Abstract
Aim
Renal diseases in diabetes mellitus (DM) patients, include diabetic nephropathies (DN) and non-diabetic renal diseases (NDRD). The clinical differentiation among them is usually not so clear and effective. Aim of this study which examined renal biopsies in patients with type-2 DM was to identify the prevalence and the nature of NDRD.
Materials and Methods
We recorded the clinical and laboratory finding alongside with the histopathological examination of the renal biopsies obtained from 71 type-2 DM patients who underwent renal biopsy in our center. Based on the renal biopsy findings patients were classified into two groups (DN and NDRD) and data was compared between the two groups.
Results
There were 42 women and 29 men; aged 55 ± 12 years. In patients with DN (n: 34), diabetic retinopathy was more common [16 (47.1 %) vs. 6 (16.2 %) respectively, p =0.01], duration of DM was longer (108.8 ± 58.8 months vs 57.8 ± 55.9 months respectively, p <0.001) and the degree of proteinuria was more severe (6 ± 4.3 g/day vs. 4.5 ± 4.6 g/day respectively, p =0.04) compared to the patients with NDRD. Regression analysis revealed that diabetes duration >60 months, presence of diabetic retinopathy and proteinuria >3.5 g/day were independent predictors of DN with 79.4 % sensitivity and 86.5% specificity. Focal segmental glomerulosclerosis was the most frequent diagnosis in patients with NDRD.
Conclusions
The prevalence of NDRD is remarkably frequent in DM patients in whom nephrologists consider renal biopsy an appropriate measure. Short duration of DM, degree of proteinuria and absence of retinopathy were predictors of NDRD. Hippokratia 2015; 19 (2):148-152.
Keywords: Diabetes mellitus, non-diabetic renal disease, renal biopsy
Introduction
Diabetes mellitus (DM) has increasing prevalence all over the world, while the increased life span of diabetic patients leads to an increase in prevalence of diabetic nephropathy (DN). Among all the patients suffering from DM, approximately 20-40 % will eventually develop diabetic renal disease1.
Apart from DN, non-diabetic renal diseases (NDRD) are also common disorders in diabetic population and these require different treatment and follow-up regimes rather than DN. With current knowledge, it is not clear whether NDRD is a co-incidental event on the basis of DN or a clinical condition which results from immuno-physiological abnormalities of glomerular basement membrane. It is believed that glomerulosclerosis is a predisposing factor and the existing glomerular changes facilitate the subepithelial immune reactions2. In patients with over 10-year history of type-1 DM, NDRD is a rare clinical condition with a rate of 2-3 %3. Thus, the decision to perform diagnostic biopsy should be considered very carefully. In patients with type-2 DM, there might be varied time interval between the onset of the disease and the time of the diagnosis; hence the exact age of the diabetes’s onset is generally not known. Clinical findings such as proteinuria could be attributed either to a different renal pathology been superimposed on DN or be the manifestation of NDRD itself. Many clinical features have been considered as predicting factors for NDRD: diabetic neuropathy or retinopathy not associated with nephropathy4, hematuria5, short duration of diabetes6, deterioration of renal functions more rapidly than expected7, and the presence of acanthocyturia8 but none of them is 100 % sensitive or specific. Differential diagnosis between the various NDRD is important due to differences in treatment and in clinical outcome regarding renal function and patients’ survival.
Aim of the present study was to evaluate the results of renal biopsies, performed on patients with type-2 DM for clinical suspicion of NDRD and to correlate the histopathological findings with the clinical presentation and laboratory parameters.
Material and Methods
After obtaining permission from the Ethical Board of Ankara Numune Education and Research Hospital, located in Ankara, Turkey, we included in this study 71 patients with type-2 DM who were submitted to renal biopsy for clinical suspicion of NDRD from January 2010 to December 2011. Data regarding age, gender, duration of diabetes, presence or absence of diabetic retinopathy and arterial hypertension were recorded for all patients. We determined as indications for renal biopsy the following: i) unexplained rapid deterioration of renal function [decrease in glomerular filtration rate (GFR) more than 1 ml/min/1.73m2/month], ii) proteinuria not accompanied by retinopathy, and iii) unexplained hematuria (2 or more red blood cells per high-power field in centrifuged urine sample) after elimination of possible obstructive pathology of the lower urogenital tract, and in the absence of infection, renal stones, tumor and trauma. Presence of acute/chronic infection, acute/chronic liver disease, and active malignancy at the time of renal biopsy (with exception of skin malignancies), functional proteinuria and secondary DM consisted our exclusion criteria.
