Abstract
Global synthesis indicates that limitation of plant fecundity by pollen receipt (pollen limitation) is positively related to regional plant diversity and is higher for self-incompatible than self-compatible species. While self-incompatible species are always dependent on pollinating agents, self-compatible species may be pollinator-dependent or autofertile. This should cause variation in pollen limitation among self-compatible species, with lower pollen limitation in autofertile species because they do not depend on pollinators. We hypothesized that the intensity of pollen limitation in self-incompatible compared with pollinator-dependent self-compatible species should depend on whether pollen limitation is determined more by quantity than quality of pollen received. We compared pollen limitation between these three groups using a dataset of 70 biotically pollinated species from biodiverse regions of South Africa. Comparison with a global dataset indicated that pollen limitation in the South African biodiversity hotspots was generally comparable to other regions, despite expectations of higher pollen limitation based on the global plant diversity–pollen limitation relationship. Pollen limitation was lowest for autofertile species, as expected. It was also higher for pollinator-dependent self-compatible species than self-incompatible species, consistent with increased pollen-quality limitation in the former group due to negative consequences of pollinator-mediated self-pollination. However, there was a higher frequency of plants with zygomorphic flowers, which were also more pollen-limited, among pollinator-dependent self-compatible species. Thus, we could not attribute this difference in pollen limitation exclusively to a difference in pollen quality. Nevertheless, our results indicate that comparative studies should control for both pollinator dependence and self-incompatiblity when evaluating effects of other factors on pollen limitation.
Keywords: autofertility, fynbos, plant breeding system, phylogenetic comparative analysis, pollen-quality limitation, pollen supplementation
1. Introduction
Experiments comparing naturally pollinated flowers with those receiving hand cross-pollination (pollen supplementation) infer that reproduction is pollen-limited when pollen supplementation increases fecundity [1]. Pollen limitation arises when stigmas receive inadequate quantity or quality of pollen or both [2]. Despite methodological criticisms [2,3], hundreds of such experiments have now been reported [4]. Comparative analyses based on these studies indicate that pollen limitation is greater for self-incompatible than self-compatible species, for trees than herbs and shrubs, for pollinator-specialized than pollinator-generalized species and increases with regional plant diversity [4–7].
The global pollen limitation–plant diversity relationship suggests that high diversity regions should have above average pollen limitation. Consistent with this is a perception of low pollinator visitation and high pollen limitation in South Africa's Greater Cape Floristic Region (GCFR), arising from anecdotal accounts and data from certain plant groups and sites [8–10]. However, the hypothesis of regionally high pollen limitation in the GCFR has not been tested [11].
Biotically pollinated self-incompatible plants depend on pollinators by definition, while self-compatible ones may be able to produce seeds by self-fertilizing after autonomous self-pollination (autofertile species [12]) or rely on pollinators to effect pollen transfer before self-fertilization can take place (pollinator-dependent species). This suggests that three breeding system classes should be considered in analyses of pollen limitation: (i) autofertile self-compatible (AF), (ii) pollinator-dependent self-compatible (PD-SC) and (iii) pollinator-dependent self-incompatible (SI). We hypothesize that differences in pollen limitation between groups will depend on differences in both pollen-quality limitation and -quantity limitation [2].
As flowers frequently receive mixed pollen loads of self- and cross-pollen [13], self-pollen receipt should be an important source of pollen-quality limitation, although effects should vary between breeding system classes. In SI species, self-pollen is prevented from fertilizing ovules while in self-compatible species, self-fertilized ovules may form seeds less successfully than outcrossed ones due to inbreeding depression. Pollen limitation should be lowest for AF species, which should have low pollen-quantity limitation by definition. Self-fertilization should also result in low pollen-quality limitation in this group on average, as many species will have high rates of self-fertilization, which leads to evolution of reduced inbreeding depression [14].
