Abstract
Coordinated landing requires anticipating the timing and magnitude of impact, which in turn requires sensory input. To better understand how cane toads, well known for coordinated landing, prioritize visual versus vestibular feedback during hopping, we recorded forelimb joint angle patterns and electromyographic data from five animals hopping under two conditions that were designed to force animals to land with one forelimb well before the other. In one condition, landing asymmetry was due to mid-air rolling, created by an unstable takeoff surface. In this condition, visual, vestibular and proprioceptive information could be used to predict asymmetric landing. In the other, animals took off normally, but landed asymmetrically because of a sloped landing surface. In this condition, sensory feedback provided conflicting information, and only visual feedback could appropriately predict the asymmetrical landing. During the roll treatment, when all sensory feedback could be used to predict an asymmetrical landing, pre-landing forelimb muscle activity and movement began earlier in the limb that landed first. However, no such asymmetries in forelimb preparation were apparent during hops onto sloped landings when only visual information could be used to predict landing asymmetry. These data suggest that toads prioritize vestibular or proprioceptive information over visual feedback to coordinate landing.
Keywords: Bufo marinus, landing, control, sensory feedback, asynchronous
1. Introduction
Coordinated landing involves the proper positioning of limbs and the development of appropriate levels of underlying limb muscle forces prior to impact. Such limb positioning and muscle force production depend on anticipating when and how hard one is going to hit the ground [1]. While anticipating and coordinating landing in a number of mammals involves complex sensory integration [2–4], it is less clear how sensory feedback is prioritized and integrated during landing preparation in the vertebrate lineage perhaps best known for jumping: anurans (frogs and toads). Here we present a study using a simple apparatus to perturb landing conditions in cane toads (Bufo marinus) to better understand how they prioritize sensory feedback to prepare for landing. In this study, we emphasize visual versus vestibular feedback, but acknowledge that proprioceptive feedback from the hindlimbs is also potentially of critical importance.
Cane toads are well known for coordinated landings [5], and two hallmarks of their in-air preparation are (i) large degrees of humeral protraction and elbow extension [6] and (ii) pre-landing electromyographic (EMG) activity in elbow extensors (m. anconeus heads; [7]) to brace for impact. Studies of anuran landing to date have used stable and level takeoff and landing surfaces during jumping trials, which typically lead to landings in which both forelimbs make ground contact nearly simultaneously at impact. To investigate roles of vestibular versus visual feedback for landing preparation, we designed experiments that forced toads to land with one forelimb well before the other. In one condition (roll treatment), asymmetry at impact was the result of long-axis rotation (roll) in mid-air (figure 1a), during which visual and vestibular feedback could be used to appropriately predict an asymmetrical landing. In the other condition (slope treatment), toads hopped off a level platform onto a sloped landing surface such that visual feedback provided conflicting information from vestibular feedback about landing conditions (figure 1a).
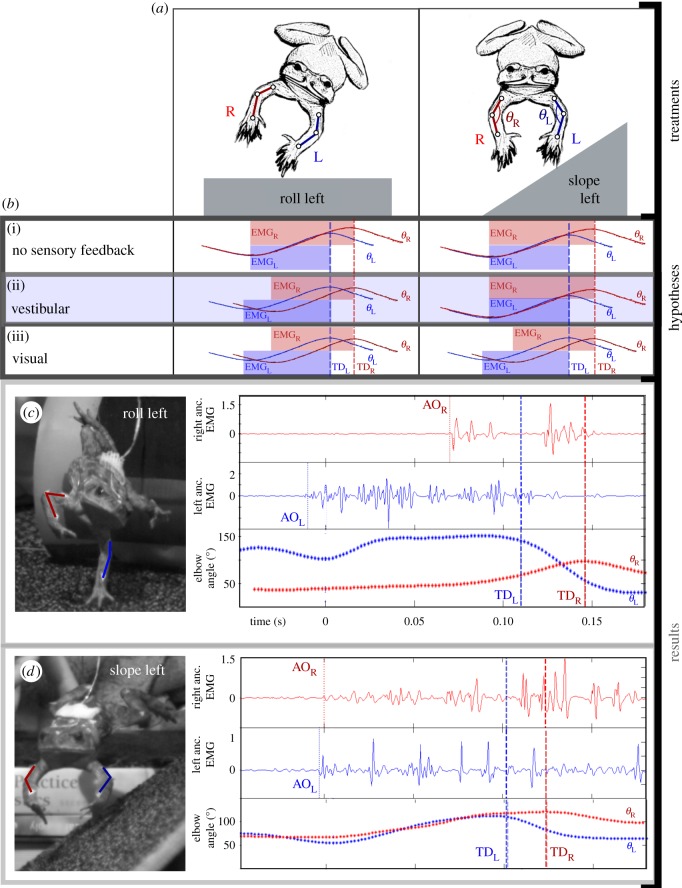
Figure 1.
