Abstract
Background
Non-dipper hypertensive patients have a higher risk of cardiovascular disease (CVD) than dipper hypertensive patients. Inflammation plays an important role in the pathogenesis and progression of CVD. This study aimed to determine the relationship between the platelet-to-lymphocyte ratio (PLR), and dipper and non-dipper hypertension.
Materials and Methods
This prospective study included 199 consecutive patients that were diagnosed with primary hypertension. According to ambulatory blood pressure monitoring measurements, non-dipper and dipper group were determined. PLR was determined based on the platelet count and lymphocyte count in the complete blood count.
Results
The non-dipper group included 103 patients (74 females and 29 males; mean age: 52.37 ± 10.7 years) and the dipper group included 96 patients (65 females and 31 males; mean age: 48.40 ± 11.1 years). Mean systolic blood pressure was significantly higher in the non-dipper group than in the dipper group (124 ± 15.1 mmHg versus 120 ± 11.2 mmHg, p =0.032) and the median PLR was significantly higher in the non-dipper group than in the dipper group [132.15 (range: 69.64-400) versus 117.0 (range: 53.52-192.26), p = 0.001], whereas the mean white blood cell count (6.86 ± 1.43 × 10³/ μL versus 7.24 ± 1.26 × 10³/μL, p =0.046) and median lymphocyte count [2.09 (range: 0.95-3.92) × 10³/μL versus 2.24 (range: 0.97-3.98) × 10³/μL, p =0.001) were significantly lower in the non-dipper group.
Conclusion
Median PLR was significantly higher in the non-dipper hypertensive patients than in the dipper hypertensive patients. We think this finding further supports the role of an increase in inflammatory response in non-dipper hypertension. Hippokratia 2015; 19 (2):114-118.
Keywords: Platelet-to-lymphocyte ratio, PLR, non-dipper hypertension, inflammation
Introduction
Hypertension is a major cause of cardiovascular morbidity and mortality1. Although its etiopathogenesis remains unclear, primary hypertension is thought to be closely associated with inflammation2. Systolic and diastolic blood pressure are expected to drop >10% during the night, as compared to daytime, varying in accordance with circadian rhythm in normal and hypertensive individuals (dipper); in non-dipper hypertensive individuals systolic and diastolic blood pressure do not decrease3. The risk of cardiovascular morbidity and mortality is higher in non-dipper hypertensive individuals, independent of mean blood pressure4-5. This increased risk is suggested to be associated with the accelerated atherosclerotic process in non-dipper hypertensive individuals6.
Inflammation is thought to play an important role in the pathogenesis of atherosclerotic cardiovascular disease (CVD)7. Such markers as high-sensitivity C-reactive protein (CRP), cytokines, matrix metalloproteinase-9, myeloperoxidase, intercellular adhesion molecule 1 (ICAM-1), soluble cluster of differentiation 40 (CD40) ligand, etc. have been reported to be indicative of inflammatory status8. Indices derived from hemogram parameters have recently been defined as inflammatory markers, including the red cell distribution width (RDW), mean platelet volume (MPV), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR)9-13. PLR is the newest of these markers and was first studied in patients with malignancy14. Subsequent research suggests that higher PLR is related with worse outcome in patients with atherosclerosis-related diseases, such as coronary artery disease (CAD)12,15 heart valve diseases16 and peripheral arterial disease17. The present study aimed to determine the relationship between PLR (as a marker of inflammation), and dipper and non-dipper hypertension.
Materials and Methods
This prospective study was conducted in an internal medicine clinic between May and December 2013, and included 199 consecutive patients diagnosed with primary hypertension. Demographic characteristics were recorded. All patients were assessed for cardiovascular risk factors (including smoking status, body mass index, cholesterol levels, duration of hypertension, medications used) and systemic diseases. Diabetes mellitus was defined as a new diagnosis according to 2011 American Diabetes Association (ADA) diagnostic criteria or receiving anti-diabetic therapy. Patients with diabetes mellitus, CAD, acute or chronic renal disease, secondary hypertension, cerebrovascular disease, acute or chronic infection, fever, collagen tissue disease, malignancy, hematological disease, thrombocytopenia (platelet count <150×10³ μL-1), thrombocytosis (platelet count >450 ×10³ μL-1), or use of anti-platelet, anti-coagulant, or immunosuppressive agents were excluded from the study.
