Abstract
The capacity to store and return energy in legs and feet that behave like springs is crucial to human running economy. Recent comparisons of shod and barefoot running have led to suggestions that modern running shoes may actually impede leg and foot-spring function by reducing the contributions from the leg and foot musculature. Here we examined the effect of running shoes on foot longitudinal arch (LA) motion and activation of the intrinsic foot muscles. Participants ran on a force-instrumented treadmill with and without running shoes. We recorded foot kinematics and muscle activation of the intrinsic foot muscles using intramuscular electromyography. In contrast to previous assertions, we observed an increase in both the peak (flexor digitorum brevis +60%) and total stance muscle activation (flexor digitorum brevis +70% and abductor hallucis +53%) of the intrinsic foot muscles when running with shoes. Increased intrinsic muscle activation corresponded with a reduction in LA compression (−25%). We confirm that running shoes do indeed influence the mechanical function of the foot. However, our findings suggest that these mechanical adjustments are likely to have occurred as a result of increased neuromuscular output, rather than impaired control as previously speculated. We propose a theoretical model for foot–shoe interaction to explain these novel findings.
Keywords: barefoot running, intrinsic foot muscles, longitudinal arch, leg-spring mechanics
1. Introduction
It has been suggested that humans may have evolved to run and have done so for millions of years [1,2]. Hard surfaces have been encountered by humans when running throughout evolution; however, the modern running environment, characterized by stiff, invariant substrates such as roads and footpaths, has transformed at a far greater rate than evolution can progress [1–3]. The apparent lack of natural variability in surface terrain and compliance that is endemic in our modern running world is believed to have altered the biomechanical demands of running [4,5], possibly contributing to the high injury rate in those who habitually partake in this activity [6].
The human foot is the interface between the body and the ground. The unique structure of the foot allows force produced by muscles of the lower limb to be transmitted to the ground, to support body weight (BW) and also generate forward propulsion [7,8]. A pronounced structural feature of the human foot is the longitudinal arch (LA), which allows the foot to function in a spring-like manner [1,2,9,10] in series with the entire lower limb [11,12]. The LA compresses during early stance, absorbing mechanical energy as the ground reaction force (GRF) increases. Presumably, the energy absorbed is stored within elastic structures supporting the arch [9,13,14]. In late stance, when GRF decreases, the LA recoils, returning elastic energy to deliver power for propulsion [9]. Stiffness of the LA is provided by passive ligamentous structures [9,14,15] acting in parallel with the intrinsic foot muscles whose relative contribution is continually adjusted by the central nervous system (CNS) in response to mechanosensory stimuli [10,16]. This elegant arrangement allows the mechanical characteristics of the foot to be rapidly adapted to loading or task demands [10] and is thought to improve the efficiency of human running, returning between 8% and 17% of the mechanical energy required for one stride, via passive mechanisms alone [9,13].
Footwear has provided mechanical and thermal protection for human feet when running, for thousands of years [17]. The contemporary running shoe, however, was not invented until the 1970s [18] and has evolved in parallel with the surge in popularity of running as a recreational pursuit. A defining characteristic of the modern running shoe is the thick viscoelastic midsole that is designed to compress and rebound when cyclically loaded and unloaded during running [19,20]. This design feature, generally referred to as cushioning, allows the shoe to function in a similar ‘spring-like’ manner to the lower limb and foot, absorbing the potentially harmful impact transients that are encountered when the foot impacts with the ground [21–24], while also returning some of this energy to aid power generation for propulsion [25]. Another key feature of the modern running shoe is the contoured midsole, designed to provide external support and reduce excessive strain on the muscles and ligaments of the LA [21].
However, despite the huge financial investment in the development of running shoes, running injury rates remain relatively unchanged over the last 40 years [6,26,27], leading some to question the efficacy of modern running shoes in preventing injury [3,28–31]. Some scholars have gone as far to suggest that cushioned midsoles may actually hinder our running performance [3,28–30,32]. These scholars have speculated that a thick cushioned interface between the runner and the ground impairs mechanosensory feedback and therefore, the inherent capacity of the CNS to contend with large impact force transients via adjustments in leg- and foot-spring stiffness [3,29,33]. Furthermore, it has been speculated that an apparent reliance on the shoe to attenuate impact and provide mechanical support for the LA may reduce the required contributions from the foot and ankle musculature, precipitating foot and ankle muscle weakness and predisposing a runner to injury [28,31,34]. While there is some evidence that runners tend to land differently when they run without shoes [28,35–38], there is no evidence that shoes have a detrimental influence on the spring-like function of the foot, or the contributions to this function from foot and ankle musculature.
Despite the ongoing speculation as to the potential benefits and detrimental effects that modern running shoes may have on running mechanics, it is apparent that there is a dearth of information pertaining to how the CNS regulates the spring-like function of the foot during shod running. Therefore, the aim of this study was to test the hypothesis that running shoes impair the spring-like function of the foot, thereby altering the required force contribution from the intrinsic foot muscles to actively support the LA during running. In order to test this hypothesis, we had participants run on a force-instrumented treadmill barefoot and wearing running shoes. In addition to the GRF, electromyograms (EMG) were recorded from the intrinsic foot muscles and ankle plantar flexors, whereas motion capture data were recorded to assess foot and ankle kinematics during multiple consecutive strides.
2. Methods
2.1. Participants
Sixteen healthy participants (seven females mean ± standard deviation (s.d.) for age 19 ± 1 years; height: 165 ± 4 cm; mass: 59 ± 7 kg, nine males age 24 ± 5 years; height: 172 ± 4 cm; mass: 73 ± 10 kg) with no history of lower limb injury in the previous six months or known neurological impairment volunteered to participate in the study. All participants were habitually shod recreational runners. Foot-strike technique (i.e. rear-foot or forefoot) was not applied as an inclusion or exclusion criteria; however, none of the participants recruited for this study displayed a forefoot running technique when either shod or barefoot. Written informed consent was obtained from each subject.
3. Experimental protocol
Following a 3 min warm up period and familiarization procedure, participants ran on a force-instrumented treadmill (AMTI, force-sensing tandem treadmill, Watertown, MA) at 14 km h−1 while barefoot and shod. The running shoe chosen for this study is described by the manufacturer as a ‘cushioned stability’ shoe, with a heel height of 30 mm and forefoot height of 20 mm (Asics GT2000, Asics Corp. Japan). The inner lining was made of soft, flexible foam. In order to prevent rubbing against the intramuscular electrodes, the raised edges of the inner lining were trimmed flat and had no contact with the skin of the LA. Kinetic, kinematic and EMG data were collected simultaneously with approximately 15–20 strides (toe-off to ipsilateral toe-off) being recorded for each condition (barefoot and shod).