Fasting blood glucose, serum urea, creatinine, sodium, potassium levels, glycosylated haemoglobin (HbA1c), urine examination, protein and creatinine levels in spot urine, serum complement C3 and C4, anti-nuclear antibody (ANA), anti-ds DNA, and p/c anti-neutrophil cytoplasmic antibody (ANCA) levels were evaluated for all patients included in the study. Blood and urine samples were analyzed on the same day. The first urine sampled in the morning was examined. Glomerular filtration rate was estimated for each participant using the equation of the 4-variable modification of diet in renal disease (MDRD) study9.
Nephrotic range proteinuria was considered as 3.5 gr/day or higher. Renal failure was defined as serum creatinine above 1.3 mg/dl. Hypoalbuminemia was defined as serum albumin value below 35 g/L. Diabetic retinopathy was defined as presence of proliferative findings and/or background retinopathy (microaneurysm, hemorrhage, soft-hard exudate) in fundus examination performed by an ophthalmologist.
Histopathological examinations were reviewed by the same pathologists. For light microscopic examination, all the specimens were processed with periodic acid schiff, hematoxylin-eosin, methenamine silver and trichrome. Immunofluorescence staining was performed for detection of antibodies to IgG, IgA, IgM, C1q and C3. Diabetic nephropathy was defined as findings of mesangial expansion, diffuse intercapillary glomerulosclerosis and/or nodular Kimmelstiel-Wilson formation, basement membrane thickening, presence of fibrin cap, or capsular drops. Determination of specific renal histopathological findings was accepted as NDRD. As electron microscope was not routinely used, no electron microscope related records existed for every patient.
Based on the renal biopsy findings, patients were divided into two groups: DN and NDRD group. Demographic features and laboratory findings were compared between the groups.
Statistical Methods
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS Inc, Chicago, Ill, USA). Student’s t-test and chi-square test were used to compare the two groups. Multiple logistic regression analysis was performed for the investigation of factors related to DN or NDRD. Variables with p values smaller than 0.20 in univariate analysis are retained to multiple logistic regression analysis. Statistical significance was set at p value of <0.05 (2-tailed).
Results
Patient general characteristics and laboratory findings are shown in Table 1. Among all the patients, 29 (40.8 %) were male whose mean age (± standard deviation) was 54 ± 12 years, and mean duration of DM was 82 ± 62 months. Indications for renal biopsy were absence of retinopathy in 31 (43.7 %) patients, rapid deterioration in 26 (36.6 %), and hematuria in the remaining 14 (19.7 %).
Table 1. Demographic features and biochemical parameters of the 71 patients with type-2 diabetes mellitus, included in the study, that were divided into two groups based on the renal biopsy findings: diabetic nephropathy (DN) and nondiabetic renal disease (NDRD) groups.
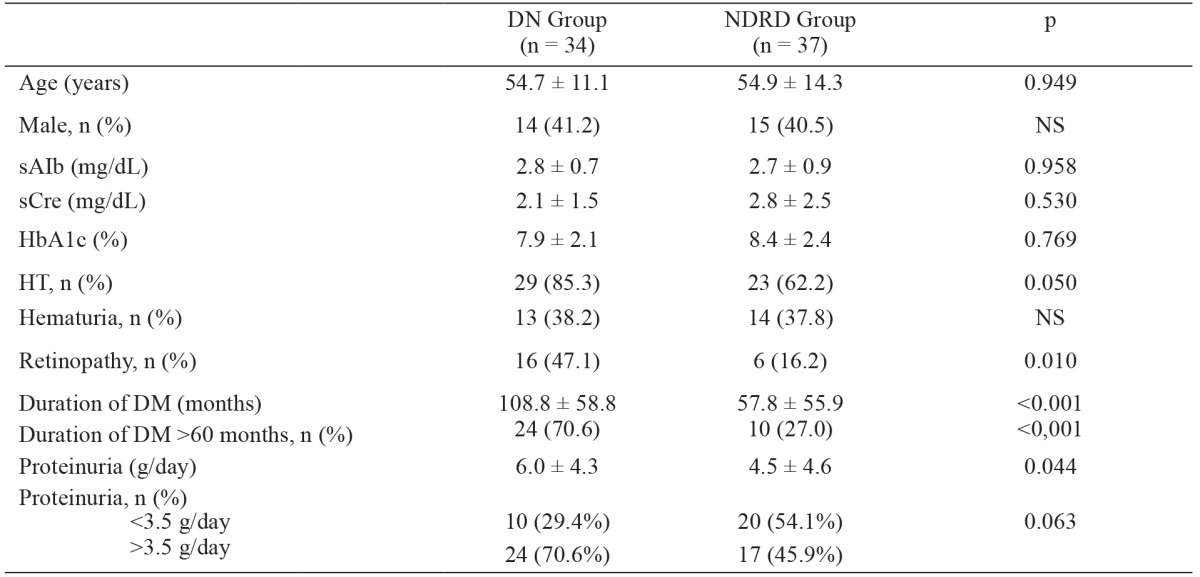
Values are expressed as means ± standard deviation or number (n) and percentage (%). DM: diabetes mellitus, sAlb: serum albumin, sCre: serum creatinine, HbA1c: hemoglobin A1c, HT: hypertension, NDRD: non-diabetic renal disease, DN: diabetic nephropathy.