Renal failure was defined according to the glomerular filtration rate (GFR), which was calculated using the simplified version of the Modification of Diet in Renal Disease study prediction equation formula, GFR=186 x Creatinine-1.154 x Age-0.203 x 1.212 (if African-American) x 0.742 (if female)18. Patients diagnosed with chronic kidney disease were excluded according to the criteria defined by KDIGO (Kidney Disease Improving Global Outcomes) 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease19.
Infection and acute illness were determined based on the anamnesis and physical examination. Among the few patients with elevated CRP, in those in which clinical findings and symptoms were not consistent with acute illness, CRP was not interpreted in favor of infection or acute inflammation.
The 24-hour ambulatory blood pressure monitoring (ABPM) was performed using a WatchBP 03 device (Microlife WatchBP AG, Switzerland); the cuff was placed on the non-dominant arm. The devices were programmed to perform measurements every 15 min between 07:00 and 23:00 (daytime) and every 20 min between 23:00 and 07:00 (nighttime). The method was considered reliable if >70% of measurements were valid. Patients with a drop in the mean systolic (SBP) and diastolic blood pressure (DBP) >10% during the night were grouped as dipper hypertension and those without such a drop were grouped as non-dipper hypertension. Blood samples were obtained between 08:00 and 10:00 following overnight fasting. Blood biochemistry and complete blood count (CBC) were determined. PLR was determined based on the platelet count and lymphocyte count in the CBC. Twenty-four hour urine was collected to measure 24 hour-urine protein and creatinine excretion. The study protocol was approved by the Ankara Numune Education and Research Hospital Ethics Committee (decision number: 614/2013, 10-04-2013) and was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent in order to participate in the study.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 20.0 for Windows (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to determine if data were distributed normally. Numerical variables with normal distribution are shown as mean ± SD and those not normally distributed are shown as median (range). The independent samples t-test was used to analyze normally distributed parameters, whereas the Mann-Whitney U test was used to analyze parameters that were not normally distributed. The relationship between parameters was analyzed via Spearman’s correlation analysis. Multivariate logistic regression analysis was used to determine which factors were associated with non-dipper hypertension; variables that were significant based on univariate analysis (p <0.05) were included in multiple regression analysis. Receiver operating characteristic (ROC) curves were plotted to determine the optimal cutoff values for PLR for the prediction of non-dipper in hypertension and to establish optimal cutoff points for use in clinical practice. The true positive rate (sensitivity) and the false-positive rate (100-specificity) were plotted for each measurement, and the area under the curve (AUC) was determined. Cutoff values were determined based on the Youden index.
Results
The study included 199 consecutive patients diagnosed with primary hypertension. Mean age of the patients was 50.93 ± 10.9 years and the median duration of hypertension was 3 years (1-20 years). The dipper group included 96 patients and the non-dipper group included 103 patients. There were not any differences in the demographic and clinical characteristics, or anti-hypertensive therapy between the two groups. In all, 184 of the 199 patients were taking antihypertensive agents; 112 were taking one drug, 61 were taking two drugs, and 11 were taking three drugs. All patients that were taking one pill (one drug or a fixed-dose combination of two drugs) took their pills in the morning; those that were taking two pills took one pill in the morning and the other 12 hours later at night. There were no patients on more than two pills.
Mean 24-hour SBP was significantly higher in the non-dipper group than in the dipper group (124 ± 15.1 mmHg versus 120 ± 11.2 mmHg, p=0.032) Mean asleep SBP was significantly higher in the non-dipper group than in the dipper group (119.25 ± 10.1mmHg versus 113.28 ± 8.2 mmHg, p <0.001). Mean asleep DBP was significantly higher in the non-dipper group than in the dipper group (73.82 ± 6.56 mmHg versus 70.32 ± 7.2 mmHg, p <0.001) (Table 1). Among all patients, the mean creatinine levels were 0.8 ± 0.14 mg/dl; the mean white blood cell (WBC) count was 7.04 ± 1.36 × 10³/μL, the median lymphocyte count was 2.18 (0.95-3.98) ×10³/μL; the mean platelet count was 279.7 ± 57.4 ×10³/μL, and the median PLR was 124 (53.5-400) (Table 2). The median PLR was significantly higher in the non-dipper group than in the dipper group [132.15 (69.64-400) versus 117.0 (53.52-192.26), p =0.001]. The WBC count was significantly lower in the non-dipper group than in the dipper group (6.86 ± 1.43 × 10³/μL versus 7.24 ± 1.26 × 10³/μL, p =0.046). Similarly, the lymphocyte count was significantly lower in the non-dipper group than in the dipper group [2.09 (0.95-3.92) × 10³/μL versus 2.24 (0.97-3.98) × 10³/μL, p =0.001) (Table 2).