Experimental set-up, hypotheses and results. (a) Treatments to produce asynchronous landings. Throughout figure, right forelimb data are depicted in red, left in blue. (b) Graphical depiction of hypotheses. Red and blue solid lines represent elbow angle over time (increases = extension; decreases = flexion). Red and blue boxes designate the hypothetical onset and duration of pre-landing EMG activity in m. anconeus (anc.) of each limb. Vertical dashed lines designate limb touchdown times. (c,d) Results: images of toads in slope-left and roll-left landings with right and left arms designated by colour. EMG activity and elbow angle, θ, are shown for each limb. In roll-left treatment (c), note the asymmetries in EMG onset timing and elbow extension kinematics, with the left limb preparing for impact earlier than the right limb. (d) In slope treatments, EMG timing and elbow kinematics parallel one another between forelimbs despite the earlier left forelimb touchdown. This pair of results is only consistent with the vestibular control of landing hypothesis (shaded blue box). AO, m. anconeus onset; TD, forelimb touchdown time.
We measured forelimb kinematics and pre-landing m. anconeus EMG activity bilaterally as toads prepared for landing under both conditions. Our null hypothesis was that toads use no sensory feedback to prepare for impact, and thus exhibit bilaterally similar forelimb movements and muscle activity patterns during hopping, regardless of landing condition (figure 1bi). If instead sensory feedback is used, we predict that asymmetries in landing preparation should be present, depending upon which arm is anticipated to make ground contact first. Specifically, if vestibular feedback is prioritized over vision under these experimental conditions, we predict landing preparation will begin earlier in the limb to touch down first during trials in which animals roll in mid-air, but not when they takeoff normally and land on sloped surfaces (figure 1bii). By contrast, if visual information is prioritized, or is integrated with vestibular feedback, then we predict left–right asymmetries under both landing conditions (figure 1biii).
2. Material and methods
(a). Animals
Five adult Bufo marinus (44–131 g; mean = 69.3 g) were used for kinematic analysis and EMG recordings. Toads were obtained and housed as described in Cox & Gillis [6].
(b). Electromyography and data collection
The EMG data were collected bilaterally from the m. anconeus, an elbow extensor that is activated consistently before impact [8]. Electrodes were implanted and EMG signals were amplified as in the earlier work [7]. EMG signals were digitized at 5000 Hz using a National Instruments A/D converter (NIcDAQ-9178).
Forelimbs were marked bilaterally as described in Cox & Gillis [6]. Animals were placed in a rectangular glass tank (89 × 43 × 43 cm) and hopped in two conditions: (i) roll treatments involved unstable takeoffs achieved by hopping toads off a weighted cylinder (12 cm diameter) that rolled as animals took off and reliably led to long-axis rotation in mid-air (figure 1a and d)—in these trials, animals landed on a flat surface, but because of the roll, forelimbs touched down at different times; (ii) slope treatments involved stable takeoffs off a 6 cm platform onto surfaces angled at 45°, which also led to different forelimb touchdown times (figure 1a,c). Videos of 12–15 hops were recorded at 500 fps and calibrated as described in Cox & Gillis [6] for each toad in each of the two conditions (131 total hops recorded, table 1).
Table 1.