Under pollen-quantity limitation and mixed pollen loads, SI species should have higher pollen limitation than PD-SC species. This is because only cross-pollen will fertilize ovules in SI species but additional ovules will be fertilized by self-pollen in PD-SC species, increasing fruit and seed set despite any abortion due to inbreeding depression. However, higher pollen limitation in SI than PD-SC species could also be an artefact. If pollinator-mediated self-pollination occurs before the experimenter applies supplementation and self-pollen fertilizes some ovules, inbreeding depression will reduce seed set in the supplementation treatment, reducing estimated pollen limitation in PD-SC species [2]. By contrast, when pollen quantity is not limiting, mixed pollen loads should result in higher pollen limitation in PD-SC than SI species because, in the former, self-pollen may fertilize ovules that could have been fertilized by cross-pollen, reducing seed set via inbreeding depression [2].
We compiled a dataset of pollen limitation from biodiverse regions of South Africa and compared it with a global dataset to test for regionally high pollen limitation. Using the South African dataset, we tested for hypothesized differences in pollen limitation between AF, PD-SC and SI species.
2. Material and methods
Data on pollen limitation of fruit set were obtained by searching published and unpublished literature (see the electronic supplementary material) and Rodger et al. [15] for studies reporting fruit set from natural and supplemental pollination (flowers either cross-pollinated and exposed to natural pollination or cross-pollinated and pollinators excluded) for biotically pollinated species in highly biodiverse regions of South Africa (greater than or equal to 3000 species per 10 000 km2) [16]. This included the GCFR [17] and a region that we call the southeastern summer rainfall region (SESRR; figure 1 inset) [18]. Data from the rest of the world were obtained from the global dataset of Vamosi et al. [6]. Pollen limitation was calculated from fruit set as PL = ln[supplemental pollination/natural pollination] [6].
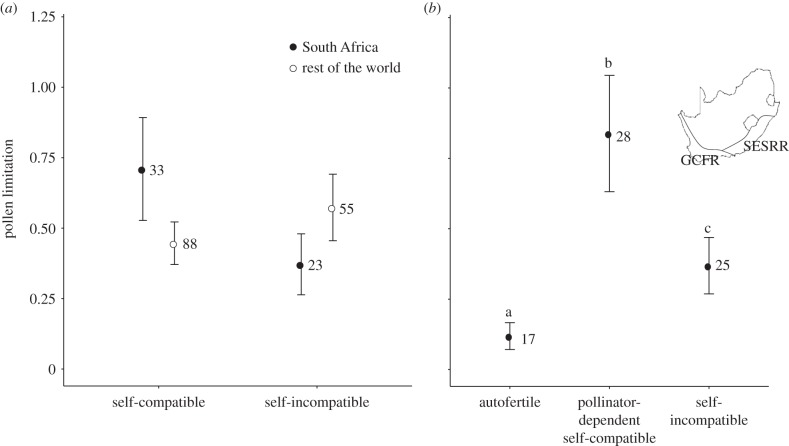
Figure 1.
Pollen limitation (mean ± s.e. log response ratio) for (a) self-compatible and self-incompatible species in South Africa and the rest of the world, (b) autofertile, pollinator-dependent self-compatible and self-incompatible species in South Africa. Different letters above bars indicate that treatments are significantly different. Numbers above bars indicate sample size (number of species). Slightly different datasets were used for South Africa in (a) and (b) (see Material and methods section). Inset shows position of study region, comprising the Greater Cape Floristic Region (GCFR) and South-Eastern Summer Rainfall Region (SESRR), within South Africa.
South African species were classified according to self-incompatibility, pollinator dependence and a combined variable incorporating both these components, where data were available (table 1). Species were also categorized for the following pollination specialization variables: floral symmetry (actinomorphic; zygomorphic), denoting phenotypic specialization, with zygomorphic regarded as specialized; pollinator richness (one to five species—few; greater than five—many), denoting ecological specialization and pollinator orders (one; greater than one—many), denoting functional specialization [20]. For the global dataset, the classification of Vamosi et al. [6] for the same variables was used. Trees were excluded from all analyses due to poor representation in the South African dataset.