Based on histopathological examination of renal biopsy specimens, 34 (47.9 %) patients had DN and 37 (52.1 %) had NDRD. Mean age of the patients, and serum creatinine, serum albumin and HbA1C levels were similar in the DN and NDRD groups. Diabetic retinopathy was noted in 22 (31 %) of the 71 patients included in the study. The prevalence of diabetic retinopathy was higher in the DN group than in the NDRD group [n: 16 (47.1 %) vs. n: 6 (16.2 %); p =0.01].
In the DN group proteinuria was significantly more severe (6.0 ± 4.3 g/day vs. 4.5 ± 4.6 g/day; p =0.04) and the duration of DM was significantly longer (108.8 ± 58.8 months vs. 57.8 ± 55.9 months; p <0.001) than in the NDRD group. The prevalence of nephrotic-range proteinuria was higher in the DN group than in the NDRD group, but this difference was not significant [n: 24 (70.6 %) vs. n: 17 (45.9 %); p =0.06]. There wasn’t significant difference in terms of hematuria between the DN and NDRD groups [n: 9 (26.5 %) vs. n: 5 (13.5 %); p =0.29). Multiple logistic regression analysis showed that the risk factors for DN included duration of DM >60 months (p =0.001), diabetic retinopathy (p =0.07), and proteinuria >3.5 g/day (p =0.06), with sensitivity of 79.4 % and specificity of 86.5 % (Table 2).
Table 2. Logistic regression analysis for diabetic nephropathy as dependant variable.
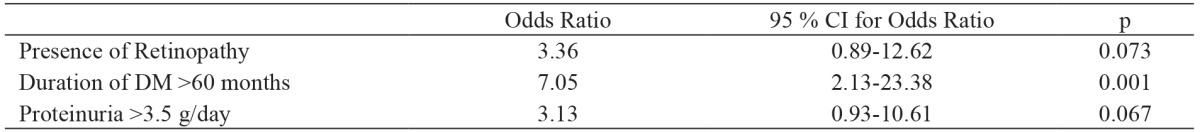
DM: Diabetes mellitus
The distribution of pathological findings in the NDRD is shown in Table 3. Grouping the pathologic findings in the NDRD group as glomerular and non-glomerular diseases showed that 24 (65 %) patients had glomerular and 13 (35 %) had non-glomerular diseases. The prevalence of diabetic retinopathy was 4.2 % and 38.5 % in the glomerular and non-glomerular disease subgroups of the NDRD group, respectively (p =0.007); however, there wasn’t a significant difference in the severity of proteinuria between these two subgroups (5.08 ± 4.84 g/day and 3.36 ± 3.91 g/day, respectively).
Table 3. Histopathologic findings of the renal biopsies of the 37 patients included in the non-diabetic renal disease (NDRD) group.