The difference in the forelimb touchdown times, TDL-R, between forelimbs and the duration of elbow extension for left, IpDurL, and right, IpDurR, forelimbs in each condition. All variables are given as means of individual means ± s.d.
| slope left | slope right | roll left | roll right | |
|---|---|---|---|---|
| total hops | 37 | 31 | 28 | 35 |
| TDL-R (ms) | −19 ± 17 | 17 ± 12 | −13 ± 8 | 15 ± 17 |
| IpDurL (ms) | 79 ± 30 | 75 ± 16 | 110 ± 28 | 79 ± 39 |
| IpDurR (ms) | 73 ± 36 | 74 ± 15 | 71 ± 32 | 101 ± 32 |
(c). Data analysis
Videos were analysed to identify the time of landing of each forelimb. Elbow kinematics were analysed bilaterally as in Cox & Gillis [6]. EMG data were analysed as described in Schnyer et al. [9]. Differences between left and right forelimbs in touchdown times, TDL-R, m. anconeus onset timing, AOL-R, and intensity, AIL-R, were calculated by subtracting the value for the right limb from that of the left. The duration of impact preparation for each limb, IpDur, was defined as the time between touchdown of the first limb and the time when elbow extension began in each limb (figure 1c,d).
ANOVAs with individual ID as a factor were performed to test for a linear relationship with a non-zero slope between the onset and intensity of pre-landing EMG activity and onset timing and duration of elbow extension across limbs, within slope and roll treatments. Differences in pre-landing EMG intensity, AIL-R, and onset time between forelimbs, AOL-R, for each individual were regressed against differences in forelimb touchdown times, TDL-R, for hops in roll conditions.
3. Results
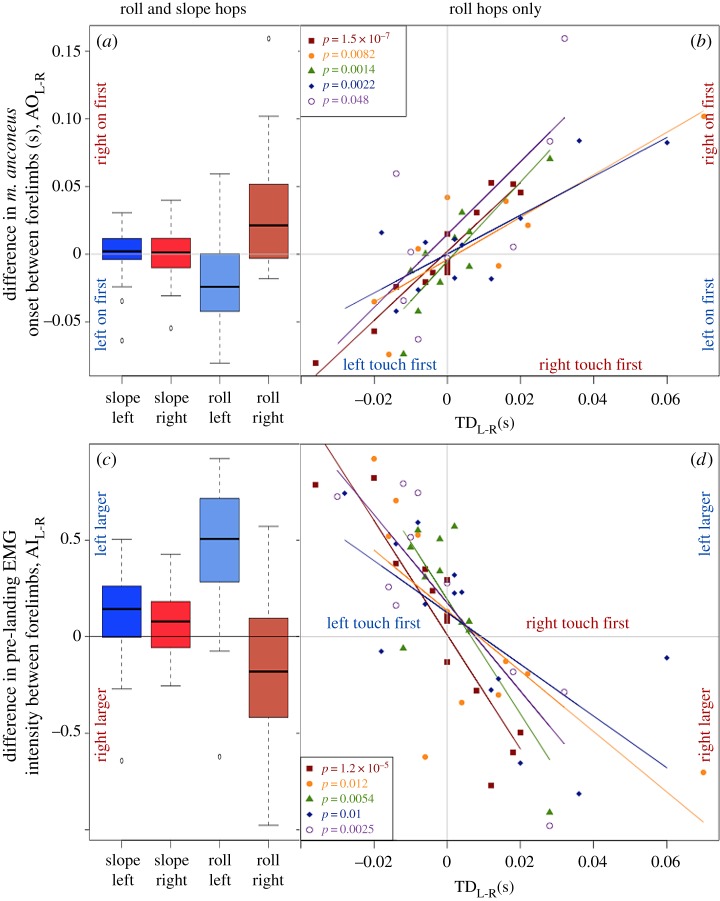
We found that toads prepared appropriately for asymmetrical landings during roll, but not slope, treatments. For example, when animals rolled in mid-air, pre-landing EMG activity timing and intensity in m. anconeus depended upon which forelimb hit the ground first (figure 1d). Specifically, pre-landing EMG activity began earlier (p = 7.9 × 10−6; figure 2a), continued for a longer duration (p = 7.9 × 10−6; table 1) and was more intense (p < 8.1 × 10−6; figure 2c) in the forelimb that touched down first. Furthermore, for every animal, the intensity of pre-landing EMG activity, as well as the time difference between the EMG onsets in the left versus right forelimb, increased linearly with the difference between forelimb touchdown times (figure 2b,d). In other words, the leading limb's m. anconeus activity began earlier and was more intense, relative to the trailing limb, the more asymmetrical the landing. But there was no significant difference between forelimbs in onset timing (p = 0.98; figure 2a), duration (p = 0.98; table 1) or intensity of EMG activity (p = 0.67; figure 2b) during slope treatments.