4. Data acquisition
4.1. Kinematic and kinetic measurements
Three-dimensional motion of the foot and shank, and GRF data were collected during each running trial. Retroreflective markers (9.0 mm diameter) were secured on the skin of the right foot, overlying the medial and lateral malleoli, posterior calcaneus, navicular tuberosity and head of the first and fifth metatarsals, in order to quantify motions of the foot segments and the LA (figure 1). Additional markers were applied to the medial and lateral femoral condyles, and a rigid cluster of four markers was placed on the anterolateral aspect of the shank. During a standing calibration trial, markers located on the segment endpoints were used to generate a two-segment model of the shank and foot. Following the calibration trial, the medial and lateral knee markers were removed, and the motion of the shank was tracked with the rigid marker cluster. In order to allow foot marker positions to be captured during the shod condition, circular holes of 25 mm diameter were cut in the shoe upper in positions corresponding to the foot marker locations. This allowed visualization of the markers, while still allowing markers to be adhered to the skin. Markers were adhered with double-sided adhesive and further secured with cohesive bandage, allowing secure positioning for both the shod and barefoot conditions.
Figure 1.

Depiction of the lower limb marker set employed for collection of kinematic data. White markers are removed following a static calibration trial, with the cluster of four markers on a rigid plastic shell used to track the motion of the shank. The posterior calcaneus marker cannot be viewed in this image. (Online version in colour.)
Kinematic data were captured at 200 Hz using an eight camera three-dimensional optoelectronic motion capture system (Qualisys, Gothenburg, Sweden), whereas GRF and EMG data were synchronously captured at 4000 Hz via a 14-bit analogue-to-digital converter (Qualisys). Kinematic, force and EMG data were collected simultaneously and synchronized using the Qualisys Track Management software from the same company.
4.2. Electromyography
Identification of the abductor hallucis (AH) and flexor digitorum brevis (FDB) muscles was conducted using real-time B-mode ultrasound imaging (10 MHz linear array, Ultrasonix RP, USA) in the right foot of each subject. Subsequently, bipolar fine-wire electrodes (0.051 mm stainless steel, Teflon-coated, Chalgren, USA) with a detection length of 2 mm and interelectrode distance of 2 mm were inserted using delivery needles (0.5 × 50 mm) into the muscle tissue of AH and FDB under ultrasound guidance, in accordance with previously described methods [39]. Sterile techniques were used for the insertion of all wires. Surface EMG data were collected from the medial gastrocnemius (MG) and soleus (SOL) from the right leg of all participants using Ag–AgCl electrodes with a diameter of 10 mm and an interelectrode distance of 20 mm (Tyco Healthcare Group, Neustadt, Germany). A surface reference electrode (10 mm diameter, Ag/AgCl, Tyco Healthcare Group) was placed over the right fibula head. Prior to electrode placement, the areas of the leg corresponding to the electrode placement sites were shaved, lightly abraded and cleaned with isopropyl alcohol.
All EMG signals were amplified 1000 times and recorded with a bandwidth of 30–1000 Hz (MA300, Motion Labs, LA). In order to minimize movement artefacts, the fine-wire electrodes, surface electrodes, connectors, cabling and pre-amplifiers were secured with cohesive bandage around the foot and shank.
Prior to data collection, each participant was asked to perform foot manoeuvres known to activate each foot muscle separately [16,40]. When predicted EMG patterns could be detected, it was concluded that the fine-wire electrodes were in the correct location. If not, the electrodes were withdrawn by approximately 1 mm until appropriate activation patterns could be detected and possible crosstalk excluded. In order to ensure quality of the intramuscular EMG signal throughout the experiment, signal quality was assessed following each experimental condition using the same technique described above. A Velcro strap was secured around the participant's waist, which enabled the EMG amplifier box to be secured to the subject without interfering with their gait. A lightweight optical cable connected the amplifier box to the analogue-to-digital converter.
5. Data analysis
Kinetic and kinematic data files were exported to Visual3D (C-motion Inc., Germantown, MD) for analysis. Force plate data recorded during each experimental trial was digitally filtered with a recursive 35 Hz low-pass, fourth-order Butterworth filter. A vertical GRF threshold was set to define each toe-off as occurring when vertical GRF fell below 50 N, whereas foot contact was defined as occurring when vertical force rose above 50 N. Swing phase was defined as the period from right toe-off to right foot contact, whereas stance phase was defined as occurring between right foot contact and right toe-off. One stride cycle was defined as occurring from right toe-off to the subsequent right foot toe-off.
Subsequently, the magnitude of the vertical and anteroposterior (A–P) components of the GRF was calculated and normalized to body weight for each participant. Peak loading rate was defined as the maximum value obtained from the first derivative of the vertical GRF in the first 50 ms following foot contact, whereas peak propulsive force was defined as the peak positive value of the A–P component of the GRF.
Marker trajectories were digitally filtered with a recursive 20 Hz low pass, fourth-order Butterworth filter. Assumed rigid segments were created for the shank and foot. Joint rotations were calculated in accordance with International Society of Biomechanics recommendations (+y, up; +z, medial; +x, anterior) with rotation about the z-axis—sagittal plane motion, rotation about the x-axis—frontal plane motion and rotation about the y-axis—transverse plane motion [41]. Ankle angle was defined as the angle of the foot segment relative to the shank, with plantar flexion reported as a positive angular rotation. Ankle angle at contact was calculated as the sagittal plane ankle angle at foot contact and ankle excursion was calculated by subtracting the minimum ankle angle during stance phase from the ankle contact angle. The LA angle was defined as a sagittal planar angle created by the bisection of a vector projecting from the medial malleolus marker to the navicular marker and another vector projecting from the head of the first metatarsal to the navicular marker (figure 2). Thus, a decrease in LA angle is indicative of a reduction in LA height. In order to describe the spring-like behaviour of the LA during stance phase, measures of compression and recoil were calculated. Compression of the LA was defined as the reduction in LA angle (height) that occurs owing to the application of load and was calculated by subtracting the minimum LA angle during stance phase from the LA angle at foot contact. LA recoil was defined as the increase in LA angle (height) that occurs during unloading and was calculated by subtracting the minimal LA angle during stance phase from the LA angle at toe-off.
Figure 2.
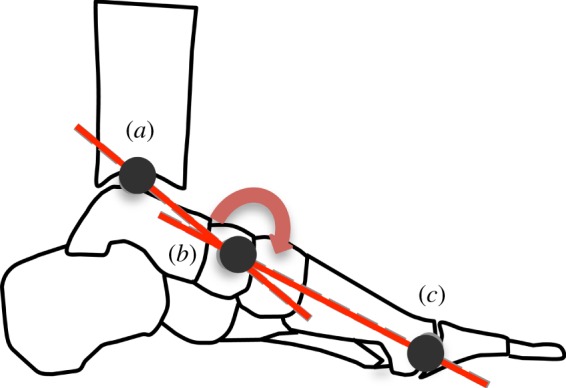
Longitudinal arch (LA) angle is defined as the angle created by the bisection of a vector projecting from a marker located on the medial malleolus (a) to a marker located on the navicular tuberosity (b), with a vector projecting from a marker located on the head of the first metatarsal (c) to a marker on the navicular tuberosity (b). A reduction in LA angle indicates arch compression, while an increase in arch angle indicates arch recoil. (Online version in colour.)