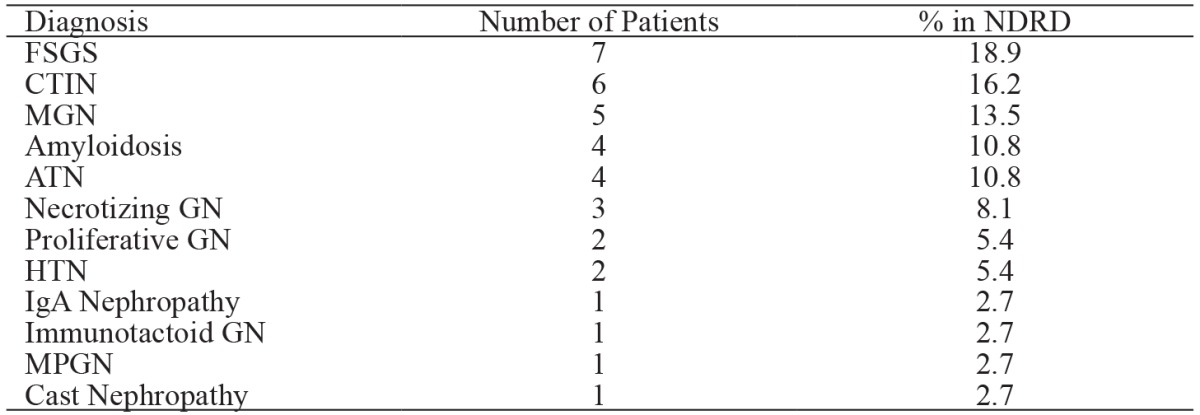
FSGS: focal segmental glomerulosclerosis, CTIN: chronic tubulointerstitial nephritis, MGN: membranous glomerulonephritis, ATN: acute tubular necrosis, GN: glomerulonephritis; HTN: hypertensive nephropathy, IgA: immunoglobulin A, MPGN: membranoproliferative glomerulonephritis, NDRD: non-diabetic renal disease.
Discussion
Worldwide, DN is the leading cause of end-stage renal disease (ESRD), with a reported frequency of 10-15 % in type-2 DM patients. In addition to DNs in diabetic patients, NDRDs are also important entities that require specific management approaches. In retrospective series, the prevalence of NDRDs in DM patients varies between 7 and 44 % in accordance with patient selection criteria4,10,11. In different studies, the prevalence of NDRDs was reported as 22 % in Caucasians, 26.7 % in Asians12, 3 % in Denmark13, and 12 % in Italy14; however, there is lack of data for the Turkish population. The present findings show that the prevalence of NDRDs in type-2 DM patients was 52.1 %, based on histopathological examination, which is in accordance with earlier studies that reported prevalence rates of 45-57 %15-18, but is higher than other earlier reports, reporting prevalence of 10-30 %5,19. We think the differences in prevalence rates are due to differences in study methodologies and biopsy selection criteria.
It is clear that the development of DN and diabetic retinopathy are closely associated with each other20. In cases of DN, diabetic retinopathy has been noted in 90-95 % of patients with type-1 DM and in 40-75 % of those with type-2 DM21,22. In the present study more than 50 % of the patients with DN did not have retinopathy and NDRD was the most common biopsy finding in patients without retinopathy. A meta-analysis that included 154 patients reported that 48.7 % of type-2 DM patients without retinopathy had NDRDs12. The prevalence of NDRDs in patients with retinopathy in two other studies was 27.2 % and 13.6 %, respectively23,24. Based on these earlier findings and those of the present study, the absence of retinopathy seems to be a predictor in terms of the underlying renal disease being related to NDRD.
The general consensus is that the duration of DM is an important predictor for development of DN and the increase in disease duration increases the risk of nephropathy25. In diabetic patients with short duration of disease and persistent proteinuria NDRD should be strongly suspected26 and kidney biopsy should be performed in those who developed proteinuria within five years of DM onset27. In the present study the duration of DM was shorter in the NDRD group. In addition, there was a significant correlation between DN and duration of DM >60 months, indicating that patients with short duration of DM should be examined in accordance with the suspicion of NDRD.
In accordance to previous studies5,18,28, in the present study there weren’t any significant differences in age, GFR, or the serum albumin level between the DN and NDRD groups. Also in the present study urinary protein excretion rates were higher in the DN group compared to the NDRD group, as reported earlier5,29. Moreover, most of the patients (70.6 %) in the DN group had nephrotic-range proteinuria, which might have been associated with the duration of DM.