Table 1.
Indices and cut-off points used for breeding system classification of South African data.
| breeding system variable | index | formula (using fruit set from pollination treatments) | categories |
||
|---|---|---|---|---|---|
| self-incompatibility | index of self-incompatibility (ISI) | 1−(hand-self/hand-cross) [12] | self-compatible ISI < 0.8 |
self-incompatible ISI ≥ 0.8 |
|
| pollinator dependence | autofertility index (AFI) | autonomous self/hand-cross [19] | autofertile AFI ≥ 0.2 |
pollinator-dependent AFI < 0.2 |
|
| combined variable | both of the above | both of the above | autofertile (AF) | pollinator-dependent self-compatible (PD-SC) | self-incompatible (SI) |
Pollen limitation was compared between South Africa and the rest of the world in a one-way analysis and, for species with self-incompatibility information, in an analysis including self-incompatibility and self-incompatibility × region. Data from a South African study, where selection of species was biased towards autofertile ones with low pollen limitation (M van Kleunen 2008, unpublished data), were excluded from this analysis, because a lack of pollinator dependence/autofertility information for the rest of the world prevented us from controlling for this bias (see the electronic supplementary material). As residuals of generalized least squares (GLS) showed significant phylogenetic signal (p = 0.008) [21], phylogenetically controlled least-squares analysis (PGLS) with the function pgls in the caper package in R [22] was performed (phylogenetic tree and additional details in the electronic supplementary material). The analysis was repeated (i) excluding the SESRR to compare the GCFR specifically with the rest of the world and (ii) comparing South Africa only with lower biodiversity regions of the rest of the world (fewer than 3000 species per 10 000 km2).
Effects of breeding system on pollen limitation were assessed for South African data in separate GLS analyses for self-incompatibility, pollinator dependence and the combined breeding system variable (table 1). These models were compared with Akaike information criterion with correction for finite sample sizes (AICc) values. This analysis was repeated for the GCFR alone. Additionally, we tested whether species with more specialized pollination systems were more pollen-limited and if the GCFR was more pollen-limited than the SESRR within South Africa in GLS analyses also including breeding system (electronic supplementary material). Phylogenetic analyses were not applied as GLS residuals showed no phylogenetic signal.
3. Results
Pollen limitation data were obtained for 94 non-tree species from high plant diversity regions of South Africa, but only 70 had breeding system information. The total included 75 herbs, 19 shrubs, 70 GCFR species and 24 SESRR species. In total, 19 families were represented, with Iridaceae (37), Orchidaceae (14) and Amaryllidaceae (8) being most frequent (electronic supplementary material, table S1). As patterns of pollen limitation within Iridaceae were similar to the rest of the dataset (JG Rodger 2016, unpublished data), dominance of Iridaceae is unlikely to bias findings.
Pollen limitation did not differ between South Africa and the rest of the world, in either the one-way analysis (LR = 0.27, p = 0.607) or two-way analysis (LR = 0.64, p = 0.425), in which the interaction with breeding system was also not significant (LR = 2.05, p = 0.152) (figure 1a; electronic supplementary material, tables S2 and S3). These findings were unchanged when comparing (i) only the GCFR with the rest of the world and (ii) South Africa with lower diversity regions of the rest of the world (electronic supplementary material, tables S2 and S3).
All three breeding system variables significantly affected pollen limitation in South Africa (electronic supplementary material, table S4), but the combined breeding system variable (LR = 14.04, p < 0.001) was far superior to either self-incompatibility or pollinator dependence alone (ΔAICc > 10; electronic supplementary material, table S4). Pollen limitation was highest for self-compatible pollinator-dependent species, lowest for autofertile species and intermediate for self-incompatible species (figure 1b; electronic supplementary material, table S5). Results were the same for the analysis on data from the GCFR alone (electronic supplementary material, tables S6 and S7).