Owing to technical difficulties associated with collecting intramuscular EMG data from the foot muscles within a running shoe, complete sets of muscle activation data from AH and FDB was only obtainable from 10 of the 16 participants, whereas surface EMG data from MG and SOL were collected from all participants. The EMG data were exported to Spike2 software (Cambridge Electronic Design, Cambridge, UK) prior to analysis. All signals were high-pass filtered using a recursive fourth-order Butterworth filter at 35 Hz to remove any unwanted low-frequency movement artefact. The EMG signals were then visually inspected in order to identify any remaining artefact, which was defined as an abnormal spike in the signal, typically associated with foot contact. Any such remaining artefacts resulted in the EMG data for that particular stride being excluded from further analysis. Following DC-offset removal, root mean square (RMS) signal amplitude was calculated using a moving window of 50 ms to generate an EMG envelope. Subsequently, the EMG envelope for each muscle was normalized to its peak amplitude found across all conditions. Normalized peak EMG amplitude and total stance activity (based on the EMG envelope) were calculated during the stance phase for each stride cycle, allowing comparisons in magnitude of stance phase muscle activation between shod and unshod conditions. In order to provide insights into the magnitude of activation relative to the time that a muscle is generating force, total stance phase activity (%max s) was calculated by multiplying the mean normalized RMS signal amplitude during stance (%max) by the mean stance phase duration (s) for each muscle and condition [42,43].
For each individual, the kinematic, kinetic and EMG data were averaged across a minimum of 10 stride cycles to form individual variable means for each condition.
5.1. Statistics
Paired t-tests were used to describe the influence of running shoes on stride temporal characteristics, peak vertical GRF, peak loading rate, peak propulsive force, ankle contact angle, ankle excursion, LA compression and recoil and peak muscle activation. Statistical differences were established at p ≤ 0.05. Results are presented as mean ± s.d., unless otherwise stated.
6. Results
6.1. Running mechanics
Shod running was typified by a longer stride duration (shod 0.68 ± 0.03 s versus barefoot 0.65 ± 0.03 s, p ≤ 0.05) and ground contact times (shod 0.21 ± 0.01 s versus barefoot 0.18 ± 0.01 s, p ≤ 0.05). When running shod and barefoot, participants produced comparable magnitudes of vertical GRFs (shod 2.75 ± 0.24 BWs versus barefoot 2.75 ± 0.22 BW, p = 0.6); however, mean peak loading rate (shod 74.5 ± 10.0 BW s−1 versus barefoot 86.4 ± 14.2 BW s−1) and mean peak propulsive force (shod 0.41 ± 0.05 BW versus barefoot 0.44 ± 0.05 BW) were both reduced when running with shoes (both p ≤ 0.05, figure 3). Participants adjusted the angular orientation of the ankle at foot contact depending on the running condition (p ≤ 0.05), adopting a position of slight dorsiflexion when running in shoes (2.0 ± 2.8°, range −7.1° to 1.9°, figure 4), while they landed in a position of slight plantar flexion when running barefoot (1.8 ± 2.3°, range −5.3° to 4.7°).
Figure 3.
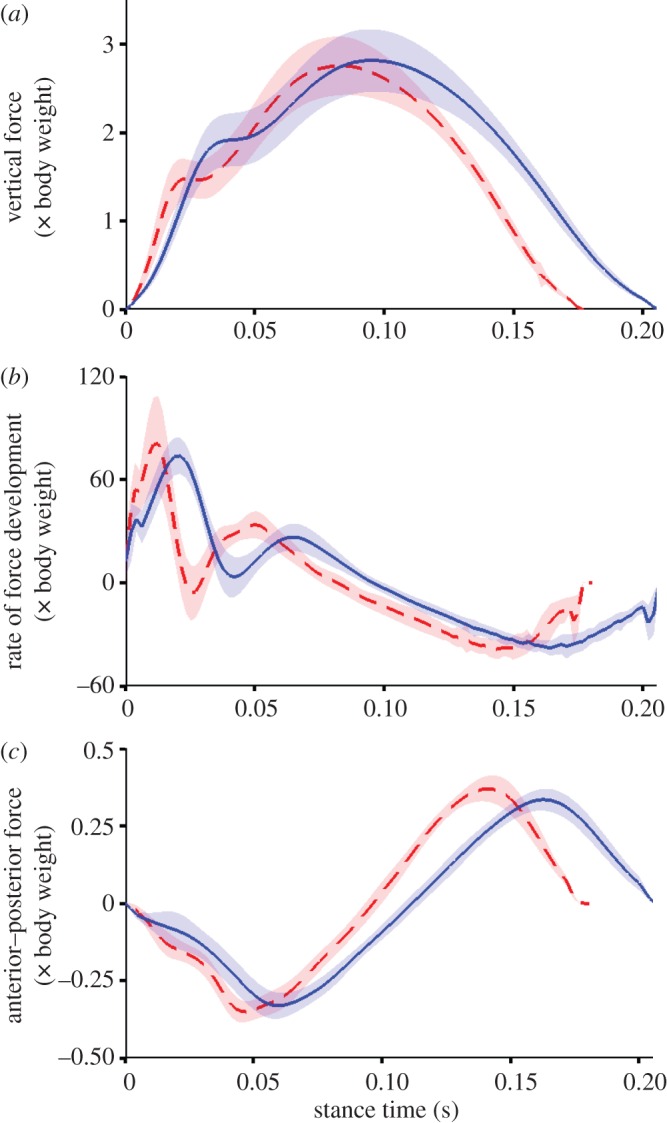
Group mean ± s.d. (shaded area) for vertical force (a), vertical force loading rate (b) and anteroposterior force (c). Data are recorded from each participant running barefoot (red) and shod (blue) at 3.89 ms−1 and presented from foot contact to toe-off from the right foot. Loading rate is defined as the first derivative of the vertical ground reaction force signal. All data are normalized to body weight. (Online version in colour.)
Figure 4.
