy, Dijon, France, and maintained in C57 BL/6 mice at our facility. Animals were allowed to acclimatize to feeding conditions one week before the start of the experiment. All mice were male and 6–7 weeks old at the beginning of the experiment. We used males because they are normally less resistant [14]; this should make it easier to detect any effects of infection. We used relatively young mice to be able to study the effects of infection on growth.
We conducted the experiment in a balanced, fully factorial set-up with two strains (CBA and BALB/c) × 2 diets × 3 parasite doses (five mice in each group). The different treatments were as follows. Diet: half of all animals were fed ad libitum with low-protein food (6% protein; similar to [11]), whereas the other half got standard mouse feed with 18.5% protein (Lantmännen, Kimstad, Sweden). Parasite dose: on days 1, 7, 14 and 21 mice were given 0, 50 or 200 infective L3s in 0.1 ml distilled and deionized H2O by oral gavage.
The experiment was terminated on day 28. We used absorption of FITC-dextran 4000 (FD4, 4 kDa; TdB, Uppsala, Sweden) as a measure of intestinal permeability. Mice were fed a solution containing 0.2 mg FD4 per gram of bodyweight. Three hours later, animals were anaesthetized by isoflurane inhalation, whereafter blood was collected via direct heart puncture into syringes containing 1.5 mg EDTA. Plasma was obtained by blood centrifugation at 3000g for 15 min at +4°C and stored at −20°C until analysis. Concentrations of FD4 passed into the blood circulation were measured spectrophotometrically in plasma. The oral gavage of marker solution failed on two individuals (uptake from respiratory tract as indicated by unphysiologically high levels of markers in the blood in 1 BALB/c given low protein and 200 L3 week−1 and 1 BALB/c given normal food and 50 L3 week−1), which means there are missing values for intestinal permeability.
To quantify parasite loads, small intestines were opened longitudinally and the number of adult worms was counted. Growth was measured as relative weight change between days 0 and 28 [(weight d28 − weight d0)/weight d0]. Relative weight change and FD4 concentrations were analysed by general linear models (proc glm in SAS 9.3; SAS Inc., Cary, NC, USA), while worm loads were analysed by generalized linear models (proc genmod) with negative binomial distribution. In analyses of effects of diet and parasite dose on worm load, relative weight change and FD4 concentration we included diet, parasite dose and mouse strain, and their two- and three-way interactions as fixed effects. In analyses of tolerance, we included diet, mouse strain, worm load and their two- and three-way interactions as fixed effects. Non-significant effects were eliminated in a step-wise manner at p > 0.05 (interactions first).
3. Results
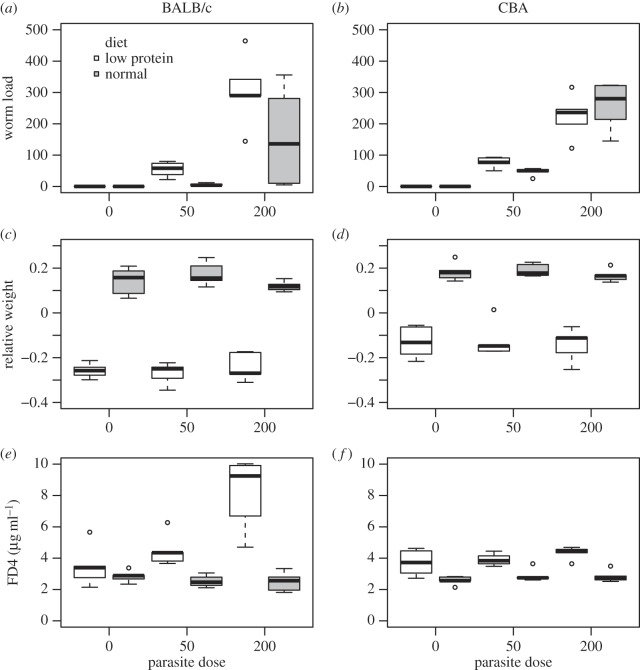
The counts of adult worms in infected mice were dependent on the combination of strain and diet (figure 1a,b; strain × diet: F1,35 = 4.40, p = 0.036; uninfected controls were not included in this analysis but are indicated in figure 1a,b), while controlling for parasite dose (F1,35 = 39.2, p < 0.0001). Post hoc tests showed that there was an effect of diet on resistance in BALB/c mice (Tukey: p = 0.009), but not in CBA mice (p = 0.84).