4. Discussion
Overall, we did not find evidence of particularly high pollen limitation in South African biodiversity hotspots relative to the rest of the world, although there was a non-significant trend for higher pollen limitation in self-compatible species in South Africa (figure 1a; electronic supplementary material, table S3). This trend may be related to a higher frequency of pollination specialization (phenotypic and ecological specialization) among self-compatible species in South Africa than in the rest of the world (electronic supplementary material, tables S8–S10), as more pollination-specialized species are more pollen-limited, both in South Africa (electronic supplementary material, figures S1a–c and tables S11,S12) and in other regions [6,7]. Nevertheless, together with a study focusing on the Brazilian Atlantic Rainforest [7], South African results show that biodiversity hotspots do not necessarily have exceptional pollen limitation.
The combined breeding system variable incorporating both pollinator dependence and self-incompatibility (figure 1b) proved superior for explaining pollen limitation in South Africa to either component separately. AF species had the lowest pollen limitation, consistent with expectations of low pollen-quantity and -quality limitation in this group. Only the abovementioned Brazilian study previously assessed the relationship between autofertility and pollen limitation, but did not find a significant effect [7].
There was also higher pollen limitation in PD-SC than SI species in South Africa (figure 1b). This is consistent with increased pollen-quality limitation due to negative consequences of pollinator-mediated self-pollination in PD-SC species. However, we could not attribute this difference exclusively to pollen-quality limitation because there was also a higher frequency of plants with zygomorphic flowers, which were also more pollen-limited, among PD-SC species (electronic supplementary material figure S1 and table S13) and the effect of breeding system was no longer significant when floral symmetry (phenotypic specialization) was controlled for in an analysis including only these two groups (electronic supplementary material table S14). Thus, studies distinguishing between pollen-quantity and -quality components [2,23,24] are needed to resolve the functional relationship between breeding system and pollen limitation. Future comparitive studies of pollen limitation should also take into account effects of both pollinator dependence and self-incompatibility, for instance by using the combined three-category breeding system variable presented here.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Andre Vermeulen, Stuart Hall and Carina Wessells for helping to compile the South African dataset, Jana Vamosi for advice on analyses, Ross Turner and Mark van Kleunen for access to unpublished data, Peter Goldblatt for breeding system information on Iridaceae, and Renate Wesselingh and an anonymous reviewer for their comments. Global pollen limitation data were provided by members of the Pollen Limitation Working Group, supported by the National Centre for Ecological Analysis and Synthesis, a centre funded by NSF (DEB 9421535).
Data accessibility
Data have been deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.pt553.
Authors' contributions
J.G.R. and A.G.E. conceived the study. J.G.R. executed it and wrote the paper with input from A.G.E. Both authors gave final approval for publication and take joint responsibility for the content.
Competing interests
We have no competing interests.
Funding
This study was supported by an NRF innovation postdoctoral fellowship to J.G.R.