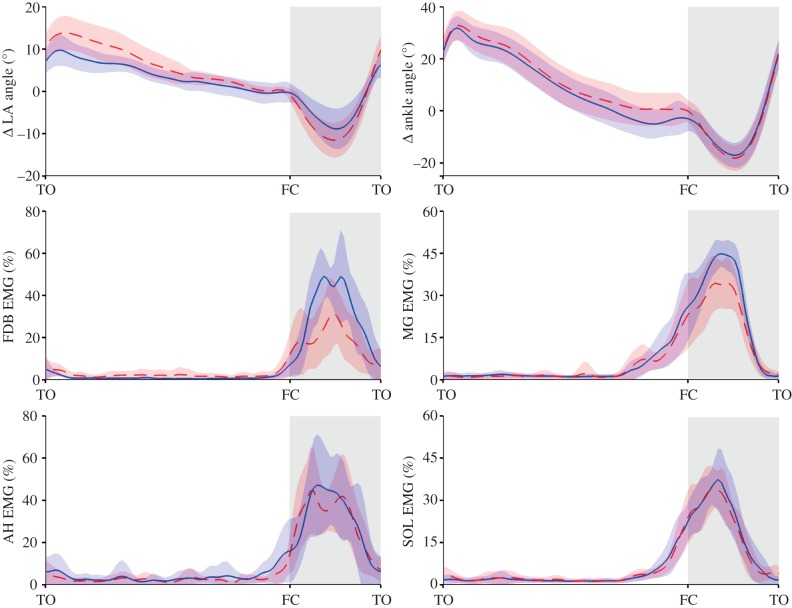
Group mean ensembles ± s.d. (shaded area) for changes (Δ) in longitudinal arch (LA) and ankle angle (degrees, °, top), electromyography (EMG) normalized root mean square signal amplitude for flexor digitorum brevis (FDB) and abductor hallucis (AH), gastrocnemius medilais (MG) and soleus (Sol). Group mean ensembles are defined from toe-off (TO) to ipsilateral TO for the right foot. Data recorded during running at 3.89 ms−1. For each muscle, EMG data are normalized to the maximal amplitude recorded for all trials. Change in LA and ankle angle was calculated by subtracting the angle at foot contact in the barefoot condition from the angle–time data from both shod and barefoot conditions. FC, foot contact. The barefoot condition is the red dashed lines and shod the solid blue lines. (Online version in colour.)
For shod and barefoot conditions, ankle dorsiflexion occurred following forefoot contact in early stance, until late stance when the ankle underwent rapid plantar flexion. Ankle dorsiflexion excursion was significantly less when running with shoes (shod 14.8 ± 4.6° versus barefoot 20.3 ± 6.8°, p ≤ 0.05), owing to a more plantar flexed position of the ankle at initial foot contact and similar peak dorsiflexion angles during mid- to late-stance (figure 4).
The LA compressed during early to mid-stance as the vertical GRF was rising and recoiled during late stance as the vertical GRF subsided (figure 4). The LA angle at foot contact was similar for both conditions (shod 150.4 ± 9.9° versus barefoot 151.0 ± 9.6°, p = 0.4). However, when running with shoes, participants displayed reduced magnitudes of both LA compression (shod 8.6 ± 4.6° versus barefoot 11.5 ± 4.0° p ≤ 0.05) and recoil (shod 15.4 ± 5.7° versus barefoot 21.5 ± 5.5°, p ≤ 0.05) primarily owing to a combination of a lower minimum LA angle at mid-stance and a higher LA angle at propulsion (figure 4). When considered together, the reduction in LA compression and similar peak GRFs, intimate that the LA is stiffer in the shod condition.
6.2. Muscle activation
The FDB and AH muscles recorded intramuscularly, displayed similar patterns of activation within each condition. Both showed the periods of relative inactivity during swing and large bursts of activity during stance (figure 4). Peak activation generally occurred during mid-stance for both muscles. Total stance activity was higher when running with shoes, for both FDB (shod 7.1 ± 2.7%max s versus barefoot 4.2 ± 3.4%max s, p ≤ 0.05) and AH (shod 6.3 ± 2.0%max s versus barefoot 4.1 ± 1.8%max s, p ≤ 0.05). Peak FDB activation was greater when running with shoes, compared with barefoot (shod 64.8 ± 25.9% versus barefoot 40.7 ± 19.0%, p ≤ 0.05, figure 4), whereas no consistent differences were observed between the shod and unshod conditions for AH (shod 56.2 ± 19.3% versus barefoot 45.4 ± 19.3%, p = 0.17, figure 4).
Soleus and MG muscles were both relatively quiescent during early swing phase, with a large burst of activity that commenced during terminal swing and peaked prior to mid-stance (figure 4). Total stance activity was higher when running with shoes, for both MG (shod 7.1 ± 2.4%max s versus barefoot 5.9 ± 3.3%max s, p ≤ 0.05) and SOL (shod 6.1 ± 1.2%max s versus barefoot 5.0 ± 0.7%max s, p ≤ 0.05). Peak MG activity was greater when running with shoes (shod 65.6 ± 15.4% versus barefoot 57.6 ± 16.2%, p ≤ 0.05, figure 4), whereas no significant differences were observed in SOL activity between the shod and unshod conditions (shod 64.8 ± 15.4% versus barefoot 59.0 ± 14.6%, p = 0.09).
7. Discussion
This study provides us novel evidence of adjustments in the mechanical function of the foot when comparing running in shoes to barefoot. In line with our first hypothesis, running with shoes led to a reduction in the magnitude of LA compression and recoil, suggesting that running shoes influence foot-spring function. Of particular interest was the underlying mechanism for the observed alterations in LA motion when running in shoes, which we believe is at least partially driven by an increase in neuromuscular output, rather than a decrease, as we originally hypothesized.
7.1. Stance phase
During stance, the lower limbs of human runners behave in a spring-like manner, ‘compressing and recoiling’ via a sequence of hip, knee and ankle joint flexion then extension in phase with the increasing and decreasing magnitude of the vertical GRF [12,44,45]. This highly efficient mechanism allows recycling of elastic and kinetic energy during each foot contact [11,46], while also allowing a relatively stable centre of mass trajectory [45]. The CNS has the capacity to adjust the stiffness of the lower limb in order to minimize centre of mass vertical motion when running across terrains with varying undulations [47] and compliance [45,48,49]. The foot is considered a key contributor to leg-spring function [9,10,12,13]; however, to date, we believe, the influence of running shoes on the spring-like function of the foot has not been reported.
Runners in our experiment displayed substantially less arch compression and recoil when running with shoes, when compared with barefoot. This finding is in line with the key design features of running shoes that aim to provide support for the LA and reduce strain on plantar soft-tissue structures [50,51]. However, this finding also highlights that running shoes may actually limit the capacity for the foot to store and return energy via elastic mechanisms, owing to a reduction in the magnitude of arch compression and recoil [13]. Recent critiques of modern running footwear have argued that cushioning and support characteristics of the shoe potentially impair foot-spring function, with a likely consequence of reduced activation from muscles that support the arch, leading to their weakness and disuse atrophy [3,29,34]. Our findings partially support this notion. However, the observed concomitant increase in intrinsic foot muscle activation in shod running appears to indicate that the reduced arch compression observed when running with shoes may be driven by an increase in muscle activation, rather than via the cushioning and external support features of the running shoes.
In a recent series of experiments, we provided novel evidence that the intrinsic foot muscles function in parallel with the plantar aponeurosis, actively tuning the stiffness of the LA in response to load during stance and locomotion [10,16,39]. Employing intramuscular electrical stimulation to activate individual intrinsic foot muscles, it was observed that contraction of these muscles could produce a 5% increase in arch height, reversing the compression of the LA that occurred when the foot was loaded with forces equivalent to body weight [16]. Given that the intrinsic foot muscles are known to act in unison as a functional group [10,52], it is likely that their combined action and the action of the extrinsic muscles [53,54] may have a profound effect on LA function. Therefore, when considering the findings of this study with those of our earlier studies, it becomes apparent that the observed increase in intrinsic foot muscle activation when running with shoes, compared with barefoot, is likely to be partially responsible for the concomitant reduction in LA compression during stance.