An increase in glomerular basement membrane thickness leads to hematuria in 33 % of patients with typical diabetic glomerulosclerosis4,17. Nevertheless, many researchers consider hematuria as atypical in patients with DN. Although numerous studies have shown that microscopic hematuria is more common in DM patients with NDRDs and have suggested that there is a significant correlation between hematuria and NDRDs5,29, presence of hematuria has low sensitivity and specificity. In the present study the frequency of hematuria was similar in the DN and NDRD groups, as previously reported18,20,24.
In clinical practice a rapid increase in the serum creatinine concentration or a decrease in GFR >1 mL/min/month is associated with the development of NDRDs30,31; however, in the present study, 29.4 % of the patients who underwent renal biopsy due to unexpectedly increasing serum creatinine values had the diagnosis of DN. This high incidence was attributable to the fact that a great many of our patients were not aware of diabetes and not being followed-up for end-organ damage.
In patients with type-2 DM, primary glomerulonephritis is the most common NDRD and all types of glomerulonephritis may be seen32. In the present study 64.8 % of patients in the NDRD group had glomerular disease, of which focal segmental glomerulosclerosis (FSGS) was most common, which is accordance with earlier reports23,28. The effect of NDRDs on renal outcome in DM patients is not known. Although it was reported that renal outcome was not affected by NDRDs33, Wong et al24 followed-up DM patients for a mean of 123 months and reported that renal outcome was worse in patients with DNs, as compared to those with NDRDs. Lesions such as FSGS can be successfully treated with steroids and immunosuppressive agents, which is why in such patients it is clear that early renal biopsy findings can result in treatment that positively affects renal prognosis; however, the literature is devoid of any large-scale studies on the effect of NDRD on long-term renal outcome in DM patients.
Conclusion
The present findings show that NDRDs are a very common clinical condition in type-2 DM patients. Duration of DM, severity of proteinuria, and absence of retinopathy might be predictive of renal involvement in DM patients. The differential diagnosis of DNs and NDRDs is of considerable importance because their management approaches and renal prognoses are unique.
Conflict of Interest
Authors declare no conflicts of interest.
References
- 1.Kikkawa R, Koya D, Haneda M. Progression of diabetic nephropathy. Am J Kidney Dis. 2003;41:S19–S21. doi: 10.1053/ajkd.2003.50077. [DOI] [PubMed] [Google Scholar]
- 2.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322–328. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 3.Olsen S. Identification of non-diabetic glomerular disease in renal biopsies from diabetics--a dilemma. Nephrol Dial Transplant. 1999;14:1846–1849. doi: 10.1093/ndt/14.8.1846. [DOI] [PubMed] [Google Scholar]
- 4.Amoah E, Glickman JL, Malchoff CD, Sturgill BC, Kaiser DL, Bolton WK. Clinical identification of nondiabetic renal disease in diabetic patients with type I and type II disease presenting with renal dysfunction. Am J Nephrol. 1988;8:204–211. doi: 10.1159/000167584. [DOI] [PubMed] [Google Scholar]
- 5.Mak SK, Gwi E, Chan KW, Wong PN, Lo KY, Lee KF, et al. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12:2588–2591. doi: 10.1093/ndt/12.12.2588. [DOI] [PubMed] [Google Scholar]
- 6.Kveder R, Kajtna-Koselj M, Rott T, Bren AF. Nephrotic syndrome in patients with diabetes mellitus is not always associated with diabetic nephropathy. Nephrol Dial Transplant. 2001;16:86–87. doi: 10.1093/ndt/16.suppl_6.86. [DOI] [PubMed] [Google Scholar]
- 7.Serra A, Romero R, Bayes B, Lopez D, Bonet J. Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract. 2002;58:149–153. doi: 10.1016/s0168-8227(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 8.Heine GH, Sester U, Girndt M, Kohler H. Acanthocytes in the urine: useful tool to differentiate diabetic nephropathy from glomerulonephritis? Diabetes Care. 2004;27:190–194. doi: 10.2337/diacare.27.1.190. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 10.Richards NT, Greaves I, Lee SJ, Howie AJ, Adu D, Michael J. Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant. 1992;7:397–399. [PubMed] [Google Scholar]