References
- 1.Bierzychudek P. 1981. Pollinator limitation of plant reproductive effort. Am. Nat. 117, 838–840. ( 10.1086/283773) [DOI] [Google Scholar]
- 2.Aizen MA, Harder LD. 2007. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271–281. ( 10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- 3.Wesselingh RA. 2007. Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol. 174, 26–37. ( 10.1111/j.1469-8137.2007.01997.x) [DOI] [PubMed] [Google Scholar]
- 4.Knight TM, et al. 2005. Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 36, 467–497. ( 10.1146/annurev.ecolsys.36.102403.115320) [DOI] [Google Scholar]
- 5.Larson BMH, Barrett SCH. 2000. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 69, 503–520. ( 10.1111/j.1095-8312.2000.tb01221.x) [DOI] [Google Scholar]
- 6.Vamosi JC, Knight TM, Steets JA, Mazer SJ, Burd M, Ashman TL. 2006. Pollination decays in biodiversity hotspots. Proc. Natl Acad. Sci. USA 103, 956–961. ( 10.1073/pnas.0507165103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolowski M, Ashman TL, Freitas L. 2014. Meta-analysis of pollen limitation reveals the relevance of pollination generalization in the Atlantic forest of Brazil. PLoS ONE 9, e89498 ( 10.1371/journal.pone.0089498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marloth R. 1908. Some observations on entomophilous flowers. S. Afr. J. Sci. 4, 110–113. [Google Scholar]
- 9.McCall C, Primack RB. 1992. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am. J. Bot. 79, 434–442. ( 10.2307/2445156) [DOI] [Google Scholar]
- 10.Johnson SD, Bond WJ. 1997. Evidence for widespread pollen limitation of fruiting success in Cape wildflowers. Oecologia 109, 530–534. ( 10.1007/s004420050113) [DOI] [PubMed] [Google Scholar]
- 11.Anderson B, Allsopp N, Ellis AG, Johnson SD, Midgley JJ, Pauw A, Rodger JG. 2014. Biotic interactions. In Fynbos: ecology, evolution, and conservation of a megadiverse region, pp. 224–247. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Schoen DJ, Lloyd DG. 1992. Self-fertilization and cross-fertilization in plants III. Methods for studying modes and functional aspects of self-fertilization. Int. J. Plant Sci. 153, 381–393. ( 10.1086/297042) [DOI] [Google Scholar]
- 13.De Jong TJ, Waser NM, Klinkhamer PGL. 1993. Geitonogamy—the neglected side of selfing. Trends Ecol. Evol. 8, 321–325. ( 10.1016/0169-5347(93)90239-L) [DOI] [PubMed] [Google Scholar]
- 14.Duminil J, Hardy OJ, Petit RJ. 2009. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evol. Biol. 9, 177 ( 10.1186/1471-2148-9-177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodger JG, Balkwill K, Gemmill B. 2004. African pollination studies: where are the gaps? Int. J. Trop. Insect Sci. 24, 5–28. ( 10.1079/IJT20045) [DOI] [Google Scholar]
- 16.Barthlott W, Biedinger N, Braun G, Feig F, Kier G, Mutke J. 1999. Terminological and methodological aspects of the mapping and analysis of global biodiversity. Acta Bot. Fenn. 162, 103–110. [Google Scholar]
- 17.Born J, Linder HP, Desmet P. 2007. The Greater Cape Floristic Region. J. Biogeogr. 34, 147–162. ( 10.1111/j.1365-2699.2006.01595.x) [DOI] [Google Scholar]
- 18.Mittermeier R, et al. 2005. Hotspots revisited. Mexico City, Mexico: Cemex. [Google Scholar]
- 19.Raduski AR, Haney EB, Igic B. 2012. The expression of self-incompatibility in angiosperms is bimodal. Evolution 66, 1275–1283. ( 10.1111/j.1558-5646.2011.01505.x) [DOI] [PubMed] [Google Scholar]
- 20.Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. 2007. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56, 717–728. ( 10.2307/25065856) [DOI] [Google Scholar]
- 21.Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods Ecol. Evol. 1, 319–329. ( 10.1111/j.2041-210X.2010.00044.x) [DOI] [Google Scholar]
- 22.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. caper: comparative analyses of phylogenetics and evolution in R. caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5.2. https://CRAN.R-project.org/package=caper.
- 23.Alonso C, Herrera CM, Ashman TL. 2012. A piece of the puzzle: a method for comparing pollination quality and quantity across multiple species and reproductive events. New Phytol. 193, 532–542. ( 10.1111/j.1469-8137.2011.03932.x) [DOI] [PubMed] [Google Scholar]
- 24.Vaughton G, Ramsey M. 2010. Floral emasculation reveals pollen quality limitation of seed output in Bulbine bulbosa (Asphodelaceae). Am. J. Bot. 97, 174–178. ( 10.3732/ajb.0900183) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited in the Dryad repository: http://dx.doi.org/10.5061/dryad.pt553.