Given that the LA acts as a spring with an actively adjustable stiffness [9] and running shoes with viscoelastic midsoles also behave in a spring-like manner [23,25], the foot and shoe can be modelled to behave as two springs acting either in parallel, or in series, during the stance phase (figure 4). Modelling the interaction between running shoes and the LA, potentially allows us to reveal the underlying mechanism for the observed increase in muscle activity when running in shoes. Within this model, the LA behaves as a single spring of given stiffness (kfoot) that is continually adjusted via activation of the muscles that span the arch of the foot [16], in order to optimize forces acting between the body and the ground. For example, intrinsic foot muscle activation increases when running at faster velocities, stiffening the LA and thereby allowing greater forces to be transmitted between the body and ground during shorter ground contact periods [10]. When a runner wears shoes with a viscoelastic midsole, the shoe will behave as an additional spring, also with a given stiffness (kshoe, figure 5) and the two form a foot–shoe system that has a stiffness (kFS) that is dependent on the configuration of the two springs.
Figure 5.
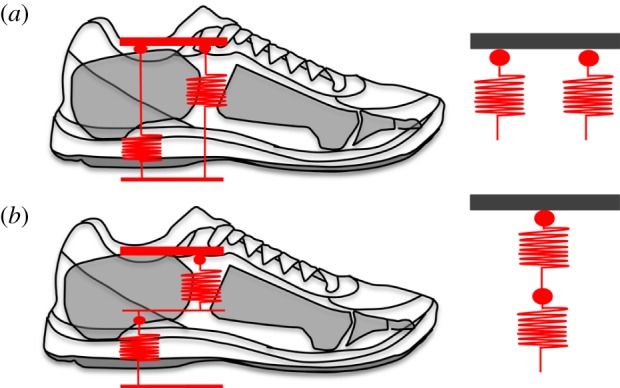
Depiction of a parallel (a) and in-series (b) spring arrangement between the longitudinal arch (LA) and running shoe. Both the LA and running shoe will behave in a spring-like manner during running, compressing and recoiling as force is increased and decreased. If the LA and running shoe act in-parallel, wearing a running shoe will increase the overall stiffness of the foot–shoe system. If the LA and running shoe act in-series, wearing a running shoe will increase the overall compliance of the foot–shoe system. Based on the assumption that constant foot–shoe system stiffness is favoured during steady-state running, the response of the intrinsic foot muscles in regulating the stiffness of the arch will vary depending on whether the longitudinal arch and running shoe behave in-parallel or in-series. (Online version in colour.)
If the arch and shoe springs are modelled to be in parallel, the net stiffness of the foot–shoe system (kFS) is the summed stiffness of the LA (kfoot) and shoe (kshoe) springs acting together.
Alternatively, if we model the foot and shoe as springs acting in series, the net compliance (inverse of stiffness) of the foot–shoe system (1/kFS) will be the common compliance of the LA (1/kfoot) and shoe (1/kshoe) springs acting together.
To interpret both of these models with our data, we will assume that the neuromuscular system seeks to maintain a constant overall lower limb stiffness, including a constant KFS. This assumption is based on a wealth of prior studies showing that humans adjust muscle activations to maintain constant system stiffness on surfaces of varied compliance [45,48,55,56] and also when wearable devices are added to the limb that influence system stiffness [57–59].
For our model of springs in parallel (kFS = kfoot + kshoe) if a runner wears running shoes of a given stiffness, the addition of the shoe spring will lead to an overall increase in kFS. Thus, under the assumption that constant system stiffness is beneficial during constant velocity running [45,48,60], a reduction in LA stiffness is required in order to offset the additional stiffness added by the shoe. Reduced LA stiffness would be achieved by allowing greater arch compression, presumably through a reduction in force output from the arch musculature; neither of which were observed here.
If the model of springs in series is considered (1/kFS = 1/kfoot + 1/kshoe) running in shoes with a viscoelastic midsole will decrease kFS owing to the presence of an additional spring. Therefore, an increase in LA stiffness is necessary to increase overall system stiffness, maintaining constant kFS. An increase in LA stiffness would require a reduction in LA compression, which is achievable via an increase in force output from the intrinsic foot muscles (increased activation) [10]. This is in line with our observations that intrinsic foot muscle activation increased and LA compression decreased, when running in shoes.
According to the above-mentioned scenarios that describe the potential interactions between human feet and running shoes, it seems that running shoes act as an additional spring in-series with the foot. While we cannot discount that deformation of the shoes may act to provide supporting forces to LA, an in-series spring model provided a sound mechanical rationale for our finding that running in shoes induced an increase in muscle activation from two of the primary muscles within the LA. The incorporation of intrinsic foot muscle activation data has therefore provided a unique insight into the underlying mechanism for the observed changes in LA function when running in shoes. Most importantly, these findings highlight that the alterations in lower limb biomechanics observed when running in shoes are not a result of reduced or impaired neuromuscular function.
The increase in ankle plantar flexor activation and reduction in ankle dorsiflexion observed when our runners were shod indicates that our runners may have also exhibited an increase in ankle stiffness in response to the increased compliance provided by the running shoe. Increased knee and ankle stiffness has previously been observed when running in shoes with viscoelastic midsoles [20,23] indicating that the cushioning properties of shoes may induce similar mechanical adaptations across the entire lower limb. This finding provides further support for our model that describes running shoes as springs acting in-series with the foot and leg. Furthermore, these findings are in line with previous research describing the in-series interaction between the lower limb and running support surface [48], and the apparent increase in leg stiffness that is observed when running on compliant surfaces [45,61].
7.2. Impact phase
The initial impact phase can be described to occur over the first 50 ms of ground contact and involves the rapid deceleration of the lower limb that occurs when the foot and ground collide [62] with forces up to twice body weight being transmitted at rates of up to 200 body weights per second [28,63]. Impact loading rates are considerably higher on stiff surfaces such as concrete, which are endemic in our modern running environment [64] and possibly contribute to the high prevalence of repetitive stress injury in runners [65]. The modern running shoe been designed to reduce the rate of force increase during the impact phase, thereby reducing the risk of injury to the runner. However, a counter argument has been raised that suggests that the presence of a cushioned midsole lends to the adoption of a marked heel-first landing pattern and a reliance on the shoe to attenuate impact, rather than via the body's natural shock absorbers: muscle and tendon [28,29]. Within the current experiments, our runners adopted a heel-strike pattern when running in shoes, while they contacted the ground with their mid-foot when barefoot. This finding is consistent with a number of previous comparisons of shod and un-shod running in runners who habitually wear shoes, further confirming that runners generally impact the ground differently when running in shoes when compared with barefoot [4,5,28,63,66]. It has been reported that barefoot runners tend to strike the ground with a forefoot first contact, allowing the body to effectively damp the large impact transients [37,67,68] via controlled dorsiflexion of the ankle and the associated stretch of the Achilles tendon [37]. Interestingly, despite our runners adopting a more plantar flexed ankle position when running without shoes, peak loading rates still remained considerably higher. Thus, despite the modification in landing mechanics, the magnitude of adaptation in our habitually shod runners was insufficient to damp impacts in a manner comparable to the cushioned running shoe. Further research may elucidate if these observations persist across habitually barefoot running populations.