Table 1. Baseline demographic and clinical characteristics of the study population consisting of 199 hypertensive patients, of whom 103 non-dipper and 96 dipper.
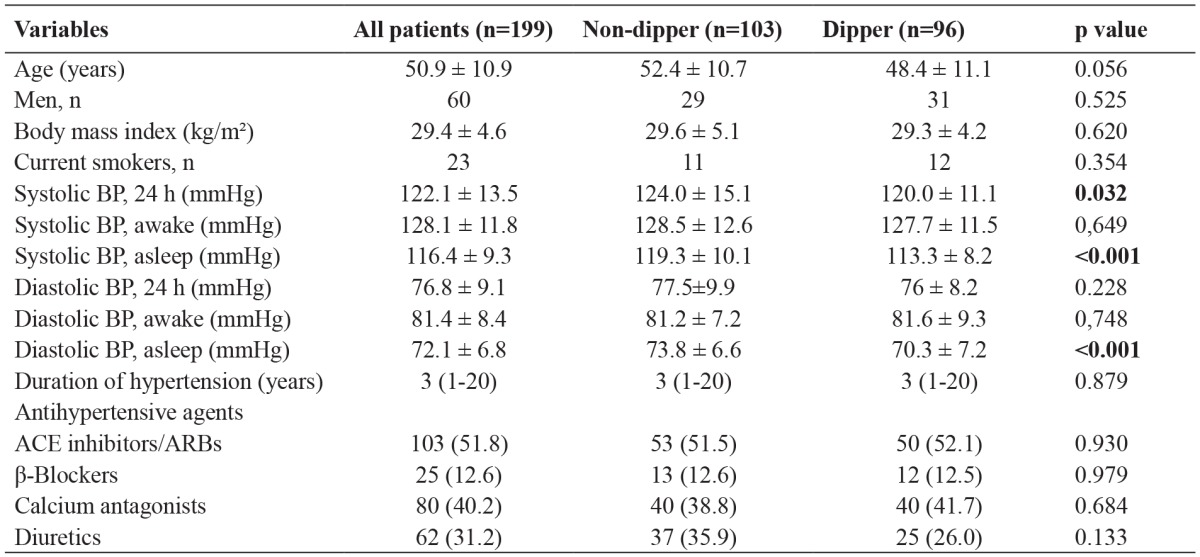
Data are presented as the number of patients or mean value ± standard deviation, p <0.05 is considered statistically significant for all tests, n: number, SBP: blood pressure, DBP: diastolic blood pressure, ACE: angiotensin converting enzyme, ARBs: angiotensin receptor blockers.
Table 2. Laboratory findings of the study population consisting of 199 patients, of whom 103 non-dipper and 96 dipper.
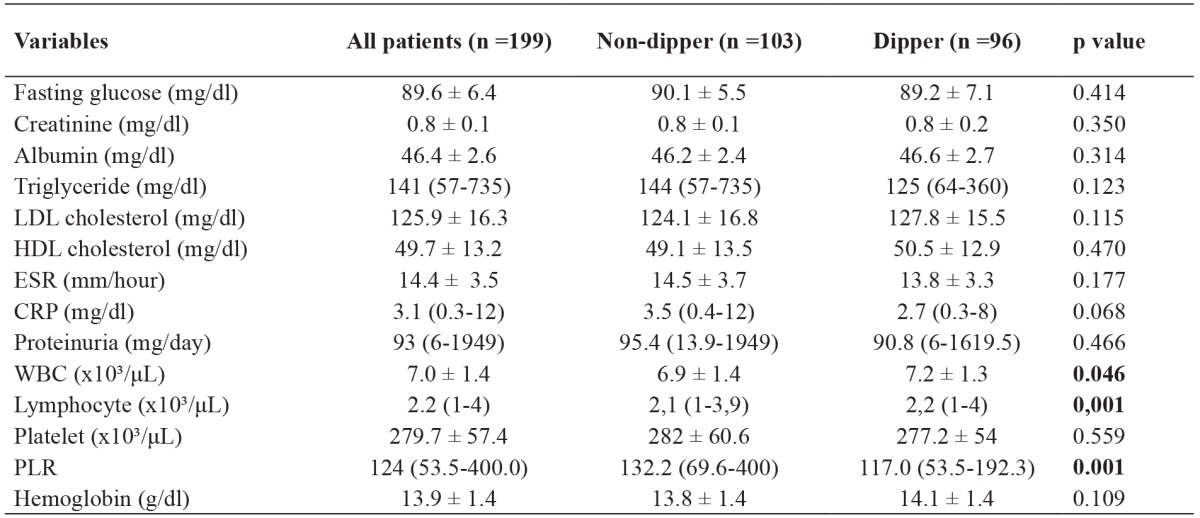
Data are presented as the number of patients or mean value ± standard deviation, p <0.05 is considered statistically significant for all tests, n: number, LDL: low-density lipoprotein, HDL: high-density lipoprotein, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, WBC: White blood cell, PLR: Platelet-to-lymphocyte ratio, SD: standard deviation.