Figure 1.
Effects of diet (low-protein food or normal food) and parasite dose (0, 50 or 200 L3 week−1) on worm load, relative weight change and intestinal permeability (absorption of FD4) at day 28 post-infection (pi) in BALB/c and CBA mice. The box plots indicate the median, first and third quartiles and range of the data. (a) Number of adult H. polygyrus worms in BALB/c (a) and CBA mice (b). Relative weight change day 0–28 in BALB/c (c) and CBA mice (d). FD4 concentration in BALB/c (e) and CBA mice (f).
There was no significant effect of parasite dose on relative weight change between day 0 and day 28 pi (figure 1c,d; F2,54 = 0.53, p = 0.59). Relative weight change was, however, dependent on the combination of strain and diet (strain × diet: F1,56 = 11.0, p = 0.016); both strains were affected by diet but the effect of low-protein food was more pronounced in BALB/c mice (figure 1c,d).
The concentration of FD4 was influenced by a three-way interaction between strain, diet and parasite dose (figure 1e,f; F2,46 = 8.86, p = 0.0006). Post hoc comparisons between all subgroups showed that the significant three-way interaction was mainly a result of BALB/c mice fed low protein and 200 L3 week−1 having higher FD4 concentration than all other groups (Tukey, p < 0.0001; figure 1e).
The observed pathologic effect of H. polygyrus infection in this experiment was increased intestinal permeability (figure 1e,f). To test for effects of diet and mouse genotype on tolerance, we therefore focused on this trait. There was an overall positive correlation between FD4 concentration and worm load (F1,50 = 39.0, p < 0.0001) and a significant three-way interaction between mouse strain, diet and worm load (F1,50 = 6.30, p = 0.015; figure 2). This interaction was due to FD4 concentration increasing with worm load in BALB/c mice fed low-protein diet, but not in other groups.
Figure 2.
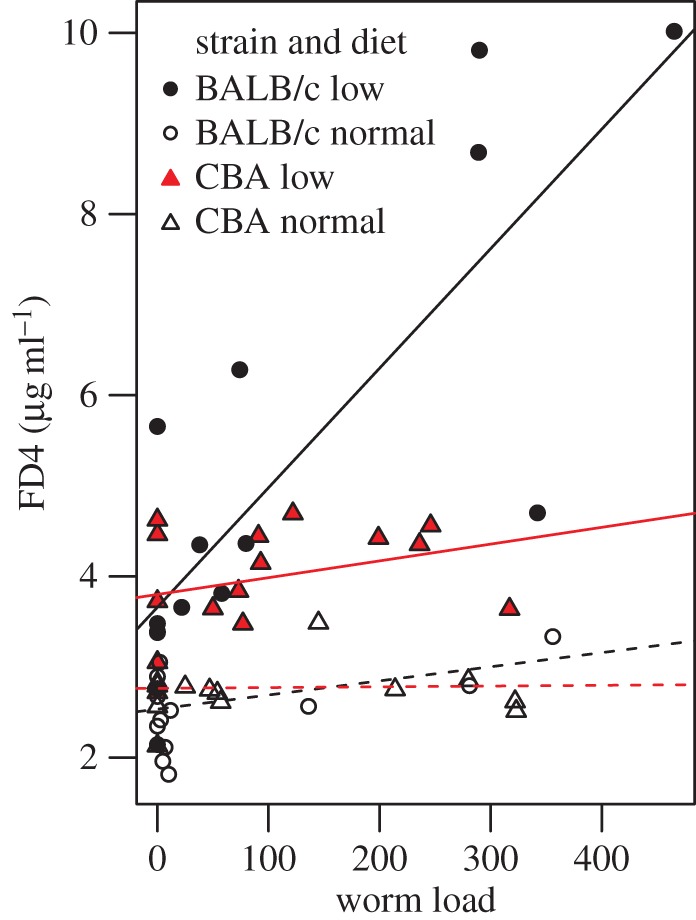
Intestinal permeability (as measured by FD4 concentration in plasma) against worm load in BALB/c and CBA mice fed either normal or low-protein diet.