7.3. Methodological considerations
There are some methodological considerations that should be taken into account when considering these data. Within this study, we have attributed the observed increase in LA stiffness when running with shoes to an increase in intrinsic foot muscle activation. It is likely that other muscles such as the tibialis posterior and the long digital flexors may have also contributed to the observed alteration in LA mechanics, as these muscles are also known to provide active support for this structure [53,54,69,70].
Our measure of ‘total stance activation’ was calculated by multiplying the average of the RMS signal envelope during stance, by the stance phase duration for each condition. This calculation was adopted to provide an indication of the cost of muscle activation per step, taking into account the differences in stance phase duration between conditions [42,43]. Participants ran with a reduced cadence (longer stride duration) when shod, and thus, it may be suggested that the observed increase in total stance phase activation when shod may be offset by fewer strides in a minute. However, within this study, this is not the case, as the difference in cadence between the two conditions is considerably smaller in magnitude than the difference in total stance activation. Based on the data presented in the manuscript, the average strides per minute for the barefoot condition is 92.3, whereas for the shod condition, it is 88.2. If the total strides in a minute are multiplied by the total stance activity for the muscle with the smallest difference (AH), the shod condition has approximately 46% more activation per minute than the barefoot condition (shod 555.1 total stance activity min−1 v barefoot 378.4 total stance activity min−1).
In our discussion of the foot and shoe interaction, we have made the assumption that constant leg stiffness is ideal during steady-state running. This assumption is based on a growing body of evidence that indicates the CNS will adjust knee and ankle stiffness in order to maintain constant COM trajectory [45,48,55,56]. Further research is now required to determine whether the foot behaves in series with the ankle and knee to govern overall limb stiffness during running, while also examining whether the observed changes in LA stiffness during running are primarily owing to an alteration in surface compliance.
8. Conclusion
In summary, we have described a novel mechanism for how human feet interact with modern running shoes. An in-series, spring-like arrangement of the foot and shoe dictates that the reduction in system stiffness that occurs when wearing a running shoe will need to be offset by an increase in the stiffness of the LA, in order to maintain constant foot–shoe system stiffness. The observed increase in LA stiffness in response to mechanosensory stimuli, is likely achieved via the observed increase in activation from the intrinsic muscles of the arch. These findings further highlight the highly adaptable nature of the human foot and its importance in upright bipedal locomotion.
Acknowledgements
The acknowledge Mr Zachary Goodchild for his contributions to data collection and processing.
Ethics
The study protocol was approved by the institutional human research ethics committee and conducted in accordance with the Declaration of Helsinki.
Authors' contributions
L.K. performed study design, data collection and analysis, manuscript preparation. A.G. and G.L. performed study design, data analysis and manuscript preparation. D.F. performed data analysis and manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
Funding for this study was provided by Asics Oceania Pty Ltd. Grant ID number 2014000885.
References
- 1.Bramble DM, Lieberman DE. 2004. Endurance running and the evolution of Homo. Nature 432, 345–352. ( 10.1038/nature03052) [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DE, Bramble DM. 2007. The evolution of marathon running. Sports Med. 37, 288–290. ( 10.2165/00007256-200737040-00004) [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DE. 2012. What we can learn about running from barefoot running: an evolutionary medical perspective. Exerc. Sport Sci. Rev. 40, 63–72. ( 10.1097/JES.0b013e31824ab210) [DOI] [PubMed] [Google Scholar]
- 4.Hardin EC, van Den Bogert AJ, Hamill J. 2004. Kinematic adaptations during running: effects of footwear, surface, and duration. Med. Sci. Sports Exerc. 838–844. ( 10.1249/01.MSS.0000126605.65966.40) [DOI] [PubMed] [Google Scholar]
- 5.Gruber AH, Silvernail JF, Brueggemann P, Rohr E, Hamill J. 2013. Footfall patterns during barefoot running on harder and softer surfaces. Footwear Sci. 5, 39–44. ( 10.1080/19424280.2012.742141) [DOI] [Google Scholar]
- 6.Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. 2002. A retrospective case–control analysis of 2002 running injuries. Br. J. Sports Med. 36, 95–101. ( 10.1136/bjsm.36.2.95) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang WJ, Crompton RH. 2004. Analysis of the human and ape foot during bipedal standing with implications for the evolution of the foot. J. Biomech. 37, 1831–1836. ( 10.1016/j.jbiomech.2004.02.036) [DOI] [PubMed] [Google Scholar]
- 8.Crompton RH, Sellers WI, Thorpe SKS. 2010. Arboreality, terrestriality and bipedalism. Phil. Trans. R. Soc. B 365, 3301–3314. ( 10.1098/rstb.2010.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM. 1987. The spring in the arch of the human foot. Nature 325, 147–149. ( 10.1038/325147a0) [DOI] [PubMed] [Google Scholar]
- 10.Kelly LA, Lichtwark G, Cresswell AG. 2014. Active regulation of longitudinal arch compression and recoil during walking and running. J. R. Soc. Interface 12, 20141076. ( 10.1098/rsif.2014.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander RM. 1984. Elastic energy stores in running vertebrates. Integr. Comp. Biol. 24, 85–94. ( 10.1093/icb/24.1.85) [DOI] [Google Scholar]
- 12.Alexander RM. 1991. Energy-saving mechanisms in walking and running. J. Exp. Biol. 160, 55–69. [DOI] [PubMed] [Google Scholar]