Age, gender, body mass index, current smokers, antihypertensive agents, duration of hypertension, SBP, DBP, fasting glucose, creatinine, GFR, albumin, uric acid, triglyceride, low-density lipoprotein (LDL), high-density lipoprotein (HDL), erythrocyte sedimentation rate (ESR), CRP, proteinuria, WBC, PLR, hemoglobin levels were included in a stepwise regression model. According to the results from multivariable regression analysis, SBP (OR =1.027, p =0.024) and PLR (OR =1.014, p =0.001) were identified to be independent predictors for non-dipper in hypertension patients (Table 3).
Table 3. Significant predictors of non-dipper pattern in hypertensive patients.
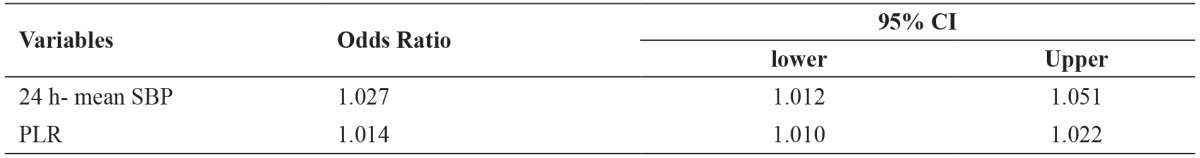
p <0.05 is considered significant for statistical analyses, SBP: systolic blood pressure, PLR: platelet-to-lymphocyte ratio, CI: confidence intervals.
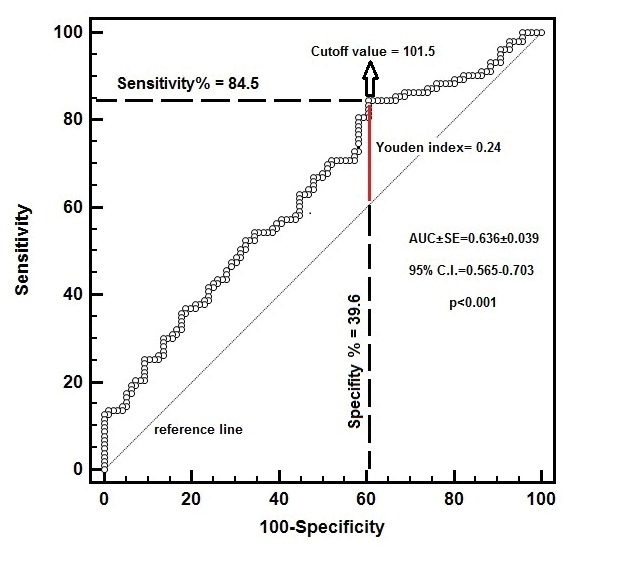
A PLR of 101.5 or higher predicted non-dipper status with 84.5% sensitivity and 39.6% specificity [AUC ± SE (standard error) = 0.636 ± 0.039, 95% CI =0.565-0.703, p <0.001] (Figure 1).
Figure 1. Receiver operating characteristic (ROC) curve for platelet-to-lymphocyte ratio (PLR) to predict non-dipper status.