4. Discussion
We found that mice vary in tolerance to H. polygyrus as a result of interactive effects of host genotype and diet. Specifically, worm load had a stronger effect on intestinal permeability in mice fed a low-protein diet than mice fed normal food, but only in one of two strains (BALB/c but not CBA mice). Previous studies of tolerance have focused on genetic variation. The present study shows that environmental factors like nutritional status can also contribute to phenotypic variation in tolerance, and that sensitivity of tolerance to malnutrition may differ between host genotypes (i.e. a host genotype-by-environment interaction for tolerance).
Increased intestinal permeability to macromolecules is most likely a result of changes in tight junction function, leading to paracellular leakage. This damage could be a result of H. polygyrus feeding on host tissue [19], or host responses to infection mediated by IL-4 and mast cells [20]. Impaired barrier function may affect nutrient absorption. Moreover, permeability to macromolecules may initiate inflammatory responses because of leakage of microbial or food antigens. For instance, in humans, increased intestinal permeability is thought to be a cause of the inflammatory bowel diseases (IBD) Crohn's disease and ulcerative colitis [18]. Thus, maintaining intestinal barrier function despite high worm loads is clearly a potentially important aspect of tolerance to helminth infection.
BALB/c mice showed reduced tolerance of H. polygyrus infection when fed low-protein food, whereas the tolerance of CBA mice was unaffected by diet. The growth of BALB/c mice was also more sensitive to low-protein diet than that of CBA (figure 1c,d); apparently, CBA mice were better able to use resources from the low-protein diet. Thus, in this system, the ability to extract resources from protein-poor food seems to enhance tolerance to an intestinal parasite. This pattern contrasts with a recent selection experiment with fruit flies, where populations of Drosophila melanogaster adapted to larval malnutrition had lower tolerance to an opportunistic intestinal bacterium than control flies maintained on normal food [21]. This effect was mediated by higher risk of loss of intestinal integrity upon infection in flies adapted to malnutrition, indicating a trade-off between the ability of the gut to extract nutrients and withstand infection-induced damage in this system, rather than a positive association as in our study.
Acknowledgements
We thank Camilla Björklöv and Agnieszka Czopek for help with animal maintenance. We are grateful to Gabrielle Sorci and Cédric Lippens for providing Heligmosomoides polygyrus larvae.
Ethics
The study was approved by the Malmö/Lund ethical board for animal experiments (M141-14).
Data accessibility
All supporting data are available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.r540f.
Authors' contributions
D.C., O.P. and L.R. designed and performed the experiment. D.C. and O.P. conducted laboratory analyses. L.R. analysed the data. D.C., O.P. and L.R. wrote the manuscript. All authors approved the final version of the manuscript, and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
The study was funded by a grant from Department of Biology, Lund University (120711).