- 13.Stearne SM, McDonald KA, Alderson JA, North I, Oxnard CE, Rubenson J. 2015. The foot's arch and the energetics of human locomotion. Sci. Rep. 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdemir A, Hamel AJ, Fauth AR, Piazza SJ, Sharkey NA. 2004. Dynamic loading of the plantar aponeurosis in walking. J. Bone Joint Surg. Am. 86-A, 546–552. [DOI] [PubMed] [Google Scholar]
- 15.Hicks J. 1954. The mechanics of the foot: II. The plantar aponeurosis and the arch. J. Anat. 88, 25. [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly LA, Cresswell AG, Racinais S, Whiteley R, Lichtwark G. 2014. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 11, 20131188. ( 10.1098/rsif.2013.1188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuttruff J, DeHart S, O'Brien M. 1998. 7500 years of prehistoric footwear from Arnold research cave, Missouri. Science 281, 72–75. ( 10.1126/science.281.5373.72) [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh PR. 1980. The running shoe book. Mountain View, CA: Anderson World. [Google Scholar]
- 19.Nigg BM, Stefanyshyn D, Cole G, Stergiou P, Miller J. 2003. The effect of material characteristics of shoe soles on muscle activation and energy aspects during running. J. Biomech. 36, 569–575. ( 10.1016/S0021-9290(02)00428-1) [DOI] [PubMed] [Google Scholar]
- 20.Bishop M, Fiolkowski P, Conrad B, Brunt D, Horodyski M. 2006. Athletic footwear, leg stiffness, and running kinematics. J. Athl. Train. 41, 387–392. [PMC free article] [PubMed] [Google Scholar]
- 21.Butler RJ, Davis IS, Hamill J. 2006. Interaction of arch type and footwear on running mechanics. Am. J. Sports Med. 34, 1998–2005. ( 10.1177/0363546506290401) [DOI] [PubMed] [Google Scholar]
- 22.Nigg BM, Bahlsen HA, Luethi SM, Stokes S. 1987. The influence of running velocity and midsole hardness on external impact forces in heel–toe running. J. Biomech. 20, 951–959. ( 10.1016/0021-9290(87)90324-1) [DOI] [PubMed] [Google Scholar]
- 23.Baltich J, Maurer C, Nigg BM. 2015. Increased vertical impact forces and altered running mechanics with softer midsole shoes. PLoS ONE 10, e0125196 ( 10.1371/journal.pone.0125196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paquette MR, Zhang S, Baumgartner LD. 2013. Acute effects of barefoot, minimal shoes and running shoes on lower limb mechanics in rear and forefoot strike runners. Footwear Sci. 5, 9–18. ( 10.1080/19424280.2012.692724) [DOI] [Google Scholar]
- 25.Brückner K, Odenwald S, Schwanitz S, Heidenfelder J, Milani T. 2010. Polyurethane-foam midsoles in running shoes—impact energy and damping. Proc. Eng. 2, 2789–2793. ( 10.1016/j.proeng.2010.04.067) [DOI] [Google Scholar]
- 26.Van Middelkoop M, Kolkman J, Van Ochten J, Bierma-Zeinstra SMA, Koes B. 2007. Prevalence and incidence of lower extremity injuries in male marathon runners. Scand. J. Med. Sci. Sports 18, 140–144. ( 10.1111/j.1600-0838.2007.00683.x) [DOI] [PubMed] [Google Scholar]
- 27.van Gent RN, Siem D, Van Middelkoop M, van Os AG, Bierma-Zeinstra SMA, Koes BW, Taunton JE. 2007. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br. J. Sports Med. 41, 469–480. ( 10.1136/bjsm.2006.033548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman DE, Venkadesan M, Werbel WA, Daoud AI, D'Andrea S, Davis IS, Mang'Eni RO, Pitsiladis Y. 2010. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature 463, 531–535. ( 10.1038/nature08723) [DOI] [PubMed] [Google Scholar]
- 29.Robbins S. 2006. Running-related injury prevention through barefoot adaptations. Med. Sci. Sports 19, 148–156. [PubMed] [Google Scholar]
- 30.Collier R. 2011. The rise of barefoot running. Can. Med. Assoc. J. 183, E37–E38. ( 10.1503/cmaj.109-3745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis IS. 2014. The re-emergence of the minimal running shoe. J. Orthop. Sports Phys. Ther. 44, 775–784. ( 10.2519/jospt.2014.5521) [DOI] [PubMed] [Google Scholar]
- 32.Jungers W. 2010. Biomechanics: barefoot running strikes back. Nature 463, 433–434. ( 10.1038/463433a) [DOI] [PubMed] [Google Scholar]
- 33.Perl DP, Daoud AI, Lieberman DE. 2012. Effects of footwear and strike type on running economy. Med. Sci. Sports Exerc. 44, 1335–1343. ( 10.1249/MSS.0b013e318247989e) [DOI] [PubMed] [Google Scholar]
- 34.Miller EE, Whitcome KK, Lieberman DE, Norton HL, Dyer RE. 2014. The effect of minimal shoes on arch structure and intrinsic foot muscle strength. J. Sport Health Sci. 3, 74–85. ( 10.1016/j.jshs.2014.03.011) [DOI] [Google Scholar]
- 35.Thompson MA, Lee SS, Seegmiller J, McGowan CP. 2015. Kinematic and kinetic comparison of barefoot and shod running in mid/forefoot and rearfoot strike runners. Gait Posture 41, 957–959. ( 10.1016/j.gaitpost.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 36.Bonacci J, Saunders PU, Hicks A, Rantalainen T, Vicenzino BT, Spratford W. 2013. Running in a minimalist and lightweight shoe is not the same as running barefoot: a biomechanical study. Br. J. Sports Med. 47, 387–392. ( 10.1136/bjsports-2012-091837) [DOI] [PubMed] [Google Scholar]
- 37.Divert C, Mornieux G, Baur H, Mayer F, Belli A. 2005. Mechanical comparison of barefoot and shod running. Int. J. Sports Med. 26, 593–598. ( 10.1055/s-2004-821327) [DOI] [PubMed] [Google Scholar]
- 38.Hall JPL, Barton C, Jones PR, Morrissey D. 2013. The biomechanical differences between barefoot and shod distance running: a systematic review and preliminary meta-analysis. Sports Med. 43, 1335–1353. ( 10.1007/s40279-013-0084-3) [DOI] [PubMed] [Google Scholar]
- 39.Kelly LA, Kuitunen S, Racinais S, Cresswell AG. 2012. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. JCLB 27, 46–51. ( 10.1093/icb/24.1.85) [DOI] [PubMed] [Google Scholar]
- 40.Jung D-Y, Kim M-H, Koh E-K, Kwon O-Y, Cynn H-S, Lee W-H. 2011. A comparison in the muscle activity of the abductor hallucis and the medial longitudinal arch angle during toe curl and short foot exercises. Phys. Ther. Sport 12, 30–35. ( 10.1016/j.ptsp.2010.08.001) [DOI] [PubMed] [Google Scholar]
- 41.Leardini A, Benedetti MG, Berti L, Bettinelli D, Nativo R, Giannini S. 2007. Rear-foot, mid-foot and fore-foot motion during the stance phase of gait. Gait Posture 25, 453–462. ( 10.1016/j.gaitpost.2006.05.017) [DOI] [PubMed] [Google Scholar]