Figure 2.
(a) Difference in m. anconeus onset, AOL-R, between forelimbs for each treatment. (b) For roll hops, the difference in m. anconeus onset between forelimbs, AOL-R, versus time difference between forelimb touchdown (TDL-R) colour coded by animal. (c) Difference in normalized pre-landing EMG activity between forelimbs, AIL-R. (d) For roll hops, the difference in normalized pre-landing EMG activity between forelimbs, AIL-R, versus the time difference between forelimb touchdown, TDL-R, colour coded by animal. Linear regression lines for each animal are included for significant relationships with non-zero slopes.
Differences in elbow kinematics during landing preparation paralleled those found for EMG activity. In the slope treatment, when vision alone could predict asymmetrical forelimb touchdown times, there was no significant difference in the onset timing (p = 0.67; figure 1c) or duration (p = 0.67; table 1) of elbow extension between left and right forelimbs. By contrast, during roll hops toads began to extend the elbow of the leading forelimb significantly earlier than the trailing forelimb (p = 1.1 × 10−6; table 1 and figure 1d).
4. Discussion
By manipulating hopping conditions and forcing toads to land with one arm before the other, we have demonstrated that toads use sensory feedback in preparation for certain kinds of asymmetrical landings. Toads exhibit left–right forelimb asymmetries during roll treatments when vestibular and visual information (and potentially hindlimb proprioception) could be useful for predicting the asymmetry, but not during slope treatments, when visual feedback alone could be used to predict landing conditions. These results suggest that toads do not prioritize visual information during landing.
This apparent neglect of visual information might appear surprising given that toads have excellent visual systems that they rely on to plan routes [10] and locate prey [11–13] and as visual feedback seems critical for smoothly navigating uneven terrain. Indeed, vision is used in a variety of animals to modulate limb kinematics and mechanics when adjusting to running over uneven ground [14,15] or stepping onto a sloped or raised surface [16]. Given this, we propose several questions that open directions for future work.
First, are our results generalizable? Our study probed toad landing preparation under a very narrow set of conditions. Are there other experimental set-ups—involving greater visual contrast or larger features—in which toads would use visual cues to modulate landing preparation? Or are toads using a sensory conflict mechanism [17] that might prioritize vision under other conditions? Second, future work could explore why toads might not rely on visual feedback for landing. Anurans typically cover their eyes with a nictitating membrane at the onset of a jump, and little is known about whether this might obscure vision. Perhaps toads rely less on vision to coordinate landing because it can be unreliable. Lastly, what limitations are there on landing without vision? Humans, monkeys and cats are able to tune landing preparation to drop height in the absence of visual information, but only when landing conditions are predictable [1]. While toads do not appear to use visual information during in-air rolling to make predictions about the relative timing and magnitude of impact between forelimbs, it is difficult to understand how, in the absence of vision, they could accurately predict the absolute time of forelimb impact, especially when navigating variable landing environments.
Thus, toads appear to use a landing strategy that relies on predictable conditions and vestibular and/or proprioceptive rather than visual feedback to prepare for impacts at landing.
Acknowledgement
Thanks to Ariela Schnyer and Sarah Crocker for their assistance in data collection.
Ethics
All experiments were approved by Mount Holyoke College's IACUC committee under project BR-47-1210.
Data accessibility
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.qt48g.
Authors' contributions
S.M.C. designed the experiment and acquired the data. Both authors participated in analysing the data and the design and writing of this manuscript. Both authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by National Science Foundation (NSF) grant 1051603 to G.B.G.