Discussion
Non-dipper hypertension is considered an independent risk factor for all-cause mortality1,5. Signs of renal damage, including microalbuminuria and rapid GFR loss20 as well as such cardiovascular signs as left ventricle hypertrophy and heart failure are reported to be more common in non-dipper hypertensive individuals than dippers21-22. Its etiology is not clearly known, but non-dipper hypertension is more common in cases with secondary causes of hypertension such as endocrine system diseases23 sleep apnea syndrome24 and chronic renal failure25. There is evidence indicating that endothelial repair is adversely affected and the inflammatory process is more severe in non-dipper hypertensive individuals than dippers26.
Inflammation plays a key role in most chronic diseases, particularly CVD, diabetes mellitus, connective tissue diseases, cancer, and chronic kidney disease7,27-31. Neutrophils, known as the primary cells that release inflammatory cytokines, closely interact with other blood cells - including platelets - during inflammation32. Increased platelet activity has been reported to correlate with an increase in the severity of inflammation33. Increases in the platelet count, MPV, and RDW are indicators of increased platelet activity34. Research has shown that a higher PLR in the absence of absolute thrombocytosis is associated with increased thrombosis and inflammation, which might be associated with an increase in platelet activity13,15,17. Additionally, it was posited that relative lymphopenia in the presence of a high PLR might be indicative of the effect of an elevated endogenous cortisol level due to inflammatory response35. Among a group of patients with obstructive peripheral artery disease, critical vascular stenosis and wounds due to vascular insufficiency were more common in those with a high PLR17. A study that examined the relationship between PLR and inflammation in cardiovascular diseases reported that a pre-procedural PLR >150, in patients diagnosed with ST segment elevation myocardial infarction that underwent primary percutaneous coronary stent placement, was predictive of no-reflow, with sensitivity of 75% and specificity of 74%15. A similar study that included patients diagnosed with non-ST segment elevation myocardial infarction reported a significantly higher mortality rate in the patients with a PLR >176 and a relative decrease in the mortality rate in response to double antiplatelet therapy36.
In the present study, the PLR (used as a marker of inflammation) was significantly higher in the non-dipper hypertension group than in the dipper hypertension group. Moreover, PLR was found to be an independent predictive factor. A PLR of 101.5 or higher predicted non-dipper status with 84.5 % sensitivity and 39.6 % specificity. The fact that both of the present study’s patient groups were similar in terms of smoking status, body mass index, cholesterol levels, duration of hypertension, medications used, and lack of other known chronic diseases strengthens the validity of the findings. Furthermore, the present findings are similar to those of an earlier relevant study that reported the PLR was significantly higher in non-dipper hypertensive patients, a PLR ≥107 was predictive of non-dipper hypertension (with sensitivity of 66.3 % and specificity of 68.7 %), and that NLR and hs-CRP levels were higher in non-dipper hypertension group37. Although specificity of PLR cut-off value was low our study, the values were similar37. Limitations of the present study include lack of evaluation of the relationship between markers of inflammation other than the PLR and signs of target organ damage, and the lack of follow-up to identify the effect of a high PLR level on the prognosis of hypertension. In conclusion, the present findings suggest that the PLR can be used in daily practice as a marker of inflammation because it is easy to calculate using hemogram parameters and is a cost-effective index. The present study also shows that a high PLR might be indicative of high atherosclerotic risk in hypertensive patients and a predictive value can be determined in the future.
Conflict of Interest Statement
The authors report no conflicts of interest, financial or otherwise.