- 42.Brennan SF, Cresswell AG, Farris DJ, Lichtwark GA. 2016. The effect of cadence on the muscle–tendon mechanics of the gastrocnemius muscle during walking. Scand. J. Med. Sci. Sports. ( 10.1111/sms.12656) [DOI] [PubMed] [Google Scholar]
- 43.Carrier DR, Anders C, Schilling N. 2011. The musculoskeletal system of humans is not tuned to maximize the economy of locomotion. Proc. Natl Acad. Sci. USA 108,18 631–18 636. ( 10.1073/pnas.1105277108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMahon TA, Cheng GC. 2003. The mechanics of running: how does stiffness couple with speed? J. Biomech. 23(Suppl 1), 65–78. ( 10.1016/0021-9290(90)90042-2) [DOI] [PubMed] [Google Scholar]
- 45.Ferris DP, Louie M, Farley CT. 1998. Running in the real world: adjusting leg stiffness for different surfaces. Proc. R. Soc. Lond. B 265, 989–994. ( 10.1098/rspb.1998.0388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander RM, Bennet-Clark HC. 1977. Storage of elastic strain energy in muscle and other tissues. Nature 265, 114–117. ( 10.1038/265114a0) [DOI] [PubMed] [Google Scholar]
- 47.Daley MA, Biewener AA. 2006. Running over rough terrain reveals limb control for intrinsic stability. Proc. Natl Acad. Sci. USA 103,15 681–15 686. ( 10.1073/pnas.0601473103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferris DP, Farley CT. 1997. Interaction of leg stiffness and surfaces stiffness during human hopping. J. Appl. Physiol. 82, 15–22; discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 49.Ferris DP, Liang K, Farley CT. 1999. Runners adjust leg stiffness for their first step on a new running surface. J. Biomech. 32, 787–794. ( 10.1016/S0021-9290(99)00078-0) [DOI] [PubMed] [Google Scholar]
- 50.Hoffman SE, Peltz CD, Haladik JA, Divine G, Nurse MA, Bey MJ. 2015. Dynamic in vivo assessment of navicular drop while running in barefoot, minimalist, and motion control footwear conditions. Gait Posture 41, 825–829. ( 10.1016/j.gaitpost.2015.02.017) [DOI] [PubMed] [Google Scholar]
- 51.Peltz CD, Haladik JA, Hoffman SE, McDonald M, Ramo NL, Divine G, Nurse M, Bey MJ. 2014. Effects of footwear on three-dimensional tibiotalar and subtalar joint motion during running. J. Biomech. 47, 2647–2653. ( 10.1016/j.jbiomech.2014.05.016) [DOI] [PubMed] [Google Scholar]
- 52.Mann R, Inman VT. 1964. Phasic activity of intrinsic muscles of the foot. J. Bone Joint Surg. Am. 46, 469–481. [PubMed] [Google Scholar]
- 53.Semple R, Murley GS, Woodburn J, Turner DE. 2009. Tibialis posterior in health and disease: a review of structure and function with specific reference to electromyographic studies. J. Foot Ankle Res. 2, 24 ( 10.1186/1757-1146-2-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Péter A, Hegyi A, Stenroth L, Finni T, Cronin NJ. 2015. EMG and force production of the flexor hallucis longus muscle in isometric plantarflexion and the push-off phase of walking. J. Biomech. 48, 3413–3419. ( 10.1016/j.jbiomech.2015.05.033) [DOI] [PubMed] [Google Scholar]
- 55.Hobara H, Inoue K, Muraoka T, Omuro K, Sakamoto M, Kanosue K. 2010. Leg stiffness adjustment for a range of hopping frequencies in humans. J. Biomech. 43, 506–511. ( 10.1016/j.jbiomech.2009.09.040) [DOI] [PubMed] [Google Scholar]
- 56.Moritz CT, Farley CT. 2003. Human hopping on damped surfaces: strategies for adjusting leg mechanics. Proc. R. Soc. Lond. B 270, 1741–1746. ( 10.1098/rspb.2003.2435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawicki GS, Lewis CL, Ferris DP. 2009. It pays to have a spring in your step. Exerc. Sport Sci. Rev. 37, 130–138. ( 10.1097/JES.0b013e31819c2df6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins SH, Wiggin MB, Sawicki GS. 2015. Reducing the energy cost of human walking using an unpowered exoskeleton. Nature 522, 212–215. ( 10.1038/nature14288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferris DP. 2006. Neuromechanical adaptation to hopping with an elastic ankle–foot orthosis. J. Appl. Physiol. 100, 163–170. ( 10.1152/japplphysiol.00821.2005) [DOI] [PubMed] [Google Scholar]
- 60.Farley CT, Ferris DP. 1998. Biomechanics of walking and running: center of mass movements to muscle action. Exerc. Sport Sci. Rev. 26, 253–285. ( 10.1249/00003677-199800260-00012) [DOI] [PubMed] [Google Scholar]
- 61.Farley CT, Houdijk HH, Van Strien C, Louie M. 1998. Mechanism of leg stiffness adjustment for hopping on surfaces of different stiffnesses. J. Appl. Physiol. 85, 1044–1055. [DOI] [PubMed] [Google Scholar]
- 62.Cavanagh PR, Lafortune MA. 1980. Ground reaction forces in distance running. J. Biomech. 13, 397–406. ( 10.1016/0021-9290(80)90033-0) [DOI] [PubMed] [Google Scholar]
- 63.Hamill J, Russell EM, Gruber AH, Miller R. 2011. Impact characteristics in shod and barefoot running. Footwear Sci. 3, 33–40. ( 10.1080/19424280.2010.542187) [DOI] [Google Scholar]
- 64.Lafortune M, Hennig E. 1996. Dominant role of interface over knee angle for cushioning impact loading and regulating initial leg stiffness. J. Biomech. 29, 1523–1529. ( 10.1016/S0021-9290(96)80003-0) [DOI] [PubMed] [Google Scholar]
- 65.Milner CE, Hamill J, Davis I. 2007. Are knee mechanics during early stance related to tibial stress fracture in runners? Clin. Biomech. 22, 697–703. ( 10.1016/j.clinbiomech.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 66.Bonacci J, Vicenzino B, Spratford W, Collins P. 2013. Take your shoes off to reduce patellofemoral joint stress during running. Brit. J. Sports Med. 48, 425–428. ( 10.1136/bjsports-2013-092160) [DOI] [PubMed] [Google Scholar]
- 67.Daoud AI, Geissler GJ, Wang F, Saretsky J, Daoud YA, Lieberman DE. 2012. Foot strike and injury rates in endurance runners. Med. Sci. Sports Exerc. 44, 1325–1334. ( 10.1249/MSS.0b013e3182465115) [DOI] [PubMed] [Google Scholar]
- 68.Divert C, Baur H, Mornieux G. 2005. Stiffness adaptations in shod running. J. Appl. Biomech. 21, 311–321. [DOI] [PubMed] [Google Scholar]
- 69.Kirane YM, Michelson JD, Sharkey NA. 2008. Evidence of isometric function of the flexor hallucis longus muscle in normal gait. J. Biomech. 41, 1919–1928. ( 10.1016/j.jbiomech.2008.03.040) [DOI] [PubMed] [Google Scholar]
- 70.Gray EG, Basmajian JV. 1968. Electromyography and cinematography of leg and foot (‘normal’ and flat) during walking. Anat. Rec. 161, 1–15. ( 10.1002/ar.1091610101) [DOI] [PubMed] [Google Scholar]