References
- 1.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936–941. ( 10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Råberg L. 2014. How to live with the enemy: understanding tolerance to parasites. PLoS Biol. 12, e1001989 ( 10.1371/journal.pbio.1001989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaible UE, Kaufmann SHE. 2007. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 4, e115 ( 10.1371/journal.pmed.0040115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor AK, Cao W, Vora KP, De La Cruz J, Shieh W-J, Zaki SR, Katz JM, Sambhara S, Gangappa S. 2013. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J. Infect. Dis. 207, 501–510. ( 10.1093/infdis/jis527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ing R, Su Z, Scott ME, Koski KG. 2000. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proc. Natl Acad. Sci. USA 97, 7078–7083. ( 10.1073/pnas.97.13.7078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Råberg L, Sim D, Read AF. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814. ( 10.1126/science.1148526) [DOI] [PubMed] [Google Scholar]
- 7.Mazé-Guilmo E, Loot G, Páez DJ, Lefèvre T, Blanchet S, Maze E. 2014. Heritable variation in host tolerance and resistance inferred from a wild host–parasite system. Proc. R. Soc. B 281, 20132567 ( 10.1098/rspb.2013.2567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regoes R, et al. 2014. Disentangling human tolerance and resistance against HIV. PLoS Biol. 12, e1001951 ( 10.1371/journal.pbio.1001951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sternberg ED, Lefèvre T, Li J, de Castillejo CLF, Li H, Hunter MD, de Roode JC. 2012. Food plant-derived disease tolerance and resistance in a natural butterfly-plant-parasite interactions. Evolution 66, 3367–3376. ( 10.1111/j.1558-5646.2012.01693.x) [DOI] [PubMed] [Google Scholar]
- 10.Behnke JM, Menge DM, Noyes H. 2009. Heligmosomoides bakeri: a model for exploring the biology and genetics of resistance to chronic gastrointestinal nematode infections. Parasitology 136, 1565–1580. ( 10.1017/S0031182009006003) [DOI] [PubMed] [Google Scholar]
- 11.Coltherd JC, Babayan SA, Bünger L, Kyriazakis I, Allen JE, Houdijk JGM. 2011. Interactive effects of protein nutrition, genetic growth potential and Heligmosomoides bakeri infection pressure on resilience and resistance in mice. Parasitology 138, 1305–1315. ( 10.1017/S0031182011000990) [DOI] [PubMed] [Google Scholar]
- 12.Keymer AE, Hiorns RW. 1986. Heligmosomoides polygyrus (Nematoda): the dynamics of primary and repeated infection in outbred mice. Proc. R. Soc. Lond. B 229, 47–67. ( 10.1073/pnas.97.13.7078) [DOI] [PubMed] [Google Scholar]
- 13.Behnke JM, Mugambi JM, Clifford S, Iraqi FA, Baker RL, Gibson JP, Wakelin D. 2006. Genetic variation in resistance to repeated infections with Heligmosomoides polygyrus bakeri, in inbred mouse strains selected for the mouse genome project. Parasite Immunol. 28, 85–94. ( 10.1111/j.1365-3024.2005.00810.x) [DOI] [PubMed] [Google Scholar]
- 14.Reynolds LA, Filbey KJ, Maizels RM. 2012. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin. Immunopathol. 34, 829–846. ( 10.1007/s00281-012-0347-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Råberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49. ( 10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Llorente C, Hartmann P, Yang A-M, Chen P, Schnabl B. 2015. Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 421, 44–53. ( 10.1016/j.jim.2014.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JR. 2009. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. ( 10.1038/nri2653) [DOI] [PubMed] [Google Scholar]
- 18.Nalle SC, Turner JR. 2015. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 8, 720–730. ( 10.1038/mi.2015.40) [DOI] [PubMed] [Google Scholar]
- 19.Bansemir AD, Sukhdeo MVK. 1994. The food resource of adult Heligmosomoides polygyrus in the small intestine. J. Parasitol. 80, 24–28. ( 10.2307/3283340) [DOI] [PubMed] [Google Scholar]
- 20.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF. 2001. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J. Immunol. 167, 2234–2239. ( 10.4049/jimmunol.167.4.2234) [DOI] [PubMed] [Google Scholar]
- 21.Vijendravarma RK, Narasimha S, Chakrabarti S, Babin A, Kolly S, Lemaitre B, Kawecki TJ. 2015. Gut physiology mediates a trade-off between adaptation to malnutrition and susceptibility to food-borne pathogens. Ecol. Lett. 18, 1078–1086. ( 10.1111/ele.12490) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data are available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.r540f.