- 11.John GT, Date A, Korula A, Jeyaseelan L, Shastry JC, Jacob CK. Nondiabetic renal disease in noninsulin-dependent diabetics in a south Indian Hospital. Nephron. 1994;67:441–443. doi: 10.1159/000188019. [DOI] [PubMed] [Google Scholar]
- 12.Zukowska-Szczechowska E, Tomaszewski M. Renal affection in patients with diabetes mellitus is not always caused by diabetic nephropathy. Rocz Akad Med Bialymst. 2004;49:185–189. [PubMed] [Google Scholar]
- 13.Olsen S, Mogensen CE. How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia. 1996;39:1638–1645. doi: 10.1007/s001250050628. [DOI] [PubMed] [Google Scholar]
- 14.Brocco E, Fioretto P, Mauer M, Saller A, Carraro A, Frigato F, et al. Renal structure and function in non-insulin dependent diabetic patients with microalbuminuria. Kidney Int Suppl. 1997;63:S40–S44. [PubMed] [Google Scholar]
- 15.Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3:1458–1466. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 16.Waldherr R, Ilkenhans C, Ritz E. How frequent is glomerulonephritis in diabetes mellitus type II? Clin Nephrol. 1992;37:271–273. [PubMed] [Google Scholar]
- 17.Tone A, Shikata K, Matsuda M, Usui H, Okada S, Ogawa D, et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69:237–242. doi: 10.1016/j.diabres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Ghani AA, Al Waheeb S, Al Sahow A, Hussain N. Renal biopsy in patients with type 2 diabetes mellitus: indications and nature of the lesions. Ann Saudi Med. 2009;29:450–453. doi: 10.4103/0256-4947.57167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719–1731. doi: 10.1046/j.1523-1755.2000.00333.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanauchi M, Kawano T, Uyama H, Shiiki H, Dohi K. Discordance between retinopathy and nephropathy in type 2 diabetes. Nephron. 1998;80:171–174. doi: 10.1159/000045162. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz A, Vaeth M. Microalbuminuria: a major risk factor in non-insulin-dependent diabetes. A 10-year follow-up study of 503 patients. Diabet Med. 1988;5:126–134. doi: 10.1111/j.1464-5491.1988.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 22.Marshall SM, Alberti KG. Comparison of the prevalence and associated features of abnormal albumin excretion in insulin-dependent and non-insulin-dependent diabetes. Q J Med. 1989;70:61–71. [PubMed] [Google Scholar]
- 23.Soni SS, Gowrishankar S, Kishan AG, Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–537. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 24.Wong TY, Choi PC, Szeto CC, To KF, Tang NL, Chan AW, et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25:900–905. doi: 10.2337/diacare.25.5.900. [DOI] [PubMed] [Google Scholar]
- 25.Rogowicz A, Litwinowicz M, Piłaciński S, Zozulińska D, Wierusz-Wysocka B. Does early insulin treatment decrease the risk of microangiopathy in non-obese adults with diabetes? Arch Med Sci. 2007;3:129–135. [Google Scholar]
- 26.Powers AC. Diabetes Mellitus. Harrison’s Principles of Internal Medicine, 16th Edition, Kasper DL, Fauci AS, Braunwald E, Hauser SL, Longo DL, Jameson JL (eds). McGraw Hill, New York. 2005:2152–2179. [Google Scholar]
- 27.Lee EY, Chung CH, Choi SO. Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J. 1999;40:321–326. doi: 10.3349/ymj.1999.40.4.321. [DOI] [PubMed] [Google Scholar]
- 28.Matias P, Viana H, Carvalho F, Santos JR. Diabetes mellitus and renal disease: when to perform a renal biopsy? Port J Nephrol Hypert. 2009;23:167–173. [Google Scholar]
- 29.Zhou J, Chen X, Xie Y, Li J, Yamanaka N, Tong X. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23:1940–1945. doi: 10.1093/ndt/gfm897. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen CE. Renal function changes in diabetes. Diabetes. 1976;25:872–879. [PubMed] [Google Scholar]
- 31.Viberti GC, Bilous RW, Mackintosh D, Keen H. Monitoring glomerular function in diabetic nephropathy. A prospective study. Am J Med. 1983;74:256–264. doi: 10.1016/0002-9343(83)90624-1. [DOI] [PubMed] [Google Scholar]
- 32.Mou S, Wang Q, Liu J, Che X, Zhang M, Cao L, et al. Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;87:354–359. doi: 10.1016/j.diabres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Chihara J, Takebayashi S, Taguchi T, Yokoyama K, Harada T, Naito S. Glomerulonephritis in diabetic patients and its effect on the prognosis. Nephron. 1986;43:45–49. doi: 10.1159/000183717. [DOI] [PubMed] [Google Scholar]