References
- 1.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 2.Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant. 2006;21:850–853. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- 3.White WB. Ambulatory blood-pressure monitoring in clinical practice. N Engl J Med. 2003;348:2377–2378. doi: 10.1056/NEJMp030057. [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 5.Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. doi: 10.1097/HJH.0b013e32833b49fe. [DOI] [PubMed] [Google Scholar]
- 6.von Kanel R, Jain S, Mills PJ, Nelesen RA, Adler KA, Hong S, et al. Relation of nocturnal blood pressure dipping to cellular adhesion, inflammation and hemostasis. J Hypertens. 2004;22:2087–2093. doi: 10.1097/00004872-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 9.Okyay GU, Inal S, Onec K, Er RE, Pasaoglu O, Pasaoglu H, et al. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35:29–36. doi: 10.3109/0886022X.2012.734429. [DOI] [PubMed] [Google Scholar]
- 10.Ordu S, Ozhan H, Caglar O, Alemdar R, Basar C, Yazici M, et al. Mean platelet volume in patients with dipper and non-dipper hypertension. Blood Press. 2010;19:26–30. doi: 10.3109/08037050903416402. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan F, Turak O, Durak A, Isleyen A, Ucar F, Ginis Z, et al. Red cell distribution width and inflammation in patients with non-dipper hypertension. Blood Press. 2013;22:80–85. doi: 10.3109/08037051.2012.707336. [DOI] [PubMed] [Google Scholar]
- 12.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Acar G, Kalkan ME, Avci A, Alizade E, Tabakci MM, Toprak C, et al. The relation of platelet-lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Clin Appl Thromb Hemost. 2015;21:462–468. doi: 10.1177/1076029613508599. [DOI] [PubMed] [Google Scholar]
- 14.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Yildiz A, Yuksel M, Oylumlu M, Polat N, Akyuz A, Acet H, et al. The Utility of the Platelet-Lymphocyte Ratio for Predicting No Reflow in Patients With ST-Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost. 2015;21:223–228. doi: 10.1177/1076029613519851. [DOI] [PubMed] [Google Scholar]
- 16.Gursoy OM, Karakoyun S, Kalcik M, Gokdeniz T, Yesin M, Gunduz S, et al. Usefulness of novel hematologic inflammatory parameters to predict prosthetic mitral valve thrombosis. Am J Cardiol. 2014;113:860–864. doi: 10.1016/j.amjcard.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013;8:e67688. doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Summary of Recommendation Statements. Kidney Int Suppl (2011) 2013;3:5–14. doi: 10.1038/kisup.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta R, Drawz PE. Is nocturnal blood pressure reduction the secret to reducing the rate of progression of hypertensive chronic kidney disease? Curr Hypertens Rep. 2011;13:378–385. doi: 10.1007/s11906-011-0217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens Suppl. 2003;21:S17–S23. doi: 10.1097/00004872-200307006-00004. [DOI] [PubMed] [Google Scholar]
- 22.Seo HS, Kang TS, Park S, Choi EY, Ko YG, Choi D, et al. Non-dippers are associated with adverse cardiac remodeling and dysfunction (R1) Int J Cardiol. 2006;112:171–177. doi: 10.1016/j.ijcard.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Zelinka T, Strauch B, Pecen L, Widimsky J., Jr Diurnal blood pressure variation in pheochromocytoma, primary aldosteronism and Cushing’s syndrome. J Hum Hypertens. 2004;18:107–111. doi: 10.1038/sj.jhh.1001644. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki N, Ozono R, Yamauchi R, Teramen K, Munemori M, Hamada H, et al. Age-related differences in the mechanism of nondipping among patients with obstructive sleep apnea syndrome. Clin Exp Hypertens. 2012;34:270–277. doi: 10.3109/10641963.2012.681083. [DOI] [PubMed] [Google Scholar]
- 25.Elung-Jensen T, Strandgaard S, Kamper AL. Longitudinal observations on circadian blood pressure variation in chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2008;23:2873–2878. doi: 10.1093/ndt/gfn126. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Kim NH, Kim YK, Yoo JH, Shin SN, Ko JS, et al. The Number of Endothelial Progenitor Cells is Decreased in Patients With Non-Dipper Hypertension. Korean Circ J. 2012;42:329–334. doi: 10.4070/kcj.2012.42.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329–337. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 28.Garcia C, Feve B, Ferre P, Halimi S, Baizri H, Bordier L, et al. Diabetes and inflammation: fundamental aspects and clinical implications. Diabetes Metab. 2010;36:327–338. doi: 10.1016/j.diabet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 30.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462–477. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 33.Projahn D, Koenen RR. Platelets: key players in vascular inflammation. J Leukoc Biol. 2012;92:1167–1175. doi: 10.1189/jlb.0312151. [DOI] [PubMed] [Google Scholar]
- 34.Kaito K, Otsubo H, Usui N, Yoshida M, Tanno J, Kurihara E, et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol. 2005;128:698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 35.Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17:506–514. doi: 10.1016/0090-1229(80)90146-4. [DOI] [PubMed] [Google Scholar]
- 36.Azab B, Shah N, Akerman M, McGinn JT., Jr Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012;34:326–334. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 37.Sunbul M, Gerin F, Durmus E, Kivrak T, Sari I, Tigen K, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2014;36:217–221. doi: 10.3109/10641963.2013.804547. [DOI] [PubMed] [Google Scholar]