References
- 1.Santello M. 2005. Review of motor control mechanisms underlying impact absorption from falls. Gait Posture 21, 85–94. ( 10.1016/j.gaitpost.2004.01.005) [DOI] [PubMed] [Google Scholar]
- 2.Keller EL, Precht W. 1979. Visual-vestibular responses in vestibular nuclear neurons in the intact and cerebellectomized, alert cat. Neuroscience 4, 1599–1613. ( 10.1016/0306-4522(79)90023-X) [DOI] [PubMed] [Google Scholar]
- 3.Vidal PP, Lacour M, Berthoz A. 1979. Contribution of vision to muscle responses in monkey during free-fall: visual stabilization decreases vestibular-dependent responses. Exp. Brain Res. 37, 241–252. ( 10.1007/BF00237711) [DOI] [PubMed] [Google Scholar]
- 4.Santello M, McDonagh MJ, Challis JH. 2001. Visual and non-visual control of landing movements in humans. J. Physiol. 537, 313–327. ( 10.1111/j.1469-7793.2001.0313k.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillis G, Ekstrom L, Azizi E. 2013. Biomechanics and control of landing in toads. Integr. Comp. Biol. 54, 1136–1147. ( 10.1093/icb/icu053) [DOI] [PubMed] [Google Scholar]
- 6.Cox SM, Gillis GB. 2015. Forelimb kinematics during hopping and landing in toads. J. Exp. Biol. 218, 3051–3058. ( 10.1242/jeb.125385) [DOI] [PubMed] [Google Scholar]
- 7.Akella T, Gillis GB. 2011. Hopping isn't always about the legs: forelimb muscle activity patterns during toad locomotion. J. Exp. Zool. A Ecol. Genet. Physiol. 315, 1–11. ( 10.1002/jez.643) [DOI] [PubMed] [Google Scholar]
- 8.Gillis GB, Akella T, Gunaratne R. 2010. Do toads have a jump on how far they hop? Pre-landing activity timing and intensity in forelimb muscles of hopping Bufo marinus. Biol. Lett. 6, 486–489. ( 10.1098/rsbl.2009.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnyer A, Gallardo M, Cox S, Gillis G. 2014. Indirect evidence for elastic energy playing a role in limb recovery during toad hopping. Biol. Lett. 10, 20140418 ( 10.1098/rsbl.2014.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collett S. 1982. Do toads plan routes—a study of the detour behavior of Bufo viridis. J. Comp. Physiol. 146, 261–271. ( 10.1007/BF00610246) [DOI] [Google Scholar]
- 11.Ewert J-P. 2010. Neuroethology of releasing mechanisms: prey-catching in toads. Behav. Brain Sci. 10, 337 ( 10.1017/S0140525X00023128) [DOI] [Google Scholar]
- 12.Robins A, Rogers L. 2006. Complementary and lateralized forms of processing in Bufo marinus for novel and familiar prey. Neurobiol. Learn Mem. 86, 214–227. ( 10.1016/j.nlm.2006.03.002) [DOI] [PubMed] [Google Scholar]
- 13.Vallortigara G, Rogers LJ, Bisazza A, Lippolis G, Robins A. 1998. Complementary right and left hemifield use for predatory and agonistic behaviour in toads. Neuroreport 9, 3341–3344. ( 10.1097/00001756-199810050-00035) [DOI] [PubMed] [Google Scholar]
- 14.Müller R, Grimmer S, Blickhan R. 2010. Running on uneven ground: leg adjustments by muscle pre-activation control. Hum. Mov. Sci. 29, 299–310. ( 10.1016/j.humov.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 15.Birn-Jeffery AV, Daley MA. 2012. Birds achieve high robustness in uneven terrain through active control of landing conditions. J. Exp. Biol. 215, 2117–2127. ( 10.1242/jeb.065557) [DOI] [PubMed] [Google Scholar]
- 16.Rossignol S. 1996. Visuomotor regulation of locomotion. Can. J. Physiol. Pharmacol. 74, 418–425. ( 10.1139/y96-041) [DOI] [PubMed] [Google Scholar]
- 17.Brandt T, Glasauer S, Stephan T, Bense S, Yousry TA, Deutschlander A, Dieterich M. 2002. Visual-vestibular and visuovisual cortical interaction: new insights from fMRI and PET. Ann. N Y Acad. Sci. 956, 230–241. ( 10.1111/j.1749-6632.2002.tb02822.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.qt48g.